Abstract
Salvia deserta Schang (SDS) belongs to the same family as Salvia miltiorrhiza bunge, one of the antithrombotic Chinese herbal medicines. In our study, EtOAc root extracts were analyzed for their effects on adenosine diphosphate (ADP)-induced platelet aggregation in rabbits and FeCl3-induced rat common carotid artery thrombosis as well as on rat blood plasma concentrations of thromboxane B2 (TXB2), 6-keto-prostaglandin F1 alpha (6-keto-PGF1α), antithrombin-III (AT-III), protein C (PC), plasminogen (PLG), plasminogen activator inhibitor (PAI-1), von Willebrand factor (vWF) and tissue-type plasminogen activator (t-PA). EtOAc extracts from SDS roots had significant inhibitory effects on ADP-induced maximum platelet aggregation rate (10.2 ± 2.6 vs control 35.7 ± 5.2; P < 0.05), reduced the FeCl3-induced rat common carotid artery thrombus weight and thrombus area ratio (P < 0.05), significantly decreased plasma TXB2, vWF and PAI-1 levels and increased 6-keto-PGF1α and t-PA levels in a dose dependent manner (all P < 0.05). Thus, the ratio of TXB2/6-keto-PGF1α was significantly decreased (P < 0.05), while the ratio of t-PA/PAI-1 was significantly increased (P < 0.05). In addition, enhanced AT-III and PC activities indicated coagulation inactivation effects of EtOAc SDS root extracts. EtOAc extraction from SDS showed antithrombotic effects, which are likely due to platelet adhesion and aggregation inhibition as well as anticoagulant activities.
Introduction
Numerous genetic, acquired and environmental factors can tip the homeostatic balance in favor of coagulation and thus lead to the formation of thrombi, which is a common pathology underlying ischemic heart disease, stroke and venous thromboembolism. It has been reported that ischemic heart disease and stroke collectively are responsible for one in four deaths worldwide1,2. Thus, despite the existing available antithrombotic agents, new effective drugs are still urgently required.
Salvia deserta Schang (SDS) is a perennial plant belonging to the Lamiaceae family and is widely distributed in the Gobi wilderness of Xinjiang province. SDS is a species of the Salvia genus like Salvia miltiorrhiza bunge, whose root extracts are an important Chinese herbal medicine called Danshen, which can affect hemostasis by several mechanisms including inhibition of platelet aggregation, interference with extrinsic blood coagulation, antithrombin III-like activity and promotion of fibrinolytic activity. Therefore, it is commonly used in Chinese clinics as antithrombotic therapy3–6. However, whether SDS extracts also have anti-thrombotic effects has rarely been investigated.
The chemical composition of whole plant SDS was previously systematically studied. About 30 different compounds including phenolic acids, diterpenoid quinones, flavonoids, triterpenoids and others were isolated of which the hydrosoluble phenolic acids and liposoluble terpenoids were also found in Salvia miltiorrhiza bunge7–9. The diterpenoid quinones 6,7-dehydroxyleanone and 6,7-dehydroroyleanone have effects in preventing myocardial ischemia, inhibiting platelet aggregation and inducing nitric oxide synthase in vitro10,11, and the triterpenoid oleanolic acid can significantly inhibit collagen and ADP-induced platelet aggregation to protect the heart12,13. In the present study, we hypothesized that SDS might have similar antithrombotic effects to Salvia miltiorrhiza bunge and we compared the SDS extract and Danshen application outcomes on thrombosis and related factor patterns in rabbit and mouse models.
Methods and Materials
Experimental animal ethics
The study was approved by the ethical committee of Xinjiang Medical University and all procedures involving animals were performed in accordance with the ethical standards of the Guidelines for the Humane Treatment of Laboratory Animals (Ministry of Science and Technology of the People’s Republic of China, Policy No. 2006 398).
Rabbits
Two hundred SPF grade healthy New Zealand white rabbits were provided by the animal research center of the Xinjiang Medical University (certificate number: SCXK (Xin) 2011-0004). The animals were male and female, weighing 2.0 ± 0.2 kg, and were kept at 21 ± 2 °C, with a light cycle of 12 hours/day at 40–45% humidity and free access to water and food.
Rats
One hundred and thirty healthy male Sprague-Dawley (SD) rats (SPF grade), weighing 250–300 g were provided by the experimental animal center of Xinjiang Medical University (license number: SCXK (Xin) 2011-0004) and kept under the same conditions as the rabbits but in separate holding rooms.
Preparation of different SDS components for different solvent extractions
SDS plants were separated into roots, stems, leaves and flowers, and then dried in the shade and finally pulverized.
H2O extraction
Roots, stems, leaves and flowers were respectively extracted 3 times for 1 hour by a reflux extraction method in water at 80 °C. The extracts were combined, concentrated and freeze-dried to obtain the water extracts of roots, stems, leaves and flowers.
Ethanol extraction
Roots, stems, leaves and flowers were extracted 3 times using a method involving 95% ethanol, 1 hour per extract; the 3 extracts were combined, concentrated in a low-temperature vacuum under reduced pressure and dried to obtain ethanol extracts of roots, stems, leaves and flowers.
EtOAc soluble fraction (ESF)
Roots, stems, leaves and flowers were reflux extracted 3 times for 1 hour with acetate (EtOAc); the 3 extracts were combined, concentrated under atmospheric pressure and dried to obtain ESFs of roots, stems, leaves and flowers.
Adenosine diphosphate (ADP)-induced platelet aggregation test
For each SDS plant component (root, stem, leaves and flower) 50 New Zealand white rabbits were randomly divided into aspirin (10 mg/kg), high (40–50 mg/kg), middle (20–25 mg/kg) and low (10–12.5 mg/kg) SDS extract doses as well as control groups; each group was comprised of 10 rabbits. Intragastric administration was carried out 3 times a day for the controls and SDS low/middle/high dose groups, and once a day for the aspirin group for 3 consecutive days. An additional dose was administered 1 hour before the operation.
For the horminone, 7-O-acetylhorminone and 6,7-dehydeoroyleanone experiments, 1,000 µg/mL, 100 µg/mL and 10 µg/mL of each chemical dissolved in 5% methyl alcohol-saline water was administered to 7 rabbits in each dosage group, with one group for each chemical acting as the control (n = 7) (5% methyl alcohol-saline water only).
Blood was collected by cardiac puncture and coagulation prevented by 3.8% sodium citrate (the volume ratio of blood with anticoagulant was 9:1), centrifuged at 1,000 rpm at room temperature for 10 min, after which the upper plasma layer was aspirated as platelet-rich plasma (PRP). The remaining sample was centrifuged again at 4,000 rpm at room temperature for 15 min and the upper plasma layer was aspirated as the platelet-poor plasma (PPP) fraction.
The PRP was adjusted to a platelet concentration of 4 ~ 5 × 108/mL with PPP. The ADP-induced platelet maximum aggregation rate (MAR) was determined using Born’s turbidimetric method14. ADP was purchased from Chrono-Log Corp. (Havertown, PA, US: lot number 3427) and data are presented as the maximum aggregation inhibition rate (MAIR) according to the following formula: MAIR (%) = platelet aggregation rate of the control group%- platelet aggregation rate of test drug treated group%/platelet aggregation rate of the control group%.
FeCl3-induced rat common carotid artery thrombosis experiment
Different root extraction method measurements
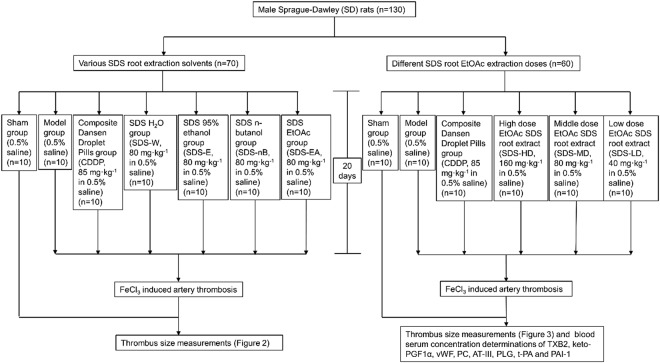
Seventy male rats were randomly divided into 7 groups with 10 rats in each group, including a group that did not receive FeCl3 (0.5% saline) (Sham), a control group (0.5% saline) (Model), a composite Danshen droplet pills group (Tianjin Tasly Pharmaceutical Group Co., Ltd., batch number: 20130522) dissolved as 85 mg/kg in 0.5% saline as a positive control (CDDP), a SDS water extraction group (SDS-W), 80 mg/kg in 0.5% saline, a SDS 95% ethanol extraction group (SDS-E), 80 mg/kg in 0.5% saline, a SDS n-butanol soluble fraction group (SDS-BuSF), 80 mg/kg in 0.5% saline) and a SDS EtOAc soluble fraction group (SDS-ESF), 80 mg/kg in 0.5% saline. All the animals underwent intragastric administration for 20 days, once daily (Fig. 1).
Figure 1.
Flow chart of the rat experiments.
Dose-dependent SDS ESF measurements
For dose-dependent measurements, rats were treated with high (160 mg/kg, SDS-HD), middle (80 mg/kg, SDS-MD) and low (40 mg/kg, SDS-LD) doses of SDS root ESF dissolved in 0.5% saline as well as a no FeCL3 group (0.5% saline) (Sham), a control group (0.5% saline) (Model) and a 85 mg/kg composite Danshen droplet pills group dissolved in 0.5% saline as a positive control (CDDP). Again, all the animals underwent intragastric administration for 20 days, once daily (Fig. 1).
Common carotid artery thrombosis rat model after 20 days of treatment
A common carotid artery thrombosis model was established by reference to previous studies15–17. In order to determine the time to blood vessel occlusion (TTO), in preliminary experiments the necessary FeCl3 concentration and exposure duration of the common carotid artery have been measured with Doppler ultrasound (Supplementary Table 1, Supplementary Figure 1). Isolated 2 cm segments of the left common carotid artery, removed under deep general anesthesia induced by intraperitoneal injection of urethane (1.5 g/kg), had a small piece of plastic film (3 cm × 1.5 cm) placed under them to protect tissues surrounding the blood vessels. Then a small piece of filter paper (1 cm × 1 cm) soaked in 2.16 mol/L FeCl3 solution was applied onto the exposed surface of the artery, which was replaced with saline soaked (filter paper in the sham group. The filter paper was positioned close to the blood vessel wall. The filter paper was removed after 20 min (the start time was when the filter paper was positioned on the artery). The left blood vessel on the site of thrombosis was removed and the residual blood blotted on filter paper and weighted. In addition, the thrombosis arteries were fixed in 10% formalin solution for 24 hours and washed in running water for 4 hours, followed by paraffin sections, HE staining and thrombosis analysis using light microscopy. The thrombus area determination has been performed with image pro plus 6.0 software (Silver Spring, MD, USA)
Detecting levels of thrombus related factors
Blood was collected via the abdominal aorta of FeCl3-induced common carotid artery thrombosis rats and anticoagulated with sodium citrate (1:9) after removal of the thrombosis arteries. The blood was centrifuged at 3,000 rpm at 4 °C for 15 min and the supernatant plasma collected for thrombus factor determinations. TXB2 and 6-keto-PGF1α radioimmunoassay kits were purchased from North Biotechnology Institute (Beijing, China). PC-C, AT-III, vWF ELISA kits as well as PAI-1 and t-PA test kits, and a PLG immunoassay quantitative test kit were purchased from West Tang Biological Technology Co., Ltd. (Shanghai, China). All measurements were carried out according to the manufacturer’s instructions.
High performance liquid chromatography (HPLC)/gas chromatography mass spectrometry (CG-MS) SDS extract analysis
Chromatographic separation of SDS root extracts was performed using a Surveyor HPLC system (ThermoFisher Scientific, San Jose, CA, USA) composed of an autosampler and an HPLC pump. The column used was an Atlantis® HILIC Silica, 4.6 mm × 250 mm, 5 µm (Waters Corporation, Milford, MA). The analytes were separated with isocratic elution: mobile phase water (A) methanol solution (B) gradient (0~19 min, 25% A, flow rate 0.8 mL/min; 19~22 min; 25%~13% A, flow rate 0.8~1.0 mL/min; 22~30 min, 13% A, flow rate 1.0 mL/min. Measurement wavelength was 272 nm (0~22.00 min for hominone, 7-O-acetylhorminone), 330 nm (22.01~30.00 min for 6,7-dehydeoroyleanone) and the column temperature was 40 °C with a sample injection volume of 10 μL. For the MS/MS analysis, a TSQ Quantum Ultra triple quadrupole mass spectrometer (ThermoFisher Scientific, San Jose, CA, USA) was used.
Statistical analysis and data processing
Stataistical analyses were pereformed with SPSS for Windows (Ver. 16.0. Chicago, SPSS Inc.). Continuous variables are presented as mean ± standard deviation ( ± SD), Differences between different doses were analysed using one-way ANOVA and comparisons between two groups were performed using bonferroni post hoc-test. The ratio variables were analysed with chi-square test. P < 0.05 was considered to be statistically significant.
Results
SDS root EtOAc extract significantly inhibited platelet aggregation
First, we investigated whether the different plant parts and extraction methods yielded effective treatments to inhibit platelet aggregation in rabbits (data not shown), when we found that only root ESF inhibited platelet aggregation. Further investigations revealed that MAR/% in high doses of root extracts was 10.2 ± 2.6, compare to MAR/% in control (35.7 ± 5.2), which showed significant inhibition of platelet aggregation (P < 0.05) to a similar extent as aspirin (P > 0.05) (Table 1).
Table 1.
Effect of EtOAc extracts of indicated SDS parts on New Zealand white rabbit platelet aggregation ( mean ± SD, n = 10).
| Faction | Groups | Dose/mg·kg | ADP | |
|---|---|---|---|---|
| MAR/% | MAIR/% | |||
| Root | Aspirin | 10 | 15.2 ± 6.5 | 57.4 ± 12.9 |
| Control | 35.7 ± 5.2Δ | |||
| STD Low Dose | 10 | 40.8 ± 11.2Δ | 0.0 ± 0.0Δ | |
| STD Middle Dose | 20 | 29.8 ± 8.7Δ | 16.5 ± 7.9Δ | |
| STD High Dose | 40 | 10.2 ± 2.6* | 71.4 ± 4.1Δ | |
| p-value (STD dose) | <0.0001 | |||
| p-value (Aspirin + STD dose) | <0.0001 | <0.0001 | ||
| Stem | Aspirin | 10 | 13.3 ± 8.1 | 67.2 ± 11.7 |
| Control | 40.6 ± 11.53Δ | |||
| STD Low Dose | 10 | 42.9 ± 17.3Δ | 0.0 ± 0.0Δ | |
| STD Middle Dose | 20 | 43.1 ± 9.5Δ | 0.0 ± 0.0Δ | |
| STD High Dose | 40 | 35.5 ± 7.4Δ | 12.6 ± 5.8Δ | |
| p-value (STD dose) | 0.4667 | |||
| p-value (Aspirin + STD dose) | <0.0001 | <0.0001 | ||
| Leaf | Aspirin | 10 | 8.2 ± 3.6 | 70.8 ± 5.1 |
| Control | 28.1 ± 8.1Δ | |||
| STD Low Dose | 10 | 30.4 ± 10.7Δ | 0.0 ± 0.0Δ | |
| STD Middle Dose | 20 | 24.5 ± 7.3Δ | 12.8 ± 1.8Δ | |
| STD High Dose | 40 | 22.8 ± 6.3Δ | 18.8 ± 2.4Δ | |
| p-value (STD dose) | 0.1780 | |||
| p-value (Aspirin + STD dose) | <0.0001 | <0.0001 | ||
| Flower | Aspirin | 10 | 8.2 ± 3.6 | 70.8 ± 5.1 |
| Control | 28.1 ± 8.1Δ | |||
| STD Low Dose | 12.5 | 35.6 ± 6.6Δ | 0.0 ± 0.0Δ | |
| STD Middle Dose | 25 | 27.1 ± 7.6Δ | 3.5 ± 1.6Δ | |
| STD High Dose | 50 | 20.8 ± 2.7Δ | 25.9 ± 12.3Δ | |
| p-value (STD dose) | 0.0002 | |||
| p-value (Aspirin + STD dose) | <0.0001 | <0.0001 | ||
*P < 0.05 significant difference compared to control group; ΔP < 0.05 significant difference compared to aspirin group.
MAIR(%) = platelet aggregation rate of the control group% - platelet aggregation rate of test drug treated group% / platelet aggregation rate of the control group%.
Effects of H2O, ethanol, n-butanol and EtOAc SDS root extracts on rat carotid artery thrombosis
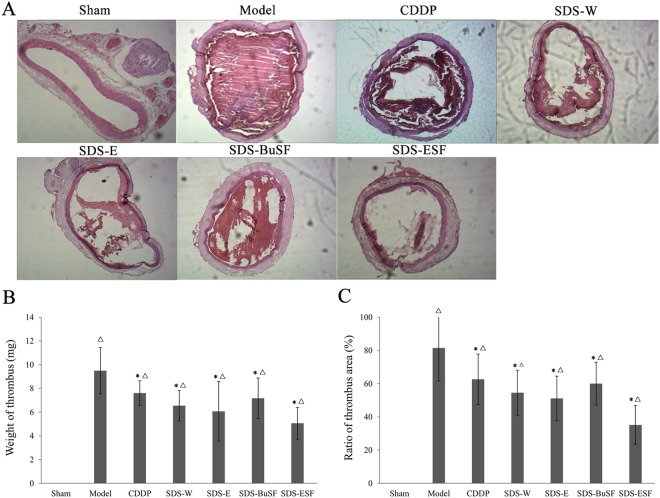
Rats in the sham group showed no common carotid endovascular thrombosis, while FeCl3 successfully induced rat endovascular thrombosis in the other groups, with weights up to 9 times that of the sham group. (Fig. 2). Compared with the model group, the thrombi in the CDDP and SDS groups were significantly smaller and the thrombus bodies were looser, especially in the SDS root ESF group. Consistent with the morphological results, thrombus weights in each test and CDDP group were significantly lower than that in the model group and thrombosis was the least in the SDS root ESF group (P < 0.05, Fig. 2B). In addition, the ratio of thrombus area in each test and CDDP were all significantly lower than that in the model group, expecially SDS root ESF group showed lowest arear ratio (*P < 0.05, Fig. 2C).
Figure 2.
Extracts of SDS roots significantly inhibited FeCl3-induced rat carotid artery thrombosis. (A) HE staining of rat carotid artery thrombosis in each group; (B) comparison of the thrombus weight in each group; (C) comparison of the ratio of thrombosis area in each group. Before the establishment of FeCl3-induced rat carotid artery thrombosis models, the rats in each group were treated with the indicated solvent extract of SDS roots or CDDP for 20 days, once a day. n = 10, data are presented as the mean ± SD; Significant difference compare to model group *P < 0.05, and compre to sham group ΔP < 0.05.
Effects of different SDS root ESF doses on rat carotid artery thrombosis
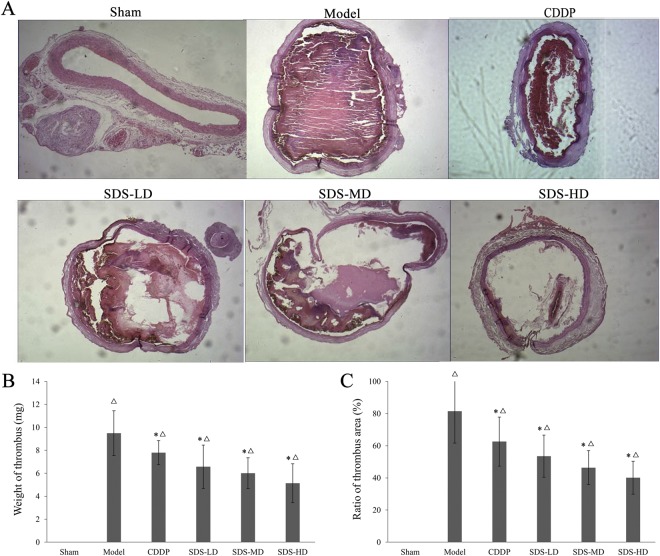
We further compared the inhibitory effects on thrombosis of different doses of SDS root ESF. As shown in Fig. 3A, the low, middle and high dose groups (40 mg/kg, 80 mg/kg and 160 mg/kg, respectively) of SDS root ESFs all significantly inhibited FeCl3-induced thrombosis in a dose-dependent manner, losing and/or reducing the weight and area ratio of thrombus bodies (P < 0.05, Fig. 3B,C). It is noteworthy that thrombus weight and area ratio in the high dose SDS group was significantly lower than that in the CDDP group, suggesting that its inhibitory effect on thrombosis was stronger than that in the CDDP group (Fig. 3B,C).
Figure 3.
Different doses of SDS root ESF suppressed FeCl3-induced rat carotid artery thrombosis. (A) HE staining of rat carotid artery thrombosis in each group; (B) comparison of the rat thrombus weight in each group; (C) comparison of the ratio of thrombosis area in each group. Before the establishment of FeCl3-induced rat carotid artery thrombosis models, rats in each group were treated with different doses of SDS root ESF or CDDP for 20 days, once a day, n = 10; data are presented as the mean ± SD; *P < 0.05, significant difference compared to the model group; ΔP < 0.05 compared to the sham group.
Influences of SDS root ESF on rat plasma ET-1, TXB2, 6-keto-PGF1α and vWF levels
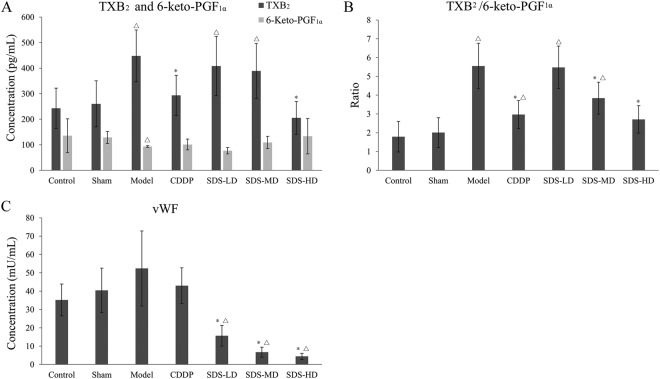
TXB2 and 6-keto-PGF1α are stable metabolites of TXA2 and prostaglandin I2 (PGI2), respectively. TXA2 is mainly produced by platelets and is a vasoconstrictor that also stimulates platelet aggregation in vivo; vascular endothelial cells mainly produce PGI2. The function of PGI2 is opposite to that of TXA2 with a strong effect on expanding capillaries and inhibiting platelet aggregation. TXA2/PGI2 imbalance is one cause of platelet aggregation, vascular spasm or thrombosis. As shown in Fig. 4A, compared with the sham group, plasma TXB2 levels were significantly higher (P < 0.05) and 6-keto-PGF1α levels significantly lower (P < 0.05) in the model group, which resulted in a significantly increased TXB2/6-keto-PGF1α ratio in the model group (P < 0.05, Fig. 4B). CDDP significantly reduced plasma TXB2 levels (P < 0.05) and the TXB2/6-Keto-PGF1α ratio (P < 0.05) compared to the model group. Similarly, the high dose group of SDS significantly reduced plasma levels of TXB2 (P < 0.05) and its effects were somewhat stronger than CDDP.
Figure 4.
Effects of SDS root ESF on FeCl3 induced rat plasma levels of TXB2 and 6-keto-PGF1α. (A) TXB2/6-keto-PGF1α ratio; (B) and vWF (C). Before the establishment of FeCl3-induced rat carotid artery thrombosis models, rats in each group were lavaged with different doses of SDS root EtOAc extract or CDDP for 20 days, once a day. n = 10; data are presented as the mean ± SD; *P < 0.05, significant difference compared to the model group; ΔP < 0.05 compared to the sham group.
von Willebrand factor (vWF) is a multimeric plasma protein that mediates platelet adhesion as well as platelet aggregation at sites of vascular injury and acts as a carrier of factor VIII18. Compared with the sham group, the vWF level in the model group was significantly increased (P < 0.05). CDDP treatment appeared to reduce the increase in vWF concentration compared to the model group, but statistical significance was not achieved, whereas SDS treatment significantly suppressed the increase of vWF concentrations (P < 0.05) in a dose-dependent manner (Fig. 4C).
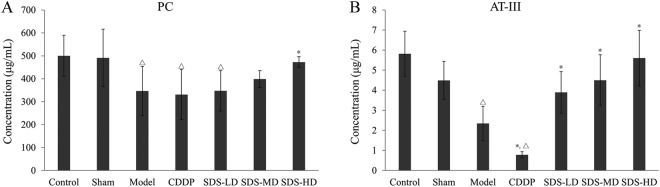
Influence of SDS root ESF on rat plasma PC and AT-III levels
Protein C (PC) system and AT-III are important physiological anticoagulants in vivo. Compared with the sham group, PC (P < 0.05, Fig. 5A) and AT-III (P < 0.05, Fig. 5B) were significantly reduced in the model group. Compared with the model group, the AT-III concentration decreased significantly in the CDDP group (P < 0.05), but the PC concentration did not change. In contrast, compared to the model group the PC (P < 0.05, Fig. 5A) and AT-III (P < 0.05, Fig. 5B) serum concentrations were significantly increase particularly in the high dose group.
Figure 5.
Effects of SDS root EtOAc extracts on FeCl3-induced rat plasma levels of PC. (A) and AT-III (B). Before the establishment of the FeCl3-induced rat carotid artery thrombosis models, rats in each group were treated with different doses of SDS root EtOAc extract or CDDP for 20 days, once a day, n = 10; data are presented as the mean ± SD; *P < 0.05, significant difference compared to the model group; ΔP < 0.05 compared to the sham group.
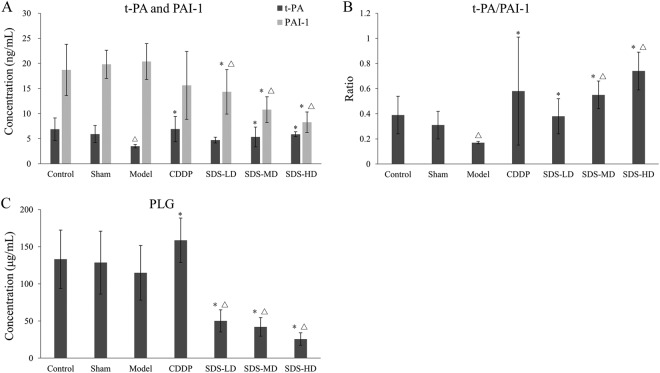
Influences of SDS root ESF on rat plasma PLG, t-PA and PAI-1
Plasminogen (PLG) is the precursor of plasmin, which is a fibrin hydrolase and mainly produced by the actions of the serum tissue-type plasminogen activator (t-PA). The resulting plasmin dissolves fibrin in blood clots. In contrast, the plasminogen activator inhibitor (PAI-1) can inhibit the activation of t-PA. Compared with the sham group, the plasma t-PA/PAI-1 ratio showed significant decrease (P < 0.05, Fig. 6B), while the PLG concentration did not show a significant decrease in the model group (Fig. 6C) and the t-PA concentration was significantly reduced (P < 0.05, Fig. 6A). Compared with the model group, PLG (P < 0.05) and t-PA (P < 0.05) concentrations in the CDDP group were increased and the PAI-1 concentration was decreased, resulting in a significantly increased t-PA/PAI-1 ratio (P < 0.05, Fig. 6B). Although in contrast to the CDDP group, PLG concentrations showed no enhancement but rather significant decreases (P < 0.05), the PAI-1 concentrations in each SDS dose group were significantly decreased and the concentration of t-PA significantly increased in a dose-dependent manner, resulting in significantly increased t-PA/ PAI-1 ratios in all dose groups (Fig. 6C).
Figure 6.
Effect of SDS root ESF on the activity of the fibrinolytic system. Influences of each dose of EtOAc extract on FeCl3-induced rat plasma levels of (A) t-PA and PAI-1, (B) t-PA/PAI-1 ratios and (C) PLG. Before the establishment of FeCl3-induced rat carotid artery thrombosis models, rats in each group were treated with different doses of SDS root EtOAc extract or CDDP for 20 days, once a day, n = 10; data are presented as the mean ± SD; *P < 0.05, significant difference compared to the model group; ΔP < 0.05 compared to the sham group.
Horminone, 7-O-acetylhormione and 6,7-dehydeoroyleanone were active ingredients in SDS root extracts
In order to further analyze active ingredients in SDS, we subjected SDS extracts to a HPL-C/CG-MS analysis and found that horminone, 7-O-acetylhormione and 6,7-dehydroroyleanone were compounds (Supplementary Figure 2). As shown in Table 2, apart from low doses of 6,7-dehydroroyleanone and 7-O-acetylhorminone all other doses of the 3 tested chemicals led to significant reductions in MAIR% compared to the controls.
Table 2.
Effects of 6,7-dehydroroyleanone, horminone and 7-O-acetylhorminone on platelet aggregation (n = 7, ± s).
| Group | Concentration/µg/mL | MAR/% | MAIR/% | |
|---|---|---|---|---|
| 6,7-dehydroroyleanone | Aspirin | 100 | 16.7 ± 4.31 | 62.3 ± 11.5 |
| Control | — | 97.1 ± 1.86Δ | — | |
| High | 1000 | 50.6 ± 10.20*Δ | 47.8 ± 10.5Δ | |
| Medium | 100 | 73.0 ± 5.90*Δ | 24.7 ± 6.07Δ | |
| Low | 10 | 94.4 ± 6.08Δ | 1.20 ± 5.31Δ | |
| p-value (Dose) | <0.0001 | |||
| p-value (Aspirin + Dose) | <0.0001 | <0.0001 | ||
| Horminone | Aspirin | 100 | 17.35 ± 4.63 | 61.70 ± 5.82 |
| Control | — | 91.00 ± 2.89Δ | — | |
| High | 1000 | 66.10 ± 4.80*Δ | 27.50 ± 5.28Δ | |
| Medium | 100 | 79.10 ± 1.21*Δ | 13.00 ± 1.36Δ | |
| Low | 10 | 85.80 ± 1.68*Δ | 6.10 ± 2.13Δ | |
| p-value (Dose) | <0.0001 | |||
| p-value (Aspirin + Dose) | <0.0001 | <0.0001 | ||
| 7-O-acetylhorminone | Aspirin | 100 | 18.03 ± 5.06 | 58.63 ± 8.91 |
| Control | — | 65.1 ± 7.15Δ | — | |
| High | 1000 | 42.60 ± 7.02*Δ | 34.50 ± 10.80Δ | |
| Medium | 100 | 51.80 ± 6.25*Δ | 17.80 ± 4.70Δ | |
| Low | 10 | 63.40 ± 4.93Δ | 2.40 ± 7.60Δ | |
| p-value (Dose) | <0.0001 | |||
| p-value (Aspirin + Dose) | <0.0001 | <0.0001 |
*P < 0.05 significant difference compared to control group; ΔP < 0.05 significant difference compared to aspirin group.
MAIR (%) = platelet aggregation rate of the control group% - platelet aggregation rate of test drug treated group% / platelet aggregation rate of the control group%.
Discussion
In the present study, we extracted different plant parts of SDS using a number of polar solvents, including water, ethanol n-Butanol and EtOAc, and then determined the antithrombotic effects of SDS extracts in New Zealand white rabbit anti-platelet aggregation and FeCl3-induced rat carotid artery thrombosis models.
The anti-platelet aggregation effective substances were rich in root SDS and EtOAc was the effective extraction solvent for it. In the New Zealand white rabbit anti-platelet aggregation model, SDS root ESF (40 mg/kg) significantly inhibited ADP-induced platelet aggregation, which was equivalent to the anti-platelet aggregation effects of aspirin (10 mg/kg). These findings suggested that SDS root extracts have similar anti-platelet aggregation effects as Salvia miltiorrhiza bunge19. Also in the FeCl3-induced rat common carotid artery thrombosis model group, particularly high doses of SDS root extracts inhibited thrombus development, which is in agreement with a previous study in which anti-platelet drugs could extend the time until occlusion in a FeCl3 common carotid artery thrombosis model20. It is noteworthy that this effect was more pronounced with SDS root ESF than with CDDP (Figs 2 and 3). Consistent with a previous study, we observed that the serum concentration of TXB2 increased in the FeCl3 common carotid artery thrombosis model group, while the activities of 6-keto-PGF1α, t-PA, AT-III and PC were reduced21. Moreover, treatment particularly with high doses SDS root ESF reduced plasma TXB2 and vWF, and increased 6-keto-PGF1α, leading to a significantly reduced TXB2/6-keto-PGF1α ratio, which was similar to the effect of CDDP and underlined the anti-platelet aggregation effects of SDS extract shown in the rabbit and rat models. However, in contrast to the CDDP formulation, vWF expression was significantly and dose-dependently reduced in SDS treated rats, indicating that SDS might have a stronger antithrombotic effect than CDDP (Fig. 4). As an indicator of coagulation inactivation, PC and AT-III serum levels were increased in SDS treated rats with the highest levels found in the SDS-HD group, indicationg a trigger of anti-coagulation by SDS root extracts.
These data are contrary to the CDDP-induced changes, since AT-III serum concentrations were significant lower and PC serum levels remained the same as the model group in CDDP treated rats (Fig. 5). Taken together, the antithrombotic effect of SDS root ESF can be attributed to anti-platelet activity and anti-coagulation actions, similar to but not the same as CDDP. However, these findings supported our hypothesis that SDS can induce similar effects on thrombus development as Salvia miltiorrhiza bunge.
In order to confirm the platelet aggregation inhibitory effect of 6,7-dehydroroyleanone11 and the two other diperpenoid quinones, horminone and 7-O-acetylhormione, which we isolated from SDS roots and which have been described as constituents in a previous study9, we carried out a dose increasing experiment in rabbits and found that all 3 chemicals significantly inhibited platelet aggregation in a dose-dependent manner (Table 2). Tanshinone IIA is a diperpenoid quinone in Salvia miltiorrhiza bunge and has been attributed to be a major active compound of Danshen with antiplatelet and anticoagulant effects via tubulin acetylation and Erk-2 phosphorylation inhibition22. However, whether the same mechanisms are valid for 6,7-dehydroroyleanone, horminone and 7-O-acetylhormione actions requires further investigation, while other compounds may also have an effect on blood coagulation.
One drawback of our study was that the time between injury and data collection was short, which may have had an influence, particularly on fibrinolytic enzyme data.
Conclusion
In conclusion, the present study demonstrated that SDS root ESF had significant antithrombotic effects, with EtOAc being the most effective extraction solvent. The antithrombotic effect could be attributed to enhanced anti-platelet aggregation and coagulation inactivation. Thus, further studies on SDS ESF as an antithrombotic traditional Chinese medicine are warranted.
Electronic supplementary material
Acknowledgements
This study was supported by the Scientific Research Program of the Higher Education Institution of XinJiang (Grant No. XJEDU2012I23).
Author Contributions
R.K. and X.L.W. designed the experiments; R.K., X.L.W., X.M.W. and Y.M. performed the experiments; All authors analyzed the data; R.K. and X.L.W. obtained funding and wrote the manuscript; all authors have read and approved the final version of the manuscript.
Data Availability
The datasets supporting the conclusions of this article is included within the article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rena Kasimu is deceased.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-36026-7.
References
- 1.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Day ISCFWT. Thrombosis: a major contributor to the global disease burden. J Thromb Haemost. 2014;12:1580–1590. doi: 10.1111/jth.12698. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, et al. A Platelet/CMC coupled with offline UPLC-QTOF-MS/MS for screening antiplatelet activity components from aqueous extract of Danshen. J Pharm Biomed Anal. 2016;117:178–183. doi: 10.1016/j.jpba.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Wang CS, Chen CS, Yang TT, Lou FC. In vitro Radix salviae miltiorrhizae action on blood anticoagulation and fibrinogenolysis. Chin Med J (Engl). 1978;4:123–126. [PubMed] [Google Scholar]
- 5.Ren-an Q, et al. Study of the protective mechanisms of Compound Danshen Tablet (Fufang Danshen Pian) against myocardial ischemia/reperfusion injury via the Akt-eNOS signaling pathway in rats. J Ethnopharmacol. 2014;156:190–198. doi: 10.1016/j.jep.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Cao H, et al. Salvia miltiorrhiza prevents deep vein thrombosis via antioxidative effects in endothelial cells. Mol Med Rep. 2015;11:3593–3600. doi: 10.3892/mmr.2015.3153. [DOI] [PubMed] [Google Scholar]
- 7.Wang XL, Kasimu R, Niyazi Z. Studies on the chemical constituents of the flowers of salvia deserta schang. Jounal of Xinjiang medical university. 2003;26:583–585. [Google Scholar]
- 8.Ma Y, Kasimu R. Studies on the chemical constituents of the stems of salvia deserta schang. Jounal of Xinjiang medical university. 2004;27:577–579. [Google Scholar]
- 9.Tezuka Y, et al. Constituents of Roots of Salvia deserta SCHANG (Xinjiang-Danshen) Chem. Pharm. Bull. 1998;46:107–112. doi: 10.1248/cpb.46.107. [DOI] [Google Scholar]
- 10.He X, Chang JM, Dilinuer D. Protect effects of 6,7-dehydroxyleanone on experimental myocardial ischemia injury. Chinese Pharmacological Bulletin. 2000;16:596–597. [Google Scholar]
- 11.Pairidaimu W, Wang XL, Kasimu R. Inhibition of Platelet Aggregation Investigation and Optimization of Extracting Technology of 6,7-Dehydroroyleanone in Roots of Salvia deserta Schang. Chinese journal of experimental traditional medical formulae. 2013;19:15–18. [Google Scholar]
- 12.Liu, Y. L. & Wang, H. S. Effect of oleanolic acid on platelet function. Jounal of Shenyang Pharmaceutical University., 275–278 (1993).
- 13.Mapanga RF, et al. Oleanolic acid: a novel cardioprotective agent that blunts hyperglycemia-induced contractile dysfunction. PLoS One. 2012;7:e47322. doi: 10.1371/journal.pone.0047322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 15.Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990;60:269–280. doi: 10.1016/0049-3848(90)90106-M. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Xu L. An optimized murine model of ferric chloride-induced arterial thrombosis for thrombosis research. Thromb Res. 2005;115:95–100. doi: 10.1016/j.thromres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Du H, Zawaski JA, Gaber MW, Chiang TM. A recombinant protein and a chemically synthesized peptide containing the active peptides of the platelet collagen receptors inhibit ferric chloride-induced thrombosis in a rat model. Thromb Res. 2007;121:419–426. doi: 10.1016/j.thromres.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Franchini M, Lippi G. The role of von Willebrand factor in hemorrhagic and thrombotic disorders. Crit Rev Clin Lab Sci. 2007;44:115–149. doi: 10.1080/10408360600966753. [DOI] [PubMed] [Google Scholar]
- 19.Lin TH, Hsieh CL. Pharmacological effects of Salvia miltiorrhiza (Danshen) on cerebral infarction. Chin Med. 2010;5:22. doi: 10.1186/1749-8546-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surin WR, Prakash P, Barthwal MK, Dikshit M. Optimization of ferric chloride induced thrombosis model in rats: effect of anti-platelet and anti-coagulant drugs. J Pharmacol Toxicol Methods. 2010;61:287–291. doi: 10.1016/j.vascn.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Tang YH, Liang Y, Yang J. Changes of TXA2 and PGI2 Content and Anticoagulative and Fibrinolytic Activity in the Plasma in a Rat Model of Topical FeCl3-induced Carotid ArteryThrombosis. Chinese Pharmacological Bulletin. 2006;22:1353–1356. [Google Scholar]
- 22.Maione F, et al. Tanshinone IIA, a major component of Salvia milthorriza Bunge, inhibits platelet activation via Erk-2 signaling pathway. J Ethnopharmacol. 2014;155:1236–1242. doi: 10.1016/j.jep.2014.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article is included within the article.