Abstract
Wiedemann-Rautenstrauch syndrome (WRS), also known as neonatal progeroid syndrome, is a rare disorder of unknown etiology. It has been proposed to be autosomal-recessive and is characterized by variable clinical features, such as intrauterine growth restriction and poor postnatal weight gain, characteristic facial features (triangular appearance to the face, convex nasal profile or pinched nose, and small mouth), widened fontanelles, pseudohydrocephalus, prominent scalp veins, lipodystrophy, and teeth abnormalities. A previous report described a single WRS patient with bi-allelic truncating and splicing variants in POLR3A. Here we present seven additional infants, children, and adults with WRS and bi-allelic truncating and/or splicing variants in POLR3A. POLR3A, the largest subunit of RNA polymerase III, is a DNA-directed RNA polymerase that transcribes many small noncoding RNAs that regulate transcription, RNA processing, and translation. Bi-allelic missense variants in POLR3A have been associated with phenotypes distinct from WRS: hypogonadotropic hypogonadism and hypomyelinating leukodystrophy with or without oligodontia. Our findings confirm the association of bi-allelic POLR3A variants with WRS, expand the clinical phenotype of WRS, and suggest specific POLR3A genotypes associated with WRS and hypomyelinating leukodystrophy.
Keywords: POLR3A, RNA polymerase 3A; neonatal progeroid syndrome; Wiedemann-Rautenstrauch syndrome
Main Text
Neonatal progeroid syndrome, also known as Wiedemann-Rautenstrauch syndrome (WRS) [MIM: 264090], is an extremely rare, heterogeneous disorder characterized by variable clinical features such as intrauterine growth restriction (IUGR) and poor postnatal weight gain, characteristic facial features (triangular face, convex nasal profile or pinched nose, and small mouth), widened fontanelles, pseudohydrocephalus (i.e., the head appears enlarged, but head circumference is normal for the person’s age), prominent scalp veins, lipodystrophy, and teeth abnormalities.1, 2, 3, 4 WRS was first described by Rautenstrauch and Snigula in 1977 in two sisters1 and by Wiedemann in 1979 in two unrelated individuals.2 Subsequently, Devos and colleagues reported a fifth unrelated patient born to consanguineous parents, proposed the name WRS, and suggested autosomal-recessive inheritance.5 Since these early reports, over 40 additional individuals with variable phenotypes have been reported, and were recently reviewed.4
Recent evidence suggests that WRS is genetically heterogeneous. For example, some individuals diagnosed with WRS have been reported to have de novo mutations in fibrillin 1 (FBN1, [MIM: 134797]),6, 7 caveolin 1 (CAV1, [MIM: 601047])8 and, more recently, in solute carrier family 25 member 24 (SLC25A24, [MIM 608744]).9 In 2016, Jay and colleagues reported an infant with a clinical diagnosis of WRS and bi-allelic POLR3A (RNA polymerase 3A, [MIM: 614258]) null variants.10 However, phenotypic features of this patient could not be conclusively attributed to POLR3A variants. Here we provide compelling evidence for POLR3A as the major locus of autosomal-recessive WRS by reporting on seven additional infants, children, and adults with WRS and bi-allelic POLR3A loss-of-function variants that alter splicing and/or truncate translation. We further expand the scope of phenotypic findings in WRS; for example, we describe near-total loss of permanent dentition, congenital fractures, lower-limb contractures, patellar dislocation, mandibular hypoplasia, thyroglossal cyst, and adult-onset thyroid papillary carcinoma.
We identified seven unrelated infants, children, and adults (ranging in age from birth to 21 years) with clinical features of WRS (Table 1, Figures 1 and 2). Clinical features of two of these individuals (subjects 2 and 3) were briefly reported previously.8 The adult subjects as well as the parents of all children provided written informed consent for participation in the current study. This study was reviewed and approved by the Human Research Protection Office at Washington University, St. Louis, Missouri, and by the Institutional Review Board of University of Texas Southwestern Medical Center, Dallas, Texas.
Table 1.
Clinical Characteristics of Infants, Children, and Adults with Wiedemann-Rautenstrauch Syndrome (WRS) and Bi-allelic POLR3A Variants
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | Subject 6 | Subject 7 | |
|---|---|---|---|---|---|---|---|
| Current age | 2 years | 20 years | 13 years | 3 years | 3 years | 21 years | 5 years |
| Sex | female | female | female | female | male | female | male |
| Pregnancy and delivery | born at 37 wks, IUGR, antenatal findings of abnormal skull shape | born at 30 wks | born at 34 wks, IUGR | born at 38 wks, IUGR | born at 38 wks, IUGR, antenatal findings of abnormal skull shape | born at 38 wks, IUGR | born at 33 wks, IUGR, antenatal findings of abnormal skull shape |
| Birth parameters | BW: 1,700 g; (< 1%, −2.9 SD) | BW: 960 g; (13%. −1.1 SD) | BW: 1,160 g; (1%, −2.5 SD) | BW: 2,400 g; (7%, −1.5 SD) | BW: 1,810 g; (< 1%, −3.3 SD) | BW: 1,970 g; (< 1%, −2.7 SD) | BW: 1,210 g; (2%, −2SD) |
| length: 43 cm; (3%, −1.8 SD) | NA | NA | length: 45.7 cm; (12%, −1.2SD) | NA | length: unable to assess due to knee flexion | NA | |
| OFC: 31 cm; (9%, −1.3 SD) | NA | NA | OFC (3 wks): 34.7 cm; (36%, −0.4 SD) | NA | OFC (8 wks): 36 cm; (16%, −1.0 SD) | OFC: 27.3 cm; (2%, −2SD) | |
| Craniofacial features | relative macrocephaly, large anterior and posterior fontanelles, prominent forehead, wide-spaced eyes, short nose with anteverted nares, small low-set ears, micrognathia | macrocephaly, prominent forehead, triangular appearance to face, thin nose, small mouth, sparse eyelashes and eyebrows, low-set ears, mandibular hypoplasia, thyroglossal cyst (post resection), sparse scalp hair | macrocephaly and hydrocephalus requiring ventriculo-peritoneal shunt at 6 mos, prominent forehead, triangular appearance to face, small palpebral fissures, thin nose, small mouth, mandibular hypoplasia, thin scalp hair | triangular appearance to face, broad forehead, sparse eyebrows, hooding of bilateral eyelids, broad nasal root and pointed nasal tip with upslanting anteverted nares, low-set ears, full head of sparse hair | triangular appearance to face, pointed chin, fullness of lateral eyebrows, bitemporal narrowing, thin lips, small mouth, low-set ears, sparse hair | prominent bitemporal narrowing, fullness of lateral eyebrows, slightly upslanting palpebral fissures, pointed chin, thin lips, small mouth, convex nasal ridge, nasal tip below level alanasae, decreased buccal fat, mandibular prognathism, columella collapse | triangular appearance to face, prominent chin, broad tall forehead, bitemporal narrowing, upslanting palpebral fissures, thin lips, convex nasal ridge, protuberant eyes, atrophy of buccal fat pad (thin appearing face), low-set ears, sparse hair |
| Dental abnormalities | natal teeth | natal teeth, absence of permanent teeth | natal teeth, absence of permanent teeth | first tooth erupted at 2 yrs and then spontaneously lost, 2 teeth at age 3 yrs | natal teeth | natal teeth, 3 permanent teeth (molars) | natal teeth |
| Postnatal growth | gastrostomy tube placed at 6 mos | poor weight gain; recurrent pneumonias and swallowing concerns; gastrostomy tube since age 13 yrs; adult weight: 30 kg; adult height: 130 cm | poor weight gain; gastrostomy tube at 5 yrs; 14.5 kg at 10 yrs, (< 1%, −6.1 SD); height: 122 cm, (1%, −2.5SD) | slow weight gain; 2nd percentile at 2 yrs | poor weight gain; 8.6 kg at 2 yrs, (< 1%, −3.7SD) | 6.1 kg at 1 yrs, (< 1%, −4.3 SD); 7.8 kg at 2 yrs, (< 1%, −4.8 SD); adult weight: 26 kg; adult height: 135 cm | nasogastric tube fed until 1 yr, then gastrostomy tube; also feeds orally; 16.8 kg at 5 yrs, (10%–25%); height: 107.4 cm, (25%–50%) |
| Fat tissue distribution | decreased subcutaneous fat, localized fat distribution over posterior iliac region and buttocks | generalized decreased subcutaneous fat | decreased subcutaneous fat, fat present in dorsum and plantar aspect of feet, abnormal fat pad over buttocks | decreased subcutaneous fat especially over extremities, thin extremities | decreased subcutaneous fat, localized fat distribution over posterior iliac region | localized fat distribution over posterior iliac region- present at birth and less prominent with age | decreased subcutaneous fat, abnormal fat distribution over buttocks |
| Skin findings | prominent veins over forehead | prominent veins, dry skin | prominent veins on forehead | NA | prominent veins on forehead | prominent veins on extremities and forehead, keloid formation, severe eczema as a child, dry skin as adult | prominent veins over forehead |
| Extremity and joint findings | left tibial and fibular fractures at birth; metaphyseal flaring of humeri, femurs and tibias; long fingers and toes with overlapping toes of right foot; left congenital patellar dislocation, underwent patellar realignment at 7 and 26 mos | contractures in bilateral ankles and knees, normal wrists and elbows. | left knee contracture at birth, absent left patella, hamstring lengthening and knee capsulotomy at 6 yrs, hip release at 8 yrs | fingers appear proportionately long to overall length of hand, normal size of hands (50th %) and feet (10th–25th %) | prominent knees | fixed flexion of knees, unable to walk unassisted as unable to fully extend knees | no abnormalities |
| Neurologic and developmental abnormalities | sits unassisted, walked at 22 mos, several words | intention tremors, cerebellar signs present, muscle weakness, numbness in toes and feet, unintelligible speech, inability to walk since 9 yrs | normal intellectual development, performs well in mainstream classroom, motor development limited by contractures | normal motor and speech development | walked at 10 mos, says several words | rolled at 8 mos, crawled at 12 mos, sat unassisted at 12 mos, walks with assistance, wheelchair proficient | walked at 14 mos;at 3 yrs, able to run, jump but not ride a bicycle, scribbles, says 50 single words |
| Vision and hearing | astigmatism, myopia | myopia and astigmatism, nystagmus on lateral gaze, nocturnal lagophthalmos | entropion, severe astigmatism, severe corneal scarring | normal vision and hearing | normal vision and hearing | hyperopia, nystagmus, recurrent otitis media as child with tympanostomy tubes, moderate hearing loss, hearing aids | normal vision and hearing |
| Additional findings | NA | papillary thyroid carcinoma at 20 yrs, menarche at 13 yrs, secondary amenorrhea at 19 yrs | heart murmur, tonsillectomy at 3 yrs | NA | NA | NA | undescended testes, repaired at 2 yrs |
| Family history | unaffected male sibling | unaffected male sibling | unaffected male and female siblings | unaffected half-sister | unaffected male and female siblings | two unaffected female siblings | affected male sibling |
| Chromosomal microarray | non-diagnostic | NA | NA | non-diagnostic | NA | NA | non-diagnostic |
| Pathogenic POLR3A variants | c.490+1G>A (C); c.3337−5T>A (C) | c.3337−11T>C (C); c.2005C>T (p.Arg669∗) | c.3337−5T>A (C); c.760C>T (p.Arg254∗) | c.1572+1G>A; c.3337−5T>A (C) | c.3243−2A>G; c.3337-5T>A (C) | c.2617-1G>A; c.3337-11T>C (C) | c.3G>T (p.Met1∗); c.∗18 C>T |
Abbreviatins are as follows: IUGR, intrauterine growth restriction; BW, birth weight; SD, standard deviation; OFC, occipitofrontal circumference; wks, weeks; mos, months; yrs, years; (C), confirmed at cDNA level; NA, not available. POLR3A GenBank: NM_007055.3.
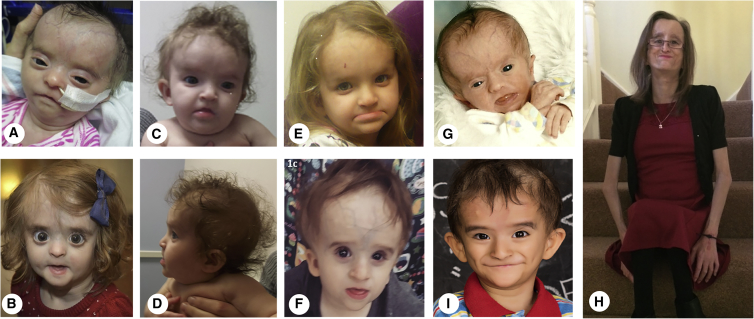
Figure 1.
Images of Infants, Children, and Adults with Wiedemann-Rautenstrauch Syndrome and Bi-allelic POLR3A Variants
Subject 1 as an infant (A) and at 17 months (B); subject 4 as an infant (C and D) and at 3 years (E); subject 5 at 3 years (F); subject 6 as an infant (G) and adult (H); and subject 7 at 5 years (I). Physical features include relative macrocephaly, a prominent forehead, a triangular-appearing face, hypertelorism, anteverted nostrils, bitemporal narrowing, upslanting palpebral fissures, thin lips, and sparse hair.
Figure 2.
Physical Features of Subjects with Wiedemann-Rautenstrauch Syndrome and Bi-allelic POLR3A Variants
(A) Decreased subcutaneous fat; localized fat distribution over posterior iliac region and buttocks in an infant.
(B and C) Anterior (B) and posterior (C) views demonstrate marked loss of subcutaneous fat from the trunk and extremities, intact fat over the buttocks, and contractures in the hips, left elbow, and knees.
(D) A lateral view demonstrates decreased subcutaneous fat in the upper and lower extremities, intact fat over the trunk, and a gastrostomy tube in the abdomen.
Using whole-exome sequencing (WES) (subjects 1–4) or Sanger sequencing of the POLR3A locus (subjects 5–7), we identified bi-allelic, rare, compound-heterozygous variants in POLR3A in all seven individuals (Table 1). The c.3337−5T>A (GenBank: NM_007055.3, dbSNP: rs368905417) variant identified in four unrelated subjects (subjects 1, 3, 4, and 5), is present in only one heterozygous individual of European descent in gnomAD (minor allele-frequency [MAF] of 0.000004, gnomAD browser, see Web Resources),11 and results in in-frame skipping of amino acids coded by exon 26, p.Ile1113_Glu1143del (Figure 3). The c.3337−11T>C variant is novel, was identified in two subjects (2 and 6), and like the c.3337−5T>A variant, also results in the skipping of exon 26 (Figure 3). The c.490+1G>A variant (subject 1) is novel, results in aberrant splicing (Figure S1), and is predicted to result in a premature termination codon 10 amino acids into intron 4. Subjects 2 and 3 carry novel nonsense variants, c.2005C>T (p.Arg669∗) and c.760C>T (p.Arg254∗), respectively. Subjects 4, 5, and 6 were found to have intronic variants within 2 base pairs of the exon-intron splice junction (c.1572+1G>A (MAF 0.000008, dbSNP: rs141484643), c.3243−2A>G [novel] and c.2617−1G>A [MAF 0.00002, dbSNP: rs181087667]), respectively, that are predicted to alter splicing in silico (Alamut, Interactive Biosoftware).12 Subject 7 was found to have a c.3G>T variant (MAF 0.000004) that results in the loss of the putative methionine start codon (p.Met1?) and a variant in the 3′ untranslated region c.∗18C>T (MAF 0.000004). Deletion and duplication analysis of POLR3A was performed in a clinical laboratory (GeneDx) for subject 7 and was negative. We speculate that the c.∗18C>T variant might alter post-transcriptional modification of POLR3A (e.g., at a microRNA binding site). Analyses of parental samples for all subjects confirmed that the POLR3A variants were inherited in trans.
Figure 3.
Intronic POLR3A Variants, c.3337−5T>A and c.3337−11T>C Result in Abnormal Splicing and an In-Frame Exon 26 Deletion, Resulting in p.Ile1113_Glu1143del
(A) A partial gene structure for POLR3A. Exons are boxed, intronic nucleotide alterations c.3337−5T>A and c.3337−11T>C are marked, and the primer pairs used for amplification are shown as arrows above and below the exons (F = forward primer; R = reverse primer).
(B) Peripheral-blood RNA samples from subject 1, her parents, and a healthy control subject demonstrate two bands (474 bp, 381 bp) for subject 1 and the mother but only one for the father and control.
(C) Amplified peripheral-blood RNA samples obtained from subject 2, shown in comparison to samples from a healthy control, demonstrate two bands (243 bp, 150 bp). The dashed line indicates that the gel image has been truncated.
(D and E) Gel purification, PCR, and Sanger sequencing of the two bands from subject 1 demonstrate wild-type sequence (D, larger band) and exon 26 skipping (E, smaller band).
(F and G) Gel purification, PCR, and Sanger sequencing of the two bands from subject 2 demonstrate wild-type sequence (F, larger band) and exon 26 skipping (G, smaller band).
Of the eight previously reported WRS-affected subjects at UT Southwestern,7, 8 two (subjects 2 and 3) had bi-allelic POLR3A variants, whereas, two others each had de novo variants in FBN1 and CAV1, and the genetic basis for the disorder found in the other two remains unclear. At Washington University, all five WRS-affected subjects had bi-allelic POLR3A variants. Thus, our report of seven infants, children, and adults with bi-allelic truncating mutations in POLR3A provides compelling evidence that POLR3A is the major locus for the autosomal-recessive WRS phenotype.
Previously recognized phenotypic heterogeneity among individuals with WRS is associated with more recently recognized genetic heterogeneity. Eight individuals with WRS and Marfan syndrome (MFS [MIM: 154700]) features, such as IUGR, preterm birth, generalized lack of subcutaneous fat except in the breast and iliac region, aged appearance of the face at birth, hyper-extensible joints, arachnodactyly, and severe myopia, have been reported with de novo heterozygous-null mutations in the penultimate exon of FBN1.6, 7, 13, 14, 15, 16 Our group has previously reported de novo heterozygous-null mutations in CAV1 in two children with neonatal-onset lipodystrophy, WRS, pulmonary artery hypertension, and advanced bilateral cataracts.8 More recently, de novo heterozygous missense variants in SLC25A24 were reported in five girls with Gorlin-Chaudhry-Moss syndrome (also called Fontaine progeroid syndrome) (FPS [MIM: 612289]; the girls presented with IUGR; short stature; coronal craniosynostosis and severe midface hypoplasia; body and facial hypertrichosis; microphthalmia; short distal phalanges; variable lipodystrophy; and cutis laxa.9 Two of these girls were also initially clinically diagnosed with WRS.9 In contrast to individuals with de novo heterozygous variants in FBN1, CAV1, and SLC25A24, our subjects with bi-allelic null POLR3A variants had short stature and poor weight gain despite gastrostomy-tube feeding; they also had macrocephaly, a prominent forehead, sparse scalp hair, prominent scalp veins, a triangular appearance to the face, natal teeth but total lack of or sparse permanent dentition, joint contractures, and generalized lipodystrophy. The infants, children, and adults in our report also had some unique clinical features that have not been previously described in WRS-affected individuals; such features included congenital fractures (subject 1); and mandibular hypoplasia, adult-onset thyroid papillary carcinoma, thyroglossal cyst, and cerebellar signs (subject 2). Previous reports indicate that approximately 30% of WRS-affected individuals died before six years of age,17 but of the seven individuals with WRS and bi-allelic POLR3A variants in our study and the one reported previously by Jay and colleagues, only one has died.10
The clinical phenotypes of the seven individuals in our series overlap with previous descriptions of WRS1, 2, 3, 4, 5 and include IUGR; post-natal failure to thrive; craniofacial features, including triangular appearance to the face, low-set ears, and prominent scalp veins; joint contractures; and generalized lipodystrophy. Using the phenotypes of the original individuals reported by Rautenstrauch and Snigula1 and by Wiedemann2 to identify WRS, Paolocci and colleagues reviewed the 51 total cases that had been reported in the literature and determined that only 18 of the described individuals had phenotypes consistent with the original clinical descriptions.4 Paolocci et al. defined the core features of WRS as IUGR, sparse scalp hair, a triangular face, a small mouth with a thin upper lip, natal teeth, and generalized lipodystrophy that spared some local fatty tissue.4 In addition, some WRS-affected individuals also had prominent scalp veins, wide cranial sutures, hypodontia, and a lower eyelid that covered part of cornea. Because our subjects share many overlapping clinical features with the 18 individuals identified by Paolocci et al, we speculate that some of these earlier individuals might also have had bi-allelic POLR3A variants.
In 2011, variants in POLR3A were discovered to underlie hypomyelinating leukodystrophy 7 (HDL7), with or without oligodontia and hypogonadotropic hypogonadism (HLD7 [MIM: 607694]); this disorder is an autosomal-recessive condition characterized by cognitive regression, upper-motor-neuron and cerebellar signs, hypodontia, evidence of cerebral hypomyelination involving the deep white matter, and vermian cerebellar atrophy as assessed by magnetic resonance imaging.18, 19 In the largest review of 43 individuals with HLD7 due to variants in POLR3A, none had bi-allelic loss of function variants.20 Interestingly, six individuals from a large Syrian family who presented with leukodystrophy and oligodontia were homozygous for the same intronic variant (p.Tyr637Cysfs∗23) identified in the WRS-affected patient identified by Jay and colleagues.10, 19, 21 The p.Tyr367Cysfs∗23 variant results in leaky splicing that produces both aberrant transcript (partial intron retention, addition of six amino acids, and a premature stop codon) and normal transcript.19 We speculate that the hypomyelinating-leukodystrophy phenotype reported in the individuals homozygous for p.Tyr367Cysfs∗23 results from expression of sufficient wild-type transcript for normal fetal development, but insufficient or abnormal POLR3A protein for normal neurologic function. However, when this variant is in trans with a nonsense variant,10 the WRS phenotype results. Although information regarding the neurologic and developmental progression of individuals with WRS in our series is limited, subjects 2 and 6 are now 20 and 21 years old and living with some assistance or independently, respectively, and subjects 1, 4, 5, and 7 are toddler-preschool age and making developmental progress. Subject 3 is performing well academically in a mainstream classroom. These outcomes contrast with those of individuals who have POLR3A-related leukodystrophy and develop progressive neurologic deterioration.
POLR3A is the largest subunit of RNA polymerase III (Pol III) and combines with POLR3B to form the catalytic subunit of Pol III. Pol III transcribes more than 200 small noncoding RNAs, including 5S ribosomal RNA (rRNA), 7U6 small nuclear RNA (snRNA), 7SK snRNA, RNase P, RNase MRP, short interspersed nuclear elements (SINEs), and transfer RNAs (tRNAs), that regulate transcription, RNA processing, and translation.18, 22 POLR3A is highly conserved from yeast to mammalian species22, 23 and is widely expressed in tissues.24 Homozygous Polr3a−/− mice are embryonic lethal, but heterozygous Polr3a+/− mice reproduce normally and do not display abnormal phenotypic features at 12 months of life.25 Of note, the lack of individuals reported in gnomAD (n= ∼135,000 individuals) with bi-allelic frameshift or nonsense variants in POLR3A suggests that POLR3A has critical functions in human development.
Our study has some limitations. We might have missed other precise genetic diagnoses by sequencing only POLR3A in subjects 5–7. However, their phenotypic features meet the diagnostic criteria for WRS, their phenotypes overlap, and these subjects exhibit rare, predicted, or demonstrated pathogenic, bi-allelic POLR3A variants, which makes other candidate genes less likely. In addition, Sanger sequencing might have missed deep intronic variants that could activate a cryptic splice site in subject 7.
In summary, our results strongly suggest that bi-allelic, rare POLR3A variants that alter splicing and/or truncate translation underlie the autosomal-recessive subtype of WRS. We speculate that bi-allelic splicing or truncating variants are associated with the WRS phenotype but that genotypes with bi-allelic missense or missense variants in trans with splicing or truncating variants are associated with the distinct phenotype of hypomyelinating leukodystrophy.
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
The authors thank the families and referring physicians for participation in these studies. The authors thank Hillary Heins and Ping Yang of Washington University for their assistance with DNA isolation and preparation. The authors also thank Pei-Yun Tseng, Mary Tunison, and Claudia Quittner of UT Southwestern for help with DNA isolation, RNA analysis, and nursing support. The authors thank GeneDx for sharing VCF and BAM files from a non-diagnostic, clinician-referred, whole exome for research analysis performed at Washington University School of Medicine. Some sequencing was provided by the University of Washington Center for Mendelian Genomics (UW-CMG) and was funded by the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute grant HG006493 to Debbie Nickerson, Michael Bamshad, and Suzanne Leal. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank the Exome Aggregation Consortium and the groups that provided exome variant data for comparison; a full list of contributing groups can be found at http://exac.broadinstitute.org/about. This work was also supported by grants from the National Institutes of Health (K08 HL105891 [J.A.W.], K12 HL120002 [F.S.C.], R01 HL065174 [F.S.C.], R21/33 HL120760 [F.S.C.], R01-DK105448 [A.G., A.K.A.], CTSA grants UL1RR024982, UL1TR001105 to UT Southwestern Medical Center, and U54 HG006493 to the University of Washington Center for Mendelian Genomics [D.A.N., M.J.B.]), the Children’s Discovery Institute (F.S.C.) and University of Texas Southwestern Medical Foundation (A.G.).
Published: November 7, 2018
Footnotes
Supplemental Data include three figures and one table and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.10.010.
Contributor Information
Jennifer A. Wambach, Email: wambachj@wustl.edu.
Abhimanyu Garg, Email: abhimanyu.garg@utsouthwestern.edu.
Web Resources
Basic Local Alignment Search Tool (BLAST), https://blast.ncbi.nlm.nih.gov
The Human Protein Atlas, https://www.proteinatlas.org
GnomAD, accessed October 2018, gnomad.broadinstitute.org
Supplemental Data
References
- 1.Rautenstrauch T., Snigula F. Progeria: a cell culture study and clinical report of familial incidence. Eur. J. Pediatr. 1977;124:101–111. doi: 10.1007/BF00477545. [DOI] [PubMed] [Google Scholar]
- 2.Wiedemann H.R. An unidentified neonatal progeroid syndrome: follow-up report. Eur. J. Pediatr. 1979;130:65–70. doi: 10.1007/BF00441901. [DOI] [PubMed] [Google Scholar]
- 3.Pivnick E.K., Angle B., Kaufman R.A., Hall B.D., Pitukcheewanont P., Hersh J.H., Fowlkes J.L., Sanders L.P., O’Brien J.M., Carroll G.S. Neonatal progeroid (Wiedemann-Rautenstrauch) syndrome: report of five new cases and review. Am. J. Med. Genet. 2000;90:131–140. [PubMed] [Google Scholar]
- 4.Paolacci S., Bertola D., Franco J., Mohammed S., Tartaglia M., Wollnik B., Hennekam R.C. Wiedemann-Rautenstrauch syndrome: A phenotype analysis. Am. J. Med. Genet. A. 2017;173:1763–1772. doi: 10.1002/ajmg.a.38246. [DOI] [PubMed] [Google Scholar]
- 5.Devos E.A., Leroy J.G., Frijns J.P., Van den Berghe H. The Wiedemann-Rautenstrauch or neonatal progeroid syndrome. Report of a patient with consanguineous parents. Eur. J. Pediatr. 1981;136:245–248. doi: 10.1007/BF00442991. [DOI] [PubMed] [Google Scholar]
- 6.Graul-Neumann L.M., Kienitz T., Robinson P.N., Baasanjav S., Karow B., Gillessen-Kaesbach G., Fahsold R., Schmidt H., Hoffmann K., Passarge E. Marfan syndrome with neonatal progeroid syndrome-like lipodystrophy associated with a novel frameshift mutation at the 3′ terminus of the FBN1-gene. Am. J. Med. Genet. A. 2010;152A:2749–2755. doi: 10.1002/ajmg.a.33690. [DOI] [PubMed] [Google Scholar]
- 7.Garg A., Xing C. De novo heterozygous FBN1 mutations in the extreme C-terminal region cause progeroid fibrillinopathy. Am. J. Med. Genet. A. 2014;164A:1341–1345. doi: 10.1002/ajmg.a.36449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg A., Kircher M., Del Campo M., Amato R.S., Agarwal A.K., University of Washington Center for Mendelian Genomics Whole exome sequencing identifies de novo heterozygous CAV1 mutations associated with a novel neonatal onset lipodystrophy syndrome. Am. J. Med. Genet. A. 2015;167A:1796–1806. doi: 10.1002/ajmg.a.37115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehmke N., Graul-Neumann L., Smorag L., Koenig R., Segebrecht L., Magoulas P., Scaglia F., Kilic E., Hennig A.F., Adolphs N. De novo mutations in SLC25A24 cause a craniosynostosis syndrome with hypertrichosis, progeroid appearance, and mitochondrial dysfunction. Am. J. Hum. Genet. 2017;101:833–843. doi: 10.1016/j.ajhg.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jay A.M., Conway R.L., Thiffault I., Saunders C., Farrow E., Adams J., Toriello H.V. Neonatal progeriod syndrome associated with biallelic truncating variants in POLR3A. Am. J. Med. Genet. A. 2016;170:3343–3346. doi: 10.1002/ajmg.a.37960. [DOI] [PubMed] [Google Scholar]
- 11.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spurdle A.B., Couch F.J., Hogervorst F.B., Radice P., Sinilnikova O.M., IARC Unclassified Genetic Variants Working Group Prediction and assessment of splicing alterations: implications for clinical testing. Hum. Mutat. 2008;29:1304–1313. doi: 10.1002/humu.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn D., Robinson P.N. Progeroid facial features and lipodystrophy associated with a novel splice site mutation in the final intron of the FBN1 gene. Am. J. Med. Genet. A. 2011;155A:721–724. doi: 10.1002/ajmg.a.33905. [DOI] [PubMed] [Google Scholar]
- 14.Goldblatt J., Hyatt J., Edwards C., Walpole I. Further evidence for a marfanoid syndrome with neonatal progeroid features and severe generalized lipodystrophy due to frameshift mutations near the 3′ end of the FBN1 gene. Am. J. Med. Genet. A. 2011;155A:717–720. doi: 10.1002/ajmg.a.33906. [DOI] [PubMed] [Google Scholar]
- 15.Takenouchi T., Hida M., Sakamoto Y., Torii C., Kosaki R., Takahashi T., Kosaki K. Severe congenital lipodystrophy and a progeroid appearance: Mutation in the penultimate exon of FBN1 causing a recognizable phenotype. Am. J. Med. Genet. A. 2013;161A:3057–3062. doi: 10.1002/ajmg.a.36157. [DOI] [PubMed] [Google Scholar]
- 16.Jacquinet A., Verloes A., Callewaert B., Coremans C., Coucke P., de Paepe A., Kornak U., Lebrun F., Lombet J., Piérard G.E. Neonatal progeroid variant of Marfan syndrome with congenital lipodystrophy results from mutations at the 3′ end of FBN1 gene. Eur. J. Med. Genet. 2014;57:230–234. doi: 10.1016/j.ejmg.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill B., Simha V., Kotha V., Garg A. Body fat distribution and metabolic variables in patients with neonatal progeroid syndrome. Am. J. Med. Genet. A. 2007;143A:1421–1430. doi: 10.1002/ajmg.a.31840. [DOI] [PubMed] [Google Scholar]
- 18.Saitsu H., Osaka H., Sasaki M., Takanashi J., Hamada K., Yamashita A., Shibayama H., Shiina M., Kondo Y., Nishiyama K. Mutations in POLR3A and POLR3B encoding RNA Polymerase III subunits cause an autosomal-recessive hypomyelinating leukoencephalopathy. Am. J. Hum. Genet. 2011;89:644–651. doi: 10.1016/j.ajhg.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard G., Chouery E., Putorti M.L., Tétreault M., Takanohashi A., Carosso G., Clément I., Boespflug-Tanguy O., Rodriguez D., Delague V. Mutations of POLR3A encoding a catalytic subunit of RNA polymerase Pol III cause a recessive hypomyelinating leukodystrophy. Am. J. Hum. Genet. 2011;89:415–423. doi: 10.1016/j.ajhg.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf N.I., Vanderver A., van Spaendonk R.M., Schiffmann R., Brais B., Bugiani M., Sistermans E., Catsman-Berrevoets C., Kros J.M., Pinto P.S., 4H Research Group Clinical spectrum of 4H leukodystrophy caused by POLR3A and POLR3B mutations. Neurology. 2014;83:1898–1905. doi: 10.1212/WNL.0000000000001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atrouni S., Darazé A., Tamraz J., Cassia A., Caillaud C., Mégarbané A. Leukodystrophy associated with oligodontia in a large inbred family: fortuitous association or new entity? Am. J. Med. Genet. A. 2003;118A:76–81. doi: 10.1002/ajmg.a.10019. [DOI] [PubMed] [Google Scholar]
- 22.Dumay-Odelot H., Durrieu-Gaillard S., Da Silva D., Roeder R.G., Teichmann M. Cell growth- and differentiation-dependent regulation of RNA polymerase III transcription. Cell Cycle. 2010;9:3687–3699. doi: 10.4161/cc.9.18.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 24.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 25.Choquet K., Yang S., Moir R.D., Forget D., Larivière R., Bouchard A., Poitras C., Sgarioto N., Dicaire M.J., Noohi F. Absence of neurological abnormalities in mice homozygous for the Polr3a G672E hypomyelinating leukodystrophy mutation. Mol. Brain. 2017;10:13. doi: 10.1186/s13041-017-0294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.