Abstract
The transition from outcrossing to selfing through the breakdown of distyly to homostyly has occurred repeatedly among families of flowering plants. Homostyles can originate by major gene changes at the S-locus linkage group, or by unlinked polygenic modifiers. Here, we investigate the inheritance of distyly and homostyly in Primula oreodoxa, a subalpine herb endemic to Sichuan, China. Controlled self- and cross-pollinations confirmed that P. oreodoxa unlike most heterostylous species is fully self-compatible. Segregation patterns indicated that the inheritance of distyly is governed by a single Mendelian locus with the short-styled morph carrying at least one dominant S-allele (S-) and long-styled plants homozygous recessive (ss). Crossing data were consistent with a model in which homostyly results from genetic changes at the distylous linkage group, with the homostylous allele (Sh) dominant to the long-styled allele (s), but recessive to the short-styled allele (S). Progeny tests of open-pollinated seed families revealed high rates of intermorph mating in the L-morph but considerable selfing and possibly intramorph mating in the S-morph and in homostyles. S-morph plants homozygous at the S-locus (SS) occurred in several populations but may experience viability selection. The crossing data from distylous and homostylous plants are consistent with either recombination at the S-locus governing distyly, or mutation at gene(s) controlling sex-organ height; both models predict the same patterns of segregation. Recent studies on the molecular genetics of distyly in Primula demonstrating the hemizygous nature of genes at the S-locus make it more likely that homostyles have resulted from mutation rather than recombination.
Subject terms: Genotype, Population genetics
Introduction
Determining the inheritance of reproductive traits causing shifts in mating systems can provide important insights into the genetic mechanisms governing reproductive transitions. In flowering plants, the evolution from predominant outcrossing to high rates of self-fertilization is the most widespread reproductive transition and has evolved on numerous occasions, particularly in herbaceous groups (Stebbins 1974). This change in mating system has important ecological, genetic and evolutionary consequences and has been a topic of sustained general interest since Darwin’s pioneering studies (Darwin 1876, 1877; Jain 1976; Lloyd 1980; Uyenoyama et al. 1993; Igić and Busch 2013; Wright et al. 2013; Barrett et al. 2014). Shifts from outcrossing to selfing are associated with genetic modifications to a range of reproductive characters, including the loss of self-incompatibility (Barrett 1988; Mable et al. 2005; Igić et al. 2008) and alterations to a suite of floral characters (Lloyd 1965; Morgan and Barrett 1989; Sicard and Lenhard 2011). Studies of the genetic basis of mating system modifiers have revealed a range of genetic architectures from a few large effect mutations to many genes of small effect, depending on the reproductive traits examined (e.g., Fishman et al. 2002; Good-Avila et al. 2008; Slotte et al. 2012; Sicard et al. 2011). Because the genetic basis of mating system modification influences the importance of inbreeding depression for mating system evolution (see Lande and Schemske 1985; Holsinger 1988), determining the inheritance of traits promoting selfing is a prerequisite for dissecting the complex dynamics of mating system change.
The evolutionary breakdown of the floral polymorphism heterostyly provides an excellent opportunity for investigating the evolution of selfing from outcrossing. In numerous heterostylous families, the style- and stamen-length polymorphism characterizing heterostyly is replaced by a monomorphic condition referred to as homostyly (Darwin 1877; Charlesworth and Charlesworth 1979; Ganders 1979; Barrett and Shore 2008). The floral morphs in heterostylous populations possess well separated stigmas and anthers (reciprocal herkogamy) and are usually self- and intramorph incompatible (heteromorphic incompatibility). In contrast, the stigmas and anthers of homostyles are located at similar positions within a flower and as plants are self-compatible they exhibit high rates of autonomous self-pollination. Because of their capacity for selfing, homostyly is often favored by reproductive assurance when mates or pollinators are scarce and plants suffer from outcross pollen limitation (Baker 1966; Ganders 1975; Piper et al. 1984; Barrett and Shore 1987; Barrett et al. 2009; de Vos et al. 2012). Homostyles can have either long styles and long-level anthers (‘long homostyles’) or short styles and short-level anthers (‘short homostyles’). The former condition is generally more common (see Charlesworth and Charlesworth 1979), either occurring as phenotypic variants in distylous populations (Crosby 1949; Bodmer 1960), or as the phenotype of derived monomorphic species in distylous genera (Li and Johnston 2001; Mast et al. 2006; de Vos et al. 2014). The evolution of homostyly represents the most common mechanism responsible for the dissolution of heterostyly and is often associated with reproductive isolation and speciation.
Investigations of the genetic basis of homostyly have been limited to a relatively small number of taxa two principal mechanisms appear to be involved. The first involves rare crossovers and/or mutations of large effect in the S-locus linkage group that governs the distylous syndrome (Ernst 1955; Dowrick 1956; Baker 1966; Charlesworth and Charlesworth 1979; Shore and Barrett 1985; Lewis and Jones 1992; Barrett and Shore 2008). A less common pathway to homostyly involves unlinked modifier genes of small effect that are non-allelic to the heterostyly genes (reviewed in Richards 1997, p. 260). These modifiers are often associated with considerable phenotypic variation in sex-organ length (Gander 1975, 1979; Arunkumar et al. 2017) and are more commonly reported in the less frequent cases in which heterostylous species are fully self-compatible, rather than in those possessing heteromorphic incompatibility.
Classic investigations of the inheritance of distyly and homostyly in Primula (Primulaceae) have provided numerous insights on the genetic architecture of heterostyly and have led to the supergene model to account for the suite of co-adapted floral traits that comprise the heterostylous syndrome (Mather and De Winton 1941; Ernst 1955; Dowrick 1956; reviewed in Lewis and Jones 1992; Richard 1997). Primula is comprised of c. 430 species of which 92% are distylous and 45 are monomorphic for style length; ancestral state reconstructions indicate that homostyly has been repeatedly derived from distyly (Mast et al. 2006; de Vos et al. 2014). In most Primula species, populations are either exclusively distylous or homostylous; but a few are polymorphic for the two conditions (e.g., Primula vulgaris—Crosby 1949; Bodmer 1960; P. chungensis—Zhou et al. 2017). These species provide valuable opportunities for investigating the genetics and evolution of mating system transitions. Here, we adopt this approach by exploiting the occurrence of floral polymorphisms in a third Primula species to investigate the genetic basis of the evolutionary breakdown of distyly to homostyly.
Primula oreodoxa is a poorly known perennial, subalpine herb, endemic to the mountains of western Sichuan, China (Richards 2003). Populations in this region can be of three types: distylous, homostylous, and those in which long-styled, short-styled and homostyled plants (hereafter L-morph, S-morph, H-morphs, respectively) co-occur (hereafter ‘mixed’ populations). A recent investigation of the reproductive ecology, morph ratios and genetic relationships of P. oreodoxa populations demonstrated that homostylous populations were derived from distylous populations and homostyles were favored at sites where pollinator visitation to flowers was reduced (Yuan et al. 2017). Two additional findings from this study were of particular significance. First, unlike most distylous Primula species (see Wedderburn and Richards 1990), the L- and S-morphs of P. oreodoxa were capable of high levels of autonomous self-pollination. Second, homostyles in P. oreodoxa are ‘long homostyles’ but exhibit considerable variation in sex-organ position, including both approach (stigma above anthers) and reverse (stigma below anthers) herkogamous phenotypes (Fig. 1 and see Yuan et al. 2017).
Fig. 1.

a Flowering progenies of Primula oreodoxa grown under glasshouse conditions. b Flowers of the L- and S-morphs (top row) and three homostyles illustrating variation in degree of herkogamy
These relatively unusual aspects of the floral biology of P. oreodoxa motivated us to address the following questions in this study: (1) What is the inheritance of distyly and homostyly? We were interested in determining whether homostyly has originated by major gene changes at the S-locus linkage group or by unlinked polygenic modifiers. (2) Given the self-compatible status of populations, does selfing occur and might this differ among the floral morphs depending on their sex-organ positions? We predicted the highest rates of outcrossing in the L-morph and a greater incidence of selfing in the S-morph, and particularly the H-morph. (3) Are there homozygous genotypes of the S-morph (SS) in natural populations, and is there evidence that this genotype has reduced viability as a result of ‘lethal linkage’ of deleterious genes at the S-locus (see Richards 1997)? Theoretical work on heterostylous species suggested that owing to enforced heterozygosity at the S-locus reduced viability of SS genotypes owing to linked sheltered load may occur (Strobeck 1972). We addressed these questions using controlled crosses of distylous and homostylous morphs and progeny testing of open-pollinated seed families from maternal parents in five populations.
Methods
Controlled pollinations
To investigate the compatibility status and fertility of cross- and self-pollinations of the floral morphs in P. oreodoxa, we transplanted into a glasshouse at the Biological Resources Research Station at E’mei Mountain (altitude 800 m), Sichuan Province, 15 individuals per morph from distylous population JCC and homostylous population CSQ. The two source populations are located relatively close to one another (~20 km) in the mountains of western Sichuan and their localities are mapped in Fig. 1 of Yuan et al. (2017), where more information on the natural history and reproductive biology of P. oreodoxa is available.
We conducted a total of 10 pollination treatments on the three floral morphs. These were: distylous morphs—self-pollination and cross-pollination with the alternate distylous morph and the homostylous morph; homostylous morph—self-pollination and cross-pollination with other homostylous plants and with each of the distylous morphs. We used an average of 10 plants (range 5–14) and 47 flowers (range 20–84) for each treatment using flowers in the second or third day of anthesis. All flowers were caged before and after pollinations to exclude pollinators, and for cross-pollinations, we emasculated flowers prior to anther dehiscence. We conducted pollinations in March and fruits were harvested 6–8 weeks later when mature and fruit set and seed set per fruit were recorded. We compared the fruit set and average seed number per capsule of pollination treatments using ANOVA in R 3.2.1 (R Development Core Team 2015).
Inheritance of distyly and homostyly
We used progeny from selected hand self- and cross-pollinations described in the preceding section to investigate the inheritance of distyly and homostyly in P. oreodoxa. These pollinations involved the following individuals: L-morph—L1, L3, L6, L7, L8, L10, L11; S-morph—S1, S3, S6, S10; H-morph—H4, H5, H8, H9, H11, H14. In the glasshouse, we germinated seed families of a given cross type in June 2015 on soil-filled rectangular flats and grew plants under semi-natural conditions until flowering (~10 months) (Fig. 1a). The plants were watered once a week and no supplementary lighting was provided and temperature and humidity conditions in the glasshouse was not controlled. At flowering, we recorded the floral morph of progeny and measured the stigma and anther height from the base of the ovary using digital calipers (573-S; Mitutoyo, Kawasaki, Japan) for at least 20 fresh flowers that were sampled randomly from each family.
Progeny testing of open-pollinated families
To investigate floral morph ratios, we grew open-pollinated maternal seed families to flowering (~10 months) in the glasshouse from three distylous (JCC, WWS and DWS) and two mixed (QLP and HZG) populations of P. oreodoxa. Two to three mature fruits were collected for each family in May 2016, from plants that were naturally pollinated by insects [see Yuan et al. (2017) for information on pollinators]. Sample sizes for each population were: JCC—total seed families = 25; L-morph 12, 536 progeny, S-morph 13, 957 progeny; WWS—total seed families = 25; L-morph 12, 472 progeny, S-morph 13, 766 progeny; DWS—total seed families = 25; L-morph 11, 361 progeny, S-morph 14, 515 progeny; QLP—total seed families = 22; L-morph 10, 228 progeny, S-morph 7, 174 progeny, H-morph 5, 167 progeny; HZG—total seed families = 39; L-morph 17, 1053 progeny, S-morph 15, 920 progeny, H-morph 7, 236 progeny. We also determined the floral morph ratios of flowering plants in each of the five populations, following methods described in Yuan et al. (2017). In distylous populations, we used pooled G-tests (Sokal and Rohlf 1995) to determine whether morph ratios deviated from the 1:1 equilibrium expected from intermorph (disassortative) mating between morphs. All analyses were conducted in R 3.2.1 (R Development Core Team 2015).
Results
Compatibility status of floral morphs
Controlled self- and cross-pollinations demonstrated that in contrast to most heterostylous plants, the floral morphs of P. oreodoxa are highly self-compatible. There were no significant differences in fruit set or seed set following self- and intermorph pollinations. As expected, homostylous plants were highly self-compatible and also fully cross-compatible with both distylous morphs. Among all 10 pollination treatments, there was no significant difference in the fertility of crosses (Fig S1; fruit set: F = 1.98, P = 0.91; seed set capsule: F = 1.92, P = 0.54).
Inheritance of distyly and homostyly
Selfed progeny of S-morph plants did not differ from the expected ratio of 3 S-morph:1 L-morph; whereas self-pollinations of L-morph plants gave only L-morph progeny. Intermorph crosses resulted in patterns of segregation that were not significantly different from the expected 1:1 morph ratio (Table 1). Thus, floral morph frequencies in self- and cross-pollinations of the distylous morphs in P. oreodoxa were consistent with the common genetic model for the inheritance of distyly with the S-morph heterozygous (Ss) and dominant to the L-morph (ss).
Table 1.
Floral morphs in progeny from controlled pollinations of distylous and homostylous plants of Primula oreodoxa
| Cross | Floral morph of progeny | ||||
|---|---|---|---|---|---|
| Long | Short | Homostyle | |||
| (1) Long × short | Deviation from 1 L:1 S | ||||
| L1 × S6 | 28 | 32 | G = 0.27 | P = 0.61 | |
| L11 × S10 | 36 | 40 | G = 0 .21 | P = 0.64 | |
| L3 × S3 | 33 | 27 | G = 0.60 | P = 0.44 | |
| Heterogeneity | G = 1.06 | P = 0.59 | |||
| (2) Short selfed | Deviation from 1 L:3 S | ||||
| S6 × S6 | 11 | 29 | G = 0.13 | P = 0.72 | |
| S3 × S3 | 17 | 55 | G = 0.08 | P = 0.78 | |
| Heterogeneity | G = 0.21 | P = 0.65 | |||
| (3) Long selfed | |||||
| L6 × L6 | 49 | ||||
| L7 × L7 | 59 | ||||
| L8 × L8 | 67 | ||||
| L10 × L10 | 27 | ||||
| (4) Homostyle selfed | |||||
| H4 × H4 | 50 | ||||
| H5 × H5 | 22 | ||||
| (5) Homostyle × long | |||||
| H11 × L1 | 17 | ||||
| H4 × L1 | 21 | ||||
| H4 × L11 | 13 | ||||
| L11 × H14 | 18 | ||||
| H11 × L11 | 65 | ||||
| (6) Homostyle × short | Deviation from 1 S:1 H | ||||
| H4 × S1 | 16 | 16 | G = 0 | P = 1 | |
| S6 × H8 | 16 | 12 | G = 0.57 | P = 0.45 | |
| H9 × S10 | 2 | 3 | G = 0.20 | P = 0.65 | |
| S10 × H5 | 31 | 28 | G = 0.15 | P = 0.70 | |
| Heterogeneity | G = 0.64 | P = 0.89 | |||
The distylous individuals were from distylous population JCC and the homostylous individuals were from homostylous population CSQ. G-tests were used to determine whether morph ratios deviated from the expected 1:1 and 1:3 ratios
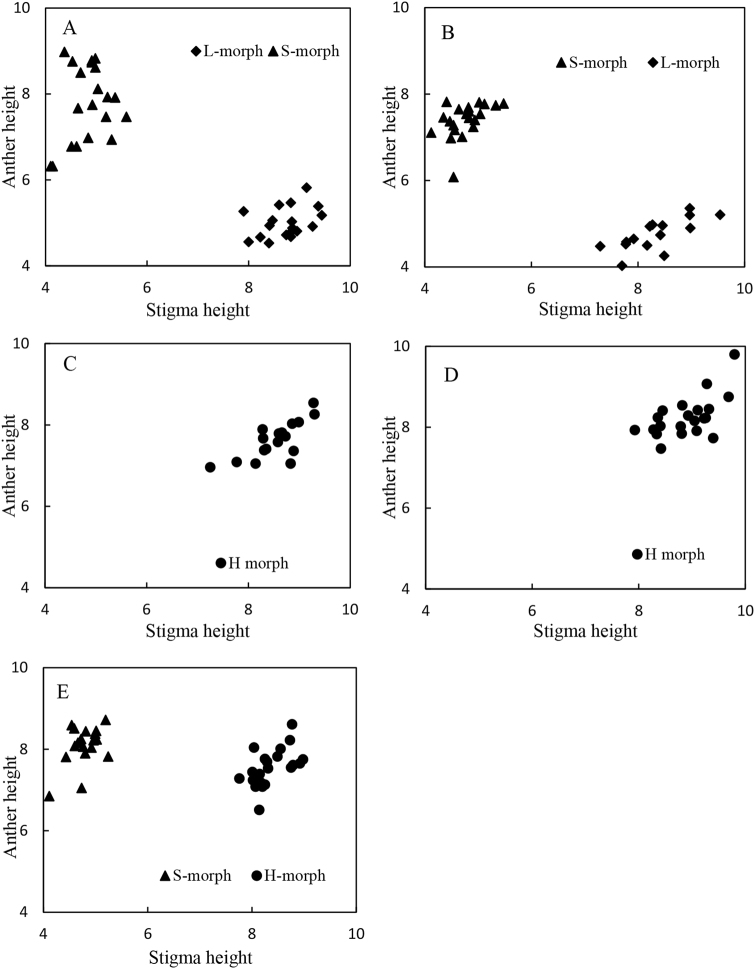
Selfing of homostylous plants gave rise to exclusively homostylous progeny and crosses between homostyles and the L-morph also resulted in only homostylous progeny, regardless of which floral morph was the pollen or ovule parent. In contrast, crosses between homostylous and S-morph plants gave rise to segregation ratios for the two morphs that did not deviate significantly from 1 H-morph:1 S-morph. The results indicate that the homostylous allele (Sh) is dominant to the long-styled allele (s), but recessive to the short-styled allele (S). Measurements of the sex organs of flowers confirmed the presence of distinct bimodal patterns in stigma and anther height where segregation occurred (Fig. 2). These patterns demonstrate that the genetic control of homostyly in P. oreodoxa is not governed primarily by polygenic modifiers and is consistent with major gene control.
Fig. 2.
Distributions of stigma and anther heights in the progeny of controlled crosses of Primula oreodoxa illustrating the discrete patterns of variation that were obtained. a S6 × S6; b L1 × S6; c H4 × L11; d H11 × L11; e S10 × H5
Progeny testing of open-pollinated plants
In the three distylous populations, progeny testing of seed families of the L-morph yielded morph frequencies not significantly different from the 1 L-morph:1 S-morph ratio expected from complete disassortative mating (Table 2). In contrast, progeny testing of S-morph families indicated a significant excess of S-morph plants in each of the three populations (Table 2). Of the 2171 plants that flowered from the 40 families, 71% were of the S-morph. In two of the three populations (JCC and DWS), morph ratios were not significantly different from 1:1, despite the significant excess of S-morph progeny. Significantly, families from four S-morph plants (JCC-S7, WWS-S4, WWS-S19 and WWS-S21) yielded between 29 and 83 S-morph progeny and no L-morph offspring. The probability of observing no L-morph plants among heterozygous (Ss) parents for a sample size of 29 is 1.86 × 10−9 (under disassortative mating) and 2.4 × 10−4 (under self-mating). Hence, these four S-morph plants are likely to be homozygous (SS) at the S-locus (Table S1).
Table 2.
Floral morphs in progeny of open-pollinated seed families of Primula oreodoxa from three distylous populations
| JCC | WWS | DWS | ||||
|---|---|---|---|---|---|---|
| L:S | G-test | L:S | G-test | L:S | G-test | |
| Population floral morph ratio |
45:55 0.45:0.55 |
G = 0.317 |
87:123 0.41:0.59 |
G = 6.20* |
14:15 0.48:0.52 |
G = 0.03 |
| L-morph families |
338:296 0.53:0.47 |
G = 2.78 |
210:248 0.46:0.54 |
G = 3.16 |
142:153 0.48:0.52 |
G = 0.41 |
| S-morph families |
198:661 0.23:0.77 |
G = 263.3*** |
217:514 0.30:0.70 |
G = 124.2*** |
219:362 0.38:0.62 |
G = 35.56*** |
| All families |
536:957 0.36:0.64 |
G = 120.3*** |
472:766 0.38:0.62 |
G = 13.58*** |
361:515 0.41:0.59 |
G = 27.21*** |
| Between morph heterogeneity | G = 145.76*** | G = 32.45*** | G = 123.69*** | |||
All G values are tested against a 1:1 morph ratio; *P < 0.05; ***P < 0.001
Progeny testing of the two mixed populations involved a total of 22 seed families and 569 offspring from QLP, and 39 seed families and 2209 progeny from HZG. The populations differed significantly in the frequency of homostylous plants (QLP = 0.51; HZG = 0.14), and in both populations S-morph plants outnumbered those of the L-morph, particularly in HZG where they comprised ~52% of the population. Pooled frequencies (L:S:H) of 0.40:0.30:0.30 were observed in the progeny of QLP and 0.47:0.42:0.11 in the progeny of HZG (Table S2). In population QLP, L-morph plants yielded L-, S- and H-morphs with a frequency of 0.54:0.25:0.21, whereas there were no homostyles in S-morph families and the L- and S-morph frequencies were 0.27:0.73. Homostyle families were almost exclusively homostylous, with only a single S-morph plant in one family (QLP-H14, Table S3). In HZG, the frequency of homostyles in families of the L- and S-morph was relatively low, 0.06 and 0.05, respectively, but homostyles dominated in homostylous families, comprising 75% of all offspring (Table S2). S-morph family HZG-S5 produced 107 S-morph plants and is therefore almost certainly of SS genotype. Three other S-morph plants QLP-S8, QLP-S11 and HZG-S29 also yielded only S-morph progeny (see Table S3), but because the family sizes were only 7, 8 and 15, respectively, more progeny would be required to unequivocally determine if these individuals were of genotype SS.
Discussion
Our study of P. oreodoxa revealed several major findings. Distyly is controlled by the common model of inheritance found in several other heterostylous families involving a single diallelic Mendelian S-locus with dominance (S-morph S-; L-morph ss), and homostyly has originated by major gene change at the distyly linkage group with the homostylous allele (Sh) dominant to the long-styled allele (s), but recessive to the short-styled allele (S). Unlike most distylous Primula species, morph-specific differences in mating are a feature of P. oreodoxa populations and appear to be associated with differences among the morphs in the degree of herkogamy. Finally, homozygous (SS) genotypes of the S-morph were evident in populations and have probably arisen by selfing and perhaps also assortative mating. We now discuss these findings in light of recent advances in understanding of the molecular genetic architecture of the Primula S-locus linkage group, and we also consider the implications of the self-compatible status of P. oreodoxa for the mating biology and genetics of populations.
Genetics of distyly and homostyly
Heterostyly has evolved on numerous occasions in the angiosperms and is reliably reported from 28 families (Lloyd and Webb 1992; Barrett et al. 2000). Studies of the inheritance of heterostyly have been conducted in 11 families, with the majority of these investigations involving distylous species (reviewed in Lewis and Jones 1992). In all distylous species examined to date, a single diallelic Mendelian locus with dominance can explain the segregation patterns obtained from controlled crosses. With two exceptions, where the dominance relations are reversed [Plumbaginaceae (Baker 1966); Hypericaceae (Ornduff 1979)], the S-morph is dominant to the L-morph. Our results for P. oreodoxa conform to the common pattern of S-morph dominance, which has also been demonstrated in a few other Primula species beginning with the seminal study of Bateson and Gregory (1905), although Darwin’s earlier crosses on P. vulgaris also conformed to these ratios (see Charlesworth and Charlesworth 2009). The uniformity of results for Primula is not unexpected because despite the large number of species in this genus, phylogenetic reconstructions are consistent with a single origin of distyly (Mast et al. 2006; de Vos et al. 2014).
The simple Mendelian inheritance of distyly is underpinned by a more complicated genetic architecture first revealed by crosses between distylous and homostylous forms of Primula (Mather 1950; Ernst 1955; Dowrick 1956). This work led to the recognition that the L- and S-morphs are genetically controlled by a chromosomal region containing several genes governing different heteromorphic traits, including style length (G locus), anther position (A locus) and pollen size (P locus) (reviewed in Richards 1997; Barrett and Shore 2008). The heterostyly genes comprise a complex supergene that functions to produce an integrated phenotype held together by tight linkage owing to suppressed recombination (Charlesworth 2016). All dominant alleles are linked on one S haplotype and all recessive alleles are linked on the alternative s haplotype. Following this model, homostyles have classically been interpreted as the product of rare recombination events within the S-locus leading to chimeric S-alleles that combine parts of both dominant and recessive alleles (Charlesworth and Charlesworth 1979; Lewis and Jones 1992). Indeed, crosses between distylous and homostylous forms of Primula species (Ernst 1957; Dowrick 1956) and Turnera ulmifolia (Shore and Barrett 1985) resulted in segregation data consistent with the origin of homostyles by recombination. Our results for P. oreodoxa also conform to these patterns and demonstrate that homostyles arise through major gene changes at the S-locus and not polygenic modifiers of sex-organ height occurring elsewhere in the genome. However, genes of small effect non-allelic to the S-locus linkage group may be involved with minor adjustments to sex-organ height once homostyles have originated, as demonstrated for homostyles of T. ulmifolia (Shore and Barrett 1990).
Recent investigations of the molecular genetic architecture of distyly in Primula cast doubt on the recombination origin of homostyles and perhaps also in other taxa. This work has established that, in contrast to the classical model of the S-locus described above, the linkage group controlling distyly is comprised of a hemizygous region comprised of at least five genes (Huu et al. 2016; Li et al. 2016; Burrows and McCubbin 2017, reviewed in Brennan 2017; Kappel et al. 2017). Of particular significance for the recombination hypothesis is that the hemizygous nature of the dominant S haplotype implies that there is no homologous sequence on the s haplotype for it to pair with and undergo crossing over (Kappel et al. 2017). Thus, it is more likely that homostyles in Primula have not arisen by recombination but rather are the result of mutation at individual loci, as originally proposed by Ernst (1936). Mutation at the style length (CYP734A50) and anther height (GLO2) genes have been proposed as possible candidate genes (Li et al. 2016). However, a model involving a mutational origin for homostyles can give the same patterns of segregation that we found in our crosses between distylous and homostylous morphs of P. oreodoxa. Therefore, based on crossing data alone it is not possible to distinguish between the recombination versus mutation hypothesis for the origins of homostyly, even though the latter seems more likely.
The finding that P. oreodoxa is thoroughly self-compatible, unlike most distylous Primula species, is of significance to current work on the molecular genetic architecture of heterostyly, which has failed to identify individual genes that specifically control heteromorphic incompatibility. Instead, several lines of evidence suggest that sex-organ length and incompatibility may be controlled by the same genes and that a classic self-recognition system, as occurs in homomorphic incompatibility (reviewed in Franklin-Tong 2008), is absent from heterostylous plants (and see Gibbs 1986; Lloyd and Webb 1992). Comparisons of sequences at CYP734A50 and GLO2 in self-incompatible Primula species with those from self-compatible species such as P. oreodoxa and P. chungensis (Zhou et al. 2017) should provide insights into the molecular basis of compatibility systems in heterostylous plants and enable definitive confirmation of the mutational origin of homostyles.
Mating patterns in natural populations
We used progeny testing of open-pollinated seed families of P. oreodoxa to provide an assessment of mating patterns in five natural populations. Although more quantitative estimates await the application of molecular markers for P. oreodoxa, by using the style length locus we were able to demonstrate striking differences among floral morphs in mating patterns. This approach using the style length alleles has been previously employed in several self-compatible heterostylous species (e.g., Ganders 1975; Barrett et al. 1987). In the three distylous populations of P. oreodoxa, open-pollinated seed families of the L-morph produced morph ratios not significantly different from 1:1. Regardless of the morph frequencies in distylous populations, this ratio is expected from disassortative mating. In contrast, seed families of the S-morph of P. oreodoxa produced mostly S-morph progeny. For example, in population JCC 77% of the total offspring from 13 S-morph families were S-morph and within each family, they outnumbered L-morph progeny. This pattern is most likely the result of significant levels of selfing and/or possibly assortative mating in the S-morph, both of which would result in S-morph biased families. Experimental studies using genetic markers have demonstrated that the contrasting sex-organ position of the floral morphs, involving approach versus reverse herkogamy, are associated with different rates of selfing, with significantly higher selfing in the S-morph compared with L-morph (Kohn and Barrett 1992; Barrett 2003, see Fig. 3). Our results are consistent with this pattern and show how the absence of self-incompatibility in heterostylous plants places an important premium on floral morphology in affecting mating patterns.
Mating in self-compatible heterostylous populations containing homostyles is more complex than in distylous populations. In P. oreodoxa, homostyles have a much higher capacity for autonomous self-pollination than the distylous morphs, owing to the close proximity of anthers and stigmas within their flowers (Yuan et al. 2017). We therefore predicted that homostyles should experience a higher incidence of selfing than either of the distylous morphs. Progeny tests of homostylous families in the two mixed populations generally supported this expectation. In mixed populations QLP and HZG, overall 99 and 75% of progeny from homostylous families were homostylous. The 24% difference between the populations likely reflects differences in mating opportunities with distylous morphs; in QLP, homostyles were the commonest morph in the population, whereas in HZG it was the least common. Our genetic study demonstrated that selfing of homostyles produced exclusively homostylous progeny, so the field data are consistent with moderate to high selfing rates in this morph. Other studies on mating patterns of homostyles in heterostylous taxa generally indicate higher rates of selfing in comparison with the distylous morphs (e.g., Primula—Piper et al. 1984; Amsinckia—Ganders et al. 1985; Eichhornia—Barrett and Husband 1990; Turnera—Belaoussoff and Shore 1995).
Sheltered load and SS genotypes in heterostylous plants
The accumulation of deleterious mutations (mutational load) is not distributed evenly across the genome. Regions under strong balancing selection or with restricted recombination may be especially susceptible to the build-up of deleterious recessive mutations. Because natural selection cannot easily purge these mutations, this phenomenon is referred to as ‘sheltered load’ and enforced heterozygosity at the S-locus is known to be associated with this form of mutational load in species with homomorphic incompatibility (Uyenoyama 1997; Stone 2004). Strobeck (1972) modeled the fitness consequences of this situation for a distylous population in which the S-locus was linked to a recessive lethal allele. Later, Richards (1997) proposed a model in which a lethal recessive allele linked to the S-locus was a key requirement for the evolution of distyly and claimed that S-morph linked lethals were “a pervasive feature of most heterostylous plants” (Richards 1997, p. 284). However, evidence for linked lethals and viability selection against homozygous genotypes of the S-morph (SS) of heterostylous species is mixed (Mather and de Winton 1941; Shore and Barrett 1985; Barrett et al. 1989; Kurian and Richards 1997). For example, homozygous S-morph plants in Primula sinensis had 70% of the viability of heterozygotes (Mather and de Winton 1941), whereas in diploid Turnera ulmifolia there was no evidence for S-linked lethals, or viability selection against SS genotypes based on 3:1 ratios following self-pollination of Ss genotypes and the occurrence of pure breeding (SS) S-morph plants.
We identified several pure breeding S-morph plants of P. oredoxa among the open-pollinated families sampled from distylous and mixed populations (see supplementary Tables S1 and S3, respectively). SS genotypes undoubtedly arise because of the capacity of the S-morph to self-fertilize and or mate with other S-morph plants. Clearly their occurrence provides evidence against S-linked lethality, as does the 3:1 rather than 2:1 ratios of the S- and L-morphs we obtained from selfing S-morph plants (Table 1). However, there is evidence that there may be viability selection against some S-morph plants in distylous populations. Progeny tests of S-morph families in distylous populations DWS and JCC indicated that overall 62 and 77% were comprised of S-morph plants. But flowering morph ratios in these populations were not significantly different from 1 L:1 S. The deficit of the S-morph in adult morph ratios could have arisen because of viability selection owing to linked load, although this pattern could also have resulted from genome-wide inbreeding depression owing to selfing.
This investigation has broadened the range of genetic and evolutionary questions that can be addressed using Primula as a model system. We have demonstrated that P. oreodoxa provides a suitable experimental system for genetic analysis as plants are easily cultured and crossed and plants can be grown to flowering in a relatively short period. The discovery of Primula species comprised of distylous, homostylous and mixed populations provides opportunities to investigate the genetic and ecological mechanisms associated with mating system transitions. Moreover, the recent discovery of two highly self-compatible Primula species (Yuan et al. 2017; Zhou et al. 2017) opens up the possibility of future comparative genomic work on the genetic architecture of the S-locus to clarify unresolved questions concerning the molecular basis of incompatibility and its breakdown in heterostylous plants.
Electronic supplementary material
Acknowledgements
We thank Dr. Shixiao Luo (SCBG) and Mr. Mingsong Wu (SCBG) for field assistance. This work was supported by National Natural Science Foundation of China (grant nos. U1202261 and U1603231) and the Ministry of Science and Technology of China (2013FY111200). Funding for SCHB was through a Discovery Grant from Natural Sciences and Engineering Research Council of Canada.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
The online version of this article (10.1038/s41437-018-0081-2) contains supplementary material, which is available to authorized users.
References
- Arunkumar R, Wang W, Wright SI, Barrett SCH. The genetic architecture of tristyly and its breakdown to self-fertilization. Mol Ecol. 2017;26:752–765. doi: 10.1111/mec.13946. [DOI] [PubMed] [Google Scholar]
- Baker HG. The evolution, functioning and breakdown of heteromorphic incompatibility systems. I. The Plumbaginaceae. Evolution. 1966;20:349–368. doi: 10.1111/j.1558-5646.1966.tb03371.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. The evolution, maintenance and loss of self-incompatibility systems. In: Doust JL, Doust LL, editors. Plant reproductive ecology: patterns and strategies. New York, NY: Oxford University Press; 1988. pp. 98–124. [Google Scholar]
- Barrett SCH. Mating strategies in flowering plants: the outcrossing-selfing paradigm and beyond. Philos Trans R Soc B. 2003;358:991–1004. doi: 10.1098/rstb.2003.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH, Arunkumar R, Wright SI. The demography and population genomics of evolutionary transitions to self-fertilization in plants. Philos Trans R Soc B. 2014;369:20130344. doi: 10.1098/rstb.2013.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH, Brown AHD, Shore SJ. Disassortative mating in tristylous Eichhornia paniculata (Pontederiaceae) Heredity. 1987;58:49–55. doi: 10.1038/hdy.1987.7. [DOI] [Google Scholar]
- Barrett SCH, Husband BC. Variation in outcrossing rate in Eichhornia paniculata: the role of demographic and reproductive factors. Plant Species Biol. 1990;5:41–56. doi: 10.1111/j.1442-1984.1990.tb00191.x. [DOI] [Google Scholar]
- Barrett SCH, Jesson LK, Baker AM. The evolution and function of stylar polymorphisms in flowering plants. Ann Bot. 2000;85:253–265. doi: 10.1006/anbo.1999.1067. [DOI] [Google Scholar]
- Barrett SCH, Morgan MT, Husband BC. The dissolution of a complex genetic polymorphism: the evolution of self-fertilization in tristylous Eichhornia paniculata (Pontederiaceae) Evolution. 1989;43:1398–1416. doi: 10.1111/j.1558-5646.1989.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Ness RW, Vallejo-Marín M. Evolutionary pathways to self-fertilization in a tristylous plant species. New Phytol. 2009;183:546–556. doi: 10.1111/j.1469-8137.2009.02937.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Shore JS. Variation and evolution of breeding systems in the Turnera ulmifolia L. complex (Turneraceae) Evolution. 1987;41:340–354. doi: 10.1111/j.1558-5646.1987.tb05802.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Shore JS. New insights on heterostyly: comparative biology, ecology and genetics. In: Franklin-Tong V, editor. Self-incompatibility in flowering plants: evolution, diversity and mechanisms. Berlin: Springer-Verlag; 2008. pp. 3–32. [Google Scholar]
- Bateson W, Gregory RP. On the inheritance of heterostylism in Primula. Proc R Soc. 1905;B76:581–586. doi: 10.1098/rspb.1905.0049. [DOI] [Google Scholar]
- Belaoussoff S, Shore JS. Floral correlates and fitness consequences of mating-system variation in Turnera ulmifolia. Evolution. 1995;49:545–556. doi: 10.1111/j.1558-5646.1995.tb02286.x. [DOI] [PubMed] [Google Scholar]
- Bodmer WF. The genetics of homostyly in populations of Primula vulgaris. Philos Trans R Soc B. 1960;242:517–549. doi: 10.1098/rstb.1960.0001. [DOI] [Google Scholar]
- Brennan AC. Distyly supergenes as a model to understand the evolution of genetic architecture. Am J Bot. 2017;104:5–7. doi: 10.3732/ajb.1600363. [DOI] [PubMed] [Google Scholar]
- Burrows BA, McCubbin AG. Sequencing the genomic regions flanking S-linked PvGLO sequences confirms the presence of two GLO loci, one of which lies adjacent to the style length determinant gene CYP734A50. Plant Repro. 2017;30:53–67. doi: 10.1007/s00497-017-0299-9. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D. The maintenance and breakdown of distyly. Am Nat. 1979;114:499–513. doi: 10.1086/283497. [DOI] [Google Scholar]
- Charlesworth B, Charlesworth D. Darwin and genetics. Genetics. 2009;183:757–766. doi: 10.1534/genetics.109.109991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth D. The status of supergenes in the 21st century: recombination suppression in Batesian mimicry and sex chromosomes and other complex adaptations. Evol Appl. 2016;9:74–90. doi: 10.1111/eva.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby JL. Selection of an unfavourable gene-complex. Evolution. 1949;3:212–230. doi: 10.1111/j.1558-5646.1949.tb00022.x. [DOI] [PubMed] [Google Scholar]
- Darwin C. The effects of cross- and self-fertilization in the vegetable kingdom. London: John Murray; 1876. [Google Scholar]
- Darwin C. The different forms of flowers on plants of the same species. London: John Murray; 1877. [Google Scholar]
- de Vos JM, Ketter B, Isham ST, Kelso S, Conti E. Reproductive implications of herkogamy in homostylous primroses: variation during anthesis and reproductive assurance in alpine environments. Funct Ecol. 2012;26:854–65. doi: 10.1111/j.1365-2435.2012.02016.x. [DOI] [Google Scholar]
- de Vos JM, Wueest R, Conti E. Small and ugly? Phylogenetic analyses of the ‘selfing syndrome’ reveal complex evolutionary fates of monomorphic primrose flowers. Evolution. 2014;68:1042–1057. doi: 10.1111/evo.12331. [DOI] [PubMed] [Google Scholar]
- Dowrick VPJ. Heterostyly and homostyly in Primula obconica. Heredity. 1956;10:219–236. doi: 10.1038/hdy.1956.19. [DOI] [Google Scholar]
- Ernst A. Weitere Untersuchungen zur Phänanalyse, zum Fertilitätsproblern und zur Genetik Primeln 2. Primula hortensis Wettstein. Arch Jul ius Klaus Stift Vercrbungsforsch Soz Rassenhyg. 1936;11:1–280. [Google Scholar]
- Ernst A. Self-fertility in monomorphic Primulas. Genetica. 1955;27:391–448. doi: 10.1007/BF01664170. [DOI] [Google Scholar]
- Ernst A. Austau sch und Mutat ion im Kornplex-Gen für Blütenplastik lind Inkornpatibilität bei. Primula Z Indukt Abstamm Vererb. 1957;88:517–599. [Google Scholar]
- Fishman L, Kelly AJ, Willis JH. Minor quantitative trait loci underlie floral traits associated with mating system divergence in Mimulus. Evolution. 2002;56:2138–2155. doi: 10.1111/j.0014-3820.2002.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Franklin-Tong V. Self-incompatibility in flowering plants: evolution, diversity, and mechanisms. Berlin: Springer; 2008. [Google Scholar]
- Ganders FR. Heterostyly, homostyly, and fecundity in Amsinckia spectabilis. Madroño. 1975;23:56–62. [Google Scholar]
- Ganders FR. The biology of heterostyly. New Zeal J Bot. 1979;17:607–635. doi: 10.1080/0028825X.1979.10432574. [DOI] [Google Scholar]
- Ganders FR, Denny SK, Tsai D. Breeding systems and genetic variation in Amsinckia spectabilis. Can J Bot. 1985;63:533–538. doi: 10.1139/b85-068. [DOI] [Google Scholar]
- Gibbs PE. Do homomorphic and heteromorphic self-incompatibility systems have the same sporophytic mechanism? Plant Syst Evol. 1986;154:285–323. doi: 10.1007/BF00990130. [DOI] [Google Scholar]
- Good-Avila SV, Mena-Ali JI, Stephenson AG, Franklin-Tong V. Genetic and environmental causes and evolutionary consequences of variations in self-fertility in self-incompatible species. In: Franklin-Tong V, editor. Self-incompatibility in flowering plants: evolution, diversity and mechanisms. Berlin: Springer-Verlag; 2008. pp. 33–52. [Google Scholar]
- Holsinger K. Inbreeding depression doesn’t matter: the genetic basis of mating-system evolution. Evolution. 1988;42:1235–1244. doi: 10.1111/j.1558-5646.1988.tb04183.x. [DOI] [PubMed] [Google Scholar]
- Huu CN, Kappel C, Keller B, Sicard A, Takebayashi Y, Breuninger H, et al. Presence versus absence of CVP734A50 underlies the style length dimorphism in primroses. eLife. 2016;5:e17956. doi: 10.7554/eLife.17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igić B, Lande R, Kohn JR. Loss of self-incompatibility and its evolutionary consequences. Int J Plant Sci. 2008;169:93–104. doi: 10.1086/523362. [DOI] [Google Scholar]
- Igić B, Busch JW. Is self-fertilization an evolutionary dead end? New Phytol. 2013;198:386–397. doi: 10.1111/nph.12182. [DOI] [PubMed] [Google Scholar]
- Jain SK. The evolution of inbreeding in plants. Annu Rev Ecol Evol Syst. 1976;7:469–495. doi: 10.1146/annurev.es.07.110176.002345. [DOI] [Google Scholar]
- Kappel C, Huu CN, Lenhard M. A short story gets longer: recent insights into the molecular basis of heterostyly. J Exp Bot. 2017;68:5719–5730. doi: 10.1093/jxb/erx387. [DOI] [PubMed] [Google Scholar]
- Kohn JR, Barrett SCH. Experimental studies on the functional significance of heterostyly. Evolution. 1992;46:43–55. doi: 10.1111/j.1558-5646.1992.tb01983.x. [DOI] [PubMed] [Google Scholar]
- Kurian V, Richards AJ. A new recombinant in the heteromorphy ‘S’ supergene in Primula. Heredity. 1997;78:383–390. doi: 10.1038/hdy.1997.61. [DOI] [Google Scholar]
- Lande R, Schemske DW. The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Lewis D, Jones DA. The genetics of heterostyly. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. pp. 129–148. [Google Scholar]
- Li J, Cocker JM, Wright J, Webster MA, McMullan M, Dyer S, et al. Genetic architecture and evolution of the S locus supergene in Primula vulgaris. Nat Plants. 2016;2:16188. doi: 10.1038/nplants.2016.188. [DOI] [PubMed] [Google Scholar]
- Li P, Johnston MO. Comparative floral morphometrics of distyly and homostyly in three evolutionary lineages of Amsinckia (Boraginaceae) Can J Bot. 2001;79:1332–1348. [Google Scholar]
- Lloyd DG. Evolution of self-compatibility and racial differentiation in Leavenworthia (Cruciferae) Contrib Gray Herb Harv Univ. 1965;195:3–134. [Google Scholar]
- Lloyd DG. Demographic factors and mating patterns in angiosperms. In: Solbrig OT, editor. Demography and evolution in plant populations. Oxford,UK: Blackwell; 1980. pp. 67–88. [Google Scholar]
- Lloyd DG, Webb CJ. The evolution of heterostyly. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. pp. 151–178. [Google Scholar]
- Mable BK, Robertson AV, Dart S, Berardo CD, Witham L, Fenster C. Breakdown of self-incompatibility in the perennial Arabidopsis lyrata (Brassicaceae) and its genetic consequences. Evolution. 2005;59:1437–1448. doi: 10.1111/j.0014-3820.2005.tb01794.x. [DOI] [PubMed] [Google Scholar]
- Mast AR, Kelso S, Conti E. Are any primroses (Primula) primitively monomorphic? New Phytol. 2006;171:605–616. doi: 10.1111/j.1469-8137.2006.01833.x. [DOI] [PubMed] [Google Scholar]
- Mather K. The genetical architecture of heterostyly in Primula sinensis. Evolution. 1950;4:340–352. doi: 10.1111/j.1558-5646.1950.tb01404.x. [DOI] [Google Scholar]
- Mather K, de Winton D. Adaptation and counter-adaptation of the breeding system in Primula. Ann Bot. 1941;5:297–311. doi: 10.1093/oxfordjournals.aob.a087394. [DOI] [Google Scholar]
- Morgan MT, Barrett SCH. Reproductive correlates of mating system evolution in Eichhornia paniculata (Spreng.) Solms (Pontederiaceae) J Evol Biol. 1989;2:183–203. doi: 10.1046/j.1420-9101.1989.2030183.x. [DOI] [Google Scholar]
- Ornduff R. The genetics of heterostyly in Hypericum aegypticum. Heredity. 1979;42:271–272. doi: 10.1038/hdy.1979.29. [DOI] [Google Scholar]
- Piper JG, Charlesworth B, Charlesworth D. A high rate of self-fertilization and increased seed fertility of homostyle primroses. Nature. 1984;310:50–51. doi: 10.1038/310050a0. [DOI] [Google Scholar]
- R Development Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical ComputingURL; 2015. [Google Scholar]
- Richards AJ. Plant breeding systems. London: Chapman and Hall; 1997. [Google Scholar]
- Richards AJ. Primula. Portland, Oregon: Timber Press; 2003. [Google Scholar]
- Shore JS, Barrett SCH. The genetics of distyly and homostyly in the Turnera ulmifolia L. (Turneraceae) Heredity. 1985;55:167–174. doi: 10.1038/hdy.1985.88. [DOI] [Google Scholar]
- Shore JS, Barrett SCH. Quantitative genetics of floral characters in homostylous Turnera ulmifolia var. angustifolia (Turneraceae) Heredity. 1990;64:105–112. doi: 10.1038/hdy.1990.13. [DOI] [Google Scholar]
- Sicard A, Lenhard M. The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants. Ann Bot. 2011;107:1433–1443. doi: 10.1093/aob/mcr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard A, Stacey N, Hermann K, Dessoly J, Neuffer B, Bäurle I, et al. Genetics, evolution, and adaptive significance of the selfing syndrome in the genus Capsella. Plant Cell. 2011;23:3156–3171. doi: 10.1105/tpc.111.088237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotte T, Hazzouri KM, Stern D, Andolfatto P, Wright AI. Genetic architecture and adaptive significance of the selfing syndrome in Capsella. Evolution. 2012;66:1360–1374. doi: 10.1111/j.1558-5646.2011.01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. San Francisco: Freeman; 1995. [Google Scholar]
- Stebbins GL. Flowering plants: evolution above the species level. Cambridge: Belknap Press; 1974. [Google Scholar]
- Stone JL. Sheltered load associated with S-alleles in Solanum carolinense. Heredity. 2004;92:335–342. doi: 10.1038/sj.hdy.6800425. [DOI] [PubMed] [Google Scholar]
- Strobeck C. Heterozygosity in pin-thrum plants or with partial sex linkage. Genetics. 1972;72:667–678. doi: 10.1093/genetics/72.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyenoyama MK. Genealogical structure among alleles regulating self-incompatibility in Angiosperms. Genetics. 1997;147:1389–1400. doi: 10.1093/genetics/147.3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyenoyama MK, Holsinger KE, Waller DM. Ecological and genetic factors directing the evolution of self-fertilization. Oxf Surv Evol Biol. 1993;9:327–381. [Google Scholar]
- Wedderburn F, Richards AJ. Variation in within-morph incompatibility inhibition sites in heteromorphic Primula L. New Phytol. 1990;116:149–162. doi: 10.1111/j.1469-8137.1990.tb00520.x. [DOI] [Google Scholar]
- Wright SI, Kalisz S, Slotte T. Evolutionary consequences of self-fertilization in plants. Proc R Soc. 2013;B280:20130133. doi: 10.1098/rspb.2013.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Barrett SCH, Duan TT, Qian X, Shi MM, Zhang DX. Ecological correlates and genetic consequences of evolutionary transitions from distyly to homostyly. Ann Bot. 2017;120:775–789. doi: 10.1093/aob/mcx098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Barrett SCH, Li HD, Wu ZK, Wang XJ, Wang H, et al. Phylogeographic insights on the evolutionary breakdown of heterostyly. New Phytol. 2017;214:1368–1380. doi: 10.1111/nph.14453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.