Abstract
The aim of this current study was to assess whether the tumour grade and molecular subtypes have influenced local control in the whole breast hypofractionated radiotherapy (HRT) over standard radiotherapy (SRT) in early breast node negative cancer patients by a retrospective control group study.
Data of 215 patients treated with hypofractionated radiotherapy at our institution from 2008 to 2011 were prospectively collected and then compared with 215 pts treated with SRT in a control group study. The local relapse free survival (LRFS) in both arms was compared on the basis of variables defined by tumour grade (Nottingham Grading System), and Molecular subtypes. Kaplan-Meier method was applied to estimate the LRFS in both groups. Chi-squared and univariate Cox proportional hazards model were conducted for all variables in both groups to assess the impact on local control. Statistical significance was assumed at P < .05. Statistical significant variables at univariate analysis were then included in multivariate Cox proportional hazards model. The median follow up duration was 9.5 years (7–13 yrs); the Kaplan Meyer 8 year LRFS did not reach any statistical significant difference between the two groups (P = . 836). At univariate Cox analysis tumour grade 3 was significantly related to local relapse only in the SRT group (P = .041) while, among molecular subtypes, no differences were found for all groups; for Her2 + noL no difference was found (P = .233). Multivariate analysis confirmed Her2 non-luminal subtype as an independent variable for local relapse regardless the fractionation arm (P = .045). Breast cancer subtypes show a different radiosensitivity, which is independent by fractionation.
Keywords: Tumour grade, Her2 non-luminal, Hypofractionation, Radiosensitivity
1. Introduction
After breast conserving surgery (BCS), adjuvant radiotherapy (ART) is indicated in the vast majority of breast cancer cases. Large clinical trials, with a follow-up of more than 20 years have clearly demonstrated the benefit of ART in terms of local control and breast cancer mortality in any age and any stage [1], [2]. To date, the most common used radiation (RT) regimen for ART has been the standard fractionation (SRT) which consists of 45–50 Gy in 25 fractions with or without a boost [3]. However, over the past two decades, large prospective trials mostly in Canada and Europe randomizing shorter RT schedules or hypofractionated RT (HypoRT) versus standard RT courses have been conducted. This has provided a result that HypoRT schedules are equally effective than longer RT ones in terms of oncological and cosmetictal outcome [4]. Furthermore HypoRT schedules have been found advantageous in terms of improved quality of life, spending time and treatment delivery resource requirements. Hypofractionated whole breast RT has been widely accepted in Canada accounting a delivery in 75–85% of patients [5]. This schedule consists of a moderate percentage as 42.5 Gy in 16 fractions administered within nearly 3 weeks without a boost. By the original randomized Ontario trial, the 10 yr recurrence rate OS and cosmetic outcome have been found similar in both groups. However in the subgroup analyses, the HRT regimen appeared to be more effective in the patients with low-intermediate grade tumours, accounting for the high grade tumours a 10 yr cumulative incidence of local relapse of 15.6% versus a 4.7% for the control arm [6]. Recently in an update analysis of this trial, following a central pathological review of specimens in 989/1234 enrolled patients, this worst relationship between high grade tumours and local relapse has not been confirmed [7]. Authors have justified these findings as a result of the different grading system applied in the original and in the last revised version. Moreover in the last version no evidence of statistical significant interaction between RT fractionation and molecular subtypes was found despite a multivariate analysis Her2 non luminal tumours showed a worse outcome. The conclusion has been that hypofractionated radiotherapy should be indicated in all grades and all molecular early breast cancer subtypes as confirmed by the ASTRO guidelines of 2018 [8]. Based on these results, we conducted a retrospective control group study to assess these correlations in our routine clinical practice outside randomized or prospective studies. Herein we present results of this retrospective control group study in which local control outcome of 215 patients treated with HypoRT has been compared with the outcome of a similar group of 215 patients identical by several characteristics but treated with standard fractionation.
2. Materials and methods
2.1. Study design
A retrospective control study group was conducted including 430 early breast cancer patients treated with ART at our institution from 2004 to 2011. Two groups consisting of 215 patients for each one were identified: the Hypofractionated radiotherapy treated group (HRT) as the study group and the standard radiotherapy treated group as the control group (SRT). Age, surgery, stage, tumour grades and molecular subtypes, systemic therapy, and duration of follow-up matched the patients of the study groups as closely as possible. The control group included patients which were previously excluded by hypofractionation due to unfavourable dosimetry as described before [9]. Results were compared and analyzed to assess as a primary endpoint the outcome in local relapse free survival (LRFS) as a function of histological grade and molecular subtypes according to the fractionation arm. Local relapse was defined as a tumour recurrence in the treated breast.
2.2. Characteristics of patients and data collection
430 patients with early breast cancer treated in our institution from 2004 and 2011 were evaluated for this study. A group of 215 patients were recruited and treated with HRT while 215 patients treated with SRT. They were matched according to these primary inclusion criteria: a) postmenopausal age greater than 60 years; b) pathological stage pT1-T2 with T < 3 cm pN0 M0 invasive breast cancer according to AJCC-UICC; c) quadrantectomy and axillary clearance or sentinel node sampling; d) systemic therapy; e) mean age e) mean duration follow up 9 years (7–13 yrs). Mean age 68 years (60–75) in the HRT group and 66 years (60–73) in the SRT group. Tumour grade was defined employing the Nottingham Grading System (NGS) in both groups. Molecular factors as Estrogen and Progesterone receptors (ER and PR), Ki 67 cut off 20% and HER2/neu were assessed by immunochemistry; HER2/neu gene amplification by fluorescence in situ hybridization was conducted in case of HER2/neu positivity [10]. Six molecular subtypes were identified on the basis of hormonal receptor status, Ki 67 value and Her2 neu expression and classified in Luminal A (LA), Luminal B+ (LB+), Luminal B− (LB−), Triple negative (TN) or basal like, Her2 luminal (Her2 + L) and He2 not luminal (Her 2 + enriched or Her2 + noL) [11]. In both groups the use of adjuvant chemotherapy, trastuzumab and hormone therapy were advised as per clinical guidelines. Patients’ characterisitics are detailed in Table 1. Written informed consent to the analysis of data was obtained by the patients. The Ethical Comitee provided the approval of the study.
Table 1.
Patient characteristics of the two groups. HRT: Hypofractionated radiotherapy. SRT: Standard radiotherapy.
| Patients | HRT | SRT |
|---|---|---|
| Mean age (years) | 68 (60–75) | 66 (60–73) |
| Stage pT1/pT2 N0 | 55/160 | 60/155 |
| HT | 135 | 136 |
| CT | 80 | 79 |
| G1 | 65 | 68 |
| G2 | 79 | 75 |
| G3 | 71 | 72 |
| LA | 50 | 45 |
| LB + | 38 | 40 |
| LB- | 41 | 44 |
| TN | 24 | 24 |
| HER 2 Lum | 42 | 40 |
| HER 2 non Lum | 20 | 22 |
2.3. Radiation treatment
Patients were treated with three dimensional conformal radiotherapy (3D-CRT) planned with Masterplan Treatment Planning® (Nucletron v./1Elekta). The planning CT scan consisted of 5 mm thick slice of the chest from the cricoid to the diaphragm; all patients were treated in a supine position on an immobilization device with shoulder inclination and both arms raised above the head. The clinical target volume (CTV) included the whole remaining breast volume defined as the palpable breast included in a tangential field, excluding the deep structures. The planning target volume of breast (PTV breast) consisted of the CTV expanded by 1 cm margins below and over the palpable breast for breathing motion and treatment set-up and 0.5 cm under the skin line and over the rib plan as seen on CT images. Whole breast was treated using two – four opposed tangential MLC customized fields-in-fields technique and 6–10 MV photon beams, depending on breast size and PTV breast coverage. The prescribed dose was 42.56 Gy in 16 fractions (266c Gy/fr 5fr/week) for the HRT arm and 50 Gy in 25 fractions (2 Gy/fr/week) for the SRT group. No boost was added in both groups.
2.4. Follow up
After completion of RT the patients in both groups were clinically evaluated every three months after radiotherapy during the first year, every six months for three years and then on a yearly basis. Post-treatment breast mammography was prescribed yearly; chest X-ray and laboratory evaluation were performed every six months for three years and then yearly. The follow up schedule was similar in both groups.
2.5. Statistical analysis
To avoid bias in the selection of patients the propensity score processing was performed. The local relapse free survival (LRFS) in both arms was obtained and compared on the basis of these variables: Nottingham Grading System (NGS) for tumour grade (G1, G2, G3) and molecular subtypes (LA, LB+, LB−, TN, Her2 + L, Her2 + noL). Survivals outcomes as LRFS, Distant metastases free survival (DFS) and overall survival (OS) were estimated using Kaplan-Meier method with log rank–test for group comparisons; patients were censored at the time of last follow up or death. Chi-squared test was used for analysis with categorical variables in both groups. Univariate Cox-proportional hazards models were performed between the fractionation groups for all variables assuming a statistical significance value at P < .05. Statistical significant variables on univariate analysis were included in multivariate Cox proportional hazards models to validate the role of prognostic variables on local control by fractionation arm. Data was processed using the SPSS Version 2.1 by normal license.
3. Results
The outcome data were obtained by analysis at 9.3 years median follow up (7–13 yrs).
In the HRT group , the median follow up was 9. 4 years. The Local relapse occurred in 14% (31/215) of treated patient. With respect to tumour grade, local relapse occurred in 12% G1, 16% G2 and 13% in G3 tumours. By Chi squared test, for grade G3 tumours no statistical correlation was recorded P = .865; OR = 0.92 [C.I. 95% 0.37–2.28]. Among molecular subtypes, local relapse was 10% for LA, 11% for LB+, 17% for LB−, 10% for Her2 + L, 20.8% for TN tumours and 30% for Her2 + noL which showed a statistical significance (P = 0.0338; OR = 0.14 [C.I. 95% 0.013–1.90]).
In the SRT group the median follow up was 9.2 years. The Local relapse in 16% (34/215) of treated patients was observed. Concerning the tumour grade, local relapse was found in 15% G1, 11% G2, and 22% G3 tumours. By Chi squared test, grade 3 tumours were significant for local relapse (P = 0.0219; OR = 0.12 [C.I. 95% 0.01–0.99]) as seen in Table 2. Among molecular subtypes, local relapse was 13% for LA, 12.5% for LB+, 18% for LB−, 21% for Her2 + L, 12% for TN tumours, 23% for Her2 + noL which was significant (P = 0.043; OR = 0.40 [C.I. 95% 0.023–0.97]). Chi squared data are detailed in Table 2 and Table 3.
Table 2.
Chi-squared test for Local Relapse (LR) by Tumor Grade in both groups.
| Grade | HRT | SRT |
|---|---|---|
| G1 | 12% (8 pts) p = 0. 28 | 15% (10 pts) p = 0.460 |
| G2 | 16% (13 pts) p = 0.345 | 11% (8 pts) p = 0.541 |
| G3 | 13% (9 pts) p = 0.865 | 22% (16 pts) p = 0.0219 |
Table 3.
Chi –squared test for LR among molecular subtypes in both fractionation groups (LA = Luminal A; LB+ = Luminal B+; LB− = Luminal B−, TN = Triple negative; HER2 L = Her2 Luminal; HER 2 non L = Her2 non Luminal).
| Subtype | HRT | SRT |
|---|---|---|
| LA | 10% (5 pts) p = 0.27 | 13% (6 pts) p = 0.35 |
| LB+ | 11% (4 pts) p = 0.12 | 12.5% (5 pts) p = 0.10 |
| LB- | 17% (7 pts) p = 0.11 | 18% (8 pts) p = 0.11 |
| TN | 20.8% (5 pts) p = 0.34 | 21% (5 pts) p = 0.32 |
| HER2 L | 10% (4 pts) p = 0.13 | 12% (5 pts) p = 0.12 |
| HER 2 non L | 30% (6 pts) p = 0.0338 | 23% (5 pts) p = 0.043 |
Comparing the outcomes of the two groups, the 8-year OS was 78% in SRT group vs 75% in HRT group (P = .572, HR 1. 15 [95% C.I. 0.90–1.55]; the 8-year DMFS was 80% in HRT group vs 75% in SRT group (P = .693, HR 0.88 [95% C.I 0.80–1.64]. Kaplan Meier log rank are shown in 4 and 5 supplementary file.
The median LRFS was 8.5 years in the whole cohort, 8.6 years in the HRT group and 8.4 years in the SRT group. The overall 8-year LRFS was 80% in HRT group vs 77% in SRT group (P = . 836); HR 0.97 [95% C.I. 0.78–1.09]). The Kaplan Meier LRFS is shown in Fig. 1.
Fig. 1.
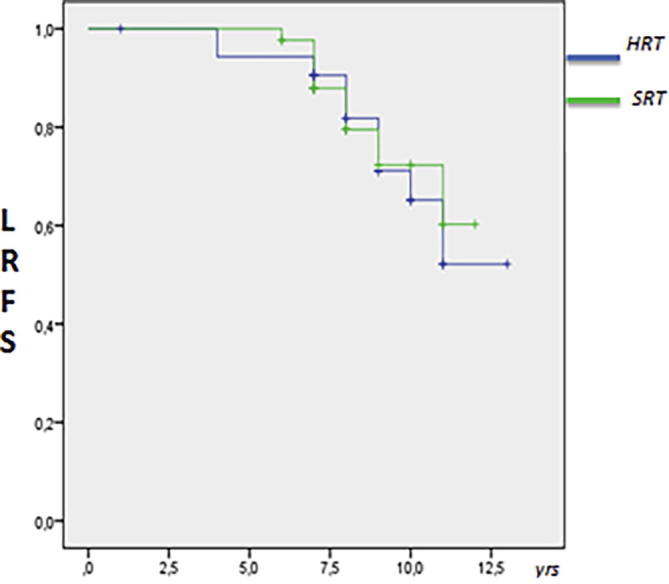
Overall estimated 8-years LRFS Kaplan Meier log –rank by fractionation group (P = . 836).
According the grading, for G3 tumours the 8 year-LRFS was 85% in HRT group vs 74% in SRT group (log–rank P = 0.035). The Kaplan Meier log-rank is shown in Fig. 2. No differences for molecular subtypes were recorded. Data and p log-rank are listed in Table 4. Interestingly the 8-year LRFS for Her2 noL did not reach a statistical difference; it was 75% in HRT group vs 78% in SRT group (log-rank P = 0.61). The Kaplan Meier log-rank is shown in Fig. 3. At univariate analysis Cox model test, tumour grade G1 and G2 were not significant for local relapse (P = .313 HR 1.24 [95% C.I 0.75–1.64] and P = .470 HR 1.15 [95% C.I 0.88–1.35]) but tumour grade 3 was significant in the SRT group (P = .0319; HR 1.75 [95% C.I. 1.53–1.93]). No interactions for molecular subtypes and local relapse according the fractionation regiment were recorded; in particular no differences were found for Her2 + noL (P = .233; HR 1.05 [95% C.I 0.73–1.28]) and TN phenotypes (P = .352; HR 1.16 [C.I. 95% 0.95–1.28]). At Multivariate Cox regression, G3 grade was not confirmed as a prognostic factor for local relapse according fractionation scheme (P = .425, HR 1.16 [C.I. 95% 0.93–1.38]).
Fig. 2.
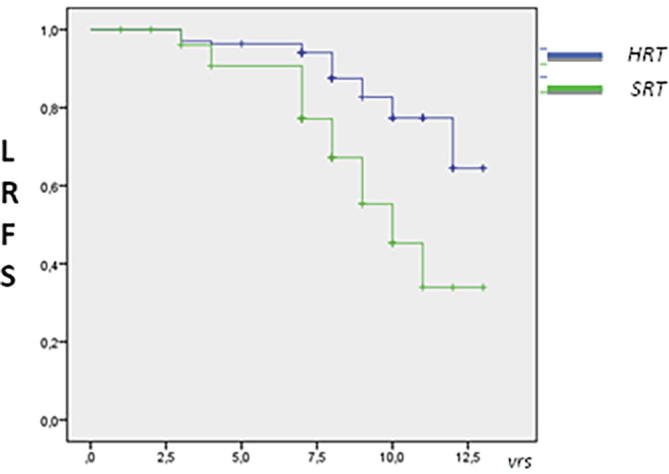
Estimated 8-year LRFS Kaplan Meier log –rank for G3 tumours by fractionation arm (P = 0.035).
Table 4.
Estimated 8-years LRFS Kaplan-Meier log-rank according grading and molecular subtypes in both groups.
| Subtype | HRT | SRT | P log-rank |
|---|---|---|---|
| G1 | 85% | 80% | 0.26 |
| G2 | 83% | 88% | 0.81 |
| G3 | 85% | 74% | 0.035 |
| LA | 88% | 85% | 0.66 |
| LB+ | 85% | 83% | 0.22 |
| LB− | 83% | 81% | 0.35 |
| TN | 78% | 80% | 0.2 |
| HER 2 Lum | 89% | 90% | 0.54 |
| HER 2 non Lum | 75% | 78% | 0.61 |
Fig. 3.
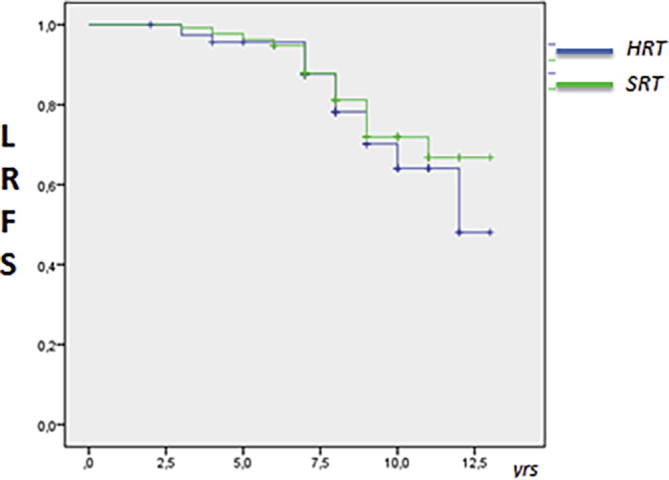
Estimated 8-year LRFS Kaplan Meier log-rank for Her2 noL by fractionation group (P = 0.6).
Among molecular subtypes, Her2 non luminal subtype was found the only indipendent factor of local relapse (P = .045 HR 1.55 [C.I. 95% 1.33–1.96]) regardless the fractionation arm. Univariate and multivariate details are listed in Table 5.
Table 5.
Univariate and Multivariate analyses Cox-model tests for local relapse among variables in both groups.
| Univariable Cox model |
Multivariable Cox model | |||||
|---|---|---|---|---|---|---|
| 215/215 | N | HR | 95% CI | % | p | HR (95%CI) |
| Her2 noL | ||||||
| HRT | 20 | ref | 30 | |||
| SRT | 22 | 1.05 | 0.73–1. 28 | 23 | 0.233 | 1.05 (0.73–1.28) p = .045 |
| Her2L | ||||||
| HRT | 42 | ref | 10 | |||
| SRT | 40 | 0.97 | 0.92–1.07 | 12 | 0.923 | – |
| LA | ||||||
| HRT | 50 | ref | 10 | |||
| SRT | 45 | 1.17 | 0.94–1.48 | 13 | 0.570 | – |
| LB+ | ||||||
| HRT | 38 | ref | 11 | |||
| SRT | 40 | 1.24 | 0.73–1.65 | 12.5 | 0.315 | – |
| LB− | ||||||
| HRT | 41 | ref | 17 | |||
| SRT | 44 | 1.13 | 0. 85–1.38 | 18 | 0.475 | – |
| TN | ||||||
| HRT | 24 | ref | 21.4 | |||
| SRT | 24 | 1.06 | 0.78–1.33 | 21 | 0.512 | – |
| G1 | ||||||
| HRT | 68 | ref | 12 | |||
| SRT | 65 | 1.24 | 0.75–1.64 | 15 | 0.313 | – |
| G2 | ||||||
| HRT | 79 | ref | 16 | |||
| SRT | 75 | 1.15 | 0.88–1.35 | 11 | 0.470 | – |
| G3 | ||||||
| HRT | 71 | ref | 13 | |||
| SRT | 72 | 1.75 | 1.53–1.93 | 22 | 0.0319 | 1.16 (0.93–1.38) p = .425 |
4. Discussion
Moderately hypofractionated radiotherapy, using schedules such as 42.5 Gy in 16 fractions administered within nearly 3 weeks, has been shown to be efficient and safe as the standard fractionated regimen for most early breast cancer patients treated with BCS.
In the ten year update of the Canadian randomized trial, the hypofractionated whole breast irradiation arm was not inferior to standard radiation in terms of local control and cosmetic outcome [6]. Nevertheless, in the subgroup analysis, a worst outcome in local control rate for G3 tumors treated in the experimental arm was found, albeit the results of UK FAST trials [12] and a population based cohort study which did not find any differences in this concern [13]. Anyway ASTRO guidelines of 2011 proceeded with caution to recommend the use of hypofractionation in this group [14].
Recently ASTRO guidelines of 2018 have revised this recommendation, indicating the choice to offer hypofractionated radiotherapy regardless the tumour grade and the molecular subtypes [8]. A contribution to this new consensus certainly comes from the update analysis at 12 years of the Canadian trial [7]. In fact, after a central pathology review with the assessment of tumour grade using the NGS [15], the update has clearly demonstrated the absence of significant differences in local control according grade and molecular subtypes between the two different fractionation schedules. Authors pointed out that in the original study the tumour grade was assessed using the Sharff Bloom Richardson (SBR) system that up to now has been considered obsolete in grading of breast cancers and less reproducible [16].
In our experience, all breast cancer specimens have been graded employing the NGS since 2003 and for all patients the prognostic factors, by which molecular classification depends, have been longer conducted by HIC procedures and fluorescence in situ hybridization for HER2/neu gene amplification.
We have demonstrated that LRFS among patients of the HRT group did not show significant difference by tumour grade, supporting the conclusions by the updated Canadian trial and other trials. Surprisingly, in the matched analysis, we found the standard fractionated RT being less effective in G3 tumours although in multivariate analysis this result was unconfirmed. However, our finding could not be a contradictive result. A probable explanation could be extrapolated by the hypofractionated studies conducted in high-risk prostate cancer because both tumours exhert the same radiobiologic rationale based on a low alpha/beta ratio in both cancers [17], [18]. For instance, in a prostate cancer hypofractionated radiotherapy randomized trial, Arcangeli et al have clearly demonstrate that high risk prostate tumours are more sensitive to hypofractionated than conventional radiotherapy [19].
In regard to the relation of molecular subtypes and radiation response by fraction sizes, it is important to remark that breast cancer is a heterogeneous disease defined by molecular distinct subtypes according to the receptor status, Ki 67 value and Her2 neu expression [20], [21].
This molecular diversity could influence the breast cancer radiosensitivity and probably it might be helpful to predict response to hypofractionated radiotherapy. Nevertheless, available data were not able to demonstrate this interaction. To this concern, in the update version, multivariate analysis provided a statistical significant worst outcome for Her2 non-luminal tumours but hypofractionation was statistical irrelevant as confirmed in the hazard ratios analysis [7]. In accordance with these findings, we too observed a high relapse rate in the Her2 non-luminal subtype in both groups.
In the matching of the two groups and in the multivariate analysis this effect did not reach any statistical significance to indicate that fractionation size mostly in this particular subtype does not influence local control.
Accumulating growing evidence suggests that the radiation response may vary significantly among molecular subtypes and that Her2 non-luminal tumours exherts more radioresistance than others as reported by Kindy [17] et al in a Danish postmastectomy study [22]. Also in the paper of Nguyen et al, the HER-2 and basal subtypes were associated with an increased risk of local recurrence [23]. Further, Voduc et al in the multivariate analysis found the Her2 enriched subtype and the young age as independent markers of local recurrence while radiation boost was statistical irrelevant [11]. In our analysis, age was not a discriminant factor because the entire studied population was older than 60 years and the boost was not delivered as properly indicated [3]. Many in vitro studies are in agreement with these clinical reports, accounting that HER2 over expression or inhibition modulates radiation resistance in breast cancer cells [24], [25]. In fact, the over-expression of HER2 is considered the hallmark of a low radiosensitivity due to some molecular mechanisms regulating cell invasivness, proliferation and stemness. There are some investigations indicating that in Her2 + noL tumours, radioresistance could be mediated by HER2-STAT3-survivin signalling, that is a crucial combination in the tumor progression and resistance to chemotherapy and radiotherapy in several tumorus, due to a key role of survivin in the inhibition of the apoptosis and promotion of mitosis in response to anticancer treatments [26]. Other studies have provided informations on the HER 2 induced breast cancer radioresistance as the effect of an activation of focal adhesion kinase (Fak) through the reduction of apoptosis and anoikis [27]. Another invoked mechanism is the modulation of FAS death receptor, which is a cell surface receptor that contains an intracellular “death domain” that plays a critical role in the initiation of the of apoptotic cell death after anticancer treatments [28]. In addition, the lack of Estrogen receptors has been associated in mediation of radiosensitivity through a modified cell cycle distribution or reduction of the radiation-induced autophagy [29].
Taken together, all these observations indicate that radiosensitivity is an intrinsic property related to specific molecular breast cancer subtype as the Her2 non-luminal breast cancer tumours; moreover different fractionation sizes seem not impact on local control according to different molecular subtypes as suggested by Bane et al [7].
5. Conclusions
To conclude, we are aware that our study presents several limitations because of a retrospective analysis and a low statistical power due to the small population sample size. However in agreement with the results of the 12 year updated Canadian trial, it supports the finding that tumour grade and breast cancer molecular subtypes are not discriminant factors to offer adjuvant hypofractionated whole breast radiotherapy in early breast cancer patients, also in Her2 enriched tumour, which is considered a radioresistant subtype by in vivo, and vitro studies. A better knowledge of mechanisms governing the response to radiation among different breast cancer subtypes could be helpful to overcome this intrinsic radioresistance.
Conflict of interest statement
Authors declare no potential conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2018.11.008.
Contributor Information
G. Lazzari, Email: lazzarigrazia@gmail.com.
G. Silvano, Email: gisilva@tin.it.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Darby S., McGale P., Correa C. Effect of radiotherapy after breast –conserving surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 10,801 women in 17 randomized trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGale P., Taylor C., Correa C. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomized trials. Lancet. 2014;383(9953):2027–2035. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartelinik H., Horiot J.C., Poortmans P.M. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881–10882 trial. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 4.Budach W., Bölke E., Matuschek C. Hypofractionated radiotherapy as adjuvant treatment in early breast cancer. A review and meta-analysis of randomized controlled trials. Breast Care. 2015;10:240–245. doi: 10.1159/000439007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasan Y., Waller J., Yao K., Kmura S.J., Huo D. Utilization trend and regimens of hypofractionated whole breast radiation therapy in the United States. Breast Cancer Res. 2017;162(2):317–328. doi: 10.1007/s10549-017-4120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whelan T.J., Pignol J.P., Levine M.N. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 7.Bane A.L., Whelan T.J., Pond G.R. Tumor factors predictive of response to hypofractionated radiotherapy in a randomized trial following breast conserving therapy. Ann Oncol. 2014;25:992–998. doi: 10.1093/annonc/mdu090. [DOI] [PubMed] [Google Scholar]
- 8.Smith B.D., Bellon J.R., Blitzblau R. Radiation therapy for the whole breast: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018;8(3):145–552. doi: 10.1016/j.prro.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Lazzari G., Terlizzi A., Della Vittoria Scarpati G. Predictive parameters in hypofractionated whole-breast 3D conformal radiotherapy according to the Ontario Canadian Trial. OncoTargets Therapy. 2017;10:1835–1842. doi: 10.2147/OTT.S127833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheang M.C., Voduk D., Bajdik C. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 11.Voduc K.D., Cheang M.C.U., Tyldesley S., Gelmon K., Nielsen T.O., Kennecke H. Breast Cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 12.Haviland J.S., Owen J.R., Dewar J.A. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 13.Herbert C., Nichol A., Olivotto I. The impact of hypofractionated whole breast radiotherapy on local relapse in Ptient with Grade 3 early breast cancer: a population – based cohort study. Int J Radiat Oncol Biol Phys. 2012;82(5):2086–2092. doi: 10.1016/j.ijrobp.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 14.Smith B.D., Bentzen S.M., Cr Correa. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81(1):59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 15.Jones H.A., Antonini N., Hart A.A. Impact of pathological characteristics on local relapse after breast –conserving therapy: a subgroup analysis of the EORTC boost versus no boost trial. J Clin Oncol. 2009;27:4939–4947. doi: 10.1200/JCO.2008.21.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloom H.J., Richardson W.W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas B.G. Superfractionation: its rationale and anticipate benefits. Int J Radiat Oncol Biol Phys. 1982;8:1143–1153. [PubMed] [Google Scholar]
- 18.Brenner D.J., Martinez A.A., Edmundson G.K., Mitchell C., Thames H.D., Armour E.P. Direct evidence that prostate tumors show high sensivity to fractionation (low alpha/beta ratio), similar to late- responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6–13. doi: 10.1016/s0360-3016(01)02664-5. [DOI] [PubMed] [Google Scholar]
- 19.Arcangeli G., Saracino B., Gomellini S., Petrongari M.G., Arcangeli S., Sentinelli S. A prospective phase III randomized trial of hypofractionation versus conventional fractionation in patients with high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:11–18. doi: 10.1016/j.ijrobp.2009.07.1691. [DOI] [PubMed] [Google Scholar]
- 20.Perou C.M., Sorlie T., Eisen M.B. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 21.Park S., Koo J.S., Kim M.S. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using himmunohistochemistry. Breast. 2012;21:50–57. doi: 10.1016/j.breast.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Kyndi M., Sorensen F.B., Knudsen H., Overgaard M., Nielsen H.M., Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen P.L., Taghian A.G., Katz M.S. Breast Cancer subtype approximated by Estrogen receptor, Progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26(14):2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 24.Pietras R.J., Poen J.C., Gallardo D., Wongvipat P.N., Lee H.J., Slamon D.J. Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells over-expressing this oncogene. Cancer Res. 1999;59:1347–1355. [PubMed] [Google Scholar]
- 25.Duru N., Fan M., Candas D. HER2–associated radioresistance of breast cancer stem cells isolated from HER-2 negative breast cancer cells. Clin Cancer Res. 2012;18:6634–6647. doi: 10.1158/1078-0432.CCR-12-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J.S., Kim H.A., Seong M.K. STAT3-survivin signaling mediates a poor response to radiotherapy in HER2-positive breast cancers. Oncotarget. 2016;7(6):7055–7065. doi: 10.18632/oncotarget.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou J., Zhou Z., Chen X. HER2 reduces breast cancer radiosensivityby activating focal adhesion Kinase in vivo and in vitro. Oncotarget. 2016;7(29):45186–45198. doi: 10.18632/oncotarget.9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horton J.K., Siamakpour-Reihani S., Lee C.T. FAS death receptor: a breast cancer subtype-specific radiation response biomarker and potential therapeutic target. Radiat Res. 2015;184:456–469. doi: 10.1667/RR14089.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X., Ma N., Zhou Z. Estrogen receptor mediates the radiosensivity of Triple-Negative Breast Cancer cells. Med Sci Monit. 2017;23:2674–2683. doi: 10.12659/MSM.904810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


