ABSTRACT
Activation of the immune system induces rapid reductions in hypothalamic-pituitary–gonadal (HPG) axis activity, which in turn decreases secretion of sex steroids. This response is likely adaptive for survival by temporarily inhibiting reproduction to conserve energy; however, the physiological mechanisms controlling this response remain unclear. The neuropeptide kisspeptin is a candidate to mediate the decrease in sex hormones seen during sickness through its key regulation of the HPG axis. In this study, the effects of acute immune activation on the response to kisspeptin were assessed in male Siberian hamsters (Phodopus sungorus). Specifically, an immune response was induced in animals by a single treatment of lipopolysaccharide (LPS), and reproductive hormone concentrations were determined in response to subsequent injections of exogenous kisspeptin. Saline-treated controls showed a robust increase in circulating testosterone in response to kisspeptin; however, this response was blocked in LPS-treated animals. Circulating luteinizing hormone (LH) levels were elevated in response to kisspeptin in both LPS- and saline-treated groups and, thus, were unaffected by LPS treatment, suggesting gonad-level inhibition of testosterone release despite central HPG activation. In addition, blockade of glucocorticoid receptors by mifepristone did not attenuate the LPS-induced inhibition of testosterone release, suggesting that circulating glucocorticoids do not mediate this phenomenon. Collectively, these findings reveal that acute endotoxin exposure rapidly renders the gonads less sensitive to HPG stimulation, thus effectively inhibiting sex hormone release. More broadly, these results shed light on the effects of immune activation on the HPG axis and help elucidate the mechanisms controlling energy allocation and reproduction.
KEY WORDS: HGP axis, LPS, Immune system, Testosterone
Summary: Within hours of an immune response in the Siberian hamster, gonadal activity is suppressed, while pituitary function remains, providing evidence for direct immune-to-gonad communication and demonstrating reproductive system–immune system trade-offs.
INTRODUCTION
Infection and disease are challenges to the energetic balance of an organism that lead to a coordinated reallocation of energy. By presenting an immediate physiological threat, inflammation can potently downregulate other costly life processes, such as reproduction (Dantzer and Kelley, 2007). Reproduction is particularly sensitive to energy perturbations, which can have substantial consequences to human and animal health; however, the mechanistic understanding of regulation of reproduction by the immune system remains incomplete.
The presence of a pathogen or foreign substance within the body induces a complex, physiological response that includes both the immune system and the hormonal stress axis. Upon recognition of a pathogen, the innate immune system induces inflammation via release of proinflammatory cytokines, including interleukin (IL)-1, IL-6 and tumor necrosis factor-alpha (TNF-α). The resulting fever and inflammation are accompanied by a suite of behavioral changes, such as social withdrawal, anorexia and depressed activity that are collectively termed sickness behavior. Sickness behavior has been proposed to be an adaptive mechanism for overcoming an immune challenge (Hart, 1988; Kent et al., 1992). The neuroendocrine hypothalamic-pituitary–adrenal (HPA) axis is also activated during infection, potentially via actions of cytokines upon the hypothalamus (Berkenbosch et al., 1987; Soto et al., 1998). Hypothalamic corticotropin-releasing hormone (CRH) induces the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary. ACTH circulates to the adrenal cortex and causes the release of the glucocorticoids cortisol and corticosterone in hamsters. The resulting rise in glucocorticoids suppresses inflammation and upregulates the release of anti-inflammatory cytokines such as IL-10 in order to restrain the rising immune response (Coutinho and Chapman, 2011).
The innate immune response can be triggered experimentally by administration of the compound lipopolysaccharide (LPS), an abundant structural component of the cell walls of Gram-negative bacteria, such as Escherichia coli. Systemic injections of LPS upregulate the release of IL-1, IL-6 and TNF-α (Rietschel et al., 1996), and injections of LPS dose-dependently increase serum corticosterone in rats (Soto et al., 1998). LPS can therefore be used to investigate the physiological effects of infection and how the immune system regulates other functions such as reproduction.
Reproduction is necessary but metabolically expensive and is controlled primarily by the neuroendocrine hypothalamic-pituitary–gonadal (HPG) axis. Upon stimulation, gonadotropin-releasing hormone (GnRH) is released from the hypothalamus and transported to the anterior pituitary, where it induces the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) into circulation. These hormones act on the gonads to induce the ovarian cycle and estrogen release in females or spermatogenesis and testosterone production in males. In addition to the gestation and birthing of offspring, female mammals also nurture their young until weaning, which requires the most energy of all the reproductive functions. Therefore, attempting reproduction at a time of high energy needs can have severe consequences on maternal investment towards and survival of offspring (Wade and Schneider, 1992). It is no surprise then that the HPG axis is highly sensitive to food shortage, psychological stress and infection. However, the exact mechanism of physiological communication between the reproductive and immune systems is not understood. Evidence from animal models points to a central action of the immune system on the HPG axis. Injection of cytokines decreases plasma levels of LH, and LPS inhibits normal preovulatory LH surges in rats, potentially due to decreased activity of GnRH neurons in the hypothalamus (Rivier and Vale, 1990; He et al., 2003).
Although the mechanisms of these effects of immune activation on reproduction remain unclear, the neuropeptide kisspeptin offers a possible route of communication between the periphery and the reproductive neuroendocrine axis. Kisspeptin is a hypothalamic neuropeptide that, when injected centrally or peripherally, potently stimulates the release of LH and, to a lesser extent, FSH across vertebrate species (Oakley et al., 2009). Neurons releasing kisspeptin are found in the arcuate nucleus (ARC) and the anteroventral periventricular nucleus (AVPV) of the hypothalamus and project to GnRH neurons (Gottsch et al., 2004). Although expression of the receptor for kisspeptin (GPR54) has been reported in the hypothalamus, pituitary gland and testes, pretreating animals with GnRH receptor antagonists eliminates the LH, FSH and testosterone responses to kisspeptin (Irfan et al., 2014; Mason et al., 2007; Mei et al., 2013). In addition, neither cultured Leydig cells nor seminiferous tubule explants respond to kisspeptin, suggesting that kisspeptin cannot induce testosterone release directly from the testis (Mei et al., 2013). These findings reveal that kisspeptin primarily acts to stimulate GnRH release from the hypothalamus. Kisspeptin neurons express receptors for sex steroids (Smith et al., 2005a,b) and thus can mediate the feedback actions of gonadal steroids. These neurons are also regulated by numerous environmental conditions, such as nutritional state, ambient conditions (such as photoperiod) and social environment (Kriegsfeld, 2006). By integrating signals from the periphery to modify GnRH release, this neuronal population may act as the gateway to HPG axis activity. Cytokines and/or glucocorticoids may decrease HPG axis activity by inhibiting kisspeptin or the response to kisspeptin during times of immune or metabolic challenge. In support of this possibility, neonatal exposure to LPS can delay puberty and down-regulate medial preoptic area (mPOA) kisspeptin mRNA expression in pre-pubertal female rats (Knox et al., 2009). Furthermore, mPOA and ARC expression of Kiss1 mRNA is reduced in response to intravenous (i.v.) injection of LPS in female rats (Kinsey-Jones et al., 2009). Three daily intraperitoneal (i.p.) injections of LPS in male rats decreased both the number of kisspeptin-immunoreactive cells in the ARC and the response of the HPG axis to intracerebroventricular (i.c.v.) kisspeptin (Castellano et al., 2010).
These studies of the effects of experimentally induced sickness on kisspeptin expression have focused on chronic administration of LPS. However, the immune response to LPS is rapid, with detectable amounts of cytokines appearing within 30 min of injection (Quan et al., 1998). In addition, LPS can lead to decreases in gonadal steroids within hours of injection (Allen et al., 2004). Investigation of the immediate effects of experimental sickness can contribute to the determination of factors that suppress HPG function and to the identification of potential candidates for the mitigation of these effects. A previous study has shown that a single dose of LPS in ovariectomized female rats can suppress serum LH within 2 h of injection and that simultaneous administration of kisspeptin can restore serum LH (Iwasa et al., 2008). However, whether kisspeptin can ameliorate the rapid effects of LPS on downstream gonadal dysfunction in males remains to be determined.
In this study, we investigated the rapid effects of an acute immune challenge on the HPG axis response to exogenous kisspeptin in male Siberian hamsters (Phodopus sungorus) and attempted to determine whether glucocorticoids constitute part of the mechanism mediating these effects. Siberian hamsters have evolved robust physiological responses to environmental perturbations as a result of inhabiting areas of the world with extreme seasonal variation, making them an ideal model in which to study the energetic trade-offs between disease, nutrition and reproduction. In the first experiment, we induced an immune response via a single, peripheral injection of LPS. After 90 min, we assessed reproductive hormone levels in response to a peripheral injection of kisspeptin. Peripherally injected kisspeptin may act on GnRH neuronal terminals that extend outside the blood–brain barrier in the median eminence and has been shown to induce a dose-dependent increase in LH and testosterone within 30 min of injection (Aparicio, 2005; Greives et al., 2011). Therefore, if endotoxin suppresses acute activation of the HPG axis, we expected to see a decrease in responsiveness to kisspeptin, shown by a decrease in the typical kisspeptin-induced rises in LH and testosterone. Following the results of the first experiment, we sought to test the hypothesis that glucocorticoids mediate the effects of LPS on the HPG axis. To do this, we blocked glucocorticoid receptors (GRs) using the GR antagonist RU-486, or mifepristone. We predicted that if glucocorticoids mediate the effects of LPS on the HPG axis, blockade of GRs would allow a kisspeptin-induced increase in testosterone despite an immune challenge via LPS.
MATERIALS AND METHODS
Animals and housing
Adult (>60 days of age) male Siberian hamsters, Phodopus sungorus (Pallas 1773), were obtained from the breeding colony at Indiana University. Breeding pairs and pre-weaned offspring were housed together in large polypropylene cages (45×23×15 cm) until weaning at 18 days of age. Post-weaning, hamsters were group-housed with same-sex individuals in smaller polypropylene cages (27.8×17.5×13 cm) until 2 weeks prior to the experiment, when animals were individually housed in polypropylene cages of the same size. Body mass of each animal was recorded 1 week after individual housing. Animals were maintained on a 16 h:8 h (light:dark; lights on at 02:00 h) photoperiod, 20±2°C temperature and 50±10% humidity. All animals were given access to food (Purina rat chow, St Louis, MO, USA) and water ad libitum throughout the study. All experimental procedures followed NIH guidelines and were in accordance with the Bloomington Institutional Animal Care and Use Committee (BIACUC).
Experiment 1: effects of LPS on kisspeptin-induced HPG activation
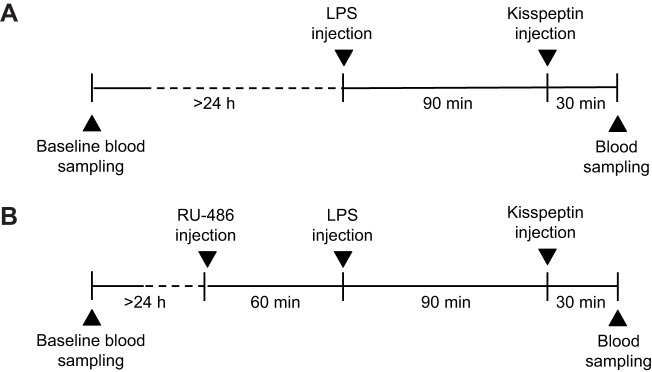
In order to test the hypothesis that acute immune system activation attenuates reproductive functioning, we examined the effects of endotoxin administration on kisspeptin-induced HPG axis activity (Fig. 1A). A blood sample was obtained prior to the day of experiments as described below for baseline determination of serum LH, testosterone and cortisol concentration. Thirty-eight adult male hamsters were randomly assigned to one of two treatment groups: i.p. injection with 10 µg LPS per animal (LPS from Salmonella enterica serotype typhimurium, Sigma-Aldrich, St Louis, MO, USA; n=20) or 0.9% saline vehicle (control; n=18). This dose of LPS was based upon a previous study from our lab demonstrating that a dose of 2.5 µg per animal was sufficient to cause a roughly 50% reduction in pregnancy success rate in female Siberian hamsters (French et al., 2013). Given that a commonly used higher dose of 25 µg per animal can sometimes induce sepsis in the hamster, our moderate dose of 10 µg per animal is high enough to induce an immune response and disrupt the HPG axis while reducing the likelihood of septic shock, which could act as a confounding variable in our experiment. Animals in each group (LPS or control) were then randomly assigned to receive i.p. injections of either 10 μmol l−1 kisspeptin-10 [KiSS-1 (112-121)/metastin (45-54) (human); Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA] or 0.1 mol l−1 phosphate-buffered saline (PBS) vehicle 90 min after LPS or vehicle injections. We previously demonstrated that this dose induces a robust increase in circulating LH in male Siberian hamsters (Greives et al., 2011). The combinations of LPS and kisspeptin treatments created four distinct groups: saline+PBS (n=9), saline+kisspeptin (n=9), LPS+PBS (n=10) and LPS+kisspeptin (n=10). Thirty minutes after kisspeptin administration, a terminal blood sample was obtained. The time points in this study were chosen to target the acute peak of the LPS response in the Siberian hamster. Fever responses are evident by 2 h after injection with LPS (Bilbo et al., 2002), and kisspeptin has been shown to induce robust increases in LH and testosterone 30 min after injection (Greives et al., 2011). All procedures were carried out in the morning (6 h after lights on). To assess the physiological response to LPS, body temperature was obtained via rectal probe and was recorded once prior to initial injection and again before the terminal blood sampling.
Fig. 1.
Experimental design. (A) Experiment 1 – animals were first injected with lipopolysaccharide (LPS) (or saline as the control) to induce an immune response and then returned to the home cage. After 90 min, animals were injected with kisspeptin [or phosphate-buffered saline (PBS) vehicle]. Blood samples were collected 30 min after kisspeptin injection. (B) Experiment 2 – animals were injected with mifepristone (RU-486) or oil control in order to block glucocorticoid receptors. After 60 min, animals were injected with LPS (or saline), followed by kisspeptin (or PBS) and blood sampling, similar to experiment 1.
Experiment 2: role of GRs in LPS-induced blockade of HPG
In order to test the hypothesis that LPS-induced blockade of HPG activity is mediated by the HPA axis and downstream release of glucocorticoids, we replicated experiment 1 with the addition of a GR antagonist, mifepristone (Fig. 1B). Sixty-four male Siberian hamsters were randomly assigned to receive i.p. injection of either 0.5 mg mifepristone (RU-486; Sigma-Aldrich) suspended in 0.3 ml sesame oil or oil vehicle (control). This dose (∼11 mg kg−1) was chosen based on work performed in Syrian hamsters and prairie voles in which doses ranging from 4 to 40 mg kg−1 could affect behavior and cortisol-dependent transcriptional activity without affecting HPA axis negative feedback (Wommack and Delville, 2007; Curtis and Wang, 2005; Jimenez et al., 1999).
One hour after mifepristone pre-treatment, animals received either 10 μg LPS or saline vehicle, followed 90 min later by either 10 μmol l−1 kisspeptin or PBS vehicle, as described previously. This timeline was modeled on work conducted in male Syrian hamsters in which injection of mifepristone 1 h before injection of cortisol blocked the glucocorticoid-induced acceleration of the development of agonistic behavior (Wommack and Delville, 2007). Body temperature was not recorded in this experiment in order to minimize animal handling and disturbance. Treatment and control injections yielded eight distinct experimental groups: oil+saline+PBS (n=11), oil+saline+kisspeptin (n=12), oil+LPS+PBS (n=12), oil+LPS+kisspeptin (n=12), mifepristone+saline+PBS (n=10), mifepristone+saline+kisspeptin (n=10), mifepristone+LPS+PBS (n=10) and mifepristone+LPS+kisspeptin (n=9). Blood samples were obtained as in experiment 1.
Blood sampling
Blood samples from all animals were collected 1 day prior to experimental procedures, within 1 h of when post-treatment samples would be collected, in order to assess baseline circulating hormone levels. Thirty minutes after kisspeptin or PBS injection, the post-treatment blood sample was taken. Animals were anesthetized with isoflurane, and samples collected via the retro-orbital sinus. Samples were allowed to clot at room temperature for 1 h before clots were removed. Samples were then centrifuged at 4°C for 30 min at 5000 g. Serum aliquots were separated and stored at −20°C until assayed for testosterone, LH and cortisol.
Hormone assays
Serum testosterone concentrations were measured via a commercial enzyme immunoassay (EIA) kit (Testosterone ELISA Kit ADI-900-065, Enzo Life Sciences, Plymouth Meeting, PA, USA). Serum samples were diluted for measurement on the linear phase of the standard curve and run in duplicate for each sample. The sensitivity of the assay was 7.81 pg ml−1, determined as the lowest concentration of standard. The intra-assay coefficient of variation (CV) was <10.8% and the inter-assay CV was 9.3%. The antiserum used in the assay was highly specific for testosterone, with low cross-reactivity with other hormones.
Serum LH concentrations were determined via a radioimmunoassay (RIA) described previously (Legan et al., 2009), with slight modifications. The standard (rat LH, RP-3) and purified LH for iodination were obtained from Dr A. F. Parlow (National Hormone and Peptide Program, Torrance, CA, USA). Post-injection samples from animals treated with kisspeptin were run in duplicate; however, because detection of basal levels required a large volume of serum (100 µl), only single aliquots of each pre-injection (all animals) and post-injection (PBS-treated animals) samples were diluted in 0.05 mol l−1 PBS containing 0.1% gelatin (gel-PBS). The primary antibody was CSU 120 (provided by Dr Terry Nett, Colorado State University, Fort Collins, CO, USA) diluted 1:10,000 in 1:100 normal rabbit serum (Millipore, St Charles, MO, USA). The tubes were incubated for 24 h at 22°C after addition of 100 µl primary antibody, again after adding radiolabeled LH (∼30,000 counts min−1 per 100 µl gel-PBS, iodinated by the iodogen method), and again following the addition of the secondary antibody (anti-rabbit gamma globulin, diluted 1:50 in gel-PBS, Millipore). The LH results reported herein were obtained from four assays for which the mean sensitivity was 0.034 ng ml−1, determined as two standard deviations below the maximum binding. Two replicates each of a standard serum pool from female hamsters that inhibited binding on average to 39.6% were analyzed at the beginning, middle and end of each assay for determination of inter- and intra-assay CV. The inter-assay CV was 5.8% and the mean intra-assay CV was 6.1%.
Serum cortisol concentration was measured for experiment 1 via a commercial EIA kit (Cortisol ELISA Kit 900-071, Enzo Life Sciences). Serum samples were diluted 1:40 and run in duplicate for each sample. The sensitivity of the assay was 6.24 ng ml−1, determined as the lowest concentration of standard. The intra-assay CV was 1.56% and the inter-assay CV was 3.44%. The antiserum used in the assay was highly specific for cortisol, with low cross-reactivity with other hormones. Limited serum samples prohibited us from re-analyzing any sample that ran beyond the highest standard (400 ng ml−1) of the assay. Therefore, these samples were conservatively assigned a value of 400 ng ml−1.
Statistical analyses
Sample sizes for each group were determined by previous studies with kisspeptin and LPS in this species (Greives et al., 2011; French et al., 2013). In experiment 1, the effects of peripheral injection of LPS and kisspeptin on circulating testosterone, cortisol and body temperature were analyzed using separate, three-way mixed-model ANOVA with time (pre- and post-treatment) as the within-subjects factor and LPS and kisspeptin treatment as between-subjects factors. In experiment 2, four-way mixed-model ANOVA were used with time as the within-subjects factor and mifepristone, LPS and kisspeptin as between-subjects factors. In our studies, we found that assumptions for homogeneity of variance were violated, and methods of transforming the data did not resolve this. We then used bootstrapping with 1000 samples to construct 95% confidence intervals (CIs) for the means of each group. This yielded the same conclusions as our ANOVA, lending confidence to our analyses. In addition, several outliers were detected in various groups; however, removal of outliers greater than 2 standard deviations from the mean had no effect on the conceptual conclusions. Therefore, we elected to retain all data points. In all cases, models were considered statistically significant if P<0.05. Overall significant ANOVA were followed up with appropriate tests (ANOVA or t-test) with Bonferroni corrections for family-wise multiple comparisons when appropriate to probe for post hoc differences between treatment groups.
To compare the effectiveness of kisspeptin and LH on inducing testosterone release in saline and LPS groups, linear regressions between the change in serum LH (ΔLH=post-injection−baseline) and the change in serum testosterone (ΔT) were performed and compared. For this regression analysis, we collapsed data collected from mifepristone- and vehicle-treated individuals because mifepristone had no effects on serum LH or testosterone (see Results). One outlier with a residual greater than 3 standard deviations was removed from the LPS-treated group. This individual, with ΔLH=1.16 and ΔT=86.11, had a large standardized residual of 3.26. To determine whether the slopes of the two models were significantly different, we used the Potthoff test for parallelism (Potthoff, 1974). Briefly, a binary variable ‘LPS’ was coded, and an analysis was performed with the variables ΔLH, LPS and LPS×ΔLH as predictors in a regression equation. Slopes are significantly different when the regression coefficient of the interaction term is a significant (P<0.05) predictor in the model. Differences in body mass between treatment groups in both experiments were investigated using a one-way ANOVA. Data are presented as means±s.e.m. All analyses were performed using IBM SPSS 19 for Windows (SPSS, Inc., Chicago, IL, USA).
RESULTS
Experiment 1: effects of LPS on kisspeptin-induced HPG activation
There were no significant differences in mean body mass between any of the treatment groups before treatment (F1,34=0.016, P=0.90; data not shown).
Body temperature
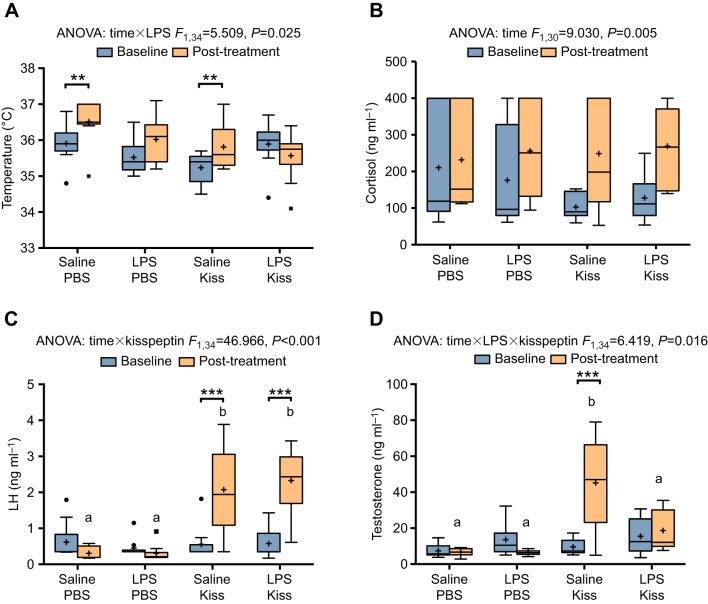
Saline-treated animals experienced an increase in body temperature over the course of the experiment (Fig. 2A), with a mean increase of 0.6±0.2°C from pre- to post-treatment (95% CI, 0.3–0.9), whereas LPS-treated animals showed no significant difference from baseline (significant time×LPS interaction: F1,34=5.51, P=0.025). Kisspeptin had no significant effect on body temperature (no significant three-way time×LPS×kisspeptin interaction: F1,34=3.52, P=0.069; no significant time×kisspeptin interaction: F1,34=3.93, P=0.056).
Fig. 2.
Acute LPS blocks the kisspeptin-induced increase in testosterone but not luteinizing hormone (LH). (A) Effects of LPS and kisspeptin (Kiss) injection on body temperature. LPS-treated animals did not experience significant rises in body temperature after injection. (B) All groups experienced increases in serum cortisol throughout the experiment. (C) Animals injected with kisspeptin experienced increases in serum LH, regardless of LPS treatment. (D) However, LPS blocked the kisspeptin-induced increase in testosterone. These results were replicated in experiment 2. Boxes denote the boundaries of the second and third quartiles with Tukey whiskers. Asterisks denote significant differences between pre- and post-injection time points. Significance was set at P<0.05 (**P<0.01, ***P<0.001). Plus signs indicate the group mean, while circles and squares represent individual values falling outside 1.5 times the interquartile range of the distribution of the pre- or post-injection time points. Letters denote differences between groups at the post-injection time point. Saline+PBS, n=9; LPS+PBS, n=10; saline+kisspeptin, n=9; LPS+kisspeptin, n=10. Group sizes for the cortisol graph in B (because of missing values): saline+PBS, n=8; LPS+PBS, n=9; saline+kisspeptin, n=7; LPS+kisspeptin, n=10.
Cortisol
There was a main effect of time in serum cortisol concentrations, which increased post-treatment when collapsed across all groups (significant main effect of time: F1,30=9.03, P=0.005). Post-treatment cortisol values were 97.2±32.4 ng ml−1 (95% CI, 31.1–163.3) greater than pre-treatment values. There were no significant differences in cortisol across groups (no significant three-way or two-way interactions: P>0.05) (Fig. 2B); however, our analysis was restricted by limited quantities of serum. Saline-treated groups experienced a mean increase of 85.3±50.8 ng ml−1 over baseline, and LPS-treated groups showed an increase of 110.6±43.7 ng ml−1 over baseline.
LH
Kisspeptin significantly increased serum LH (significant time×kisspeptin interaction: F1,34=46.97, P<0.001), with a mean difference of 1.9±0.2 ng ml−1 (95% CI, 1.4–2.4) between kisspeptin- and PBS-treated animals (Fig. 2C). However, this was not affected by LPS treatment (no three-way interaction: F1,34=0.012, P=0.91; no time×LPS interaction: P=0.46).
Testosterone
There were no significant differences between groups at the pre-injection time point. Only the saline+kisspeptin group showed an increase in testosterone, of 35.6±5.3 ng ml−1 (P<0.001). LPS treatment significantly attenuated this response to kisspeptin (significant three-way time×LPS×kisspeptin interaction: F1,34=6.42, P=0.016); thus, at the post-injection time point, mean testosterone was lower in LPS-treated animals after kisspeptin injection (18.7±3.5 ng ml−1) than in saline-treated animals after kisspeptin injection (45.2±8.5 ng ml−1), a mean difference of −26.6 ng ml−1 (95% CI, −39.3 to −13.8, P<0.001) (Fig. 2D).
Experiment 2: effects of mifepristone on blockade of kisspeptin-induced HPG activation
There were no significant differences in body mass between any of the groups before treatment (F1,78=0.22, P>0.05, data not shown).
LH
Mifepristone had no effect on serum LH (no statistically significant time×mifepristone×LPS×kisspeptin interaction: F1,78=2.19, P=0.14; no three- or two-way interactions with mifepristone; and no main effect of mifepristone, P>0.05; Fig. 3A). Similar to our first experiment, kisspeptin robustly increased serum LH regardless of LPS treatment (significant time×kisspeptin interaction: F1,78=144.90, P<0.001). Kisspeptin-treated animals experienced significant increases in LH over baseline values (P<0.001) and exhibited a mean increase of 2.0±0.2 ng ml−1 (95% CI: 1.7–2.3 ng ml−1) over PBS-treated controls (P<0.001). There was a statistically significant time×LPS×kisspeptin interaction (F1,78=4.45, P=0.038); however, after statistical correction for multiple comparisons, the LPS×kisspeptin interaction did not hold at the separate pre- and post-injection time points (F1,78=0.98, P=0.33, F1,78=2.75, P=0.10, respectively), suggesting that LPS did not significantly modify the LH response to kisspeptin.
Fig. 3.
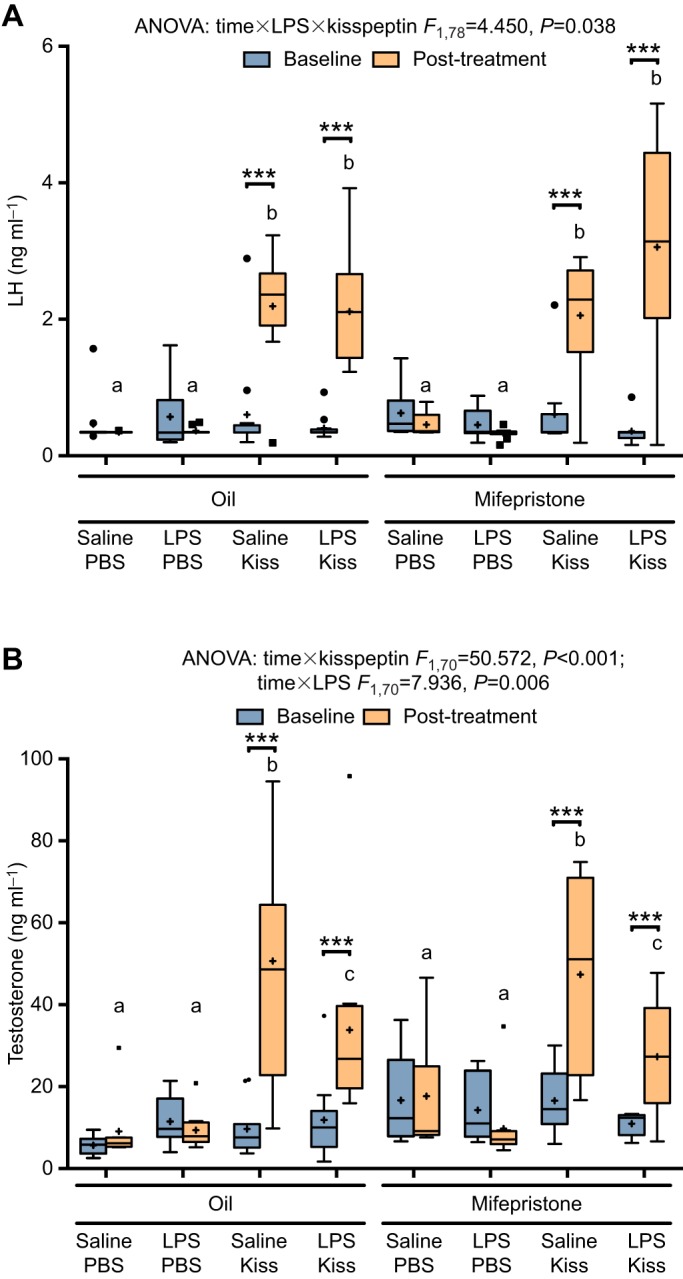
Blocking the actions of glucocorticoids does not ameliorate the effects of LPS on testosterone. (A) Animals pre-treated with mifepristone (RU-486) showed increases in serum LH after kisspeptin injection, regardless of LPS treatment. (B) Despite pre-treatment with mifepristone, LPS significantly attenuated the gonadal response to kisspeptin, similar to experiment 1. Boxes denote the boundaries of the second and third quartiles with Tukey whiskers. Asterisks denote significant differences between pre- and post-injection time points. Significance was set at P<0.05 (***P<0.001). Plus signs indicate the group mean, while circles and squares represent individual values falling within 1.5 times the interquartile range of the distribution of the pre- or post-injection time points. Letters denote differences between groups at the post-injection time point. For LH graph, oil+saline+PBS, n=11; oil+LPS+PBS, n=12; oil+ saline+kisspeptin, n=12; oil+LPS+kisspeptin, n=12; mifepristone+saline+PBS, n=10; mifepristone+LPS+PBS, n=10; mifepristone+saline+kisspeptin, n=10; mifepristone+LPS+kisspeptin, n=9. Group sizes for testosterone graph in B (because of missing values): n=10 for all groups except oil+saline+PBS, n=8; oil+saline+kisspeptin, n=11; mifepristone+LPS+kisspeptin, n=9.
Testosterone
In this experiment, it is worth noting that while no other single outlier affected our statistics in any parameter, one animal's testosterone value in the oil+LPS+kisspeptin group dramatically affected the statistical analysis. This value was 2.64 standard deviations from the mean for that group and was 3.24 interquartile ranges above the 3rd quartile. However, because this value lies within the range of expected post-kisspeptin testosterone levels and because analyses both with and without this animal yielded similar conceptual conclusions, we have elected to retain this individual in the analysis.
Mifepristone had no effect on testosterone levels (no time×mifepristone×LPS×kisspeptin interaction: F1,70=0.086, P=0.77; no three- or two-way interactions, and no main effect of mifepristone, P>0.05; Fig. 3B). When collapsed across mifepristone treatment, both kisspeptin and LPS significantly affected testosterone levels (significant time×kisspeptin interaction: F1,70=50.57, P<0.001; significant time×LPS interaction F1,70=7.94, P=0.006). Thus, at the post-injection time point, kisspeptin-treated animals had significantly higher serum testosterone (39.8±2.8 ng ml−1) than PBS-treated controls (11.5±2.9 ng ml−1; P<0.001). However, LPS-treated animals had lower post-treatment testosterone (20.1±2.9 ng ml−1) than saline-treated animals (31.2±2.9 ng ml−1), a mean difference of −11.2±4.0 ng ml−1 (95% CI, −19.2 to −3.1; P=0.007).
Relationship between LH and testosterone changes
We used the large sample sizes in our second experiment to investigate the relationship between changes in LH to changes in testosterone by performing linear regressions in saline- and LPS-treated animals. As expected, there were positive, linear relationships between ΔLH and ΔT in both groups (Pearson's r=0.78, P<0.001 and r=0.73, P<0.001, respectively). However, the slopes of these relationships were markedly different in LPS- versus saline-treated groups.
In saline-treated animals, ΔLH accounted for 61.5% of the variation in ΔT with an adjusted R2=60.4%. ΔLH significantly predicted ΔT (F1,37=59.05, P<0.001). The slope of the model was 18.98 (P<0.001; 95% CI, 13.98–23.99). In LPS-treated animals, ΔLH accounted for 53.3% of variation in ΔT with an adjusted R2=52.0%. The model was statistically significant (F1,36=41.15, P<0.001), and the slope of the model was 7.14 (P<0.001; 95% CI, 4.88–9.39). The slope of the saline group model was significantly greater than that of the LPS group model with a statistically significant difference of 11.85 (t=−4.66, P<0.001), indicating LH in saline-treated animals induced significantly more testosterone release than LH in LPS-treated animals (Fig. 4). This further reinforces our finding that the gonads of LPS-treated animals are less sensitive to stimulating peptides.
Fig. 4.
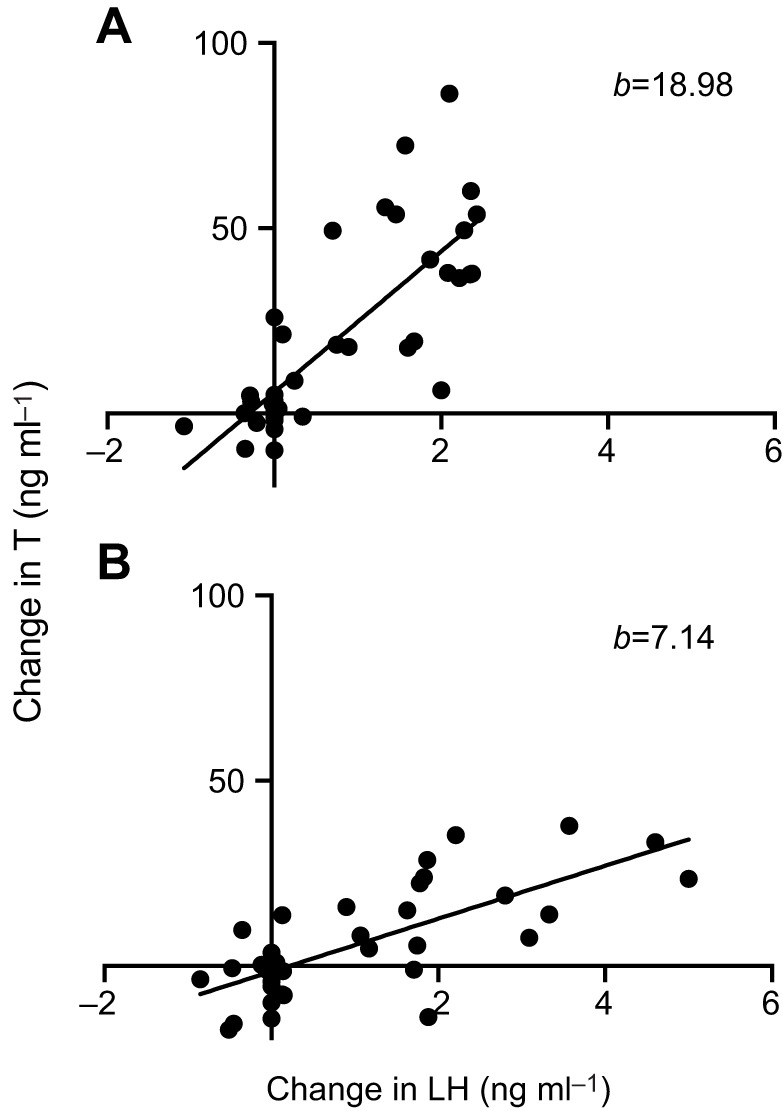
The testes are less sensitive to LH after acute LPS treatment. From our second experiment, we collapsed data across mifepristone and kisspeptin treatments and plotted the change in testosterone as a function of the change in LH. This revealed that, while the testes show a large surge in testosterone with increasing LH in saline-treated control animals (A), this response is dramatically decreased in LPS-treated animals (B). The slopes (b) of these two regressions were significantly different from each other (P<0.001), further demonstrating that acute endotoxin can dampen the testicular response to LH and kisspeptin. Saline, n=39; LPS, n=38.
DISCUSSION
In our first experiment, we analyzed the effects of acute LPS administration on the HPG axis response to kisspeptin. To do this, we determined serum LH and testosterone concentrations in response to kisspeptin injection after a single inoculation with LPS. Within 30 min, kisspeptin elicited a strong surge in LH, regardless of LPS treatment. However, LPS-treated animals showed no gonadal testosterone response to kisspeptin. Thus, during the first 2 h following LPS exposure, the pituitary remained sensitive to kisspeptin; however, LH did not induce significant testosterone release. These results suggest that the gonads were rendered insensitive to stimulatory hormones during the LPS challenge.
To understand the potential mechanisms behind this response, we analyzed body temperature and serum glucocorticoids before and after treatment to gauge the immune and HPA responses to LPS. For body temperature, our results indicated that saline-treated controls experienced increases in body temperature after treatment, while LPS-treated animals did not differ pre- to post-treatment. Increases in body temperature may have been due to the stress of repeated handling and injection or to daily variation in body temperature. During the typical response to LPS, the Siberian hamster demonstrates fever followed by a period of hypothermia (Bilbo et al., 2002; Carlton and Demas, 2017). Thus, while we were surprised to see no changes in temperature, our results may indicate that LPS initiated a hypothermic response that negated the rise in temperature seen in controls. This does not, however, eliminate the possibility that an increase in temperature occurred prior to a hypothermic response. In addition, rectal temperature may not be tightly correlated with intratesticular temperature. Increased intratesticular temperature may cause morphological damage to Leydig cells and thus affect testosterone production (Aktas and Kanter, 2009). Nonetheless, our LPS-treated animals did not exhibit increased rectal temperature, arguing against the possibility of body temperature effects on testicular function.
We also measured serum cortisol levels in our animals to gauge activation of the HPA axis. The results indicated that there was generally an increase in cortisol over the time of the experiment. While it is not surprising to see an increase in cortisol after multiple handlings and injections, there were no statistically significant differences between groups. Our measurements, however, were limited by the quantity of serum obtained and hence our inability to re-analyze samples that ran beyond the limits of the assay. Thus, while these results may suggest that cortisol does not specifically contribute to the effect, we cannot eliminate the possibility of differential HPA responses after LPS treatment. Indeed, LPS injections have been found to increase circulating glucocorticoids in male rats (Soto et al., 1998). Various stressors lead to increased circulating glucocorticoids with a concomitant decrease in testosterone (Whirledge and Cidlowski, 2010), and patients with Cushing's syndrome present with low circulating testosterone despite little change in LH (Smals et al., 1977). Furthermore, testosterone-producing Leydig cells express GRs, and glucocorticoids have been shown to decrease Leydig cell testosterone production within 30 min via rapid inhibition of cAMP production (Dong et al., 2004). Therefore, we chose to examine whether glucocorticoids mediate these rapid effects on the testes. In the second experiment, we administered mifepristone to animals to block GRs to test the hypothesis that this would ameliorate the effects of LPS and rescue sensitivity of the gonads to LH and kisspeptin.
Consistent with the first experiment, LPS had no effect on LH concentrations in this second experiment; LH levels were elevated by kisspeptin whether or not LPS was administered. Mifepristone also had no effect on LH concentrations. There was a trend of LPS to increase serum LH in kisspeptin-treated animals; however, this was not statistically significant after a correction for multiple comparisons. Thus, we showed again that the pituitary remains sensitive to kisspeptin over the course of acute LPS exposure.
Mifepristone, however, had no effect on serum testosterone. When data were collapsed across mifepristone treatment, we found that while kisspeptin increased testosterone in both saline- and LPS-treated animals, testosterone was significantly lower after LPS administration. Thus, LPS injection attenuated, rather than eliminated, testosterone release after kisspeptin injection. This replicates our original finding. Furthermore, we found that when the change in serum testosterone was examined as a function of the change in LH, animals injected with LPS showed much lower increases in testosterone per unit of LH increase, demonstrating again that the testes are less sensitive to stimulating peptides during an acute immune response. Finally, because mifepristone did not ameliorate the effect of LPS on the gonads, glucocorticoids likely do not play an important role in mediating the effects of acute inflammation on the testes. These negative results should be taken with caution, as few studies have utilized mifepristone in the Siberian hamster. Mifepristone dosage effects, general efficacy and impact upon HPA negative feedback are less understood in this species. Despite these caveats, our results align with other studies showing that glucocorticoids may not mediate suppression of testosterone production due to immobilization stress or IL-1β injection (Dong et al., 2004; Turnbull and Rivier, 1997).
Our results show that within 2 h of injection, the acute administration of LPS in the Siberian hamster can render the testes less sensitive to LH and kisspeptin, while the pituitary remains responsive. This suggests that peripheral mechanisms acting directly on the testes, rather than changes in LH levels, are responsible for the immediate inhibition of testosterone secretion in this species. In contrast, previous studies have investigated central mechanisms of HPG suppression. For example, LPS injections over several days were found to decrease the amount of kisspeptin produced within the hypothalamus (Castellano et al., 2010). However, our results indicate that LPS does not affect LH levels at 2 h post-injection in Siberian hamsters, suggesting that pituitary function remains unaffected in the time immediately after an inflammatory response. Thus, while downregulation of kisspeptin may mediate the long-term effects of LPS on the HPG axis, other more acute mechanisms may act upon the gonads to suppress testosterone release immediately following inflammation.
Work in other species has also shown that LH is decreased by 2 h after acute endotoxin administration. For example, i.c.v. administration of LPS led to decreased serum LH within 60 min of injection in gonadectomized male rats (Ebisui et al., 1992). In addition, a study in ovariectomized female rats demonstrated that LH is decreased by 2 h after peripheral LPS administration and that simultaneous kisspeptin injections rescued serum LH (Iwasa et al., 2008). Such differences in the effects on LH may arise from differences in factors such as routes of LPS administration, sex, species and gonadectomy. The timing and strength of the LPS effect may also contribute to our findings. For example, testing our animals at an hour closer to natural foraging times in this crepuscular species could reveal stronger hypothalamic inhibition. In addition, disparate effects on LPS-induced neuroinflammation have been found in studies that utilize low versus high LPS doses and different sampling time points after LPS administration (Lopes, 2016). Here, we chose a moderate LPS dose for this species that still yields HPG disruption; higher doses could conceivably initiate hypothalamic suppression earlier. In addition, several other studies have shown that kisspeptin expression, GnRH release and LH release can be decreased with long-term LPS exposure (Castellano et al., 2010; Rivier and Vale, 1990; He et al., 2003). Sampling from our animals at later time points, then, may have eventually yielded decreases in LH. These considerations, however, make our results all the more interesting, as we saw a blunting of the testosterone response with a moderate LPS dose prior to any blunting of the LH response. In our study, we focused on the acute phase of the immune response, and we tested the effects of peripherally administered LPS in gonadally intact males of a species that is exquisitely sensitive to environmental conditions. Our dosage and our timing revealed a mechanism of gonadal suppression that may be understudied in this and other species, in which LPS blocks the effects of stimulatory hormones on the gonads, suggesting a dichotomy between pituitary and gonadal responses to an immune challenge.
These results align with other studies that suggest a mechanism of direct inhibition on the testes. For example, i.c.v. and i.v. injection of IL-1β inhibits testosterone release induced by human chorionic gonadotropin (hCG) in rats (Turnbull and Rivier, 1995). Although LH was decreased in this model, hCG should act directly on the gonads to induce steroid release, and experimentally decreasing LH by inhibiting GnRH via azaline neither mimicked nor compounded the blunting effect, suggesting that decreased testis sensitivity is independent of LH changes (Turnbull and Rivier, 1997). Our data provide direct evidence for this by demonstrating that LPS-induced testosterone suppression occurs despite a kisspeptin-induced increase in LH in the Siberian hamster.
Thus far, however, the specific mechanisms mediating the acute effects of LPS on reproduction in hamsters remain unclear. Our results indicate that blockade of glucocorticoids could not rescue testosterone release, suggesting that activation of the HPA axis is not the mechanism of immune-induced testosterone suppression. While expression of GRs in the testis suggests that the gonads are capable of responding to stress-related cues independently of the hypothalamus and pituitary, our results align with research in rats showing that adrenalectomy could not ameliorate suppression of testosterone by IL-1β, and that intratesticular mifepristone only partly rescues testosterone production in mice subjected to immobilization stress (Turnbull and Rivier, 1997; Dong et al., 2004). This suggests that other, direct actions may mediate acute suppression of the testes. For example, pro-inflammatory cytokines are produced peripherally and centrally during an immune challenge and play key roles in suppression of the HPG axis (Rivier and Vale, 1990; Watanobe and Hayakawa, 2003). At the level of the gonad, IL-1 may inhibit Leydig cell steroidogenesis via decreasing expression of the testosterone biosynthesis enzyme P450 scc (Lin et al., 1998). Alternatively, evidence from retrograde tracing studies in rats suggests that a direct neural brain–testicular circuit can modulate Leydig cell steroidogenic activity independently of pituitary secretagogues and that this pathway modulates the suppression of testosterone by i.c.v. IL-1β (Lee et al., 2002).
Another potential mechanism may lie in the local expression of inhibitory neuropeptides, such as RF-amide related peptide 3 (RFRP3, the mammalian ortholog of avian gonadotropin-inhibitory hormone, GnIH). This hormone was first characterized as a hypothalamic peptide that suppresses GnRH production and release (Tsutsui et al., 2000; Ubuka et al., 2012; Ducret et al., 2009), but its expression has recently been reported in other tissues, including the testes of birds and hamsters (Bentley et al., 2008; McGuire and Bentley, 2010; Zhao et al., 2010). In the zebra finch, restraint stress has been shown to increase testicular expression of GnIH (Ernst et al., 2016), and ovarian expression of RFRP3 increases with age and reproductive decline in female rats (Geraghty et al., 2016). In addition, testicular RFRP3 has been suggested to decrease acute testosterone production via inhibition of steroidogenic enzymes (Anjum et al., 2014). Thus, although the relationship between an immune challenge and testicular RFRP3 expression remains unclear, this peptide could serve as a mechanism of inhibitory cue-induced suppression of the gonads (Bentley et al., 2017). Which of these mechanisms regulates LPS-induced suppression of the testes in Siberian hamsters remains to be determined, and these possibilities offer interesting future directions for this work.
The results of the current study demonstrate a remarkably rapid suppression of the gonads in males in response to endotoxin challenge, consistent with the idea that decreased sexual and reproductive neuroendocrine activity are components of sickness behavior (Johnson, 2002). Physiologically, this would represent a means of shunting energy away from reproduction in favor of immune function, thus conserving energy and concentrating resources on recovery from illness. This study, then, is a direct observation of immediate trade-offs between the immune and reproductive systems in seasonally breeding Siberian hamsters. Overall, the results of this study show the dramatic effects of an acute immune reaction on the HPG axis, and the suppression of testosterone release seen in this study may represent a physiological mechanism for the reallocation of energy from reproduction to immune function. This study contributes to the elucidation of the specific mechanisms at play and adds to the growing body of research on the neuroendocrine control of reproduction and its relation to immune function.
Acknowledgements
We would like to thank Elizabeth Lithio for assistance with animal work and the members of the Demas lab for helpful feedback.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.L.L., A.M.B., T.J.G., G.E.D.; Methodology: K.L.L., A.M.B., T.J.G., G.E.D.; Formal analysis: K.L.L., A.M.B.; Investigation: K.L.L., A.M.B., S.J.L., G.E.D.; Resources: S.J.L., G.E.D.; Writing - original draft: K.L.L.; Writing - review & editing: K.L.L., A.M.B., T.J.G., S.J.L., G.E.D.; Visualization: K.L.L.; Supervision: G.E.D.; Project administration: G.E.D.; Funding acquisition: G.E.D.
Funding
This work was supported by a Grant-in-Aid from the Society for Integrative Biology and a National Institutes of Health T32 training grant [HD049336-0] (T.J.G.), an Eli Lilly METACyt grant, the Indiana University Faculty Research Support Program, and a National Science Foundation grant [IOB:0543798] (G.E.D). Deposited in PMC for release after 12 months.
References
- Aktas C. and Kanter M. (2009). A morphological study on Leydig cells of scrotal hyperthermia applied rats in short-term. J. Mol. Histol. 40, 31-39. 10.1007/s10735-009-9210-9 [DOI] [PubMed] [Google Scholar]
- Allen J. A., Diemer T., Janus P., Hales K. H. and Hales D. B. (2004). Bacterial endotoxin lipopolysaccharide and reactive oxygen species inhibit Leydig cell steroidogenesis via perturbation of mitochondria. Endocrine 25, 265-275. 10.1385/ENDO:25:3:265 [DOI] [PubMed] [Google Scholar]
- Anjum S., Krishna A. and Tsutsui K. (2014). Inhibitory roles of the mammalian GnIH ortholog RFRP3 in testicular activities in adult mice. J. Endocrinol. 223, 79-91. 10.1530/JOE-14-0333 [DOI] [PubMed] [Google Scholar]
- Aparicio S. A. (2005). Kisspeptins and GPR54--the new biology of the mammalian GnRH axis. Cell Metab. 1, 293-296. 10.1016/j.cmet.2005.04.001 [DOI] [PubMed] [Google Scholar]
- Bentley G. E., Ubuka T., McGuire N. L., Chowdhury V. S., Morita Y., Yano T., Hasunuma I., Binns M., Wingfield J. C. and Tsutsui K. (2008). Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. Gen. Comp. Endocrinol. 156, 34-43. 10.1016/j.ygcen.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Bentley G. E., Wilsterman K., Ernst D. K., Lynn S. E., Dickens M. J., Calisi R. M., Kriegsfeld L. J., Kaufer D., Geraghty A. C., viviD D. et al. (2017). Neural versus gonadal GnIH: are they independent systems? A Mini-Review. Integr. Comp. Biol. 57, 1194-1203. 10.1093/icb/icx085 [DOI] [PubMed] [Google Scholar]
- Berkenbosch F., van Oers J., del Rey A., Tilders F. and Besedovsky H. (1987). Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science 238, 524-526. 10.1126/science.2443979 [DOI] [PubMed] [Google Scholar]
- Bilbo S. D., Drazen D. L., Quan N., He L. and Nelson R. J. (2002). Short day lengths attenuate the symptoms of infection in Siberian hamsters. Proc. Biol. Sci. 269, 447-454. 10.1098/rspb.2001.1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton E. D. and Demas G. E. (2017). Glucose and insulin modulate sickness responses in male Siberian hamsters. Gen. Comp. Endocrinol. 242, 83-91. 10.1016/j.ygcen.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano J. M., Bentsen A. H., Romero M., Pineda R., Ruiz-Pino F., Garcia-Galiano D., Sánchez-Garrido M. A., Pinilla L., Mikkelsen J. D. and Tena-Sempere M. (2010). Acute inflammation reduces kisspeptin immunoreactivity at the arcuate nucleus and decreases responsiveness to kisspeptin independently of its anorectic effects. Am. J. Physiol. Endocrinol. Metab. 299, E54-E61. 10.1152/ajpendo.00081.2010 [DOI] [PubMed] [Google Scholar]
- Coutinho A. E. and Chapman K. E. (2011). The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 335, 2-13. 10.1016/j.mce.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J. T. and Wang Z. (2005). Glucocorticoid receptor involvement in pair bonding in female prairie voles: the effects of acute blockade and interactions with central dopamine reward systems. Neuroscience 134, 369-376. 10.1016/j.neuroscience.2005.04.012 [DOI] [PubMed] [Google Scholar]
- Dantzer R. and Kelley K. W. (2007). Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 21, 153-160. 10.1016/j.bbi.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q., Salva A., Sottas C. M., Niu E., Holmes M. and Hardy M. P. (2004). Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J. Androl. 25, 973-981. 10.1002/j.1939-4640.2004.tb03170.x [DOI] [PubMed] [Google Scholar]
- Ducret E., Anderson G. M. and Herbison A. E. (2009). RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology 150, 2799-2804. 10.1210/en.2008-1623 [DOI] [PubMed] [Google Scholar]
- Ebisui O., Fukata J., Tominaga T., Murakami N., Kobayashi H., Segawa H., Muro S., Naito Y., Nakai Y. and Masui Y. (1992). Roles of interleukin-1 alpha and −1 beta in endotoxin-induced suppression of plasma gonadotropin levels in rats. Endocrinology 130, 3307-3313. 10.1210/endo.130.6.1597143 [DOI] [PubMed] [Google Scholar]
- Ernst D. K., Lynn S. E. and Bentley G. E. (2016). Differential response of GnIH in the brain and gonads following acute stress in a songbird. Gen. Comp. Endocrinol. 227, 51-57. 10.1016/j.ygcen.2015.05.016 [DOI] [PubMed] [Google Scholar]
- French S. S., Chester E. M. and Demas G. E. (2013). Maternal immune activation affects litter success, size and neuroendocrine responses related to behavior in adult offspring. Physiol. Behav. 119, 175-184. 10.1016/j.physbeh.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Geraghty A. C., Muroy S. E., Kriegsfeld L. J., Bentley G. E. and Kaufer D. (2016). The role of RFamide-related peptide-3 in age-related reproductive decline in female rats. Front. Endocrinol. 7, 71 10.3389/fendo.2016.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch M. L., Cunningham M. J., Smith J. T., Popa S. M., Acohido B. V., Crowley W. F., Seminara S., Clifton D. K. and Steiner R. A. (2004). A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145, 4073-4077. 10.1210/en.2004-0431 [DOI] [PubMed] [Google Scholar]
- Greives T. J., Long K. L., Burns C. M. and Demas G. E. (2011). Response to exogenous kisspeptin varies according to sex and reproductive condition in Siberian hamsters (Phodopus sungorus). Gen. Comp. Endocrinol. 170, 172-179. 10.1016/j.ygcen.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B. (1988). Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123-137. 10.1016/S0149-7634(88)80004-6 [DOI] [PubMed] [Google Scholar]
- He D., Sato I., Kimura F. and Akema T. (2003). Lipopolysaccharide inhibits luteinizing hormone release through interaction with opioid and excitatory amino acid inputs to gonadotropin-releasing hormone neurones in female rats: possible evidence for a common mechanism involved in infection and immobilization stress. J. Neuroendocrinol. 15, 559-563. 10.1046/j.1365-2826.2003.01031.x [DOI] [PubMed] [Google Scholar]
- Irfan S., Ehmcke J., Wahab F., Shahab M. and Schlatt S. (2014). Intratesticular action of kisspeptin in rhesus monkey (Macaca mulatta). Andrologia 46, 610-617. 10.1111/and.12121 [DOI] [PubMed] [Google Scholar]
- Iwasa T., Matsuzaki T., Murakami M., Shimizu F., Kuwahara A., Yasui T. and Irahara M. (2008). Decreased expression of kisspeptin mediates acute immune/inflammatory stress-induced suppression of gonadotropin secretion in female rat. J. Endocrinol. Invest. 31, 656-659. 10.1007/BF03345620 [DOI] [PubMed] [Google Scholar]
- Jimenez R., Yoburn B. C., Calvano S. E. and Franklin S. O. (1999). Preproenkephalin mRNA and enkephalin levels in the adult Syrian hamster: the influence from glucocorticoids. Brain Res. Mol. Brain Res. 66, 179-183. 10.1016/S0169-328X(99)00019-4 [DOI] [PubMed] [Google Scholar]
- Johnson R. W. (2002). The concept of sickness behavior: a brief chronological account of four key discoveries. Vet. Immunol. Immunopathol. 87, 443-450. 10.1016/S0165-2427(02)00069-7 [DOI] [PubMed] [Google Scholar]
- Kent S., Bluthé R.-M., Kelley K. W. and Dantzer R. (1992). Sickness behavior as a new target for drug development. Trends Pharmacol. Sci. 13, 24-28. 10.1016/0165-6147(92)90012-U [DOI] [PubMed] [Google Scholar]
- Kinsey-Jones J. S., Li X. F., Knox A. M. I., Wilkinson E. S., Zhu X. L., Chaudhary A. A., Milligan S. R., Lightman S. L. and O'Byrne K. T. (2009). Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J. Neuroendocrinol. 21, 20-29. 10.1111/j.1365-2826.2008.01807.x [DOI] [PubMed] [Google Scholar]
- Knox A. M. I., Li X. F., Kinsey-Jones J. S., Wilkinson E. S., Wu X. Q., Cheng Y. S., Milligan S. R., Lightman S. L. and O'Byrne K. T. (2009). Neonatal lipopolysaccharide exposure delays puberty and alters hypothalamic Kiss1 and Kiss1r mRNA expression in the female rat. J. Neuroendocrinol. 21, 683-689. 10.1111/j.1365-2826.2009.01885.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld L. (2006). Driving reproduction: RFamide peptides behind the wheel. Horm. Behav. 50, 655-666. 10.1016/j.yhbeh.2006.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Miselis R. and Rivier C. (2002). Anatomical and functional evidence for a neural hypothalamic-testicular pathway that is independent of the pituitary. Endocrinology 143, 4447-4454. 10.1210/en.2002-220392 [DOI] [PubMed] [Google Scholar]
- Legan S. J., Donoghue K. M., Franklin K. M. and Duncan M. J. (2009). Phenobarbital blockade of the preovulatory luteinizing hormone surge: association with phase-advanced circadian clock and altered suprachiasmatic nucleus Period1 gene expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1620-R1630. 10.1152/ajpregu.90914.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T., Wang D. and Stocco D. (1998). Interleukin-1 inhibits Leydig cell steroidogenesis without affecting steroidogenic acute regulatory protein messenger ribonucleic acid or protein levels. J. Endocrinol. 156, 461-467. 10.1677/joe.0.1560461 [DOI] [PubMed] [Google Scholar]
- Lopes P. C. (2016). LPS and neuroinflammation: a matter of timing. Inflammopharmacology 24, 291-293. 10.1007/s10787-016-0283-2 [DOI] [PubMed] [Google Scholar]
- Mason A. O., Greives T. J., Scotti M.-A. L. A., Levine J., Frommeyer S., Ketterson E. D., Demas G. E. and Kriegsfeld L. J. (2007). Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Horm. Behav. 52, 492-498. 10.1016/j.yhbeh.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire N. L. and Bentley G. E. (2010). A functional neuropeptide system in vertebrate gonads: gonadotropin-inhibitory hormone and its receptor in testes of field-caught house sparrow (Passer domesticus). Gen. Comp. Endocrinol. 166, 565-572. 10.1016/j.ygcen.2010.01.010 [DOI] [PubMed] [Google Scholar]
- Mei H., Doran J., Kyle V., Yeo S.-H. H. and Colledge W. H. (2013). Does Kisspeptin signaling have a role in the testes? Front. Endocrinol. 4, 198 10.3389/fendo.2013.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley A. E., Clifton D. K. and Steiner R. A. (2009). Kisspeptin signaling in the brain. Endocr. Rev. 30, 713-743. 10.1210/er.2009-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff R. F. (1974). A non-parametric test of whether two simple regression lines are parallel. Ann. Stat. 2, 295-310. 10.1214/aos/1176342664 [DOI] [Google Scholar]
- Quan N., Whiteside M. and Herkenham M. (1998). Time course and localization patterns of interleukin-1β messenger rna expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience 83, 281-293. 10.1016/S0306-4522(97)00350-3 [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Brade H., Holst O., Brade L., Müller-Loennies S., Mamat U., Zähringer U., Beckmann F., Seydel U., Brandenburg K. et al. (1996). Bacterial endotoxin: chemical constitution, biological recognition, host response, and immunological detoxification. Curr. Top. Microbiol. Immunol. 216, 39-81. 10.1007/978-3-642-80186-0_3 [DOI] [PubMed] [Google Scholar]
- Rivier C. and Vale W. (1990). Cytokines act within the brain to inhibit luteinizing hormone secretion and ovulation in the rat. Endocrinology 127, 849-856. 10.1210/endo-127-2-849 [DOI] [PubMed] [Google Scholar]
- Smals A. G. H., Kloppenborg P. W. C. and Benraad T. H. J. (1977). Plasma testosterone profiles in Cushing's syndrome. J. Clin. Endocrinol. Metab. 45, 240-245. 10.1210/jcem-45-2-240 [DOI] [PubMed] [Google Scholar]
- Smith J. T., Dungan H. M., Stoll E. A., Gottsch M. L., Braun R. E., Eacker S. M., Clifton D. K. and Steiner R. A. (2005a). Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146, 2976-2984. 10.1210/en.2005-0323 [DOI] [PubMed] [Google Scholar]
- Smith J. T., Cunningham M. J., Rissman E. F., Clifton D. K. and Steiner R. A. (2005b). Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146, 3686-3692. 10.1210/en.2005-0488 [DOI] [PubMed] [Google Scholar]
- Soto L., Martin A. I., Millan S., Vara E. and Lopez-Calderon A. (1998). Effects of endotoxin lipopolysaccharide administration on the somatotropic axis. J. Endocrinol. 159, 239-246. 10.1677/joe.0.1590239 [DOI] [PubMed] [Google Scholar]
- Tsutsui K., Saigoh E., Ukena K., Teranishi H., Fujisawa Y., Kikuchi M., Ishii S. and Sharp P. J. (2000). A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem. Biophys. Res. Commun. 275, 661-667. 10.1006/bbrc.2000.3350 [DOI] [PubMed] [Google Scholar]
- Turnbull A. and Rivier C. (1995). Brain-periphery connections: do they play a role in mediating the effect of centrally injected interleukin-1 beta on gonadal function? Neuroimmunomodulation 2, 224-235. 10.1159/000097200 [DOI] [PubMed] [Google Scholar]
- Turnbull A. V. and Rivier C. (1997). Inhibition of gonadotropin-induced testosterone secretion by the intracerebroventricular injection of interleukin-1 beta in the male rat. Endocrinology 138, 1008-1013. 10.1210/endo.138.3.5019 [DOI] [PubMed] [Google Scholar]
- Ubuka T., Inoue K., Fukuda Y., Mizuno T., Ukena K., Kriegsfeld L. J. and Tsutsui K. (2012). Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology 153, 373-385. 10.1210/en.2011-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade G. N. and Schneider J. E. (1992). Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev. 16, 235-272. 10.1016/S0149-7634(05)80183-6 [DOI] [PubMed] [Google Scholar]
- Watanobe H. and Hayakawa Y. (2003). Hypothalamic interleukin-1β and tumor necrosis factor-α, but not interleukin-6, mediate the endotoxin-induced suppression of the reproductive axis in rats. Endocrinology 144, 4868-4875. 10.1210/en.2003-0644 [DOI] [PubMed] [Google Scholar]
- Whirledge S. and Cidlowski J. A. (2010). Glucocorticoids, stress, and fertility. Minerva Endocrinol. 35, 109-125. [PMC free article] [PubMed] [Google Scholar]
- Wommack J. C. and Delville Y. (2007). Cortisol controls the pubertal development of agonistic behavior in male golden hamsters via type II corticosteroid receptors. Horm. Behav. 51, 306-312. 10.1016/j.yhbeh.2006.11.007 [DOI] [PubMed] [Google Scholar]
- Zhao S., Zhu E., Yang C., Bentley G. E., Tsutsui K. and Kriegsfeld L. J. (2010). RFamide-related peptide and messenger ribonucleic acid expression in mammalian testis: association with the spermatogenic cycle. Endocrinology 151, 617-627. 10.1210/en.2009-0978 [DOI] [PMC free article] [PubMed] [Google Scholar]