Abstract
We have investigated a distinct disorder with progressive corneal neovascularization, keloid formation, chronic skin ulcers, wasting of subcutaneous tissue, flexion contractures of the fingers, and acro-osteolysis. In six affected individuals from four families, we found one of two recurrent variants in discoidin domain receptor tyrosine kinase 2 (DDR2): c.1829T>C (p.Leu610Pro) or c.2219A>G (p.Tyr740Cys). DDR2 encodes a collagen-responsive receptor tyrosine kinase that regulates connective-tissue formation. In three of the families, affected individuals comprise singleton adult individuals, and parental samples were not available for verification of the de novo occurrence of the DDR2 variants. In the fourth family, a mother and two of her children were affected, and the c.2219A>G missense variant was proven to be de novo in the mother. Phosphorylation of DDR2 was increased in fibroblasts from affected individuals, suggesting reduced receptor autoinhibition and ligand-independent kinase activation. Evidence for activation of other growth-regulatory signaling pathways was not found. Finally, we found that the protein kinase inhibitor dasatinib prevented DDR2 autophosphorylation in fibroblasts, suggesting an approach to treatment. We propose this progressive, fibrotic condition should be designated as Warburg-Cinotti syndrome.
Keywords: keloid formation, chronic skin ulcers, corneal neovascularization, contractures, lipodystrophy, acro-osteolysis, DDR2
Main Text
In 2006, Warburg et al. described an apparently distinct connective-tissue disorder characterized by blepharophimosis, progressive corneal vascularization, retinal dystrophy, conductive hearing loss, acro-osteolysis, and wasting of subcutaneous tissue in the face, hands, and feet.1 For updated pictures of the described individual, see Figure 1. Cinotti et al. later reported an individual with similar features.2 In this report we extend the clinical description by identifying four additional individuals, three of whom are from a single family, and identify the molecular cause of this syndrome.
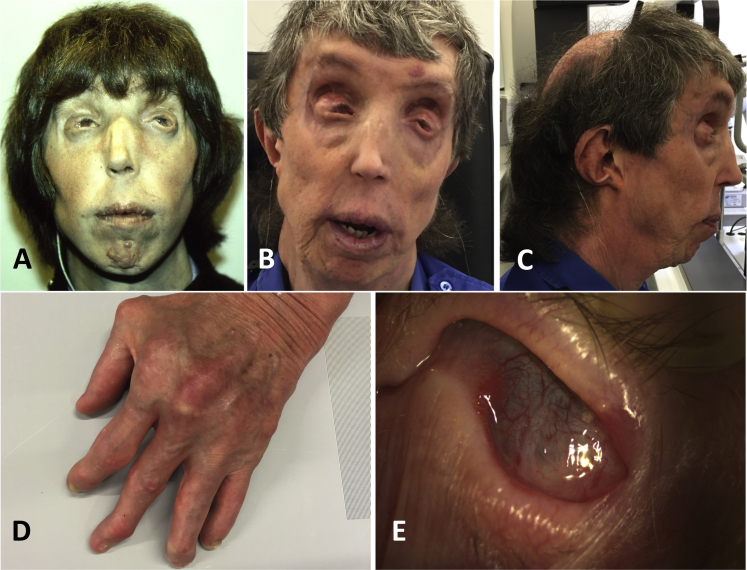
Figure 1.
Features of Individual 1, with the p.Leu610Pro DDR2 Variant
(A–C) Panels A–C show facial features, including a long philtrum, a short nose, a thin upper vermilion, midface retrusion, and narrow nostrils.
(D) Panel D shows mild cutaneous acquired syndactyly of fingers 3 and 4 and flexion contractures.
(E) Panel E shows corneal vascularization at age 54 years and development of complete corneal conjunctivalization and symblepharon formation.
Informed consent was obtained for participation and publication of clinical photographs. The study was approved by the Regional Committee for Medical and Health Research Ethics, Western Norway (IRB no. 00001872, project number 2014/59). The NIH study was reviewed and approved by the National Human Genome Research Institute institutional review board, protocol 10-HG-0065.
The clinical findings are illustrated in Figures 1 and 2 and are summarized in Tables 1 and S1. Pedigrees are provided in Supplemental Figures S1–S4. Individuals 1 and 2 have been described in detail.1, 2 The proband of the third pedigree (individual 3) is a 31-year-old female with two affected children (Figures 2 and S3). She was born with a clubfoot, which was successfully treated. When she was 5 years old, the first red, linear, and firm keloid-like plaque appeared spontaneously on her left forearm. This was slowly progressive. Over the years, several additional red papules and linear or annular plaques developed on her arms and feet, without preceding trauma or inflammation. In contrast, no keloid formations have occurred after surgical and traumatic wounds, with the exception of one instance after an ear helix piercing. At age 14, finger flexion contractures, preceded by painless, non-erythematous swelling of the involved joints, developed. This progressed to ankylosis of proximal interphalangeal (PIP) joints and cutaneous fusion between the digits and the palm (Figures 2E and 2F). Surgical correction and collagenase injections allowed the fixated digit to extend, but rapid recurrence and progression occurred. Gradual cutaneous fusion of the toes led to little or no separation of the toes (Figures 2I and 2J). She has had numerous sterile abscesses of the hands and feet, and these abscesses were often followed by scarring, cutaneous fusions, and contractures. From the age of 25, she developed corneal neovascularization and subsequent pannus and symblepharon formation on the right eye; eventually, there was complete conjunctivalization of the cornea and a reduction of her visual acuity to hand motion only. Her left eye has normal vision, but examination showed limbal stem-cell deficiency (LSCD), a superior corneal vascular pannus, reduction of the central corneal thickness to 419 μm, and endothelial-cell dysfunction with disruption of healthy hexagonal morphology (Figures 2A–2C). Fundoscopy showed normal retinal findings. Her facial features include a mild narrowing of the nasal bridge, mild lower-midface retrusion, and mild epicanthal folds. The lengths of her palpebral fissures are normal. She was diagnosed with hypothyroidism in her 20s. She has generalized thin skin and erythema.The hyperkeratosis of follicular orifices of her arms, neck, and chest resembles keratosis pilaris. Radiographs of the hands showed relatively normal phalanges with contractures. Radiographs of the feet showed small, hypodense distal phalanges and deformed middle phalanges, and in the 3-5th toe severe lateral angulation of the DIP joints could be seen (Figure 2H). She had enlarged frontal sinuses on skull films and no apparent osteolysis.
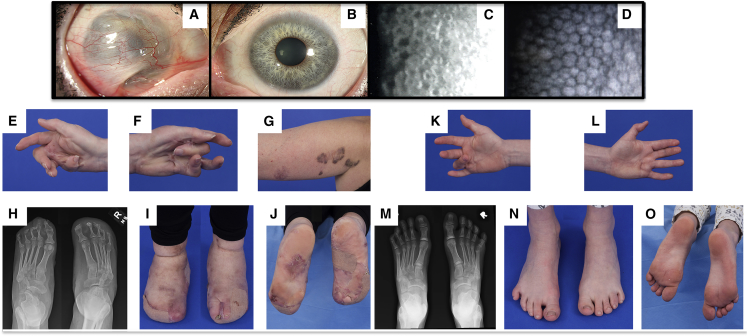
Figure 2.
Features of Individuals 3 and 4
Individual 3 (A–J) is the index patient of this family, and individual 4 (K–O) is her 8-year-old daughter. Both have the p.Tyr740Cys DDR2 variant.
(A and B) Slit-lamp microscopy images of the mother’s anterior segment. There was complete conjunctivalization of the right cornea with symblepharon formation (A) and superior vascular pannus in the left eye (B).
(C) A specular microscopy image of the cornea of individual 3 (C). The image shows disruption of the healthy hexagonal endothelial morphology.
(D) A normal image is shown for comparison.
(E and F) Extreme flexion contractures of the mother’s hands, as well as scarring and adhesions of the flexor surfaces of the digits and palm.
(G) A few of the annular plaques with features that are similar to those of keloids, though they have an atypical maroon-brown pigmentation. The more distal, lighter-pigmented lesion had been excised, after which it recurred and was treated with intralesional corticosteroids.
(H–J) Feet and radiographs featuring acro-osteolysis, lateral deviation of the digits, and extreme connective-tissue overgrowth leading to subsequent fusion of the toes.
(K–O) Images of the 8-year-old daughter show a 90-degree flexion contracture of the right fourth finger (K), scar-like lesions of the palm, mild contraction of the left fourth finger, and asubtle, scar-like lesion in the palm proximal to that digit (L). The radiograph shows an absence of major osteolysis in the feet, whichappeared largely normal aside from a deviated, deformed distal phalanx of the left fourth toe.
Table 1.
Overview of Phenotypic Features
| Individual 1 | Individual 2 | Individual 3 | Individual 4 | Individual 5 | Individual 6 | |
|---|---|---|---|---|---|---|
| Described by | Warburg et al.1 | Cinotti et al.2 | this paper | this paper | this paper | this paper |
| Age | 57 years | 58 years | 31 years | 8 years | 3 years | 35 years |
| Familial status | singleton individual | singleton individual | index mother | child of individual 3 | child of individual 3 | singleton individual |
| DDR2 variant | p.Leu610Pro | p.Tyr740Cys | p.Tyr740Cys | p.Tyr740Cys | p.Tyr740Cys | p.Leu610Pro |
| Narrow palpebral fissures | + | + | – | + | NR | + |
| Corneal vascularization | +++ | +++ | ++ | + | + | NR |
| Reduced vision | +++ | +++ | ++ | – | NR | NR |
| Thin nose ± small alae nasi | + | + | + | + | + | + |
| Long face | + | – | + | + | + | NR |
| High palate | + | NR | – | – | – | + |
| Abnormal teeth | + | + | – | – | – | + |
| Posteriorly rotated ears | + | + | + | – | – | NR |
| Thin ear cartilage | NR | NR | + | + | NR | NR |
| Conductive hearing loss | + | – | – | NR | NR | + |
| Cholesteatoma | + | NR | NR | + | NR | NR |
| Skin with little subcutaneous tissue | + | NR | + | + | NR | + |
| Keloid-like plaques | + | + | + | – | – | + |
| Follicular hyperkeratosis | NR | NR | + | – | + | + |
| Contractures | ++ | +++ | ++ | + | + | + |
| Joint swellings | NR | NR | + | + | + | + |
| Acro-osteolysis | + | + | + | – | – | + |
| Palmar fibrotic bands / cutaneous fusions | NR | + | + | + | – | – |
| Loss of toenails / toes | + | + | + | – | – | + |
| Pneumothorax | + | – | – | – | – | + |
| Mitral valve insufficiency | NR | + | – | – | – | + |
| Hypothyroidism | NR | NR | + | – | – | + |
| Intestinal problems | + | NR | – | – | – | ++ |
Abbreviations are as follows: + = present, with degree of presence indicated when appropriate; – = absent; and NR = not reported.
Her eldest daughter (individual 4) had a normal birth and health history in early childhood. She developed contractures of her fourth fingers at 4–5 years of age; these contractures were associated with painless, non-erythematous swelling of the involved joints. Her PIP contractures were ∼90 degrees on the right fourth finger, but less severe on the left third finger. On her palms, she had associated scar-like thickening overlying the flexor tendons of the fourth digits (Figures 2K and 2L). She had a right cholesteatoma removed at age 4; this recurred and was removed again at age 8. On evaluation at age 8.5, she had a faint papular erythematous rash on her arms, face, neck, and chest; a 3 mm bluish papule with a punctate eschar on her right upper arm; and a soft, slightly bluish 1.5–2.5 cm mass on the sole of her left foot. Although her vision was normal, she had a superior corneal vascular pannus and reduced central corneal thickness (right eye 491 μm, left eye 484 μm), but normal endothelial cell morphology on specular microscopy. She has a long face with midface retrusion, a narrow nose, short palpebral fissures, epicanthal folds, and thin ear cartilage. Her skin is thin and somewhat lax, similar to her mother’s, although her feet appear relatively normal.
The youngest child (individual 5) is a male with an unremarkable birth and health history, except for noticeable enlargement of several PIP finger joints and mild flexion contractures of several DIP finger joints. On evaluation at age 3, he had mild follicular hyperkeratosis, and an eye examination showed a prominent superior corneal vascular pannus. His facial features are essentially the same as those of his sister. The proband’s third and healthy child, her brother, and both of her parents reportedly have none of the features described above. All individuals have apparently normal intelligence.
The sixth affected individual (individual 6) is a 35-year-old female who presented with a complex combination of congenital and acquired symptoms. She was born to healthy, unrelated parents with an unremarkable family history, apart from the presence of polycystic kidney disease in her father and paternal uncle. She presented with pyloric stenosis and facial dysmorphism in infancy. Psychomotor development was normal, but she suffered from recurrent ear infections that led to conductive hearing loss during childhood. In addition, she also has polycystic kidney disease. Her arms had angiodermatofibromas (benign and superficial fibrous histiocytoma), and her scalp had lichenoid skin lesions. Furthermore, she has hypothyroidism and mild mitral valve insufficiency, and during adolescence a susceptibility to bruising was noted. She has had surgical corrections of dental crowding and malpositioned teeth. At age 18 years, spontaneous pneumothorax was diagnosed. At age 22 years, she underwent sigmoid resection as a result of chronic diverticulitis and developed stenotic scarring as a later complication. At age 31years she had peritonitis after surgery associated with an ovarian abscess. Wound healing was delayed, and she developed a thick, protruding scar that in some areas was keloid-like. During treatment of her abdominal illness, she sustained an ischemic stroke, and she developed cysts in the liver. She has hypermobile joints, flat feet, contractures, and generalized joint enlargements of the fingers. Chronic ulcerations on her toes eventually led to the loss of all toes and a significant portion of forefoot tissue. She has surgical wound management on a regular basis. At her latest clinical examination (at age 35), she presented with apparently normal cognition, tall stature (∼95th percentile), reduced subcutaneous body fat, enlarged and low-set-ears, widely spaced eyes, bilateral epicanthus, bilateral blepharophimosis, a thin nose with small alae nasi, and a high palate. She is able to walk unsupported but suffers from limitations due to chronic ulcerations on her feet.
Clinical testing for genomic copy-number aberrations and a connective-tissue gene panel of individuals 1 and 2 were normal (data not shown). Research exome sequencing, using both dominant and recessive models, was undertaken as described in the Supplemental Methods. We made a list of all variants shared by these unrelated individuals, then removed all variants previously classified as benign in our diagnostic pipeline, as well as all variants present in dbSNP build 137. Only four heterozygous missense variants in two genes remained. Two variants were in MUC4 [MIM: 158372] in non-conserved nucleotides and amino acids, and both were predicted to be benign by the in silico tools provided by Alamut (Interactive Biosoftware). Two variants were in DDR2 [MIM: 191311]; these variants affected conserved nucleotides and amino acids, and both were predicted to be damaging by the following in silico prediction programs: PolyPhen2, MutationTester, SIFT, and LRT. Individual 1 was heterozygous for DDR2 (GenBank: NM_001014796.1) c.1829T>C (p.Leu610Pro), and individual 2 was heterozygous for DDR2 c.2219A>G (p.Tyr740Cys). In programs provided through Alamut, neither of the variants were predicted to affect splicing. In addition, the variants had CADD scores of 30 for p.Leu610Pro and 31 for p.Tyr740Cys, also decreasing the likelihood that this was a chance finding (see Supplemental Methods for details). DDR2 is relatively intolerant to loss-of-function variants (Exac database pLI score of 0.99) and more tolerant to missense variation (Exac database Z score of 2.09). Neither of the DDR2 variants found were present in the gnomAD database, nor were any other missense changes to either the Leu610 or Tyr740 codons. Both missense changes affect conserved amino acids located in the DDR2 tyrosine kinase domain, as can be seen from the cross-species comparison made in Figure 3. The same amino acids are also conserved in DDR1 [MIM: 600408]. Subsequently, we found that individual 3 had the same p.Tyr740Cys variant previously found in individual 2 and that individual 6 had the same p.Leu610Pro variant previously found in individual 1. The variant found in individual 3 was proven to be de novo after parental testing (Figure S3). In the other individuals, samples from both parents were not available (Figures S1, S2, and S4). The two affected children of individual 3 (individuals 4 and 5) had inherited the p.Tyr740Cys variant (Figure S3). The variants were verified by Sanger sequencing (Figures S1–S3). We conclude that the identification of two unique and recurrent missense variants, p.Leu610Pro and p.Tyr740Cys, affecting conserved DDR2 amino acids, in four families with a similar clinical phenotype (one proven de novo occurrence) confirms the pathogenicity of these variants.
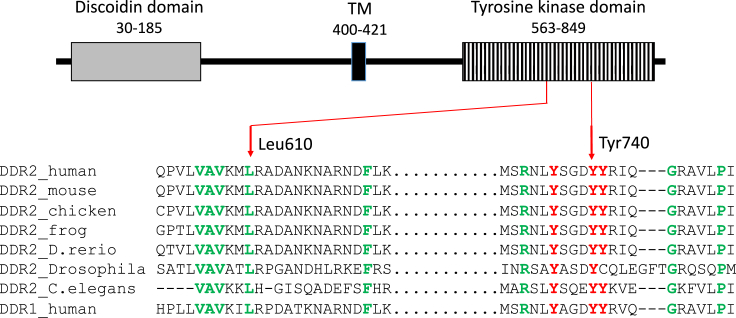
Figure 3.
DDR2 Structure and Amino Acid Conservation
Above, the DDR2 structure is sketched with variant positions indicated. Below, the degree of amino acid conservation is indicated by a comparison to organisms ranging from C. elegans to humans. The paralog DDR1 is also included for comparison. Red Y’s are tyrosines known to be phosphorylated. Green amino acids are conserved. TM = transmembrane domain.
The discoidin domain receptors, DDR1 and DDR2, are receptor tyrosine kinases (RTKs) that are stimulated by collagen in the extracellular matrix (ECM). Unlike most other RTKs, they form ligand-independent stable dimers that are non-covalently linked.3 This ECM activation induces receptor phosphorylation with slow kinetics, i.e., a slow and prolonged response, unlike the quicker responses of most other RTKs. DDRs regulate cell proliferation, differentiation, migration, and survival and control extracellular matrix homeostasis and remodeling. Dysregulated DDR function has been associated with fibrosis, arthritis, and cancer.3, 4 DDR2 is predominantly expressed in fibroblasts, chondrocytes, osteoblasts, and other connective-tissue cells of mesenchymal origin.5
Both the p.Leu610Pro and the p.Tyr740Cys substitutions are located in the kinase domain of the DDR2 receptor. To study the consequences of these substitutions, we first examined DDR2 autophosphorylation. All results were reproduced in at least two independent experiments. Control fibroblasts and fibroblasts from individuals 1 and 3, heterozygous for the p.Leu610Pro and the p.Tyr740Cys substitutions, respectively, were cultured in Dulbecco's modified Eagle's medium (DMEM)—high glucose (Lonza) supplemented with 10% fetal calf serum, penicillin, streptomycin, and glutamine. When the cells were 80%–90% confluent, fresh medium was added, and the cells were harvested the following day. The total phosphorylated DDR2 was measured with a DuoSet IC Phospho-DDR2 kit (#DYC6170, R&D Systems) according to the manufacturer’s recommendations (see Supplemental Methods). More phosphorylated DDR2 was observed in the fibroblasts from affected individuals than in those of controls, indicating that the variants were activating and caused autophosphorylation of the receptor (Figures 4A and 4B).
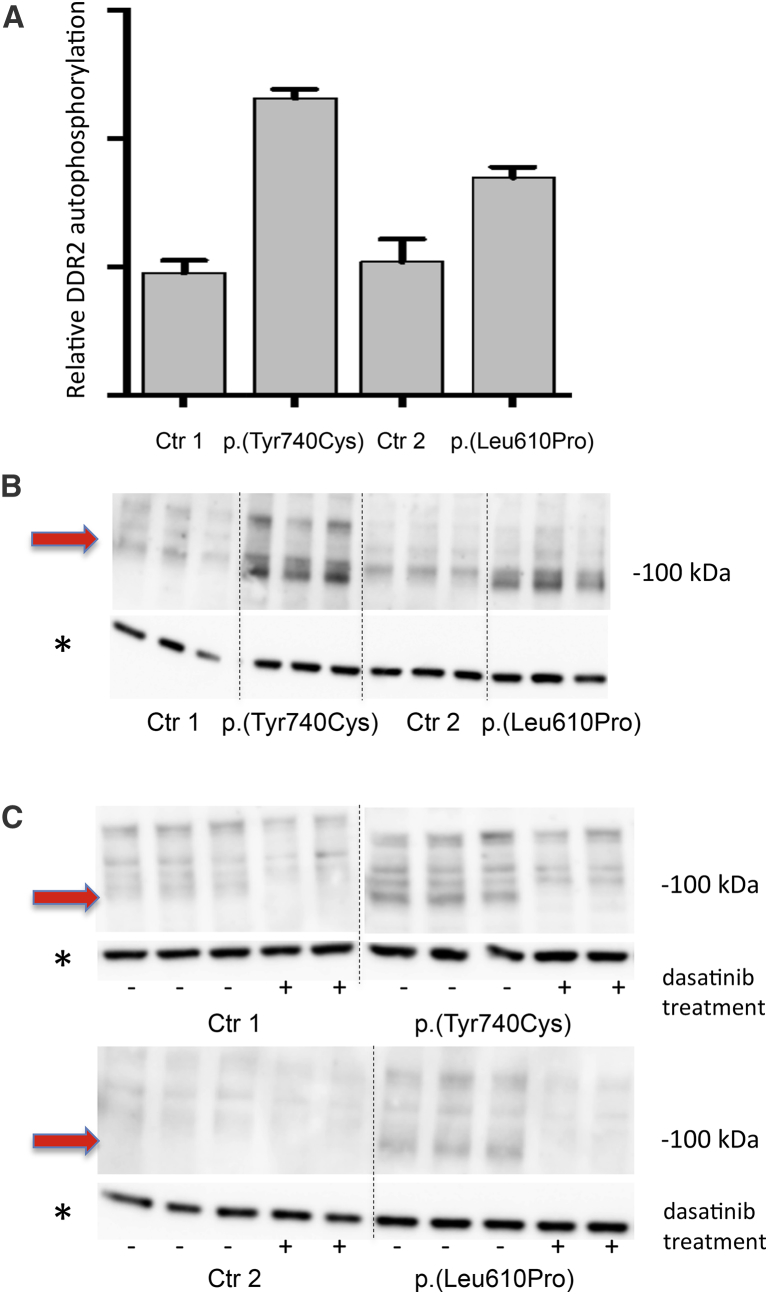
Figure 4.
Autophosphorylation of DDR2 and Effect of Dasatinib Treatment
(A and B) ELISA (A) and immunoblot (B) results show that DDR2 phosphorylation is greater in fibroblasts with the p.Tyr740Cys or p.Leu610Pro substitutions than in control fibroblasts (Ctr 1 and Ctr 2). For ELISA (A), cells were harvested, and total phosphorylated DDR2 was measured with the DuoSet IC Phospho-DDR2 kit. For immunoblot analysis (B), cells that had been serum starved overnight were harvested and examined. The antibody used targeted phospho-Tyr740-DDR2. The band representing phospho-DDR2 is marked with a red arrow.
(C) The result of treatment with 0.1 μM dasatinib (+) in control fibroblasts (left lanes), fibroblasts from individual 3 (top right lanes), and fibroblasts from individual 1 (bottom right lanes). In fibroblasts with the p.Leu610Pro and p.Tyr740Cys substitution, the band representing phospho-DDR2 (marked with a red arrow) disappeared after dasatinib treatment. All experiments were reproduced in at least two independent experiments, and representative results are shown.
Error bars illustrate the standard deviation of replicated experiments.
DDR2 has 14 tyrosine residues: four located in the extracellular juxtamembrane region, and the rest located in the kinase domain of the receptor.6 Tyr740 is thought to play a critical role for DDR2 autoinhibition and site-directed Tyr740Phe mutagenesis caused in vitro DDR2 autophosphorylation and thereby mimicked the effect of high levels of SRC.7 Because the p.Tyr740Cys variant alters this tyrosine residue, phosphorylation at this site was further examined. For immunoblot analyses, cells were starved of serum overnight before being harvested, separated on a high-resolution gel system, transferred to nitrocellulose membranes, and incubated overnight at 4°C with antibodies against phospho-Tyr740-DDR2 (#MAB25382) and DDR2 (#MAB2538) (R&D Systems, detailed description in Supplemental Methods). Anti-rabbit IgG (#7074) and anti-mouse IgG (#7076) (Cell Signaling Technology) were used as secondary antibodies. As a control for equal loading, the membranes were blocked again, incubated overnight with a GAPDH primary antibody (#G99545, Sigma-Aldrich), and visualized as described above. HEK293 cells transiently transfected with a human DDR2 expression vector were used as a positive control (see Supplemental Methods). After use of an antibody against non-phosphorylated DDR2, no DDR2 was detected in fibroblasts from affected individuals or controls (Figure S5). In contrast, the antibody against Tyr740-phosphorylated DDR2 left a clear band of expected size in fibroblasts harboring the p.Leu610Pro and p.Tyr740Cys substitutions, but not in controls (Figure 4B and Figure S6). Increased phosphorylation of Tyr740 in cells heterozygous for the p.Tyr740Cys variant suggested that autophosphorylation of the wild-type protein partner of a DDR2 dimer took place in these cells or that this antibody also binds to DDR2 if phosphorylated at nearby tyrosine residues, e.g., Tyr736 and Tyr741 (Figure 3). It is likely that both variants cause ligand-independent kinase activation, as has been described for other RTKs.8
The DDRs can interact with multiple proteins and also modulate signaling pathways initiated by other matrix receptors, cytokines,growth factors, and transmembrane receptors in a context- and cell-type-dependent manner.3, 4 We therefore evaluated the consequences of DDR2 activation on potential downstream growth-stimulatory pathways and STAT1. The latter is an important modifier in the overgrowth and tissue wasting seen in individuals with PDGFRB [MIM: 173410] gain-of-function variants.9 The following proteins were assessed with appropriate antibodies, all obtained from Cell Signaling Technology at recommended dilutions (see Supplemental Methods for details): phospho-Tyr542-PTPN11(SHP-2), phospho-Tyr580-PTPN11(SHP-2), PTPN11(SHP-2), phospho-Ser473-AKT, AKT, phospho-Thr202/Tyr204-MAPK3(ERK1), MAPK3(ERK1), phospho-Tyr416-SRC, Tyr416-SRC, phospho-Tyr527-SRC, Tyr527-SRC, SRC, and phospho-Tyr70-STAT1. We did not detect increased phosphorylation of any of these proteins (Figures S7–S21). This suggests that the consequence of DDR2 activation in Warburg-Cinotti syndrome (MIM: 618175) is targeted to a group of proteins with little signal-transduction crosstalk with well-known growth-stimulatory pathways, such as the RAS/ERK and PI3K/AKT pathways.
Our finding that activating DDR2 variants are a cause of this disease suggested that the ABL inhibitor dasatinib, a leukemia drug that also inhibits DDR2, could be used for treatment of affected individuals.10, 11 To examine the effect of dasatinib on p.Leu610Pro- and p.Tyr740Cys-induced autophosphorylation, we cultured fibroblasts from affected individuals and controls as described above. When the cells were 80%–90% confluent, the medium was replaced with serum-free DMEM. After 16 hours, cells were either left untreated or treated with 0.05 or 0.1μM dasatinib (#S1021, Selleckchem); they were then harvested after 6 hours as described above. Immunoblot analysis determined the presence of phospho-Tyr740-DDR2. At both concentrations, dasatinib abolished the observed autophosphorylation of DDR2 (Figure4C and Figures S22 and S23), providing invitro support for experimental treatment of affected individuals. Penttinen syndrome, associated with activating mutations in PDGFRB, and Warburg-Cinottisyndrome have many similarities, such as lipodystrophy, subcutaneous-tissue wasting and accompanying hypertrophic lesions, and marked acro-osteolysis.12 Of note, PDGF-targeted therapy has been effective in three reported individuals with germline activating PDGFRB mutations.13, 14
DDR2 is an important regulator of bone growth and resorption, both as a promoter of osteoblastogenesis and as an inhibitor of osteoclastogenesis.15, 16 DDR2 has been suggested as a therapeutic target for osteoporosis.16 In addition, DDR2-collagen interaction stimulates the secretion of lysyl oxidase, which cross-links collagen fibers in the ECM.3, 17 Why activating mutations in DDR2 might be associated with osteolysis in the individuals described here remains to be elucidated. However, bi-allelic loss-of-function variants in DDR2 cause spondylometaepiphyseal dysplasia accompanied by short limbs and abnormal (premature) calcifications (SMED-SL [MIM: 271665]).18, 19 This developmental disorder is associated with decreased bone formation but not increased bone destruction (or osteolysis). No skin or eye changes have been reported.18, 19, 20 The individuals described in this report had normal or tall stature and normal limb lengths, indicating normal developmental bone growth. They also had an acquired arthropathy with osteolysis that was associated with flexion contractures. Premature calcifications (as seen in SMED-SL) were not observed in any of the individuals with Warburg-Cinotti syndrome. Thus, the phenotype of the individuals reported here with gain-of-function DDR2 variants was distinct from that seen in SMED-SL, which is associated with loss-of-function DDR2 variants.
The individuals described here experienced corneal vascularization in early adult life. DDR2 is able to both drive and prevent angiogenesis under different conditions.21, 22, 23 The primary effect seems to be angiogenesis stimulation, but a rebound effect or overcompensation can counterbalance this.22 In several of the individuals, limbal stem-cell deficiency (LSCD) was detected at an early age. The corneal epithelium undergoes constant shedding and regeneration to maintain optic clarity. The surrounding limbus forms a barrier to protect the cornea from neovascularization. In LSCD this barrier is disrupted, and the corneal epithelium might be replaced with conjunctival cells, similar to what we observed in these individuals. Although symptoms of LSCD often include signs of inflammation (redness and irritation), corneal neovascularization might also develop in the absence of such signs. One example is in aniridia caused by haploinsufficiency of PAX6.24, 25
The individuals described here had multiple skin problems, including thin skin, chronic ulcers, and a tendency to form keloid-like lesions. In mice, the highest levels of phosphorylated DDR2 were found in the lungs, ovaries, and skin.26 DDR2 is thought to be both a marker and key regulator of the epithelial-mesenchymal transition.22 DDR2 signaling is important for wound healing through multiple mechanisms: chemotactic migration to the wounded area, proliferation, synthesis and remodeling of the wound matrix by collagen cross-linking, and finally, fibroblast-mediated contraction of the healing wound. Inhibition of DDR2 activity has been postulated as a therapeutic option to improve wound healing and reduce keloid formation,27 which is supported by our data.
Several of the keloid-like lesions seen in these individuals were pigmented. It is of potential relevance that genetic variants in DDR1 [MIM: 600408] have been associated with vitiligo, and DDR1 activation is involved when melanocytes are attached to collagen-IV fibers.28 Because DDR1 and DDR2 are paralogous receptors, we hypothesize that activated DDR2 has a role in the migration or function of melanocytes.
In conclusion, we have identified the cause of a fibrotic syndrome that is inherited in an autosomal-dominant pattern. This syndrome is characterized by corneal vascularization, acro-osteolysis, contractures, thin skin, keloid-like plaques, and ulcerations, particularly of the toes and feet. We suggest it should be designated Warburg-Cinotti syndrome, after the authors of the first two clinical reports. In addition, we have identified a family and another singleton individual with the same condition. All affected individuals had activating variants in DDR2, either p.Leu610Pro or p.Tyr740Cys. We show that dasatinib inhibited the ligand-independent DDR2 autophosphorylation induced by both variants in vitro, suggesting an approach for treatment.
Declaration of Interests
L.G.B. is an uncompensated advisor to the Illumina Corporation, receives royalties from Genentech, and in-kind research support from ArQule. The other authors declare no competing interests.
Acknowledgments
We thank Unni Larsen for technical assistance and Raoul C.M. Hennekam and Karen Brøndum-Nielsen for professional assistance. The work was supported by grants from the Western Norway Regional Health Authority (911977 and 912161 to C.B.), the Dr. Jon S. Larsens Foundation (to C.B.), and the Olav Raagholt and Gerd Meidel Raagholt Foundation for Research (to C.B.). L.G.B., J.J.J., and J.C.S. were supported by the Intramural Research Program of the National Human Genome Research Institute, grants HG200328 12 and HG200388 04. The NIH Intramural Sequencing Center performed exome sequencing on the family identified at the NIH (individuals 3–5).
Published: November 15, 2018
Footnotes
Supplemental Data include 23 figures, Supplemental Methods, and one table and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.10.013.
Web Resources
Exac Browser, http://exac.broadinstitute.org/
GnomAD Browser, http://gnomad.broadinstitute.org/
Online Mendelian Inheritance in Man, http://www.omim.org/
Supplemental Data
References
- 1.Warburg M., Ullman S., Jensen H., Pedersen H., Kobayashi T., Russell B., Tranebjaerg L., Richard G., Brøndum-Nielsen K. Blepharophimosis, corneal vascularization, deafness, and acroosteolysis: A “new” syndrome? Am. J. Med. Genet. A. 2006;140:2709–2713. doi: 10.1002/ajmg.a.31543. [DOI] [PubMed] [Google Scholar]
- 2.Cinotti E., Ferrero G., Paparo F., Papadia M., Faravelli F., Rongioletti F., Traverso C., Di Maria E. Arthropathy, osteolysis, keloids, relapsing conjunctival pannus and gingival overgrowth: A variant of polyfibromatosis? Am. J. Med. Genet. A. 2013;161A:1214–1220. doi: 10.1002/ajmg.a.35908. [DOI] [PubMed] [Google Scholar]
- 3.Leitinger B. Discoidin domain receptor functions in physiological and pathological conditions. Int. Rev. Cell Mol. Biol. 2014;310:39–87. doi: 10.1016/B978-0-12-800180-6.00002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borza C.M., Pozzi A. Discoidin domain receptors in disease. Matrix Biol. 2014;34:185–192. doi: 10.1016/j.matbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alves F., Vogel W., Mossie K., Millauer B., Höfler H., Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–618. [PubMed] [Google Scholar]
- 6.Valiathan R.R., Marco M., Leitinger B., Kleer C.G., Fridman R. Discoidin domain receptor tyrosine kinases: New players in cancer progression. Cancer Metastasis Rev. 2012;31:295–321. doi: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang K., Kim J.H., Kim H.J., Park I.S., Kim I.Y., Yang B.S. Tyrosine 740 phosphorylation of discoidin domain receptor 2 by Src stimulates intramolecular autophosphorylation and Shc signaling complex formation. J. Biol. Chem. 2005;280:39058–39066. doi: 10.1074/jbc.M506921200. [DOI] [PubMed] [Google Scholar]
- 8.Chen H., Marsiglia W.M., Cho M.K., Huang Z., Deng J., Blais S.P., Gai W., Bhattacharya S., Neubert T.A., Traaseth N.J., Mohammadi M. Elucidation of a four-site allosteric network in fibroblast growth factor receptor tyrosine kinases. eLife. 2017;6 doi: 10.7554/eLife.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He C., Medley S.C., Kim J., Sun C., Kwon H.R., Sakashita H., Pincu Y., Yao L., Eppard D., Dai B. STAT1 modulates tissue wasting or overgrowth downstream from PDGFRβ. Genes Dev. 2017;31:1666–1678. doi: 10.1101/gad.300384.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terai H., Tan L., Beauchamp E.M., Hatcher J.M., Liu Q., Meyerson M., Gray N.S., Hammerman P.S. Characterization of DDR2 inhibitors for the treatment of DDR2 mutated nonsmall cell lung cancer. ACS Chem. Biol. 2015;10:2687–2696. doi: 10.1021/acschembio.5b00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L., Hussain M., Luo J., Duan A., Chen C., Tu Z., Zhang J. Synthesis and biological evaluation of novel dasatinib analogues as potent DDR1 and DDR2 kinase inhibitors. Chem. Biol. Drug Des. 2017;89:420–427. doi: 10.1111/cbdd.12863. [DOI] [PubMed] [Google Scholar]
- 12.Johnston J.J., Sanchez-Contreras M.Y., Keppler-Noreuil K.M., Sapp J., Crenshaw M., Finch N.A., Cormier-Daire V., Rademakers R., Sybert V.P., Biesecker L.G. A point mutation in PDGFRB causes autosomal-dominant penttinen syndrome. Am. J. Hum. Genet. 2015;97:465–474. doi: 10.1016/j.ajhg.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mudry P., Slaby O., Neradil J., Soukalova J., Melicharkova K., Rohleder O., Jezova M., Seehofnerova A., Michu E., Veselska R., Sterba J. Case report: Rapid and durable response to PDGFR targeted therapy in a child with refractory multiple infantile myofibromatosis and a heterozygous germline mutation of the PDGFRB gene. BMC Cancer. 2017;17:119. doi: 10.1186/s12885-017-3115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pond D., Arts F.A., Mendelsohn N.J., Demoulin J.B., Scharer G., Messinger Y. A patient with germ-line gain-of-function PDGFRB p.N666H mutation and marked clinical response to imatinib. Genet. Med. 2018;20:142–150. doi: 10.1038/gim.2017.104. [DOI] [PubMed] [Google Scholar]
- 15.Lin K.L., Chou C.H., Hsieh S.C., Hwa S.Y., Lee M.T., Wang F.F. Transcriptional upregulation of DDR2 by ATF4 facilitates osteoblastic differentiation through p38 MAPK-mediated Runx2 activation. J. Bone Miner. Res. 2010;25:2489–2503. doi: 10.1002/jbmr.159. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Su J., Wu S., Teng Y., Yin Z., Guo Y., Li J., Li K., Yao L., Li X. DDR2 (discoidin domain receptor 2) suppresses osteoclastogenesis and is a potential therapeutic target in osteoporosis. Sci. Signal. 2015;8:ra31. doi: 10.1126/scisignal.2005835. [DOI] [PubMed] [Google Scholar]
- 17.Khosravi R., Sodek K.L., Faibish M., Trackman P.C. Collagen advanced glycation inhibits its discoidin domain receptor 2 (DDR2)-mediated induction of lysyl oxidase in osteoblasts. Bone. 2014;58:33–41. doi: 10.1016/j.bone.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bargal R., Cormier-Daire V., Ben-Neriah Z., Le Merrer M., Sosna J., Melki J., Zangen D.H., Smithson S.F., Borochowitz Z., Belostotsky R., Raas-Rothschild A. Mutations in DDR2 gene cause SMED with short limbs and abnormal calcifications. Am. J. Hum. Genet. 2009;84:80–84. doi: 10.1016/j.ajhg.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali B.R., Xu H., Akawi N.A., John A., Karuvantevida N.S., Langer R., Al-Gazali L., Leitinger B. Trafficking defects and loss of ligand binding are the underlying causes of all reported DDR2 missense mutations found in SMED-SL patients. Hum. Mol. Genet. 2010;19:2239–2250. doi: 10.1093/hmg/ddq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge C., Wang Z., Zhao G., Li B., Liao J., Sun H., Franceschi R.T. Discoidin receptor 2 controls bone formation and marrow adipogenesis. J. Bone Miner. Res. 2016;31:2193–2203. doi: 10.1002/jbmr.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S., Bu X., Zhao H., Yu J., Wang Y., Li D., Zhu C., Zhu T., Ren T., Liu X. A host deficiency of discoidin domain receptor 2 (DDR2) inhibits both tumour angiogenesis and metastasis. J. Pathol. 2014;232:436–448. doi: 10.1002/path.4311. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H., Bian H., Bu X., Zhang S., Zhang P., Yu J., Lai X., Li D., Zhu C., Yao L., Su J. Targeting of discoidin domain receptor 2 (DDR2) prevents myofibroblast activation and neovessel formation during pulmonary fibrosis. Mol. Ther. 2016;24:1734–1744. doi: 10.1038/mt.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu T., Zhu J., Bu X., Zhao H., Zhang S., Chang Y., Li R., Yao L., Wang Y., Su J. The anti-angiogenic role of discoidin domain receptor 2 (DDR2) in laser-induced choroidal neovascularization. J. Mol. Med. (Berl.) 2015;93:187–198. doi: 10.1007/s00109-014-1213-7. [DOI] [PubMed] [Google Scholar]
- 24.Lim P., Fuchsluger T.A., Jurkunas U.V. Limbal stem cell deficiency and corneal neovascularization. Semin. Ophthalmol. 2009;24:139–148. doi: 10.1080/08820530902801478. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez G., Sasamoto Y., Ksander B.R., Frank M.H., Frank N.Y. Limbal stem cells: identity, developmental origin, and therapeutic potential. Wiley Interdiscip. Rev. Dev. Biol. 2018;7 doi: 10.1002/wdev.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labrador J.P., Azcoitia V., Tuckermann J., Lin C., Olaso E., Mañes S., Brückner K., Goergen J.L., Lemke G., Yancopoulos G. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep. 2001;2:446–452. doi: 10.1093/embo-reports/kve094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Márquez J., Olaso E. Role of discoidin domain receptor 2 in wound healing. Histol. Histopathol. 2014;29:1355–1364. doi: 10.14670/HH-29.1355. [DOI] [PubMed] [Google Scholar]
- 28.Ricard A.S., Pain C., Daubos A., Ezzedine K., Lamrissi-Garcia I., Bibeyran A., Guyonnet-Dupérat V., Taieb A., Cario-André M. Study of CCN3 (NOV) and DDR1 in normal melanocytes and vitiligo skin. Exp. Dermatol. 2012;21:411–416. doi: 10.1111/j.1600-0625.2012.01473.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.