Abstract
Discarded feathers represent an important residue from the poultry industry and are a rich source of keratin. Bacillus subtilis LFB-FIOCRUZ 1266, previously isolated from industrial poultry wastes, was used in this work and, through random mutation using ethyl methanesulfonate, ten strains were selected based on the size of their degradation halos. The feather degradation was increased to 115% and all selected mutants showed 1.4- to 2.4-fold increase in keratinolytic activity compared to their wild-type counterparts. The protein concentrations in the culture supernatants increased approximately 2.5 times, as a result of feather degradation. The mutants produced more sulfide than the wild-type bacteria that produced 0.45 µg/ml, while mutant D8 produced 1.45 µg/ml. The best pH for enzyme production and feather degradation was pH 8. Zymography showed differences in the intensity and molecular mass of some bands. The peptidase activity of the enzyme blend was predominantly inhibited by PMSF and EDTA, suggesting the presence of serine peptidases. HPTLC analysis evidenced few differences in band intensities of the amino acid profiles produced by the mutant peptidase activities. The mutants showed an increase in keratinolytic and peptidase activities, demonstrating their biotechnological potential to recycle feather and help to reduce the environmental impact.
Keywords: Keratin, Bacillus, Biodegradation, Peptidase, Mutagenesis
Introduction
Keratin is an insoluble and hard-to-degrade protein with a stable structure that is a major constituent of skin, hair, feathers, wool and nails of various animals, and represents an important form of protection for those animals (Lange et al. 2016). Keratin contains considerable amounts of amino acids containing sulfur-forming disulfide bridges which confer the mechanical stability and resistance to common proteolytic enzymes such as pepsin, trypsin and papain. In addition to the high degree of cross-linkages formed by disulfide and hydrogen bonds, the resistance of keratins is associated with its tight packaging as chains in α-helix (α-keratin) or β-sheet (β-keratin) structures (Mazotto et al. 2017). Thus, keratinous materials can remain inert in the environment for a long time, as keratinase producing microorganisms are not widespread (Lange et al. 2016). However, despite their rigid structure, keratins are recycled in nature and can be efficiently degraded by a few microorganisms such as bacteria from the genus Bacillus (Fellahi et al. 2016), Streptomyces (Ramakrishnan et al. 2011) from several species of fungi (El-Gendy 2010; Duarte et al. 2011; Bohacz 2017) and from Archaea (Kublanov et al. 2009; Brandelli et al. 2010) that are capable of producing keratinolytic peptidases known as keratinases.
Keratinases [EC 3.4.21/24/99.11] are mostly serine or metalloproteases able to catalyze the hydrolysis of highly stable keratin proteins. When compared with other peptidases, keratinases have high nucleotide homologies and protein similarities with peptidases belonging to the subtilisin group (Gupta and Ramnani 2006). The mechanism of keratinolysis is complex and poorly understood. Currently it is accepted that the keratinolytic process involves two steps, sulfitolysis and proteolysis (Prakash et al. 2010; Lange et al. 2016). The first step is the breakdown of disulfide bonds that partially denature the keratin and expose sites for keratinase action. This sulfitolysis occurs through the activity of sulfide reductases or by secretion of reducing agents like sulfide (Navone and Speight 2018). Even in presence of reducing agents, common proteases like pepsin and papain are not capable of degrading keratin. On the other hand, some purified keratinases maintain their keratinolytic activity in the absence of a reducing agent (Kim et al. 2004; Sanghvi et al. 2016).
Keratinases from microorganisms have attracted attention due to their extensive uses in a variety of industrial applications such as in the feed, fertilizer, detergent and leather industries, as well as for pharmaceutical and biomedical applications (Brandelli et al. 2010; Gupta et al. 2013). Prion degradation by bacterial keratinases has also been reported (Yoshioka et al. 2007; Okoroma et al. 2013). However, the initial proposition for microbial keratinases was in the bioprocessing of the increasing keratinous wastes generated by the poultry industry (Daroit et al. 2009; Syed et al. 2009).
These keratin-rich wastes contain a high amount of protein and approximately 90% of the total weight of feathers is keratin, and this could be a relatively inexpensive dietary supplement for animal feedstuffs after being processed into a protein hydrolysate (Onifade et al. 1998; Mokrejs et al. 2011). Today feathers are turned into feather meal through steam pressure cooking, which requires a high-energy input, resulting in the destruction of certain essential amino acids such as methionine, lysine, histidine and tryptophan, and in the formation of non-nutritive amino acids, such as lysinoalanine and lanthionine, yielding a product with poor digestibility and variable nutrient quality (Wang and Parsons 1997). On the other hand, keratin-degrading microorganisms could be used as an important and ecofriendly biotechnique to recycle feathers into a rich hydrolysate (Mabrouk 2008; Vasileva-Tonkova et al. 2009).
There are some reports in the literature about using chemical (N-methyl-N′-nitro-N-nitrosoguanidine ethyl methane sulfonate) and physical (UV) mutagenesis to improve enzymatic activity (Ribeiro et al. 2013). The activities of endoglucanase I (EGI), β-glucosidase and carboxymethylcellulase (CMCase) have been improved two- to threefold by mutagenesis using ethyl methanesulfonate (EMS) (Ribeiro et al. 2013).
Keratinolytic activity has already been described in Bacillus licheniformis PWD1 (Manczinger et al. 2003) and Bacillus subtilis (Mazotto et al. 2011). Applying these enzymes to process industrial feather wastes offers advantages tothisindustry and to the environment. Improving the production of these enzymes with bacteria could improve the efficiency and reduce costs for the use of feather waste residues in the production of animal feed or cosmetics (Manczinger et al. 2003; Mazotto et al. 2011). Additionally, B. subtilis has been reported as a probiotic alone or in association with other bacteria, improving the immunologic response of rabbits and Artemia franciscana (Guo et al. 2017; Giarma et al. 2017).
The aim of the present work was to increase keratinase production by chemical mutagenesis. This study suggests that improved B. subtilis LFB-FIOCRUZ 1266 has potential to produce a bioaccessible protein from feathers with probiotic activity that can be used as a valuable ingredient in animal feed.
Materials and methods
Chemicals
Media constituents were obtained from Oxoid Ltd. (Hampshire, England). Reagents used in electrophoresis were purchased from Amersham Life Science (Little Chalfont, England). Gelatin and casein were purchased from Merck (Darmstadt, Germany) and bovine serum albumin from Sigma Chemical Co. (St. Louis, MO, USA). All other reagents were of analytical grade.
Feather keratin substrate
Chicken feathers obtained from poultry waste were extensively washed with water and detergent and dried at 60 °C overnight. Afterwards, the feathers were delipidated with chloroform:methanol (1:1 v/v) and dried again at 60 °C. The keratin substrate was obtained according to Wawrzkiewicz et al. (1987). Briefly, 10 g of feathers was heated in a reflux condenser at 100 °C for 80–120 min with 500 ml of DMSO. Keratin was then precipitated by the addition of two volumes of cold acetone and maintained at 4 °C for 24–48 h. The keratin precipitates were collected by centrifugation at 2000g for 15 min, washed twice with distilled water and dried at 4 °C. The powder obtained was ground to homogeneity in a mortar. This keratin was used in agar keratin medium and for keratinase analysis.
Microorganism and culture media
Bacillus subtilis LFB-FIOCRUZ 1266 was isolated from the feather residues that came from the poultry industry as described by Mazotto et al. (2010). The microorganism selected for this study was deposited at the Culture Collection of Bacillus at the Fundação Oswaldo Cruz (Rio de Janeiro, RJ, Brazil) and registered under number LFB-FIOCRUZ1266. B. subtilis strains were grown in yeast extract medium (0.5% yeast extract, 0.5% peptone, 2% KCl, 2% sucrose, pH 7.0), Luria-Bertani medium (1% peptone, 0.5% yeast extract, 1% NaCl pH 7.2) and whole feather medium (1% whole feathers, 0.1% yeast extract in 0.1 M phosphate buffer, pH 8.0). For the solid medium 1.5% agar was added.
Mutagenesis and screening
The B. subtilis strain LFB-FIOCRUZ 1266 was grown in 5 ml of yeast extract medium at 37 °C for 24 h; after that, 1% of ethyl methanesulfonate (EMS) was added and incubated at 37 °C for 2 h (Lindegren et al. 1965). Cells were then centrifuged at 2800g for 15 min, washed twice with sterile saline solution and the pellet was resuspended in 5 ml of yeast extract medium and incubated at 37 °C for 1 h. Successive serial dilutions were prepared up to 10−5, and 0.1 ml of the bacterial dilutions was spread on the Luria-Bertani agar medium. After 24 h incubation at 37 °C, mutant colonies of the B. subtilis strain LFB-FIOCRUZ 1266 were first streaked on gelatin agar (2% gelatin, 0.1% yeast extract, 2% agar) and replica plates of the colonies on yeast extract medium of the screened colonies were kept refrigerated at 4 °C. The gelatinolytic positive mutants were streaked in keratin agar media (2% keratin, 0.01% yeast extract, 2% agar) for 5 days at 37 °C to verify the keratinolytic activities. Plates were stained with Coomassie brilliant blue R-250 [0.2% Coomassie brilliant blue R-250 (for protein substrates)] in methanol: acetic acid: water (5:1:4). Clear halos indicated enzyme production and were selected for quantitative and qualitative keratinolytic assays.
Enzymatic extract
The B. subtilis strain LFB-FIOCRUZ 1266 and its mutants were grown on yeast extract medium for 48 h at 37 °C under 300 rpm agitation to obtain biomass. The cells were harvested by centrifugation at 2000g for 20 min at 4 °C, washed twice with saline solution (0.85% NaCl) and inoculated in whole feather medium. This medium was inoculated with 108 UFC/ml and incubated under the same conditions. After incubation, the media were centrifuged at 2000g for 20 min at 4 °C and the supernatant solutions were used as enzymatic extracts for keratinase and gelatinase assays. For zymographic analysis, the enzymatic extracts were concentrated 20-fold by dialysis (cut off 9 kDa) using PEG 4.000 overnight at 4 °C.
Protein determination
Protein concentration was determined as previously described by Lowry et al. (1951), using bovine serum albumin (BSA) as standard. Absorbance was measured at 660 nm.
Feather degradation
The percentage of feather degradation was calculated from the differences in residual feather dry weight between a control (feather without bacterial inoculation) and after microorganism growth at 37 °C for 24 h. Whole feather medium was composed of 1% whole feathers and 0.1% yeast extract in 0.1 M phosphate buffer, pH 8.0.
Keratinase and gelatinase activity
Keratinase activity was determined with feather keratin substrate (see “Feather keratin substrate” section above) by the method according to Grzywnowicz et al. (1989). The enzymatic reaction was composed of 0.5 mg of keratin, 750 µl of phosphate buffer, pH 7.4 and 500 µl enzymatic extract. The reaction mixture was incubated at 37 °C for 1 h, after which the reaction was stopped by adding 250 µl of 10% trichloroacetic acid (TCA). Samples were kept refrigerated at 4 °C for 30 min. Then, the solution was centrifuged at 2.500g for 15 min. The supernatant was collected, and the absorbance was measured at 280 nm. The control was obtained by adding TCA to the reaction mixture before incubation. One unit of keratinolytic activity was considered to be an increase of 0.01 in the absorbance unit at 280 nm, under standard assay conditions (1 h at 37 °C).
Gelatinase activity was measured according to the method of Jones et al. (1998) with modifications. Briefly, 100 µl of the enzyme extract and 900 µl of phosphate buffer, pH 7.4, were added to 1.5 ml of the substrate solution (1% gelatin in distilled water) and incubated at 37 °C for 30 min. After incubation, an aliquot of 375 µl of the reaction mixture was added to 500 µl of isopropanol. For the control, an aliquot of 375 µl of reaction mixture was transferred to 500 µl of isopropanol before the incubation.
All samples were centrifuged at 2.500g for 15 min. The supernatant was collected, and the soluble protein present in the supernatant was measured as described by Lowry et al. (1951). One unit of gelatinolytic activity was defined as the amount of enzyme required to produce an increase of 0.01 absorbance unit at 660 nm, under standard assay conditions (30 min at 37 °C).
In order to evaluate the enzymatic activity at different pH values, the following buffers were used: citric acid buffer (0.05 M citric acid and 0.2M Na2HPO4, pH 5.2–6.0), phosphate buffer (0.06 M Na2HPO4.7H2O and 0.04 M KH2PO4, pH 8.0) or glycine buffer (0.2 M glycine, 0.2 M NaOH, pH 10.0–10.8).
Sulfide concentration
The sulfide produced by B. subtilis strain LFB-FIOCRUZ 1266 was evaluated as previously described (Arikawa et al. 1971). Briefly, 50 µl of ρ-rosaniline solution (0.04% ρ-rosaniline in 6% HCl), 50 µl of 0.2% formaldehyde solution and 350 µl of distilled water were added to 50 µl of the culture supernatant. The solution was incubated at room temperature for 10 min and the absorbance was measured at 562 nm. Sodium sulfide (0.1 mg/ml) was used as standard.
High-performance thin-layer chromatography (HPTLC)
An aliquot of culture supernatants (15 µl) was applied on high-performance thin-layer chromatography plates (MERCK) for amino acid detection. The solvent for the HPTLC plates was n-butanol:acetone:acetic acid:distilled water (5:5:3:1 v/v/v/v) and the amino acid-specific ninhydrin reagent (7.5% in n-butanol:acetone 1:1 v/v) was used (Brenner and Niederwieser 1965; Kanaki and Rajani 2005). Commercial amino acids were used as standard.
Zymography
The concentrated culture supernatants were mixed with the sample buffer for zymography (125 mM Tris, pH 6.8, 4% SDS, 20% glycerol and 0.002% bromophenol blue) in a sample:buffer ratio of 6:4 (Nogueira De Melo et al. 2007). Keratinases and gelatinases were assayed and characterized by 10% SDS–PAGE with co-polymerized keratin feather powder or gelatin (0.1%). Additionally, other substrates such as casein, BSA or hemoglobin (0.1%) were incorporated in the gel for substrate specificity studies. Gels were loaded with 30 µg of protein from the concentrated supernatants per slot. After electrophoresis at 120 V for 2 h at 4 °C, the gels were washed twice for 15 min at room temperature with 2.5% Triton X-100 and then were incubated for 48 h at 37 °C in citric acid proteolysis buffer at pH 5.0. Then the gels were stained for 1 h with 0.2% Coomassie brilliant blue R-250 in methanol:acetic acid:water (5:1:4 v/v/v) and destained in the same solvent. For enzymatic classification, 3 mM phenylmethylsulphonyl fluoride (PMSF), 0.26 mM ethylenediamine tetra acetic acid (EDTA), 10 mM 1.10 phenanthroline (Phenan), 10 µM pepstatin A (Peps) and 5 µM trans-epoxysuccinyl l-leucylamido-(4-guanidino) butane (E-64) were used in the proteolysis buffer.
Results
Selected mutants
A culture of B. subitilis LFB-FIOCRUZ 1266 was mutagenized with 1% EMS. The mutants were selected based on the increased secretion of extracellular gelatinolytic and keratinolytic enzymes. This selection was carried out in agar plates with gelatin and keratin incorporated. After the tests, 150 mutants with gelatinolytic activity were found, but only ten mutants (degradation halo ≥ 6 mm of diameter) were selected for this study.
Enzymatic activity and feather degradation of selected mutants
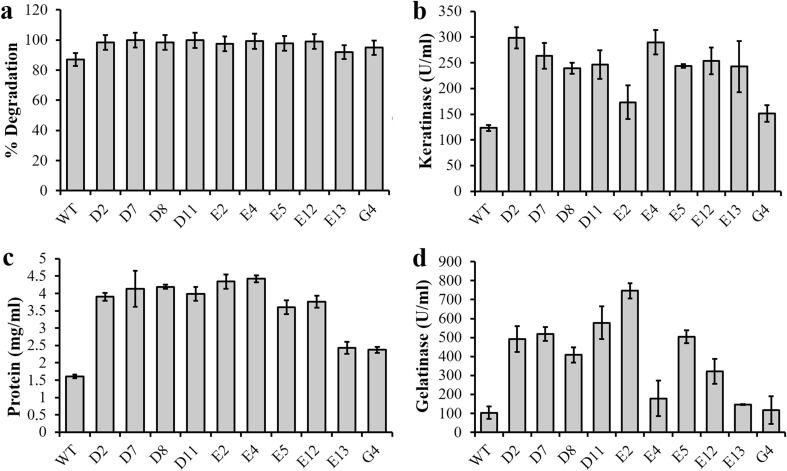
Ten strains with high gelatinolytic and keratinolytic activity were selected to analyze feather degradation and enzyme production in feather medium. The feather degradation increased 115%, reaching 99.9% of feather degradation using strain D7 when compared with the wild type that presented 87% (Fig. 1a). Corroborating this result, all mutants showed keratinolytic activity higher than the wild type (Fig. 1b). While B. subtilis LFB-FIOCRUZ 1266 presented a keratinolytic activity of 127 U/ml, all the related mutants presented a superior activity, ranging from 152 to 298 U/ml for mutant E2 and D2, respectively. As expected, the protein concentration in the culture supernatants of all mutants presented the same profiles as the feather degradation profiles, with an approximate 2.5 times increase (Fig. 1c), when compared with the wild type.
Fig. 1.
Comparative studies of feather degradation in percentage (a), keratinolytic activity (b), protein concentration (c) and gelatino lytic activity (d) of the selected mutant strains from Bacillus subtilis LFB-FIOCRUZ 1266 and the wild type (WT) cultured in feather medium at pH 8.0 and 28 °C
Gelatin substrate was used to verify the total peptidase action. While the wild type presented gelatinolytic activity of 106 U/ml, all selected mutants presented superior activity, from 180.76 U/ml for the mutant E4 to 738.33 U/ml for the mutant E2.
Sulfide concentration
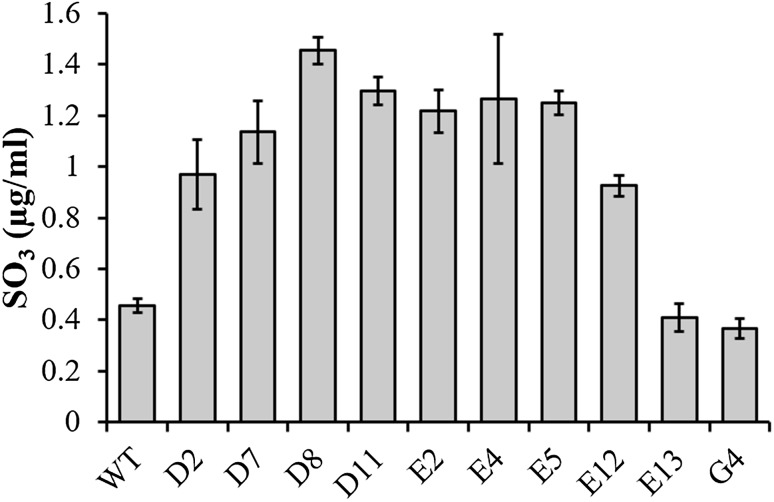
The mutagenesis also altered the sulfide secretion in the culture medium. The sulfide concentration detected in the enzymatic extract of the wild type was 0.45 µg/ml, while the mutant D8 presented a higher concentration of 1.45 µg/ml. All mutants, except mutants E13 and G4, showed significantly higher concentrations of sulfide when compared to the wild type (Fig. 2).
Fig. 2.
Sulfide concentrations in the culture supernatants of B. subtilis LFB-FIOCRUZ 1266 mutants and wild-type strains grown in feather medium
Effect of temperature and pH for enzyme expression/activity
The effect of temperature and pH on feather degradation, gelatinolytic activity and sulfide production was evaluated. The results were analyzed by ANOVA, using the significance limit of 95%. The results and analysis showed that the best feather degradation results were obtained at pH 8.0 independent of the temperature (Table 1). The pH is the variable that has the greatest effect on feather degradation. However, variations of temperature affected the gelatinolytic activity among the mutants and wild-type bacterium. A temperature of 28 °C was the best for the production of gelatinolytic peptidases. The mutant E12 presented similar dosages for temperatures of 28 and 35 °C at pH 8.0 and was the strain that best with stood temperature changes (Table 2). The highest sulfide concentrations were detected at 28 °C with pH 8.0, except for the mutant D8 (Table 3). As observed for the degradation of feathers, the pH was the variable that most affected the sulfide production, suggesting a correlation between sulfide concentration in the medium and keratin degradation.
Table 1.
Effect of different temperatures and pH on feather degradation by B. subtilis LFB-FIOCRUZ 1266 mutants and wild type strains
| T (°C) | pH | WT | D2 | D7 | D8 | D11 | E2 | E4 | E5 | E12 | E13 | G4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 28 | 8.0 | 96.3 | 91.4 | 94.1 | 98.0 | 99.5 | 98.4 | 100 | 100 | 98.2 | 73.4 | 72.6 |
| 30 | 6.0 | 7.6 | 7.0 | 8.2 | 9.1 | 9.4 | 6.9 | 8.1 | 8.0 | 6.8 | 7.0 | 4.4 |
| 30 | 10.0 | 20.6 | 23.7 | 20.4 | 20.3 | 19.3 | 19.9 | 21.2 | 20.5 | 16.5 | 18.3 | 18.8 |
| 35 | 5.2 | 1.8 | 0.2 | 2.0 | 1.8 | 2.0 | 0.7 | 3.3 | 4.4 | 0.9 | 6.1 | 3.5 |
| 35 | 8.0 | 85.7 | 96.1 | 95.1 | 99.2 | 98.9 | 98.0 | 98.1 | 98.2 | 97.1 | 93.9 | 95.5 |
| 35 | 10.8 | 51.4 | 51.1 | 50.3 | 51.0 | 35.8 | 48.1 | 42.9 | 48.0 | 38.7 | 44.4 | 69.2 |
| 40 | 6.0 | 6.6 | 9.1 | 8.8 | 7.5 | 3.1 | 7.0 | 9.4 | 11.4 | 8.4 | 6.7 | 9.6 |
| 40 | 10.0 | 24.5 | 25.7 | 23.6 | 24.4 | 28.1 | 24.1 | 24.5 | 30.0 | 54.5 | 27.0 | 23.6 |
| 42 | 8.0 | 89.5 | 89.9 | 94.8 | 38.0 | 93.4 | 97.3 | 98.8 | 98.2 | 96.9 | 70.5 | 88.0 |
Table 2.
Effect of different temperatures and pH on gelatinolytic activity of B. subtilis LFB-FIOCRUZ 1266 mutants and wild-type strains
| T (°C) | pH | WT | D2 | D7 | D8 | D11 | E2 | E4 | E5 | E12 | E13 | G4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 28 | 8.0 | 880.7 | 948.9 | 659.1 | 630.7 | 926.1 | 875.0 | 1210.3 | 1068.2 | 755.7 | 522.7 | 358.0 |
| 30 | 6.0 | 39.8 | 233.0 | 125.0 | 181.8 | 34.1 | 62.5 | 261.4 | 227.3 | 278.4 | 178.0 | 200.8 |
| 30 | 10.0 | 284.1 | 153.4 | 403.4 | 409.1 | 272.7 | 352.3 | 329.6 | 210.2 | 153.4 | 102.3 | 306.8 |
| 35 | 5.2 | 352.3 | 340.9 | 242.4 | 284.1 | 272.7 | 619.3 | 45.5 | 89.0 | 187.5 | 147.7 | 193.2 |
| 35 | 8.0 | 123.1 | 535.4 | 372.5 | 352.3 | 520.8 | 433.7 | 524.6 | 275.3 | 780.3 | 153.4 | 356.1 |
| 35 | 10.8 | 352.3 | 227.3 | 312.5 | 85.2 | 335.2 | 335.2 | 380.7 | 431.8 | 181.8 | 354.2 | 272.7 |
| 40 | 6.0 | 176.1 | 340.9 | 193.2 | 431.8 | 409.1 | 375.0 | 403.4 | 380.7 | 250.0 | 113.6 | 121.2 |
| 40 | 10.0 | 176.1 | 142.1 | 136.4 | 128.8 | 204.6 | 244.3 | 204.6 | 66.3 | 56.8 | 108.0 | 157.2 |
| 42 | 8.0 | 386.4 | 409.1 | 136.4 | 301.1 | 448.9 | 448.9 | 511.4 | 352.3 | 267.1 | 253.8 | 210.2 |
Table 3.
Effect of different temperatures and pH on sulfide (µg/ml) excreted by the strains in the culture
| T (°C) | pH | WT | D2 | D7 | D8 | D11 | E2 | E4 | E5 | E12 | E13 | G4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 28 | 8.0 | 10.9 | 19.8 | 19.3 | 2.5 | 20.4 | 18.4 | 19.1 | 20.5 | 21.4 | 5.8 | 9.0 |
| 30 | 6.0 | 10.8 | 9.1 | 10.8 | 10.5 | 10.1 | 9.6 | 10.9 | 9.4 | 9.3 | 9.0 | 8.7 |
| 30 | 10.0 | 5.0 | 4.8 | 5.5 | 4.7 | 6.2 | 4.4 | 4.1 | 4.2 | 3.6 | 3.5 | 3.6 |
| 35 | 5.2 | 7.1 | 8.7 | 9.0 | 6.0 | 6.4 | 8.8 | 10.5 | 8.3 | 10.1 | 6.1 | 11.4 |
| 35 | 8.0 | 7.9 | 16.7 | 9.0 | 12.6 | 19.4 | 12.8 | 13.4 | 23.0 | 14.5 | 10.7 | 8.2 |
| 35 | 10.8 | 10.5 | 8.8 | 7.4 | 9.3 | 7.1 | 7.2 | 7.8 | 6.5 | 8.0 | 7.6 | 9.8 |
| 40 | 6.0 | 2.2 | 2.0 | 2.3 | 11.4 | 2.5 | 2.5 | 2.7 | 0.7 | 3.2 | 1.2 | |
| 40 | 10.0 | 4.5 | 4.3 | 4.5 | 4.1 | 4.8 | 5.0 | 5.5 | 4.5 | 5.9 | 4.8 | 4.0 |
| 42 | 8.0 | 9.0 | 19.2 | 17.2 | 23.9 | 18.2 | 13.1 | 19.8 | 14.2 | 15.4 | 5.2 | 4.7 |
Zymography
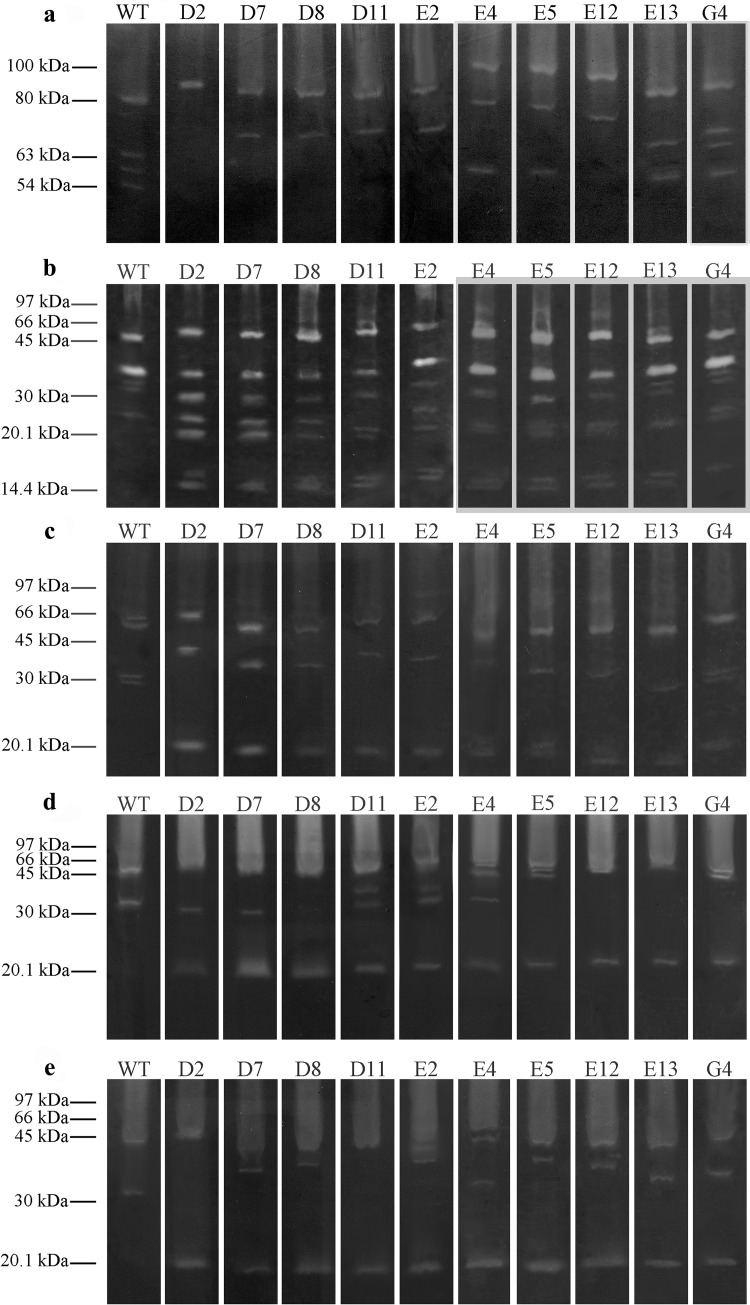
When the incorporated substrate was keratin, the electrophoresis profile of all mutants showed differences compared to the wild type. The mutant D2 only presented an 80-kDa band with a reduction of intensity. The mutants D7, D8, D11 and E2 only showed bands of 80 and 63 kDa. The mutants E4 and E5 did not present the 63-kDa band; however, they showed a higher intensity of the 100-kDa band. The E12 mutant also increased intensity of the 100-kDa and also of the 63- and 54-kDa bands (Fig. 3a). The electrophoresis profiles of the gelatin substrates were similar; however, there were differences in the intensity of some bands (Fig. 3b). When the BSA was used as the co-polymerized protein substrate, all the mutants presented similar electrophoresis profiles. Also, there was an increase in the 66-kDa band in the mutants E4, E5 and E12, and also a new band of about 20 kDa was observed in all mutants tested (Fig. 3c). The profiles of the peptidases analyzed with co-polymerized casein showed some differences: such as a weak 55 kDa band in mutants D2, D7, D8 and E12. Also, the 34-kDa band in the mutants D8 and E5 disappeared; however, a new band of about 20 kDa was seen in all mutants (Fig. 3d).
Fig. 3.
Zymographic analyses of the enzymatic extracts of strains of B. subtilisLFB-FIOCRUZ 1266 wild type and mutants using keratin (a), gelatin (b), BSA (c), casein (d) and hemoglobin (e) as substrates. The values on the left are the molecular weight pattern
In relation to the last substrate, the hemoglobin, the wild type presented two bands: one of 45 and the other of 32 kDa. The mutant D2 presented an increased intensity in the 45-kDa band, while the 32-kDa band disappeared. The mutants D7, D8 and E2 presented a lower intensity in the 45-kDa band, and a new band of about 36 kDa. The mutant D11 presented a lower intensity 45 kDa band and the 30-kDa band disappeared. The mutants E5 and E12 maintained the 45-kDa band; however, they presented a 36–37 kDa band that probably was a derivative of the 30-kDa band. The mutant E4 presented two bands with molecular weights less than 45 kDa. Again all mutants presented a 20-kDa band (Fig. 3e).
Effects of specific inhibitors on peptidase activity
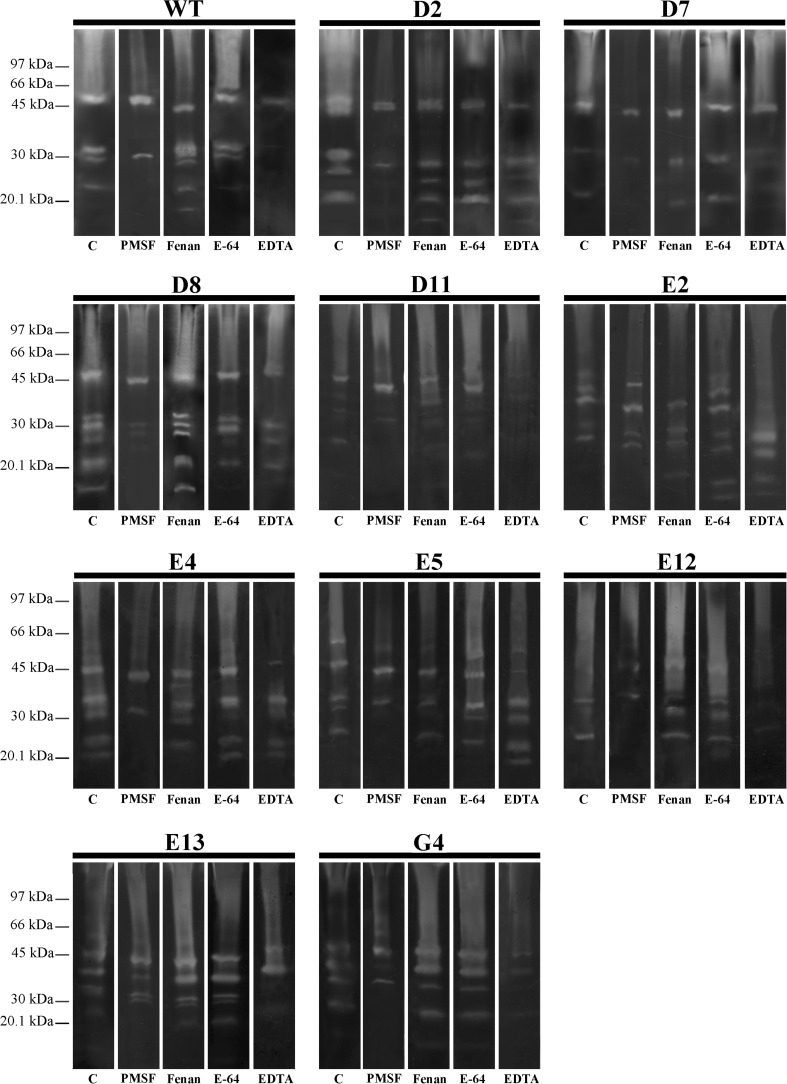
The peptidases were classified using different inhibitors by zymography in polyacrylamide gel with gelatin as the substrate. The peptidases of all the mutants, except mutant E2, were inhibited by PMSF, a serine peptidase inhibitor. The E2 mutant had two bands inhibited by phenanthroline, a metallopeptidase inhibitor. The divalent ion chelator EDTA inhibited several peptidases of mutant or wild-type strains, but not for the D2 and E5 strains. Wild-type and mutant peptidases were not affected by the treatment with E-64 (Fig. 4).
Fig. 4.
Determination of the keratinase classes of the wild-type strain LFB-FIOCRUZ 1266 and its mutants. The gels were incubated with the following inhibitors: PMSF, phenanthroline, E-64 and EDTA
Profile of feather hydrolysates
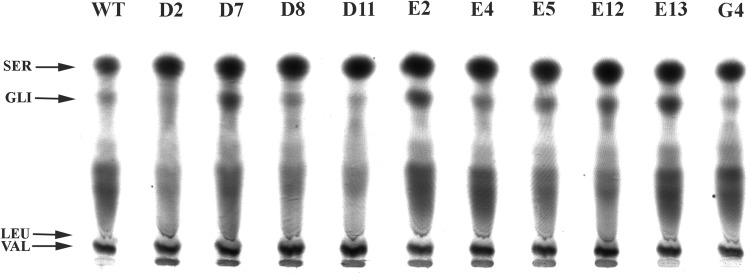
The profile of feather hydrolysates was analyzed by HPTLC. The chromatogram showed an increase of amino acid concentrations presenting similarity to the serine mobility patterns in all mutants. There was an increase in the amino acids with a retention time similar to glycine, in mutants D7, E2, E4 E5, E12 and E13. However, there was a reduction of amino acid concentrations with mobilities similar to glycine and leucine in mutants D2, D8, D11, E5 and E12 (Fig. 5).
Fig. 5.
HPTLC of feather hydrolysates obtained by microbial degradation of feathers by the wild-type (LFB-FIOCRUZ 1266) and mutant B. subtilis strains
Discussion
The Bacillus subtilis LFB-FIOCRUZ 1266 strain, a keratinolytic bacterium isolated from the agroindustrial waste of the poultry industry, was able to increase the keratinolytic activity and improved the production of extracellular peptidases by mutagenesis with EMS. The efficacy of chemical and physical mutagenesis to improve enzyme expression has already been reported (Wang et al. 2007; Villa et al. 2013; Isaac and Abu-Tahon 2015; Abt et al. 2016). In this work, eight mutants were able to degrade 97–99% of the feather in the culture media while the wild type was only able to degrade 87% (Fig. 1). The improvement in the keratinolytic activity of the mutants varied from 1.4- to 2.4-fold of the wild type. There are only two reports in the literature concerning the use of chemical mutagenesis to improve keratinolytic activity. The first was the use of 1-methyl-3-nitro-1-nitrosoguanidine for the treatment of Bacillus subtilis KD1, that produced a mutant with a keratinolytic activity double that of the wild type (Cai et al. 2008). The second one was the use of EMS to mutate Candida parapsilosis, which produced three mutants presenting 1.4-, 1.5- and 1.7-fold activity of the wild type (Duarte et al. 2011). Looking at the proportion of increase of protein expression, the results of this work are similar to those obtained in the two works mentioned above.
The hydrolysis mechanism of keratin is not fully understood, but there is a consensus that an important step of this process is to break the disulfide bridges. The role of sulfitolysis in feather degradation by Bacillus licheniformis RG1 was shown by Ramnani et al. (2005) who analyzed mutants that presented an improvement in the excretion of sulfide. Gupta and Ramnani (2006) showed that all tested peptidases were able to degrade feathers in the presence of an appropriate reducing agent. The mutant WB600, which is a peptidase mutant, was unable to degrade feathers, even after long periods of incubation. But it gained the ability to degrade feathers when the culture was supplemented with different peptidases. This study suggests that those cells probably offer the reduction power required for the reaction. To confirm the hypothesis that keratinolytic activity is a cooperative activity of reduction and proteolysis, reducers, such as β-mercaptoethanol, thioglycollate, glutathione and sodium sulfide, were inoculated together with the peptidases into feather medium. Similarly to what happened with WB600, their peptidases were able to degrade the feathers in the medium and the highest efficiency was detected when sodium sulfide was used as the reducing agent (Gupta and Ramnani 2006). Once the degradation process has started and the cells secrete sulfide, other extracellular peptidases can also hydrolyze the feathers. In this study, we observed that all strains that secreted large amounts of sulfide into the medium also presented significant degradation of the feathers.
The present work demonstrated the presence of sulfide secretion by most of the bacillus mutants studied. Sulfide detected in the extracellular broth during feather degradation indicated that sulfitolysis may also play a role in feather degradation by the bacterium.
In previous studies by our group we detected the presence of sulfide on extracellular medium that was probably participating in the breakdown of sulfide bridges of the feather keratin. Keratinase activity in this B. subtilis SLC strain is a coordinated action of two factors: the sulfide and peptidases, both of which were liberated from Bacillus sp. in the culture medium. (Cedrola et al. 2012) With the mutants obtained from Bacillus subtilis LFB-FIOCRUZ 1266 probably the same keratin hydrolysis mechanism was present considering that sulfide and peptidases were detected in all mutants. Several peptidases have been detected in Bacillus sp. with variable molecular masses. Six peptidases of B. subtilis, which have been well studied, are neutral protease A, subtilisin (also known as alkaline protease), extracellular protease, metalloprotease, bacillopeptidase F and neutral protease B (Rabbani et al. 2014). The most explored peptidase is subtilisin, coded by aprE gene and presenting a molecular mass range of 20–45 kDa, depending on the producer strain (He et al. 1991; Rabbani et al. 2014). The major peptidase with keratinolytic activity produced by B. subtilis KD-N2 has a molecular mass of 30.5 kDa and is inhibited by PMSF (Cai et al. 2008). Bacillus subtilis MTCC (9102) produces a peptidase with keratinolytic activity with a molecular mass range between 64 and 69 kDa and is inhibited by PMSF and EDTA. One of keratinolytic peptidases with the highest molecular mass has been described as the keratinase from B. subtilis DP1 with 97.4 kDa and is inhibited by PMSF (Sanghvi et al. 2016).
According to the literature, the molecular weight of bacterial keratinases ranges from 18 to 200 kDa. The smallest keratinase described is from Streptomyces albidoflavus SK 1–02 (Bressollier et al. 1999) and the largest ones are from Kokuriarosea and FervidobacteriumislandicumAW-1 (Nam et al. 2002; Bernal et al. 2003). In fungi, however, there are keratinases of about 440 kDa that can be found in the pathogenic fungi group (Kim et al. 2004). Keratinases have a broad specificity to different substrates, such as casein, gelatin, BSA and hemoglobin, as well as feather, collagen, elastin, hair and nails (Gupta and Ramnani 2006). The B. subtilis LFB-FIOCRUZ 1266 and its mutants investigated in this study were able to produce peptidases that degrade different protein-soluble substrates and powder keratin that is partially insoluble. The zymograms presented in this study showed that the EMS mutagenic agent generated mutants with superior activity, as can be seen by the more intense degradation bands. In general, the mutants presented a small variation in the intensity of the bands; however, some differences in the molecular weight of some peptidases or even the disappearance of some bands were detected. An interesting novelty was the detection of a new 20 kDa band in all selected mutants that was not present in the wild type. Some differences were observed between the analytica methods of keratinolytic activity: quantitative method (dosage) and qualitative method (zymogram). These discrepancies could be due to the differences between the methodologies. The methods used different amounts of keratin, buffers and incubation conditions. However, it was observed that the dosage method was more sensitive.
The peptidases of our samples were inhibited by PMSF and EDTA, suggesting that metallic ions are important for activity. The majority of peptidases are from classes of serine peptidases, cysteine peptidases or metallopeptidases (Rawlings et al. 2010). The mutant E2 peptidases were not inhibited by PMSF, but by EDTA and phenanthroline, suggesting a modification in the proteolytic profile. The majority of the peptidases of the mutants D2 and E5 were inhibited by PMSF, but not by EDTA.
In the recent past, waste feathers were cooked at high temperatures and pressure to be used as a feed supplement known as feather meal. This hydrothermal process destroys some amino acids such as methionine, lysine, histidine and tryptophan and produces some amino acids with no feed value: lysine alanine and lanthionine (Wang and Parsons 1997; Bertsch and Coello 2005). The bioconversion of keratin materials into amino acids, peptides and soluble proteins by keratinases produced by microorganisms can improve the nutritional value of animal feed produced from keratin residues, like feathers (Cai et al. 2008; Prakash et al. 2010). The amino acid profile of feather hydrolysates of mutants and wild type were analyzed by HPTLC. The profiles presented some differences, such as the increase of amino acids that, however, presented a retention pattern similar to the serine pattern; the appearance of amino acids with a glycine pattern and some changes in the concentration of amino acids but with the same retention pattern as leucine and glycine. These data suggest that there may have been changes in the hydrolysis of keratin by the mutants of B. subtilis LFB-FIOCRUZ 1266; however, it is not possible to verify whether all the amino acids were present in the feather hydrolysates and their concentrates.
This work demonstrated that it was possible to improve the keratinolytic activity of B. subtilis LFB-FIOCRUZ 1266 by chemical mutagenesis using EMS. The eight mutants isolated were able to hydrolyze 97% of the feather in the culture media. Some of them achieved 99–100% of feather hydrolysis. The soluble protein concentration varied from 2.37 to 4.42 mg/ml in the mutants while in the wild type it was 1.60 mg/ ml. The peptidases produced by the mutants were able to hydrolase different kinds of protein substrates.
Several works have been published, describing the production and analysis of enzyme-producing strains and selecting recombinants in order to improve the production of bacterial enzymes for industrial applications (Li et al. 2017; Deng et al. 2018; Jahromi et al. 2018; Zhu et al. 2018).Our results show that the mutants of B. subtilis with increased keratinolytic activity were able to fulfill food and feed approval standards, especially with regard to safety have a potential use (as whole cell catalysts or enzyme blends) to improve the nutritional value of food and feed, as well as uses in the cosmetic, textile and leather industries.
Acknowledgements
This work was financed in part by the Coordenação de Aperfeiçoamento Pessoal de Nível Superior-Brasil (CAPES), Financecode001, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro—FAPERJ (Daniel Pereira de Paiva: 202.941/2016).
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Arikawa Y, Ozawa T, Iwasaki I. An improved photometric method for the determination of sulfite with pararosaniline and formaldehyde. Bull Chem Soc Jpn. 1971;41:1454–1456. doi: 10.1246/bcsj.41.1454. [DOI] [Google Scholar]
- Bernal C, Vidal L, Valdivieso E, Coello N. Keratinolytic activity of Kocuria rosea. World J Microbiol Biotechnol. 2003;19:255–261. doi: 10.1023/A:1023685621215. [DOI] [Google Scholar]
- Bertsch A, Coello N. A biotechnological process for treatment and recycling poultry feathers as a feed ingredient. Bioresour Technol. 2005;96:1703–1708. doi: 10.1016/j.biortech.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Bohacz J. Biodegradation of feather waste keratin by a keratinolytic soil fungus of the genus Chrysosporium and statistical optimization of feather mass loss. World J Microbiol Biotechnol. 2017;33:1–16. doi: 10.1007/s11274-016-2177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandelli A, Daroit DJ, Riffel A. Biochemical features of microbial keratinases and their production and applications. Appl Microbiol Biotechnol. 2010;85:1735–1750. doi: 10.1007/s00253-009-2398-5. [DOI] [PubMed] [Google Scholar]
- Brenner M, Niederwieser A. Thin-layer chromatography (TLC) of amino acids. In: Hirs CHW, editor. Methods in enzymology. New York: Academic Press; 1965. pp. 39–59. [Google Scholar]
- Bressollier P, Letourneau F, Urdaci M, Verneuil B. Purification and characterization of a keratinolytic serine proteinase from streptomyces albidoflavus. Appl Environ Microbiol. 1999;65(6):2570–2576. doi: 10.1128/aem.65.6.2570-2576.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Lou B, Zheng X. Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. J Zhejiang Univ Sci B. 2008;9:60–67. doi: 10.1631/jzus.B061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedrola SML, Melo ACN, Mazotto AM, Lins U, Zingali RB, Rosado AS, Peixoto RS, Vermelho AB. Keratinases and sulfide from Bacillus subtilis SLC to recyclefeather waste. World J Microbiol Biotechnol. 2012;28:1259–1269. doi: 10.1007/s11274-011-0930-0. [DOI] [PubMed] [Google Scholar]
- Daroit DJ, Corrêa APF, Brandelli A. Keratinolytic potential of a novel Bacillus sp. P45 isolated from the Amazon basin fish Piaractusmesopotamicus. Int Biodeter Biodegrad. 2009;63:358–363. doi: 10.1016/j.ibiod.2008.11.008. [DOI] [Google Scholar]
- Den Abt T, Souffriau B, Foulquié-Moreno MR, et al. Genomic saturation mutagenesis and polygenic analysis identify novel yeast genes affecting ethyl acetate production, a non-selectable polygenic trait. Microb Cell. 2016;3:159–175. doi: 10.15698/mic2016.04.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Li J, Shin HD, Du G, Chen J, Liu L. Efficient expression of cyclodextrin glycosyltransferase from Geobacillus stearothermophilus in Escherichia coli by promoter engineering and downstream box evolution. J Biotechnol. 2017;266:77–83. doi: 10.1016/j.jbiotec.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Duarte TR, Oliveira SS, Macrae A, et al. Increased expression of keratinase and other peptidases by Candida parapsilosis mutants. Braz J Med Biol Res. 2011;44:212–216. doi: 10.1590/S0100-879X2011007500011. [DOI] [PubMed] [Google Scholar]
- El-Gendy MMA. Keratinase production by endophytic Penicillium spp. Morsy1 under solid-state fermentation using rice straw. Appl Biochem Biotechnol. 2010;162:780–794. doi: 10.1007/s12010-009-8802-x. [DOI] [PubMed] [Google Scholar]
- Fellahi S, Chibani A, Feuk-Lagerstedt E, Taherzadeh MJ. Identification of two new keratinolytic proteases from a Bacillus pumilus strain using protein analysis and gene sequencing. AMB Express. 2016 doi: 10.1186/s13568-016-0213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarma E, Amanetidou E, Toufexi A, Touraki M. Defense systems in developing Artemia franciscana nauplii and their modulation by probiotic bacteria offer protection against a Vibrio anguillarum challenge. Fish Shellfish Immunol. 2017;66:163–172. doi: 10.1016/j.fsi.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Grzywnowicz G, Lobarzewski J, Wawrzkiewicz K, Wolski T. Comparative characterization of proteolytic enzymes from Trichophyton gallinae and Trichophyton verrucosum. Med Mycol. 1989;27:319–328. doi: 10.1080/02681218980000431. [DOI] [PubMed] [Google Scholar]
- Guo M, Wu F, Hao G, Qi Q, Li R, Li N, Wei L, Chai T. Bacillus subtilis improves immunity and disease resistance in rabbits. Front Immunol. 2017;8:354. doi: 10.3389/fimmu.2017.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Ramnani P. Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biotechnol. 2006;70:21–33. doi: 10.1007/s00253-005-0239-8. [DOI] [PubMed] [Google Scholar]
- Gupta R, Rajput R, Sharma R, Gupta N. Biotechnological applications and prospective market of microbial keratinases. Appl Microbiol Biotechnol. 2013;97:9931–9940. doi: 10.1007/s00253-013-5292-0. [DOI] [PubMed] [Google Scholar]
- He XS, Brüickner R, Doi RH. The protease genes of Bacillus subtilis. Res Microbiol. 1991;142:797–803. doi: 10.1016/0923-2508(91)90058-I. [DOI] [PubMed] [Google Scholar]
- Isaac GS, Abu-Tahon MA. Enhanced alkaline cellulases production by the thermohalophilic Aspergillus terreus AUMC 10138 mutated by physical and chemical mutagens using corn stover as substrate. Braz J Microbiol. 2015;46:1269–1277. doi: 10.1590/S1517-838246420140958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi ST, Bazkar N. Future direction in marine bacterial agarases for industrial applications. Appl Microbiol Biotechnol. 2018;102(16):6847–6863. doi: 10.1007/s00253-018-9156-5. [DOI] [PubMed] [Google Scholar]
- Jones BL, Fontanini D, Jarvinen M, Pekkarinen A. Simplified endoproteinase assays using gelatin or azogelatin. Anal Biochem. 1998;263(2):214–220. doi: 10.1006/abio.1998.2819. [DOI] [PubMed] [Google Scholar]
- Kanaki NS, Rajani M. Development and validation of a thin-layer chromatography-densitometric method for the quantitation of alliin from garlic (Allium sativum) and its formulations. J AOAC Int. 2005;88:1568–1570. [PubMed] [Google Scholar]
- Kim JS, Kluskens LD, de Vos WM, Huber R, van der Oost J. Crystal structure of fervidolysin from Fervidobacterium pennivorans, a keratinolytic enzyme related to subtilisin. J Mol Biol. 2004;335(3):787–797. doi: 10.1016/j.jmb.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Kublanov IV, Bidjieva SK, Mardanov AV, Bonch-Osmolovskaya EA. Desulfurococcus kamchatkensis sp. nov., a novel hyperthermophilic protein-degrading archaeon isolated from a Kamchatka hot spring. Int J Syst Evol Microbiol. 2009;59:1743–1747. doi: 10.1099/ijs.0.006726-0. [DOI] [PubMed] [Google Scholar]
- Lange L, Huang Y, Busk PK. Microbial decomposition of keratin in nature: a new hypothesis of industrial relevance. Appl Microbiol Biotechnol. 2016;100:2083–2096. doi: 10.1007/s00253-015-7262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegren G, Hwang YL, Oshima Y, Lindegren CC. Genetical mutants induced in ethyl methanesulfonate in Saccharomyces. Can J Genet Cytol. 1965;7:491–499. doi: 10.1139/g65-064. [DOI] [PubMed] [Google Scholar]
- LiZ SuL, Duan X, Wu D, Wu J. Efficient expression of maltohexaose-forming α-amylase from Bacillus stearothermophilus in Brevibacilluschoshinensis SP3 and Its use in maltose production. J Biotechnol. 2017;266:77–83. doi: 10.1155/2017/5479762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. doi: 10.1016/0304-3894(92)87011-4. [DOI] [PubMed] [Google Scholar]
- Mabrouk MEM. Feather degradation by a new keratinolytic Streptomyces sp. MS-2. World J Microbiol Biotechnol. 2008;24:2331–2338. doi: 10.1007/s11274-008-9748-9. [DOI] [Google Scholar]
- Manczinger L, Rozs M, Vágvölgyi C, Kevei F. Isolation and characterization of a new keratinolytic Bacillus licheniformis strain. World J Microbiol Biotechnol. 2003;19:35–39. doi: 10.1023/A:1022576826372. [DOI] [Google Scholar]
- Mazotto AM, LageCedrola SM, Lins U, et al. Keratinolytic activity of Bacillus subtilis AMR using human hair. Lett Appl Microbiol. 2010;50:89–96. doi: 10.1111/j.1472-765X.2009.02760.x. [DOI] [PubMed] [Google Scholar]
- Mazotto AM, Coelho RRR, Cedrola SML, et al. Keratinase production by three Bacillus spp. using feather meal and whole feather as substrate in a submerged fermentation. Enzyme Res. 2011;2011:1–7. doi: 10.4061/2011/523780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazotto AM, Ascheri JLR, de Oliveira Godoy RL, et al. Production of feather protein hydrolyzed by B. subtilis AMR and its application in a blend with cornmeal by extrusion. LWT Food Sci Technol. 2017;84:701–709. doi: 10.1016/j.lwt.2017.05.077. [DOI] [Google Scholar]
- Mokrejs P, Svoboda P, Hrncirik J, et al. Processing poultry feathers into keratin hydrolysate through alkaline-enzymatic hydrolysis. Waste Manag Res. 2011;29:260–267. doi: 10.1177/0734242X10370378. [DOI] [PubMed] [Google Scholar]
- Nam GW, Lee DW, Lee HS, Lee NJ, Kim BC, Choe EA, Hwang JK, Suhartono MT, Pyun Y. Native-feather degradation by Fervidobacterium islandicum AW-1, a newly isolated keratinase-producing thermophilic anaerobe. Arch Microbiol. 2002;178(6):538–547. doi: 10.1007/s00203-002-0489-0. [DOI] [PubMed] [Google Scholar]
- Navone L, Speight R. Understanding the dynamics of keratin weakening and hydrolysis by proteases. PLoS One. 2018;13(8):e0202608. doi: 10.1371/journal.pone.0202608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De NogueiraMelo AC, Dornelas-Ribeiro M, De ParaguaiSouza E, et al. Peptidase profiles from non-albicans Candida spp. isolated from the blood of a patient with chronic myeloid leukemia and another with sickle cell disease. FEMS Yeast Res. 2007;7:1004–1012. doi: 10.1111/j.1567-1364.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- Okoroma EA, Purchase D, Garelick H, et al. Enzymatic formulation capable of degrading scrapie prion under mild digestion conditions. PLoS One. 2013;8:1–7. doi: 10.1371/journal.pone.0068099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifade AA, Al-Sane NA, Al-Musallam AA, Al-Zarban S. A review: potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour Technol. 1998;66:1–11. doi: 10.1016/S0960-8524(98)00033-9. [DOI] [Google Scholar]
- Prakash P, Jayalakshmi SK, Sreeramulu K. Purification and characterization of extreme alkaline, thermostable keratinase, and keratin disulfide reductase produced by Bacillus halodurans PPKS-2. Appl Microbiol Biotechnol. 2010;87:625–633. doi: 10.1007/s00253-010-2499-1. [DOI] [PubMed] [Google Scholar]
- Rabbani M, Soleymani S, Sadeghi HM, Soleimani N, Moazen F. Inactivation of aprE gene in Bacillus subtilis 168 by homologus recombination. Avicenna J Med Biotechnol. 2014;6(3):185–189. [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan J, Balakrishnan H, Raja STK, et al. Formulation of economical microbial feed using degraded chicken feathers by a novel Streptomyces sp: mitigation of environmental pollution. Braz J Microbiol. 2011;42:825–834. doi: 10.1590/S1517-83822011000300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani P, Singh R, Gupta R. Keratinolytic potential of Bacillus licheniformis RG1: structural and biochemical mechanism of feather degradation. Can J Microbiol. 2005;51(3):191–196. doi: 10.1139/w04-123. [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ, Bateman A. MEROPS: the peptidase database. Nucleic Acids Res. 2010;38(suppl_1):D227–D233. doi: 10.1093/nar/gkp971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro O, Magalhães F, Aguiar TQ, et al. Random and direct mutagenesis to enhance protein secretion in Ashbya gossypii. Bioengineered. 2013;4:322–331. doi: 10.4161/bioe.24653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghvi G, Patel H, Vaishnava D, Ozaa T, Dave G, Kunjadiac P, Shetha N. A novel alkaline keratinase from Bacillus subtilis DP1 with potential utility in cosmetic formulation. Int J Biol Macromol. 2016;8:256–262. doi: 10.1016/j.ijbiomac.2016.02.067. [DOI] [PubMed] [Google Scholar]
- Syed DG, Lee JC, Li WJ, et al. Production, characterization and application of keratinase from Streptomyces gulbargensis. Bioresour Technol. 2009;100:1868–1871. doi: 10.1016/j.biortech.2008.09.047. [DOI] [PubMed] [Google Scholar]
- Vasileva-Tonkova E, Gousterova A, Neshev G. Ecologically safe method for improved feather wastes biodegradation. Int Biodeterior Biodegrad. 2009;63:1008–1012. doi: 10.1016/j.ibiod.2009.07.003. [DOI] [Google Scholar]
- Villa ALV, Aragão MRS, dos Santos EP, et al. Feather keratin hydrolysates obtained from microbial keratinases: effect on hair fiber. BMC Biotechnol. 2013;13:1–11. doi: 10.1186/1472-6750-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Parsons CM. Effect of processing systems on protein quality of feather meals and hog hair meals. Poult Sci. 1997;76:491–496. doi: 10.1093/ps/76.3.491. [DOI] [PubMed] [Google Scholar]
- Wang HY, Liu DM, Liu Y, et al. Screening and mutagenesis of a novel Bacillus pumilus strain producing alkaline protease for dehairing. Lett Appl Microbiol. 2007;44:1–6. doi: 10.1111/j.1472-765X.2006.02039.x. [DOI] [PubMed] [Google Scholar]
- Wawrzkiewicz K, Łobarzewski J, Wolski T. Intracellular keratinase of Trichophyton gallinae. J Med Vet Mycol. 1987;25:261–268. doi: 10.1080/02681218780000601. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Miwa T, Horii H, et al. Characterization of a proteolytic enzyme derived from a Bacillus strain that effectively degrades prion protein. J Appl Microbiol. 2007;102:509–515. doi: 10.1111/j.1365-2672.2006.03080.x. [DOI] [PubMed] [Google Scholar]
- Zhu BW, Xiong QNF, Sun Y, Yao Z. High-level expression and characterization of a new κ-carrageenase from marine bacterium Pedobacterhainanensis NJ-02. Lett Appl Microbiol. 2018;66(5):409–415. doi: 10.1111/lam.12865. [DOI] [PubMed] [Google Scholar]