Adenovirus-based vectors are used today for gene transfer and vaccination. HAdV26 has emerged as a promising candidate vector for development of vaccines due to its relatively low seroprevalence and its ability to induce potent immune responses against inserted transgenes. However, data regarding the basic biology of the virus, like receptor usage or intracellular trafficking, are limited. In this work, we found that efficient infection of human epithelial cell lines by HAdV26 requires the expression of the αvβ3 integrin. By studying intracellular trafficking of fluorescently labeled HAdV26 in a cell clone with stably increased expression of αvβ3 integrin, we observed that HAdV26 colocalizes with αvβ3 integrin and confirmed that αvβ3 integrin expression facilitates efficient HAdV26 internalization. These results will allow further improvement of HAdV26-based vectors for gene transfer and vaccination.
KEYWORDS: αvβ3 integrin, adenoviruses, epithelial cells, human adenovirus type 26, receptor
ABSTRACT
Human adenoviruses (HAdVs) are being explored as vectors for gene transfer and vaccination. Human adenovirus type 26 (HAdV26), which belongs to the largest subgroup of adenoviruses, species D, has a short fiber and a so-far-unknown natural tropism. Due to its low seroprevalence, HAdV26 has been considered a promising vector for the development of vaccines. Despite the fact that the in vivo safety and immunogenicity of HAdV26 have been extensively studied, the basic biology of the virus with regard to receptor use, cell attachment, internalization, and intracellular trafficking is poorly understood. In this work, we investigated the roles of the coxsackievirus and adenovirus receptor (CAR), CD46, and αv integrins in HAdV26 infection of human epithelial cell lines. By performing different gain- and loss-of-function studies, we found that αvβ3 integrin is required for efficient infection of epithelial cells by HAdV26, while CAR and CD46 did not increase the transduction efficiency of HAdV26. By studying intracellular trafficking of fluorescently labeled HAdV26 in A549 cells and A549-derived cell clones with stably increased expression of αvβ3 integrin, we observed that HAdV26 colocalizes with αvβ3 integrin and that increased αvβ3 integrin enhances internalization of HAdV26. Thus, we conclude that HAdV26 uses αvβ3 integrin as a receptor for infecting epithelial cells. These results give us new insight into the HAdV26 infection pathway and will be helpful in further defining HAdV-based vector manufacturing and vaccination strategies.
IMPORTANCE Adenovirus-based vectors are used today for gene transfer and vaccination. HAdV26 has emerged as a promising candidate vector for development of vaccines due to its relatively low seroprevalence and its ability to induce potent immune responses against inserted transgenes. However, data regarding the basic biology of the virus, like receptor usage or intracellular trafficking, are limited. In this work, we found that efficient infection of human epithelial cell lines by HAdV26 requires the expression of the αvβ3 integrin. By studying intracellular trafficking of fluorescently labeled HAdV26 in a cell clone with stably increased expression of αvβ3 integrin, we observed that HAdV26 colocalizes with αvβ3 integrin and confirmed that αvβ3 integrin expression facilitates efficient HAdV26 internalization. These results will allow further improvement of HAdV26-based vectors for gene transfer and vaccination.
INTRODUCTION
Adenoviruses are nonenveloped double-stranded DNA viruses with an icosahedral capsid approximately 90 nm in diameter and a mass of 150 MDa (1). The major building blocks of the adenoviral capsid are the hexon and penton proteins. On each vertex, there is an extended fiber protein noncovalently attached to the penton base protein (2). A broad knowledge of adenovirus molecular biology and the relative ease with which the genome can be manipulated have made adenoviruses attractive as vectors for gene transfer and vaccination (3). Adenovirus-based vectors rapidly infect a broad range of human cells and induce strong innate responses (4) that positively influence adaptive T- and B-cell responses (5). Adenovirus-based vectors currently represent a leading choice for vectors used in gene therapy clinical trials aimed at treating inherited diseases, infections, and cancer (http://www.abedia.com/wiley/vectors.php).
Human adenoviruses (HAdVs) belong to the genus Mastadenovirus of the family Adenoviridae and comprise more than 60 distinct serotypes divided into 7 species or subgroups (A to G) (6–8). The most common and best-described HAdV so far is the species C human adenovirus type 5 (HAdV5). HAdV5 infection starts with binding to the coxsackievirus and adenovirus receptor (CAR), followed by interaction of the RGD sequence motif present on the penton base with the αv integrins on the cell surface, allowing internalization of the viral particle (9). HAdV5 has very high in vitro transduction efficiency and levels of gene expression; however, disadvantages are the high level and frequency of preexisting immunity in human populations. The seroprevalence of HAdV5 ranges from 50 to 90% depending on the geographical region (10, 11). Preexisting immunity may limit the efficiency of adenovirus-based vaccine vectors, and thus, development of new strategies to evade undesired antivector host immune responses, such as vectors based on adenoviruses that occur at low prevalence in human populations, is needed. Some rare human adenovirus types are under evaluation, including HAdV35 (species B) and HAdV26 (species D), as well as adenoviruses from nonhuman primates (12, 13). Vaccine vectors based on HAdV26 and HAdV35 have been extensively studied and are listed as interventions in more than 40 clinical trials, either alone or in prime-boost regimes (https://clinicaltrials.gov).
As mentioned above, HAdV26 belongs to species D, the largest group of HAdVs (14), which are mainly known to be responsible for eye infections and for gastrointestinal infections in immunocompromised individuals. Similarly to the majority of HAdVs, HAdV26 has RGD motifs in the penton base that can mediate integrin binding. In contrast to HAdV5, which has a long fiber containing 22 beta repeat motifs, HAdV26 has a relatively short fiber with only 8 beta repeats (15). Also, unlike HAdV5, HAdV26 does not bind coagulation factor X (16).
Although the safety and immunogenicity of HAdV26-based vaccine vectors in vivo are well established (17–20), the basic biology of the virus, such as receptor usage, is less well understood. Several molecules have been identified as cellular receptors for HAdVs (21). As discussed above, HAdV5 from species C uses CAR as the primary receptor for facilitating entry into cells (22), while HAdV35 from species B utilizes CD46 as the primary receptor (23). HAdV5 also uses αv integrins as coreceptors mediated by interaction with the RGD sequence in the penton base (24). Integrins are heterodimers of noncovalently associated α and β subunits assembled into 24 different receptors. They are major receptors for cell adhesion to extracellular matrix proteins and activate many intracellular signaling pathways after binding to cognate ligands. Several studies have reported that HAdV26 utilizes CAR, CD46, and/or integrins as receptors for infecting target cells in vitro. Abbink et al. reported that HAdV26 transduces B16F10-CD46 cells, mouse B16F10 melanoma cells that stably express the BC1 isoform of human CD46 on the membrane, more efficiently than B16F10 cells, indicating that HAdV26 is able to utilize CD46 as a receptor. However, transduction appeared to be less efficient than for HAdVs from species B, suggesting that HAdV26 may utilize other receptors in addition to CD46 (20). Recently, it has been shown that HAdV26 uses CD46 as a primary receptor in human peripheral blood mononuclear cells (PBMCs) and that HAdV26 transduction was efficiently blocked by an anti-CD46 monoclonal antibody (25).
Chen et al. compared the transduction efficiencies of HAdV5 and HAdV26 in CHO cells (a cell line originally derived from the Chinese hamster ovary) stably expressing CAR (CHO-CAR) and control cells that did not express CAR (CHO-HVEM). They observed that at the higher dose tested, the transduction efficiencies of the two viruses were similar in CHO-CAR cells, indicating that HAdV26 could utilize CAR for cell binding. At the same time, transduction in the CHO-HVEM cell line by HAdV26 was higher than that by HAdV5, suggesting that HAdV26 can enter cells upon binding to alternative receptors that HAdV5 is unable to use. In the same study, the authors investigated the agglutination of CD46-expressing red blood cells from rhesus macaques by HAdV26. A species B chimpanzee adenovirus serotype C1-based vector, which had previously been shown to bind CD46, readily agglutinated red blood cells from rhesus macaques, whereas this was not seen with HAdV26, suggesting that HAdV26 did not bind CD46 (26). Another study found that cyclic-RGD peptides partially inhibited human hepatoma Hep3B cell killing by HAdV26, indicating a role of αv integrins in HAdV26 infection. In the same study, the combination of an anti-CD46 antibody and cyclic-RGD peptides on patient myeloma cells mediated complete protection against killing by HAdV26, suggesting that both receptors, CD46 and αv integrins, are utilized by the virus to infect these target cells (27). Finally, very recently, the scavenger receptor SR-A6 has been implicated in facilitating HAdV26 entry into murine alveolar-macrophage-like MPI cells (28). HAdV26 receptor usage has also been investigated for peripheral blood mononuclear cells (25) or malignant B cells (27), while HAdV26 receptor usage in epithelial cells is less well defined.
Since HAdV26 has been reported to use different molecules for cell entry, we wished to investigate the roles of CAR, CD46, and αv integrins in mediating the entry of HAdV26 into human epithelial cells. By performing different gain- and loss-of-function studies, we found that αvβ3 integrin is necessary for the efficient infection of epithelial cells by HAdV26. At the same time, the presence of CAR or CD46 did not increase the transduction efficiency of HAdV26. By studying intracellular trafficking of fluorescently labeled HAdV26 in A549 cells and in A549 cells with increased expression of αvβ3 integrin, we observed that αvβ3 integrin expression allowed better internalization of HAdV26. Additionally, we have shown that in an A549 cell clone with increased αvβ3 integrin expression, HAdV26 colocalizes with αvβ3 integrin. Thus, we conclude that HAdV26 uses αvβ3 integrin as a receptor for infecting epithelial cells.
RESULTS
HAdV26 binds and infects A549 and SK-OV-3 cells less efficiently than HAdV5 and HAdV35.
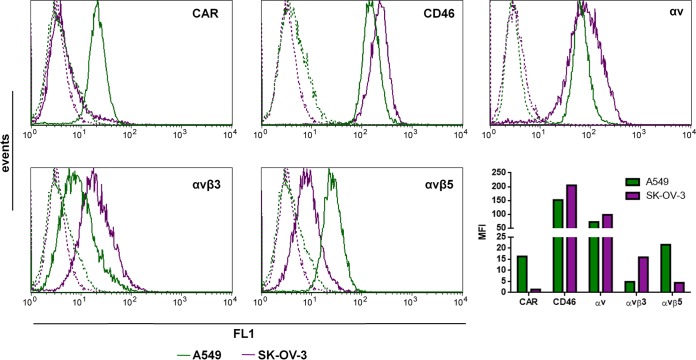
Studies regarding HAdV26 transduction efficiency and receptor usage in epithelial cells are limited. Therefore, in this work, we investigated the transduction efficiencies of HAdV26 in A549 and SK-OV-3 epithelial cell lines, which are often used in adenovirus research. Several molecules have been reported to function as HAdV26 receptors: CAR, CD46, and αv integrins. In order to determine the expression levels of these molecules on A549 and SK-OV-3 cells, we assessed the expression of CAR; CD46; and the integrins αv, αvβ3, and αvβ5 on the surfaces of the cells by flow cytometry. While SK-OV-3 cells were found to be CAR negative, A549 cells showed high expression of CAR. A549 and SK-OV-3 cells both showed high expression of CD46 and αv integrins; however, SK-OV-3 cells expressed more CD46 and αv integrin than A549 cells. Expression of αvβ3 and αvβ5 integrins, known receptors for the RGD motif that is present in the adenovirus penton base, was disparate between the two cell lines. A549 cells expressed very small amounts of αvβ3 integrin and showed expression of αvβ5 integrin, while SK-OV-3 cells expressed αvβ3 integrin, but the level of αvβ5 integrin was very low (Fig. 1).
FIG 1.
Flow cytometry analysis of known adenovirus receptors, CAR, CD46, αvβ3, αvβ5, and αv integrin, on surfaces of A549 and SK-OV-3 cells. The cells were detached and incubated with specific antibodies on ice, and cell surface expression of CAR, CD46, αv, αvβ3, and αvβ5 integrins was analyzed by flow cytometry. The following antibodies were used to detect the studied receptors: RcmB (CAR), MEM-258 (CD46), LM609 (αvβ3), P1F6 (αvβ5), and 272-17E6 (αv). Green and violet represent primary-antibody staining in the A549 and SK-OV-3 cells, respectively. Results are from two independent experiments (n = 2). MFI, mean fluorescence intensity expressed in arbitrary units.
Next, the efficiency of HAdV26 in transducing A549 and SK-OV-3 cells was investigated (Fig. 2). HAdV5 and HAdV35 were used as representatives of HAdVs known to utilize the receptors CAR and CD46, respectively. As an additional control, we used HAdV26F35, a chimeric vector based on HAdV26 that has been pseudotyped with the HAdV35 fiber.
FIG 2.
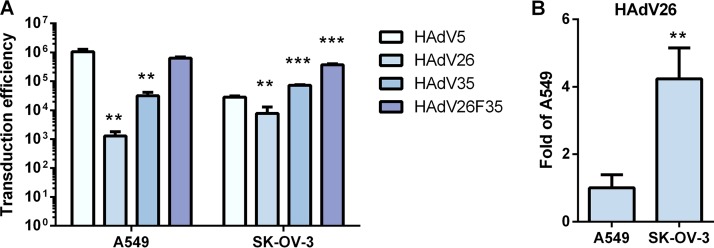
Transduction efficiencies of HAdV5, HAdV26, HAdV35, and HAdV26F35 in A549 and SK-OV-3 cells. (A) Comparison of transduction efficiencies of HAdV5, HAdV26, HAdV35, and HAdV26F35 in A549 and SK-OV-3 cells. The results are presented as absolute values in relative light units (RLU) per milligram of protein. (B) Transduction efficiencies of HAdV26 in A549 and SK-OV-3 cells. The results are presented as fold A549 transduction efficiency. Transduction efficiency was measured by luciferase activity assay 48 h after infection. The results are expressed as means and standard deviations. **, P < 0.01; ***, P < 0.001. Results are from three independent experiments (n = 3).
HAdV5, HAdV35, and HAdV26F35 were found to transduce A549 cells much better than HAdV26. HAdV26 transduced A549 cells 1,000-fold less efficiently than HAdV5. The transduction efficiency of HAdV26 was comparable to that of HAdV5 in SK-OV-3 cells, i.e., HAdV26 transduced SK-OV-3 cells only 3-fold less efficiently than HAdV5 (Fig. 2A). HAdV26 showed 4-fold higher reporter gene expression in SK-OV-3 cells than in A549 cells (Fig. 2B). This may indicate that SK-OV-3 cells express higher levels of the molecule(s) that HAdV26 uses as a receptor than A549 cells.
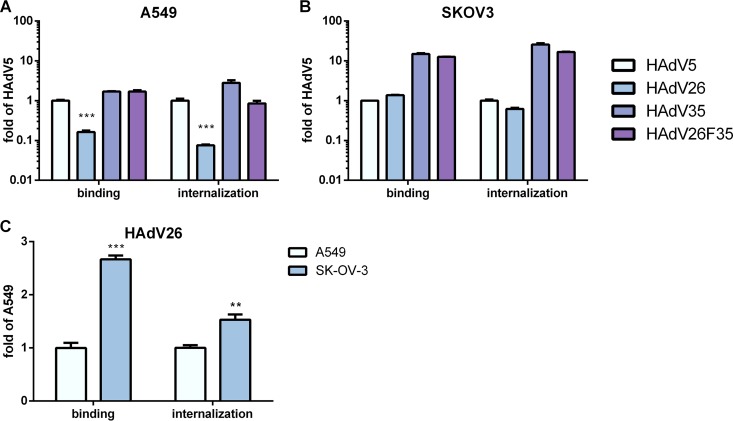
To investigate whether the relatively low level of transduction efficiency observed for HAdV26 in A549 and SK-OV-3 cells (compared to the other vectors) is caused by low levels of binding and/or inefficient internalization of the virus, we measured the binding and internalization of HAdV5, HAdV26, HAdV35, and HAdV26F35 in A549 and SK-OV-3 cells. While the levels of binding and internalization of HAdV5, HAdV35, and HAdV26F35 in A549 cells were comparable, binding and internalization of HAdV26 were found to be poor on this cell line. Compared to HAdV5, HAdV26 was found to bind 6-fold and to internalize 14-fold less efficiently in A549 cells (Fig. 3A). In SK-OV-3 cells, the amounts of both bound and internalized HAdV26 were comparable to those of HAdV5. However, in comparison to AdV35, HAdV26 was less efficient in both binding and internalization in this cell line. In the same cell line, HAdV35 and HAdV26F35 were found to bind and internalize more than 10-fold more efficiently than HAdV5 (Fig. 3B). A comparison of the binding and internalization of HAdV26 in A549 and SK-OV-3 cells is shown in Fig. 3C. It was seen that HAdV26 bound 3-fold and internalized 1.5-fold better to SK-OV-3 cells than to A549 cells. These data indicate that the low transduction efficiency of HAdV26 in A549 cells is caused by decreased binding of the virus, suggesting that it is due to comparatively small amounts of the HAdV26 receptor on A549 cells.
FIG 3.
(A and B) Binding and internalization of HAdV5, HAdV26, HAdV35, and HAdV26F35 in A549 cells (A) and in SK-OV-3 cells (B). The results are expressed as fold control value, i.e., the value obtained for HAdV5, plus standard deviations. (C) Binding and internalization of HAdV26 in A549 and SK-OV-3 cells. The results are presented relative to A549 plus standard deviations. For both binding and internalization, cells were first incubated with HAdV5, HAdV26, HAdV35, and HAdV26F35 on ice for 1 h, Multiplicity of infection (MOI), 1,000 viral particles (vp)/cell. To measure binding, unbound viruses were removed by rinsing the cells with cold trypsin and PBS and collected by scraping the cells. For internalization measurement, unbound viruses were removed as described above, and the cells were transferred to 37°C and incubated for 1 h, allowing the viruses to enter the cells. The Cells were then rinsed twice with warm trypsin, dispersed, and pelleted by centrifugation. For both binding and internalization, total (cellular plus viral) DNA was extracted from the cells and used for quantification of viral DNA by qPCR, using the CMV region as a target sequence. **, P < 0.01; ***, P < 0.001. Results are from three independent experiments (n = 3).
Downregulation of αv integrin decreases the transduction efficiency of HAdV26.
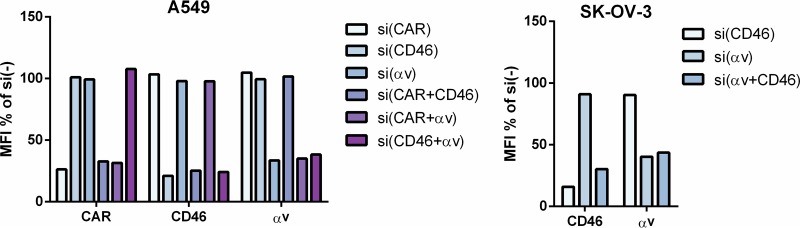
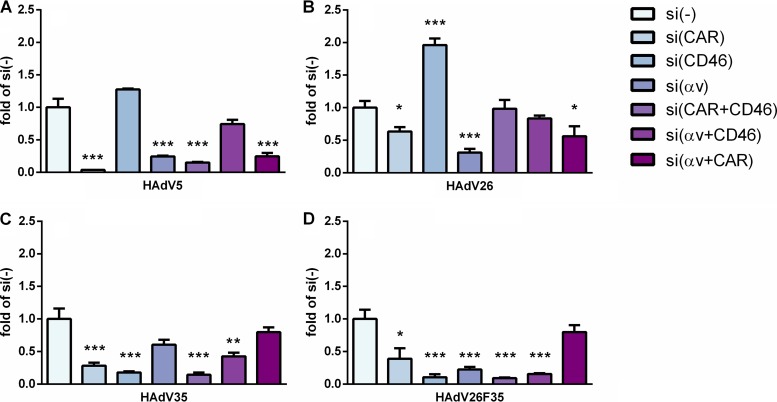
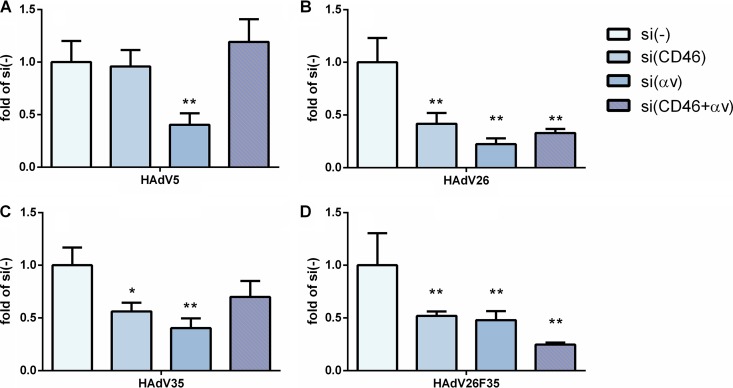
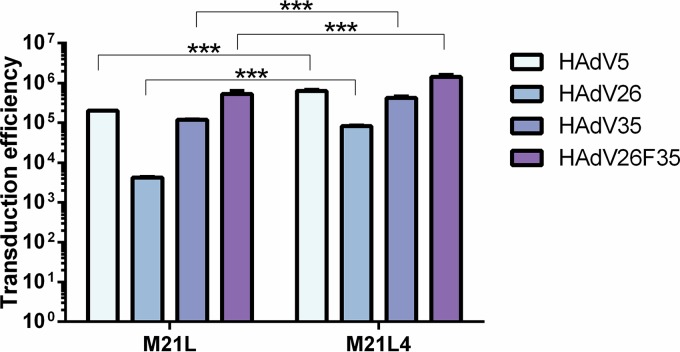
To investigate the importance of CAR, CD46, and αv integrin in contributing to the transduction efficiency of HAdV26 in A549 and SK-OV-3 cells, we decided to downregulate those molecules, alone or in combination, and measure the transduction efficiency of HAdV26. To downregulate the target receptor(s), we transfected cells with CAR-, CD46-, and/or αv integrin-specific small interfering RNA (siRNA) (50 nM) and, 48 h posttransfection, confirmed the efficiency of silencing by flow cytometry. Downregulation of CAR, CD46, and αv integrin was specific and did not influence expression of the other observed molecules (Fig. 4). As expected, downregulating CAR (alone or in combination with CD46 or αv integrin) almost abolished HAdV5 transduction of A549 cells (Fig. 5A). Silencing of CD46 significantly decreased transduction of HAdV35 (Fig. 5C) and HAdV26F35 (Fig. 5D) but increased transduction of HAdV26 (Fig. 5B). Downregulation of CAR and, to a greater extent, αv integrin decreased the transduction efficiency of both HAdV26 (Fig. 5B) and HAdV26F35 (Fig. 5D). The most prominent effect on HAdV26 transduction was observed in the case of αv integrin downregulation, which decreased HAdV26 transduction efficiency 3-fold in comparison to cells transfected with the scrambled siRNA control (Fig. 5B). These data indicate that αv integrin could be a receptor for HAdV26 in A549 cells. The same effect was observed in another CAR-positive cell line, HeLa, where downregulation of αv integrin decreased HAdV26 transduction efficiency 3-fold in comparison to cells transfected with scrambled siRNA (data not shown). Similar results were obtained in SK-OV-3 cells. Since SK-OV-3 cells are CAR negative, we downregulated only CD46 and/or αv integrins. Downregulating αv integrin in SK-OV-3 cells decreased the transduction efficiency of all 4 studied viruses (Fig. 6); however, the decrease was highest for HAdV26. Downregulating αv integrin in SK-OV-3 cells decreased HAdV26 transduction efficiency 5-fold compared to controls (Fig. 6B). That αv integrin is necessary for HAdV26 transduction efficiency was also confirmed in the M21 melanoma cell line variants M21L and M21L4. The transduction efficiency of HAdV26 was much higher in M21L4 cells, which are αv integrin positive, than in M21L cells, which are αv integrin negative (Fig. 7).
FIG 4.
Flow cytometry analysis of CAR, CD46, and αv integrin on surfaces of A549 and SK-OV-3 cells after downregulation by specific siRNA transfection. Cells were transfected with specific siRNA at a final concentration of 50 nM, and 48 h later, surface expression of CAR, CD46, and αv integrin was determined. The following antibodies were used to detect the studied receptors: RcmB (CAR), MEM-258 (CD46), and 272-17E6 (αv integrin). The results are shown as percentages of the value for the control, i.e., cells transfected with scrambled siRNA. Results are from two independent experiments (n = 2).
FIG 5.
Transduction efficiencies of HAdV5 (A), HAdV26 (B), HAdV35 (C), and HAdV26F35 (D) in A549 cells after downregulation of CAR, CD46, and/or αv integrin by specific siRNA transfection. Cells were transfected with specific siRNA at a final concentration of 50 nM and infected 48 h later with HAdV5, HAdV26, HAdV35, and HAdV26F35 at an MOI of 1,000 vp/cell. Transduction efficiency was measured by luciferase activity assay 48 h after infection. The results are presented as fold control, i.e., cells transfected with scrambled siRNA, plus standard deviations. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Results are from three independent experiments (n = 3).
FIG 6.
Transduction efficiencies of HAdV5, HAdV26, HAdV35, and HAdV26F35 in SK-OV-3 cells after downregulation of CD46 and/or αv integrin by specific siRNA transfection. Cells were transfected with specific siRNA at a final concentration of 50 nM and infected 48 h later with HAdV5, HAdV26, HAdV35, and HAdV26F35 at an MOI of 1,000 vp/cell. Transduction efficiency was measured by luciferase activity assay 48 h after infection. The results are presented as fold control, i.e., cells transfected with scrambled siRNA, plus standard deviations. *, P < 0.05; **, P < 0.01. Results are from three independent experiments (n = 3).
FIG 7.
Transduction efficiencies of HAdV5, HAdV26, HAdV35, and HAdV26F35 in M21L and M21L4 cells. Cells were infected with HAdV5, HAdV26, HAdV35, and HAdV26F35 at an MOI of 1,000 vp/cell. Transduction efficiency was measured by luciferase activity assay 48 h after infection. M21L cells are αv integrin negative, and M21L4 cells are αv integrin positive. The results are presented as absolute values in RLU per milligram of protein and are shown as means plus standard deviations. ***, P < 0.001. Results are from two independent experiments (n = 2).
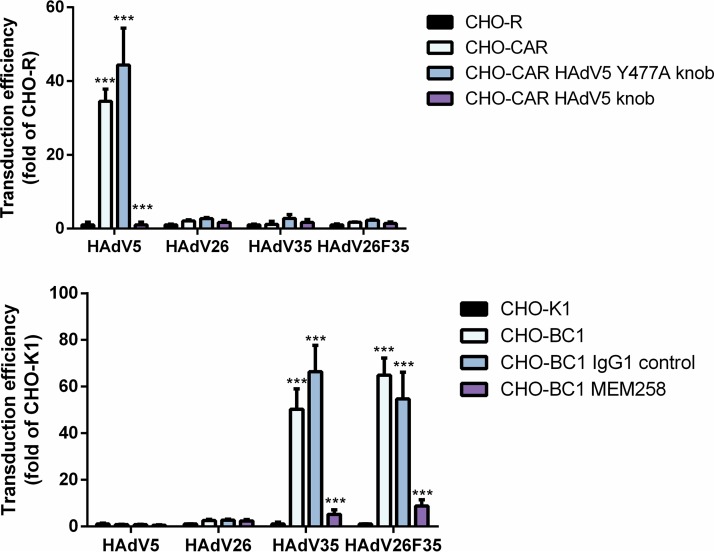
Downregulating CD46 alone or in combination with αv integrin in SK-OV-3 cells decreased the transduction efficiency of HAdV35 (Fig. 6C) and HAdV26F35 (Fig. 6D), but also HAdV26 (Fig. 6B), indicating that in this cell line CD46 could be involved in HAdV26 transduction efficiency. The roles of CAR and CD46 in HAdV26 transduction efficiency were also studied in CHO cells overexpressing CAR (CHO-CAR) or CD46 (CHO-BC1). As expected, increased expression of CAR significantly increased the transduction efficiency of HAdV5. However, there was no impact on the transduction of the HAdV26, HAdV35, or HAdV26F35 vector. Increased expression of CD46 significantly increased the transduction efficiency of HAdV35 and HAdV26F35 but did not change the transduction efficiency of HAdV5 or of HAdV26 (Fig. 8). Based on these data, we hypothesize that HAdV26 uses αv integrin as a receptor for infecting epithelial cells, while CAR and CD46 are not crucial molecules in this process.
FIG 8.
Transduction efficiencies of HAdV5, HAdV26, HAdV35, and HAdV26F35 in CHO-CAR (A) and CHO-BC1 (B) cells. CHO-CAR cells were incubated with HAdV5 knob (wild type or Y477A) and CHO-BC1 cells with anti-CD46 antibody (MEM258) or IgG1 control on ice for 1 h and then infected with HAdV5, HAdV26, HAdV35, and HAdV26F35 at an MOI of 5,000 vp/cell. Transduction efficiency was measured by luciferase activity assay 48 h after infection. CHO-CAR cells are CHO cells stably transfected with a plasmid containing CAR cDNA, and CHO-R cells are CHO cells stably transfected with empty plasmid; CHO-K1 cells are normal CHO cells, and CHO-BC1 cells are CHO cells stably transfected with a plasmid containing CD46 cDNA. The results are presented as absolute values in RLU per milligram of protein and are shown as means plus standard deviations. ***, P < 0.001. Results are from three independent experiments (n = 3).
Downregulation of αv integrin decreases binding and internalization of HAdV26 in A549 cells.
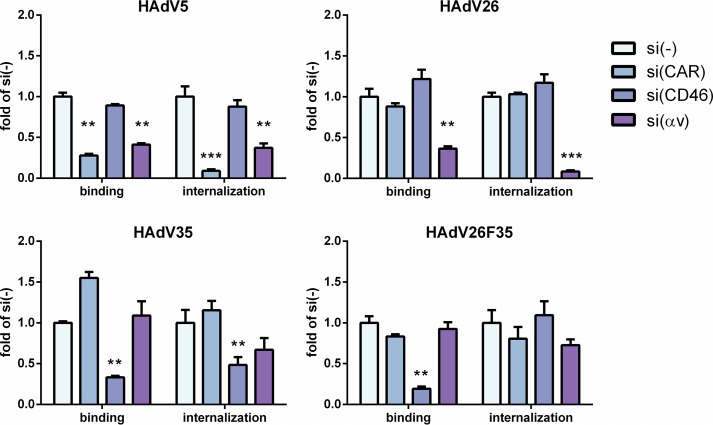
To further investigate the roles of CAR, CD46, and αv integrins in HAdV26 infection of A549 cells, we downregulated these molecules and subsequently determined their effects on the binding and internalization of HAdV26 compared to HAdV35 and HAdV26F35. Downregulation of CAR decreased both binding and internalization of HAdV5 4- and 11-fold, respectively, in comparison to cells transfected with scrambled siRNA (control). Downregulation of CD46 decreased the binding of HAdV35 3-fold and HAdV26F35 5-fold compared to controls. As expected, downregulation of CD46 also diminished internalization of HAdV35 but, surprisingly, had no effect on HAdV26F35 internalization. Downregulation of αv integrin significantly decreased binding and internalization of HAdV26. While downregulating αv integrin decreased HAdV26 binding 3-fold, it almost completely abrogated internalization of the virus in A549 cells. Downregulating CAR or CD46 had no influence on HAdV26 binding or internalization (Fig. 9). These data confirm that αv integrin plays an important role in binding and internalization of HAdV26 in A549 cells.
FIG 9.
Binding and internalization of HAdV5, HAdV26, HAdV35, and HAdV26F35 in A549 cells after downregulating CAR, CD46, and αv integrins. Cells were transfected with specific siRNA at a final concentration of 50 nM and incubated 48 h later with HAdV5, HAdV26, HAdV35, and HAdV26F35 on ice for 1 h at an MOI of 1,000 vp/cell. To measure binding, unbound viruses were removed by rinsing the cells with cold trypsin and PBS and collected by scraping the cells. For internalization measurement, unbound viruses were removed as described above, and the cells were transferred to 37°C and incubated for 1 h, allowing the viruses to enter the cells. The cells were then rinsed twice with warm trypsin, dispersed, and pelleted by centrifugation. For both binding and internalization, total (cellular plus viral) DNA was extracted from cells and used for quantification of viral DNA by qPCR, using the CMV region as a target sequence. The results are presented as fold control, i.e., cells transfected with scrambled siRNA, plus standard deviations. **, P < 0.01; ***, P < 0.001. Results are from three independent experiments (n = 3).
Blocking αv integrins decreases transduction efficiency of HAdV26 in A549 cells.
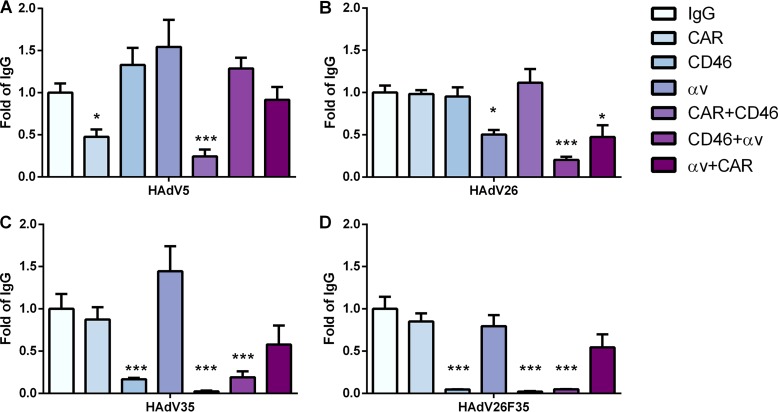
While downregulating target receptors by the use of specific siRNA removes the target mRNA and, hence, the protein from the cell, pharmacological inhibition by using a specific inhibitor or antibody blocks the function of a protein without affecting protein expression. Therefore, we decided to investigate the roles of cell surface CAR, CD46, and αv integrins in HAdV26 transduction efficiency by reducing the accessibility of these molecules with blocking antibodies. Blocking CD46 alone or in combination with CAR and αv integrins efficiently decreased transduction of HAdV35 and HAdV26F35 (Fig. 10C and D). This effect was very pronounced for HAdV26F35, where blocking CD46 almost abrogated HAdV26F35 transduction efficiency in A549 cells. Blocking CD46 had no influence on HAdV26 transduction efficiency. The transduction efficiency of HAdV5 was influenced only by blocking CAR, alone or in combination with αv integrins (Fig. 10A). Blocking the surface availability of αv integrins, alone or in combination with both CAR and CD46, significantly decreased the transduction efficiency of HAdV26. While blocking αv integrins alone or in combination with CAR decreased HAdV26 transduction efficiency 2-fold (compared to cells incubated with an irrelevant IgG), blocking αv integrins and CD46 at the same time decreased HAdV26 transduction efficiency 5-fold (Fig. 10B). Together, these results confirm that the presence of αv integrin on the surfaces of A549 cells is important for the transduction efficiency of HAdV26.
FIG 10.
Transduction efficiencies of HAdV5 (A), HAdV26 (B), HAdV35 (C), and HAdV26F35 (D) in A549 cells after incubation with anti-CAR, anti-CD46, and/or anti-αv integrin blocking antibodies. Cells were first incubated with antibodies on ice for 1 h, and then the viruses were added. The following antibodies, at a final concentration of 20 µg/ml, were used: RcmB (CAR), MEM-258 (CD46), LM609 (αvβ3), P1F6 (αvβ5), and 272-17E6 (αv). Transduction efficiency was measured by luciferase activity assay 48 h after infection. The results are presented as fold control, i.e., cells incubated with IgG, plus standard deviations. *, P < 0.05; ***, P < 0.001. Results are from two independent experiments (n = 2).
Overexpression of αvβ3 integrin in A549 cells allows better transduction efficiency and internalization of HAdV26.
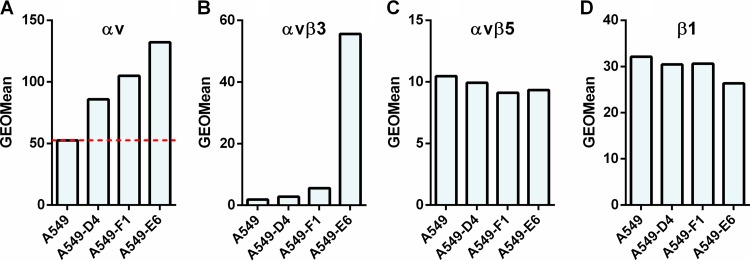
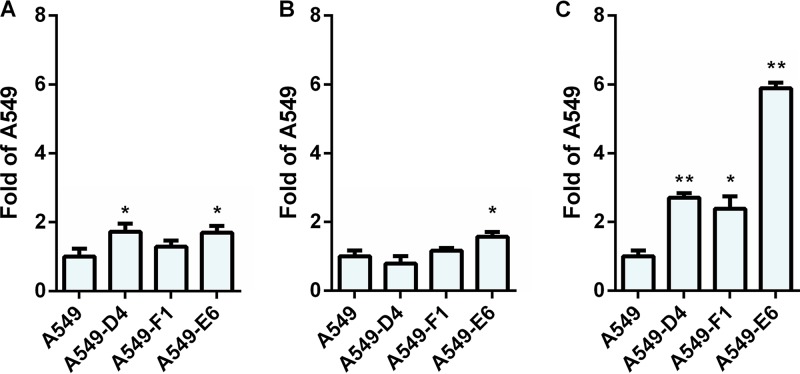
To further confirm the role of αv integrins in the transduction efficiency of HAdV26, we decided to stably transfect A549 cells with an αv integrin expression plasmid. We isolated three A549 cell clones with increased expression of αv integrin on the cell surface: A549-D4, A549-F1, and A549-E6. Among them, A549-E6 has the highest expression of αv integrins (Fig. 11A). In order to determine if this increased expression of αv integrins has an influence on HAdV26 binding, we incubated A549, A549-D4, A549-F1, and A549-E6 cells with HAdV26 and measured the binding of the virus by quantitative PCR (qPCR). In comparison to A549, HAdV26 bound slightly better to all three clones, namely, 1.7-fold better to A549-D4 and A549-E6 cells and 1.3-fold better to the A549-F1 clone (Fig. 12A). However increased internalization was observed only in clone A549-E6, in which HAdV26 internalized 1.6 times better than in A549 cells (Fig. 12B).
FIG 11.
Expression of αv, αvβ3, αvβ5, and β1 integrins in A549 cell clones obtained by stable transfection of A549 cells with a plasmid containing αv integrin subunit cDNA. Cells were detached and incubated with specific antibodies on ice, and cell surface expression of αv, αvβ3, αvβ5, and β1 integrins was analyzed by flow cytometry. The following antibodies were used: LM609 (αvβ3), P1F6 (αvβ5), and 272-17E6 (αv). Representative geometric mean (GEOMean) fluorescence intensities obtained in one of three independent experiments with similar results are shown. The dashed red line in panel A represents expression of αv integrin in A549 cells.
FIG 12.
Binding, internalization and transduction efficiency of HAdV26 in A549 cell clones with increased expression of αv integrin. (A and B) Binding (A) and internalization (B) of HAdV26 in A549 cells and A549 cell clones with increased expression of αv integrin (A549-D4, A549-F1, and A549-E6). Cells were incubated with HAdV26 on ice for 1 h at an MOI of 1,000 vp/cell. To measure binding, unbound viruses were removed by rinsing the cells with cold trypsin and PBS and collected by scraping the cells. For internalization measurement, unbound viruses were removed as described above, and the cells were transferred to 37°C and incubated for 1 h, allowing the viruses to enter the cells. The cells were then rinsed twice with warm trypsin, dispersed, and pelleted by centrifugation. For both binding and internalization, total (cellular plus viral) DNA was extracted from cells and used for quantification of viral DNA by qPCR, using the CMV region as a target sequence. The results are expressed as fold value obtained for A549 plus standard deviations. (C) Transduction efficiencies of HAdV26 in A549 cells and A549 cell clones with increased expression of αv integrin (A549-D4, A549-F1, and A549-E6). Transduction efficiency was measured by flow cytometry 48 h after infection. The results are expressed as fold value obtained for A549 plus standard deviations. *, P < 0.05; ** P < 0.01. Results are from three independent experiments (n = 3).
Next, we examined the influence of increased αv integrin expression on the transduction efficiency of HAdV26. The efficiency of HAdV26 transduction was found to be higher in all the cell clones with increased αv integrin expression than in the parental A549 cells. The most increased transduction efficiency was observed for the A549-E6 cell clone, which expressed 6 times more integrins than A549 cells. HAdV26 transduced A549-D4 and A549-F1 cells with similar efficiencies, 2.7- and 2.4-fold better than A549 cells, respectively (Fig. 12C). These data confirm that αv integrin is important for both binding and transduction of HAdV26.
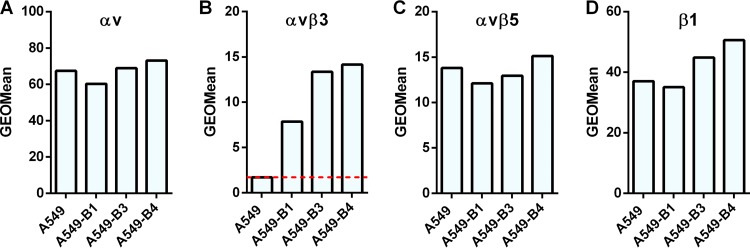
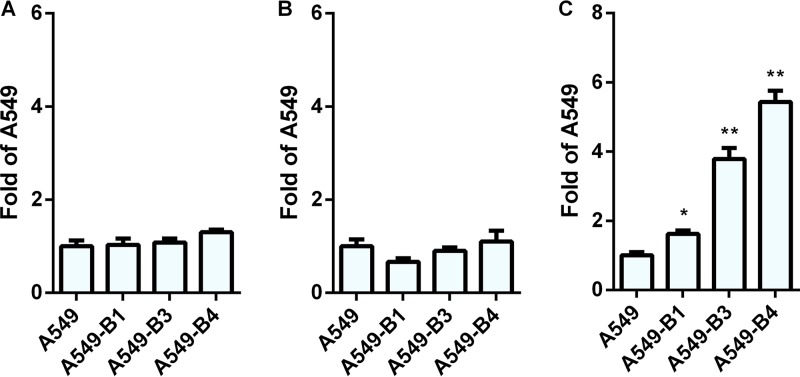
Since it is known that αv integrin most frequently forms heterodimers with β1, β3, β5, or β6 subunits, we determined the expression of αvβ3, αvβ5, αvβ6, and β1 on the surfaces of A549-D4, A549-F1, and A549-E6 cells. All three clones had the same levels of expression of the αvβ5 heterodimer (Fig. 11C) and the β1 integrin subunit (Fig. 11D) as the parental A549 cells. Neither A549, A549-D4, A549-F1, nor A549-E6 showed expression of αvβ6 integrin (data not shown). However, clone A549-E6 was found to have strikingly higher expression of αvβ3 integrin than A549, A549-D4, or A549-F1 (Fig. 11B). Since clones A549-D4, A549-F1, and A549-E6 had expression levels of αvβ5 (Fig. 11C) and β1 (Fig. 11D) comparable to those of A549 cells but showed increased transduction efficiency with HAdV26, we conclude that the expression of αvβ5 and β1 is not critical for HAdV26 binding or transduction. Based on the data with respect to the greatly increased expression of αvβ3 integrin in the A549-E6 clone, we assume that αvβ3 integrin is the molecule responsible for increased transduction efficiency of HAdV26 in this cell clone. To further confirm this hypothesis, we stably transfected A549 cells with β3 integrin subunit expression plasmid and isolated 3 clones with increased expression of αvβ3 integrin: A549-B1, A549-B3, and A549-B4 (Fig. 13B). Even though all 3 clones with increased β3 integrin subunit expression, A549-B1, A549-B3, and A549-B4, had increased expression of αvβ3 integrin, they did not show increased binding (Fig. 14A) or internalization (Fig. 14B) with HAdV26, which is different than what was observed with A549-E6. Nevertheless, the transduction efficiency of HAdV26 was increased in all three clones (A549-B1, A549-B3, and A549-B4, 1.6-, 3.7-, and 5.4-fold, respectively) (Fig. 14C). This increased transduction matched the increased expression of αvβ3 integrin. Stable transfection of the β3 integrin subunit in A549 cells did not change the expression of αvβ5 (Fig. 13C) or β1 (Fig. 13B), further confirming that their presence is not crucial for HAdV26 transduction efficiency. We obtained similar results in HEp2 cell clones with de novo expression of αvβ3 integrin (29), where high expression of αvβ3 integrin caused increased transduction efficiency of HAdV26 (data not shown). The importance of αvβ3 integrin in the transduction of A549 cells was also confirmed by preincubating cells with vitronectin and RGD peptide, known ligands for αvβ3 integrin, prior to infection with HAdV26. Incubation with both vitronectin and RGD peptide decreased the transduction efficiency of HAdV26 in A549 cells (Fig. 15). Based on our results obtained in the A549-E6, A549-B3, and A549-B4 clones, we conclude that αvβ3 integrin is required for efficient transduction of epithelial cells with HAdV26.
FIG 13.
Expression of αv, αvβ3, αvβ5, and β1 integrins in A549 cell clones obtained by stable transfection of A549 cells with a plasmid containing β3 integrin subunit cDNA. Cells were detached and incubated with specific antibodies on ice, and cell surface expression of αv (A), αvβ3 (B), αvβ5 (C), and β1 (D) integrins was analyzed by flow cytometry. The following antibodies were used: LM609 (αvβ3), P1F6 (αvβ5), and 272-17E6 (αv). Representative geometric mean fluorescence intensities obtained in one of three independent experiments with similar results are shown. The dashed red line in panel B represents expression of αvβ3 integrin in A549 cells.
FIG 14.
Binding, internalization, and transduction efficiencies of HAdV26 in A549 cell clones with increased expression of β3 integrin. (A and B) Binding (A) and internalization (B) of HAdV26 in A549 cells and A549 cell clones with increased expression of β3 integrin (A549-B1, A549-B3, and A549-B4). Cells were incubated with HAdV26 on ice for 1 h at an MOI of 1,000 vp/cell. To measure binding, unbound viruses were removed by rinsing the cells with cold trypsin and PBS and collected by scraping the cells. For internalization measurement, unbound viruses were removed as described above, and the cells were transferred to 37°C and incubated for 1 h, allowing the viruses to enter the cells. The cells were then rinsed twice with warm trypsin, dispersed, and pelleted by centrifugation. For both binding and internalization, total (cellular plus viral) DNA was extracted from cells and used for quantification of viral DNA by qPCR, using the CMV region as a target sequence. The results are expressed as fold value obtained for A549 cells plus standard deviations. (C) Transduction efficiencies of HAdV26 in A549 cells and A549 cell clones with increased expression of β3 integrin (A549-B1, A549-B3, and A549-B4). Transduction efficiency was measured by flow cytometry 48 h after infection. The results are expressed as fold value obtained for A549 cells plus standard deviations. *, P < 0.05; ** P < 0.01. Results are from three independent experiments (n = 3).
FIG 15.
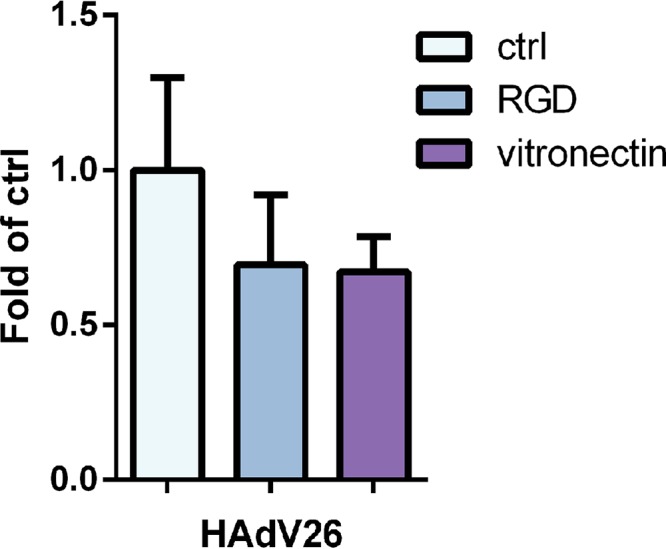
Transduction efficiency of HAdV26 in A549 cells after incubation with vitronectin and RGD peptide. Cells were first incubated with vitronectin (10 µg/ml) or RGD peptide (15 µg/ml) on ice for 1 h, and then viruses were added. Transduction efficiency was measured by luciferase activity assay 48 h after infection. The results are presented as fold control plus standard deviations. Results are from two independent experiments (n = 2).
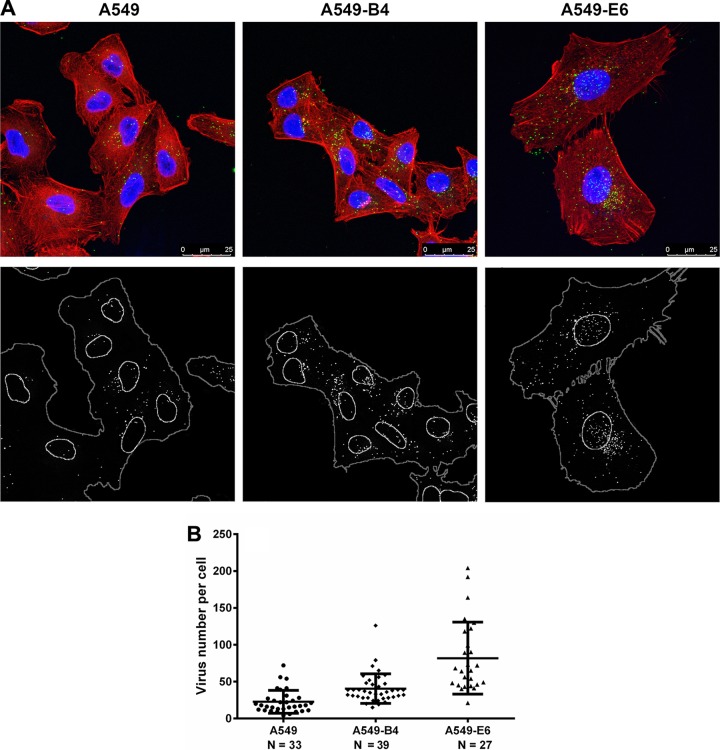
Since internalization of HAdV26 in A549 clones with increased expression of αvβ3 integrin measured by qPCR did not completely correspond to increased transduction in the cell clones, we decided to study intracellular trafficking of HAdV26 in A549, A549-B4, and A549-E6 cells by confocal microscopy. We fluorescently labeled HAdV26 and observed its localization in the cells 2 h postinfection (Fig. 16A). In both A549-B4 and A549-E6 cell clones, the average amount of HAdV26 per cell was higher than in parental A549 cells, i.e., 22 viruses per cell in A549 versus 40 and 82 viruses per cell in A549-B4 and A549-E6 cells, respectively (Fig. 16B). Based on these data, we conclude that overexpression of αvβ3 integrin in A549 cells allows better internalization and transduction efficiency of HAdV26.
FIG 16.
Intracellular trafficking of Alexa Fluor 488-labeled HAdV26 in A549, A549-B4, and A549-E6 cells. (A) Cells were incubated with Alexa Fluor 488-labeled HAdV26 (50,000 vp/cell) for 2 h at 37°C. Noninternalized viruses were rinsed away, and the cells were fixed with 2% paraformaldehyde (PFA). Green, Alexa Fluor 488-labeled HAdV26; blue, nuclei stained with DAPI; red, actin cytoskeleton stained with phalloidin. The images are maximum projections of confocal stacks. Representative confocal images are shown. Scale bars = 25 μm. (B) Quantification of virus internalization efficiency, expressed as virus number per cell. The horizontal bars represent means, and the error bars indicate standard deviations; the numbers of cells analyzed are indicated.
HAdV26 shows colocalization with αvβ3 integrin.
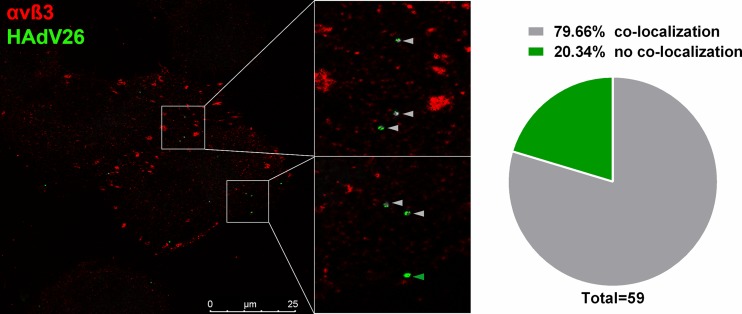
To gain further insight into the interaction between HAdV26 and αvβ3 integrin, we asked if HAdV26 colocalizes with αvβ3 integrin in A549-E6, the cell clone with the highest expression of αvβ3 integrin. Fluorescently labeled HAdV26 was incubated with A549-E6 on ice for 30 min and then transferred to 37°C for 1 min to trigger internalization. Immediately afterward, the cells were transferred to ice to stop internalization. We assumed that at this time point we should be able to capture colocalization between HAdV26 and αvβ3 integrin if there was any. About 80% of the HAdV26 virions detected under these conditions were found to colocalize either partially or completely with αvβ3 integrin, indicating that HAdV26 can use αvβ3 integrin as a receptor for infecting epithelial cells (Fig. 17).
FIG 17.
Colocalization of Alexa Fluor 488-labeled HAdV26 with αvβ3 integrin in A549-B6 cells. Cells were incubated with Alexa Fluor 488-labeled HAdV26 (50,000 vp/cell) for 1 min at 37°C, fixed with 2% PFA, and subsequently stained for αvβ3 integrin expression (LM609). A representative confocal image of HAdV26 colocalized with αvβ3 integrin is shown. The gray arrowheads indicate colocalization; the green arrowhead indicates absence of colocalization. The pie chart represents quantification of the percentage of colocalized HAdV26 with αvβ3 integrin. The data were collected from 9 cells and 59 viruses that infected the cells.
DISCUSSION
In this study, we found that HAdV26 uses αvβ3 integrin as a receptor for infecting epithelial cells. Until now, molecules that can serve as receptors for HAdV26 infection have been studied mostly in cells circulating in blood. It has been shown that HAdV26 uses CD46 as a receptor for cell entry into human peripheral blood mononuclear cells (25) and B cells (27). There are studies that show that other known adenovirus receptors, like CAR and αv integrins, could be involved in HAdV26 infection (26). So far, the only study regarding HAdV26 receptors in epithelial cells was done on HEp3 cells, where it was shown that the RGD-4C peptide partially inhibited oncolysis by the species D viruses HAdV17, HAdV24, HAdV26, and HAdV48, indicating involvement of αv integrins in species D adenovirus infection (27). Therefore, in this study, we investigated the roles of the above-mentioned molecules in HAdV26 infection of human epithelial cells.
We compared the transduction efficiencies of HAdV26 on the A549 and SK-OV-3 cell lines and observed very low transduction efficiency of HAdV26 on A549 cells and higher transduction efficiency of HAdV26 on SK-OV-3 cells, suggesting that the two cell lines differ in the expression of the HAdV26 receptor. We also observed low binding and internalization of HAdV26 in A549 cells, presumably reflecting inadequate amounts of HAdV26 receptor for efficient infection. We found that A549 cells express CAR, CD46, and αv integrins, while SK-OV-3 cells show expression only of CD46 and αv integrins and have little or no CAR on their surfaces. Expression levels of CD46 and αv integrins, namely, αvβ3 and αvβ5, known to be involved in binding the RGD sequence from the adenovirus penton base, were different in the two cell lines. To investigate the roles of the above-mentioned adenovirus receptors and to determine their importance for HAdV26 infection, we downregulated CAR, CD46, and αv integrins and studied how this downregulation influenced HAdV26 transduction efficiency. As reference viruses, we used HAdV5, which uses CAR for initial binding and αv integrins as coreceptors, and HAdV35, as a representative of a CD46 binding virus. Additionally, we used HAdV26F35, a chimeric HAdV26 vector pseudotyped with the HAdV35 fiber, which we assumed uses CD46 for cell binding. Confirmation of this assumption came from our own observation that incubation of A549 with HAdV35 and HAdV26F35 for 4 h at 37°C resulted in a significant decrease of CD46 on the cell surface, indicating that CD46 is internalized together with these viruses upon binding (data not shown), indirectly confirming that HAdV26F35 does indeed bind CD46. However, the transduction efficiency of this chimeric virus might be altered because of the HAdV35 fiber and consequent differences in the engagement between the RGD motif present in the HAdV26 penton base and integrins.
As expected, downregulation of CAR or αv integrin significantly decreased HAdV5 transduction, while downregulation of CD46 significantly decreased HAdV35 transduction efficiency in both A549 and SK-OV-3 cells, validating our cell model. Downregulation of CAR slightly decreased HAdV26 transduction in A549 cells; however, since SK-OV-3 cells have no CAR on their surfaces but are transduced better with this virus than A549 cells, we assumed that CAR is not crucial for HAdV26 infection. This assumption is further supported by results obtained in CHO-CAR cells, where increased expression of CAR had no influence on HAdV26 transduction efficiency. Downregulation of CAR in A549 cells had a very significant negative effect on HAdV35 transduction efficiency, which came as a surprise, since it is well known that HAdV35 uses CD46 for infecting cells (23). Since blocking CD46 availability with specific antibody had no influence on HAdV35 transduction efficiency and downregulating CAR did not change cell surface expression of CD46 or the binding or internalization of the virus, we could assume that some other alteration happened. Since CAR directly interacts with actin (30) and actin dynamics are needed for HAdV35 cytosol localization (31), one could imagine that downregulating CAR might influence HAdV35 infection by modifying actin dynamics and macropinocytosis, a process used by HAdV35 for cell entry. To our knowledge, there are no published data discussing the influence of siCAR on HAdV35 transduction efficiency; however, further clarification of this observation is beyond the scope of our work. Unexpectedly, downregulation of CD46 increased the transduction efficiency of HAdV26 on A549 and HeLa cells, implying that the presence of the molecule on the A549 cell surface has a negative influence on HAdV26 transduction. We observed the opposite effect in the SK-OV-3 cell line, where downregulation of CD46 decreased transduction efficiency, indicating that in SK-OV-3 cells CD46 contributes to the transduction efficiency of HAdV26. Downregulating CD46 by use of specific siRNA did not change cell surface expression of any of the other investigated receptors, namely, CAR, αv integrin, αvβ3, or αvβ5 integrin, showing that decreased HAdV26 transduction was not due to diminished abundance of cell surface receptor. This observation is consistent throughout the study, but at this point, we cannot explain the phenomenon, which seems to be cell specific. Our result is in line with data obtained in peripheral blood mononuclear cells, for which it was reported that HAdV26 transduction is CD46 dependent (25). Just like SK-OV-3 cells, peripheral blood mononuclear cells are CAR negative (32), suggesting that the role of CD46 in HAdV26 transduction efficiency might depend on other molecules present in the cells. Nevertheless, this needs further investigation. Downregulation of αv integrin significantly decreased the transduction efficiency of HAdV26 in A549, SK-OV-3, and HeLa cells, indicating that αv integrin is involved in HAdV26 transduction in these cell lines. The same effect was observed in the melanoma cell line M21 variants M21L and M21L4. The transduction efficiency of HAdV26 was much higher in M21L4 cells, which are αv integrin positive, than in M21L cells, which are αv integrin negative. Downregulation of αv integrin also decreased the transduction efficiency of HAdV35, albeit much less than HAdV26F35. Since HAdV26F35 possesses a penton base from HAdV26, it is possible that the spatial organization of the RGD loop in HAdV26F35 is different from that in HAdV35, indicating that the two viruses might use αv integrin in different manners. That αv integrin is necessary for HAdV26 transduction was also confirmed by pretreating A549 cells with specific blocking antibodies prior to infection. Blocking the surface availability of αv integrin, alone or in combination with both CAR and CD46, significantly decreased the transduction efficiency of HAdV26. Blocking CAR or CD46 alone had no effect on HAdV26 infection. At the same time, blocking CD46 alone or in combination with CAR or αv integrin abrogated HAdV26F35 transduction efficiency. Downregulation of αv integrin also decreased binding and internalization of HAdV26 in A549 cells, while downregulating CAR or CD46 had no influence on HAdV26 binding or internalization. This confirms that αv integrin plays an important role in binding and internalization of HAdV26 in A549 cells. Altogether, these data allow us to propose that αv integrin serves as a receptor for HAdV26 in human epithelial cells.
In order to further confirm the role of αv integrin in HAdV26 infection, we took a different approach. Instead of downregulating αv integrin, we decided to upregulate αv integrin in A549 cells, assuming that this would allow better HAdV26 transduction efficiency. We isolated several stably transfected A549 clones with increased αv integrin expression and measured binding, internalization, and transduction efficiency. Increased αv integrin expression in A549 cells resulted in slightly increased binding and internalization of HAdV26, which was followed by significantly increased HAdV26 transduction efficiency in the cell clone with the highest expression of αv integrin. Since it is known that αv integrin exists in interaction with integrin subunits β1, β3, β5, β6, and β8, creating the heterodimers αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8, among which αvβ3 and αvβ5 bind the RGD sequence and serve as coreceptors for adenoviruses, we wanted to determine the status of those heterodimers on the surfaces of A549 cells resulting from increased expression of the αv integrin subunit. In transfected A549 clones with different levels of expression of the αv integrin subunit, we did not observe changes in the expression of αvβ5 or of β1 (and thus αvβ1). However, in the A549 clone with the highest expression of αv, we detected a large increase in the expression of αvβ3, suggesting that in this clone the larger amount of αv integrin subunit caused augmentation of β3 integrin subunit expression. We did not detect expression of αvβ6 integrin, and due to the lack of an adequate antibody, we did not measure the expression of αvβ8 integrin. However, previous reports have stated that A549 cells lack both αvβ6 and αvβ8 integrins (33). Based on these observations, we conclude that the molecule responsible for increased HAdV26 transduction efficiency is the αvβ3 integrin. We further corroborated this conclusion by isolating A549 cell clones stably transfected with β3 subunit integrin cDNA. The integrin β3 subunit creates heterodimers only with αv and αIIb subunits. Since the αIIb subunit is a marker of hematopoietic cells (34), we assumed that in A549 cells, the β3 subunit would interact only with the αv subunit, resulting in the αvβ3 integrin. We isolated several clones with increased expression of αvβ3 and detected increased HAdV26 transduction efficiency in all of them, in accordance with αvβ3 integrin expression. We also determined the expression levels of αv, αvβ5, and β1 in these clones and verified that increase in αvβ3 integrin did not change the expression of any of them, further underlining that the transduction efficiency of HAdV26 depended on the expression of the αvβ3 integrin. Since the promiscuous integrin subunits β1 and αv are synthesized in excess, the formation of any αβ heterodimer is dependent on the availability of the other subunit; thus, formation of αv-containing heterodimers follows a hierarchical order. Therefore, the cell surface copy numbers of, for example, αvβ3 and αvβ5 integrins are dependent on the amounts of β3 and β5 subunits, respectively (29, 35). This can explain why in our A549 cell clones stably overexpressing αv integrin we saw upregulation of only αvβ3 and not αvβ5 integrin, i.e., β3 and β5 integrin subunits compete for newly synthesized αv subunit, causing differences in the expression of αvβ3 and αvβ5 heterodimers.
The discrepancy observed between transduction efficiency and binding/internalization of HAdV26 in A549 clones with increased expression of αvβ3 integrin measured by qPCR could lie in a quite high dissociation constant (Kd) between the adenovirus penton base and αvβ3 integrin (415 ± 62 nM) (36). Although this Kd refers to HAdV9, we can assume that the Kd value would be similar for HAdV26, because they belong to the same serotype. Since the binding assay is performed on ice, which does not allow integrin clustering, it is possible that some information could be lost.
Even though the overall structure of the HAdV26 capsid is mostly similar to that of HAdV5, there are some striking differences in structure between the two viruses. One difference with possible implications for αv integrin binding is present in the penton base structure. Sequence alignments between HAdV5 and HAdV26 showed that there is a 12-residue deletion at the N terminus and two deletions in the RGD-containing loop in the penton base of HAdV26 relative to species C (15). One could suspect that these changes could render RGD from the HAdV26 penton less reachable by αv integrins. HAdV26 has a relatively short fiber with only 8 beta repeats in the shaft compared to 22 repeats in the case of HAdV5. This short fiber is assumed to be fairly rigid, allowing only limited bending (37). Bending of a long fiber allows easier interaction between RGD from the penton of CAR binding adenoviruses and cell surface integrins, which otherwise would not be possible. The RGD binding site on αvβ3 integrin is situated on the top of the integrin subunits and can be reached only when the integrin molecule is activated, i.e., in the extended conformation. According to the current model, the length of the extended αvβ3 integrin is approximately 20 nm (38). The length of the adenovirus fiber with 8-shaft beta repeats is 11 nm (39). Therefore, αvβ3 integrin in its extended form should be able to span the distance between the cell surface and the HAdV26 penton base and reach the RGD peptide, i.e., a rigid fiber should not impair binding of HAdV26 to αvβ3 integrin. In order to corroborate this, further research is needed.
Adenovirus-mediated transduction efficiency reflects the sum of adenovirus binding, internalization, and intracellular trafficking. Intracellular trafficking is best understood for HAdV5 and includes clathrin-mediated dynamin-dependent endocytosis followed by endosomal escape and cytosolic transport all the way to the nucleus. This entire path is thought to be completed within approximately 90 min of infection (40). For HAdV26, there are no detailed reports regarding intracellular trafficking. Here, we studied intracellular trafficking of fluorescently labeled HAdV26 in A549 cells and two clones with increased expression of αvβ3 integrin, A549-E6 and A549-B4, 120 min postinfection. The average amounts of internalized HAdV26 virions per cell in cell clones A549-E6 and A549-B4 were 4 and 2 times larger, respectively, indicating that αvβ3 integrin allows efficient internalization of HAdV26. Additionally, we studied colocalization of HAdV26 and αvβ3 integrin in A549-E6, the cell clone with the highest expression of αvβ3 integrin, and observed that at a very early time point following binding, HAdV26 colocalizes with αvβ3 integrin, confirming that HAdV26 uses αvβ3 integrin as a receptor in epithelial cells. By studying intracellular trafficking of fluorescently labeled HAdV26 in A549 cells, we did not observe an accumulation of HAdV26 in the proximity of the microtubule-organizing center, as has been described for HAdV5 (41), indicating that HAdV26 might traffic differently from HAdV5. Further studies are needed in order to learn more about HAdV26 intracellular trafficking.
The data obtained in this study give us new insight into the HAdV26 infection pathway, confirming that αvβ3 integrin is required for efficient infection of epithelial cells by HAdV26. Recently, Casiraghi et al. reported that αvβ3 integrin strongly affects the innate immune response in epithelial cells. They showed that αvβ3 integrin greatly increased the immune response elicited by herpes simplex virus, which had previously been shown to bind αvβ3 integrin (42). This implies that HAdV26 interaction with αvβ3 integrin might also influence the innate immune response in infected cells, and therefore, it would be interesting to investigate it in more detail. Based on our data, one could wonder what the relationship of αvβ3 is to the previously reported receptor for the virus, namely, CD46, which has been reported to be involved in binding of HAdV26 to PBMCs. We point out that PBMCs have almost no expression of αvβ3 and αvβ5 integrin (43); thus, αvβ3 integrin is not available as a receptor for HAdV26 in these cells. The results obtained in this study bring us new knowledge regarding HAdV26 receptor usage and should be taken into account when using current or constructing new HAdV26-based vectors for gene transfer and vaccination purposes.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
HEK293 (human embryonic kidney; ATCC CRL-1573), A549 (human lung carcinoma; ATCC CCL-185), SK-OV-3 (human ovarian carcinoma; ATCC HTB-77), and HeLa (human cervix adenocarcinoma; ATCC CCL-2) cells were obtained from the ATCC Cell Biology Collection and were cultured according to the manufacturer’s instructions. Adherent CHO-K1 cells (Chinese hamster ovary cells; ATCC CCL-61; CAR and CD46 negative) and CHO-CAR cells (CHO cells transfected to stably express human CAR) were kind gifts from George Santis, King’s College London School of Medicine, London, United Kingdom. CHO-BC1 cells (CHO cells stably transfected to express CD46) were previously described (44). Melanoma M21 cell variants M21L and M21L4 (45) were kindly supplied by Urs Greber, University of Zurich, Zurich, Switzerland. Replication-incompetent recombinant adenoviral vectors based on adenovirus types 5, 26, and 35 were previously constructed (20, 46). Viruses were propagated on HEK293 cells and purified by CsCl gradients. They carried either an enhanced green fluorescent protein (GFP) or a luciferase gene driven by the cytomegalovirus (CMV) promoter as a reporter gene. Antibodies used for flow cytometry, immunohistochemistry, colocalization, and infection competition analyses were as follows: anti-CAR (RcmB) from Merck Millipore, anti-CD46 (MEM-258) from Thermo Fisher Scientific, anti-αvβ3 integrin (LM609) from Merck Millipore, anti-αvβ5 integrin (P1F6) from Merck Millipore, anti-αv (272-17E6) from Merck Millipore, anti-β1 (JB1A) from Merck Millipore, anti-αvβ6 integrin (E7P6) from Merck Millipore, and fluorescein isothiocyanate (FITC) goat anti-mouse IgG (catalog no. 554001) from BD Pharmingen.
Adenovirus infection assay.
Adherent cells were incubated with viruses at 37°C, and transduction efficiency was measured 48 h after infection by assaying for luciferase activity (Promega, Southampton, United Kingdom) or by flow cytometry in the case of the GFP reporter. For the measurement of transduction efficiency in the presence of function-blocking antibodies, cells were incubated with antibodies at a final concentration of 20 µg/ml for 1 h on ice prior to incubation with viruses for 1 h on ice. The cells were then rinsed and transferred to 37°C. Transduction efficiency was measured 48 h after infection. For the measurement of transduction efficiency in the presence of vitronectin or RGD peptide, cells were incubated with vitronectin or RGD peptide for 1 h on ice prior to incubation with viruses for 1 h on ice. The cells were then rinsed and transferred to 37°C. Transduction efficiency was measured 48 h after infection. For the measurement of transduction efficiency after downregulating specific receptors using siRNA, cells were transfected with the specific siRNA (50 nM final concentration) and infected with adenoviruses 48 h later. Transduction efficiency was measured 48 h after infection.
Adenovirus labeling.
After purification by banding in CsCl and dialysis against phosphate-buffered saline (PBS) buffer, adenovirus particles were incubated with a 20-fold excess of chemically reactive Alexa Fluor 488-TFP (Molecular Probes) for 2 h at room temperature in PBS buffer, pH 7.2. The labeled viral particles were then purified of excess dye by dialysis using Zeba Spin desalting columns (Pierce). The transduction efficiency of the modified vector was analyzed by transduction assay in HEK293 cells. Alexa Fluor 488-TFP labeling did not alter the transduction efficiency of the labeled viruses.
siRNA experiments.
To downregulate specific receptors, we used the following Silencer Select predesigned siRNAs: CAR siRNA (s3774), CD46 siRNA (s8604), αv integrin siRNA (s7570), and scrambled siRNA no. 1 (catalog no. 4390844), all from Thermo Fisher Scientific. Cells were transfected at a confluence of 30 to 50% using Lipofectamine RNAiMax reagent (Invitrogen) according to the manufacturer’s protocol. The efficiency of silencing was verified 48 h after transfection by flow cytometry.
Flow cytometry.
Flow cytometry was used to analyze the expression of CAR; CD46; αv integrin subunit; β1 integrin subunit; and integrin heterodimers αvβ3, αvβ5, and αvβ6. Briefly, adherent cells were grown in tissue culture dishes, detached, and washed twice with PBS. Subsequently, the cells were incubated on ice with the specific primary antibodies that recognized CAR, CD46, αv integrin, β1 integrin, αvβ3 integrin, αvβ5 integrin, and αvβ6 integrin. The binding of unlabeled primary antibodies was revealed by using FITC-conjugated anti-mouse Ig as a secondary reagent.
Binding and internalization.
Adherent cells were grown in 6-well dishes to 80% confluence. Adenoviruses (1,000 physical particles per cell) were added to the cells and incubated for 1 h on ice. To measure binding, unbound viruses were removed by washing the cells twice with cold trypsin and twice with cold PBS. The cells were then harvested with a cell scraper and pelleted by centrifugation. To measure internalization, unbound viruses were removed, warm growth medium was added, and the cells were transferred to 37°C, allowing the viruses to enter the cells. After incubation at 37°C for 1 h, the cells were washed twice with warm trypsin, dispersed, and pelleted by centrifugation. Total (cellular plus viral) DNA was extracted using commercially available materials (DNeasy kit; Qiagen) and used to quantify viral DNA. To measure the extent of viral attachment or internalization, viral DNA was quantified by qPCR on 100 ng of total DNA. Viral DNA was detected by qPCR using primers for the CMV sequence (CMV Rv, CGATCTGACGGTTCACTAAACG; CMV Fw, TGGGCGGTAGGCGTGTA; CMV probe, TGGGAGGTCTATATAAGC). The amount of viral DNA was normalized using expression of GAPDH (glyceraldehyde-3-phosphate dehydrogenase).
Isolation of A549 cells stably expressing αv or β3 integrin.
Integrin αv-expressing cell clones A549-D4, A549-F1, and A549-E6 were established from A549 cells by stable transfection with the pcDNA2004Neo(−)αv plasmid containing αv integrin subunit cDNA, which was purchased from Life Technologies. Integrin αvβ3-expressing cell clones A549-B1, A549-B3, and A549-B4 were established from A549 cells by stable transfection with the pcDNA β3 plasmid containing integrin subunit β3 cDNA (kindly provided by E. H. Danen, Amsterdam, The Netherlands). The plasmid was transfected into A549 cells using Lipofectamine (Invitrogen, La Jolla, CA). The cells were selected in the presence of G418 (0.6 mg/ml) and screened for αv or αvβ3 integrin expression by flow cytometry.
Confocal microscopy.
Cells (20,000 per coverslip) were seeded in 24-well plates. Two days later, labeled adenoviruses were added to the cells (50,000 particles/cell) and incubated on ice for 30 min to allow binding. Subsequently, the cells were transferred to 37°C for the indicated time. The cells were fixed with 2% paraformaldehyde in PBS for 12 min at room temperature. Nuclei were labeled with DAPI (4′,6-diamidino-2-phenylindole). Coverslips were slide mounted using Fluoromount (Southern Biotech). Confocal laser scanning microscopy analyses were performed using a Leica TCS SP2 AOBS. Observations were made with a 63× objective. The images showing intracellular trafficking of Alexa Fluor 488-labeled HAdVs are maximum projections of 7 confocal stacks and were processed with the Leica Application Suite X (LAS X) software platform, Adobe Photoshop CC software (Adobe Systems), and ImageJ. The colocalization analysis was performed using digital images processed with a colocalization plug-in in ImageJ.
Statistical analyses.
All experiments were performed at least three times in duplicate or triplicate, except flow cytometry experiments, which were performed twice. The results are expressed as means and standard deviations and were analyzed either by t test or by two-way analysis of variance. We used GraphPad Prism software. All P values of <0.05 were considered statistically significant.
ACKNOWLEDGMENTS
This investigation was supported by Croatian Science Foundation Installation Research Project UIP-2014-09-3912 and Marie Curie Initial Training Network ADVance Grant FP7-290002. This paper was published, in part, with the support of the Marie Curie Alumni Association.
T.G.U., J.V., and J.C. are employees of Janssen Vaccines and Prevention BV, Leiden, The Netherlands.
REFERENCES
- 1.Mangel WF, San Martin C. 2014. Structure, function and dynamics in adenovirus maturation. Viruses 6:4536–4570. doi: 10.3390/v6114536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemerow GR, Stewart PL, Reddy VS. 2012. Structure of human adenovirus. Curr Opin Virol 2:115–121. doi: 10.1016/j.coviro.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C, Zhou DM. 2016. Adenoviral vector-based strategies against infectious disease and cancer. Hum Vacc Immunother 12:2064–2074. doi: 10.1080/21645515.2016.1165908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendrickx R, Stichling N, Koelen J, Kuryk L, Lipiec A, Greber UF. 2014. Innate immunity to adenovirus. Hum Gene Ther 25:265–284. doi: 10.1089/hum.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartman ZC, Kiang A, Everett RS, Serra D, Yang XY, Clay TM, Amalfitano A. 2007. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J Virol 81:1796–1812. doi: 10.1128/JVI.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madisch I, Harste G, Pommer H, Heim A. 2005. Phylogenetic analysis of the main neutralization and hemagglutination determinants of all human adenovirus prototypes as a basis for molecular classification and taxonomy. J Virol 79:15265–15276. doi: 10.1128/JVI.79.24.15265-15276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh MP, Seto J, Liu EB, Dehghan S, Hudson NR, Lukashev AN, Ivanova O, Chodosh J, Dyer DW, Jones MS, Seto D. 2011. Computational analysis of two species C human adenoviruses provides evidence of a novel virus. J Clin Microbiol 49:3482–3490. doi: 10.1128/JCM.00156-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson CM, Singh G, Henquell C, Walsh MP, Peigue-Lafeuille H, Seto D, Jones MS, Dyer DW, Chodosh J. 2011. Computational analysis and identification of an emergent human adenovirus pathogen implicated in a respiratory fatality. Virology 409:141–147. doi: 10.1016/j.virol.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier O, Greber UF. 2003. Adenovirus endocytosis. J Gene Med 5:451–462. doi: 10.1002/jgm.409. [DOI] [PubMed] [Google Scholar]
- 10.Nwanegbo E, Vardas E, Gao WT, Whittle H, Sun HJ, Rowe D, Robbins PD, Gambotto A. 2004. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin Diagn Lab Immun 11:351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holterman L, Vogels R, van der Vlugt R, Sieuwerts M, Grimbergen J, Kaspers J, Geelen E, van der Helm E, Lemckert A, Gillissen G, Verhaagh S, Custers J, Zuijdgeest D, Berkhout B, Bakker M, Quax P, Goudsmit J, Havenga M. 2004. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J Virol 78:13207–13215. doi: 10.1128/JVI.78.23.13207-13215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy S, Medina-Jaszek A, Wilson MJ, Sandhu A, Calcedo R, Lin J, Wilson JM. 2011. Creation of a panel of vectors based on ape adenovirus isolates. J Gene Med 13:17–25. doi: 10.1002/jgm.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewer K, Rampling T, Venkatraman N, Bowyer G, Wright D, Lambe T, Imoukhuede EB, Payne R, Fehling SK, Strecker T, Biedenkopf N, Krahling V, Tully CM, Edwards NJ, Bentley EM, Samuel D, Labbe G, Jin J, Gibani M, Minhinnick A, Wilkie M, Poulton I, Lella N, Roberts R, Hartnell F, Bliss C, Sierra-Davidson K, Powlson J, Berrie E, Tedder R, Roman F, De Ryck I, Nicosia A, Sullivan NJ, Stanley DA, Mbaya OT, Ledgerwood JE, Schwartz RM, Siani L, Colloca S, Folgori A, Di Marco S, Cortese R, Wright E, Becker S, Graham BS, Koup RA, Levine MM, Volkmann A, Chaplin P, Pollard AJ, Draper SJ, Ballou WR, Lawrie A, Gilbert SC, Hill AV. 2016. A monovalent chimpanzee adenovirus Ebola vaccine boosted with MVA. The N Engl J Med 374:1635–1646. doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davison AJ, Benko M, Harrach B. 2003. Genetic content and evolution of adenoviruses. J Gen Virol 84:2895–2908. doi: 10.1099/vir.0.19497-0. [DOI] [PubMed] [Google Scholar]
- 15.Yu XD, Veesler D, Campbell MG, Barry ME, Asturias FJ, Barry MA, Reddy VS. 2017. Cryo-EM structure of human adenovirus D26 reveals the conservation of structural organization among human adenoviruses. Sci Adv 3:e1602670. doi: 10.1126/sciadv.1602670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SMK, Greig JA, Denby L, Custers J, Morita T, Francischetti IMB, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Hlavenga MJE, Nicklin SA, Baker AH. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, Plested E, Nuthall E, Voysey M, Silva-Reyes L, McElrath MJ, De Rosa SC, Frahm N, Cohen KW, Shukarev G, Orzabal N, van Duijnhoven W, Truyers C, Bachmayer N, Splinter D, Samy N, Pau MG, Schuitemaker H, Luhn K, Callendret B, Van Hoof J, Douoguih M, Ewer K, Angus B, Pollard AJ, Snape MD. 2016. Safety and immunogenicity of novel adenovirus type 26-and modified vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA 315:1610–1623. doi: 10.1001/jama.2016.4218. [DOI] [PubMed] [Google Scholar]
- 18.Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, Patel A, Johnson JA, Kleinjan J, Yanosick KE, Perry J, Zablowsky E, Abbink P, Peter L, Iampietro MJ, Cheung A, Pau MG, Weijtens M, Goudsmit J, Swann E, Wolff M, Loblein H, Dolin R, Barouch DH. 2013. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis 207:240–247. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barouch DH, Tomaka FL, Wegmann F, Stieh DJ, Alter G, Robb ML, Michae NL, Peter L, Nkolola JP, Borducchi EN, Chandrashekar A, Jetton D, Stephenson KE, Li WJ, Korber B, Tomaras GD, Montefiori DC, Gray G, Frahm N, McElrath MJ, Baden L, Johnson J, Hotter J, Swann E, Karita E, Kibuuka H, Mpendo J, Garrett N, Mngadi K, Chinyenze K, Priddy F, Lazarus E, Laher F, Nitayapan S, Pitisuttithum P, Bart S, Campbell T, Feldman R, Lucksinger G, Borremans C, Callewaert K, Roten R, Sadoff J, Scheppler L, Weijtens M, Feddes-de Boer K, van Manen D, Vreugdenhil J, Zahn R, Lavreys L, Nijs S, Tolboom J, Hendriks J, Euler Z, Pau MG, Schuitemaker H. 2018. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 392:232–243. doi: 10.1016/S0140-6736(18)31364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbink P, Lemckert AAC, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O'Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJE, Barouch DH. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol 81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnberg N. 2012. Adenovirus receptors: implications for targeting of viral vectors. Trends Pharmacol Sci 33:442–448. doi: 10.1016/j.tips.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Bergelson JM, Cunningham JA, Droguett G, KurtJones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 23.Gaggar A, Shayakhmetov DM, Lieber A. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat Med 9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 24.Nemerow GR, Stewart PL. 1999. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev 63:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li HL, Rhee EG, Masek-Hammerman K, Teigler JE, Abbink P, Barouch DH. 2012. Adenovirus serotype 26 utilizes cd46 as a primary cellular receptor and only transiently activates T lymphocytes following vaccination of rhesus monkeys. J Virol 86:10862–10865. doi: 10.1128/JVI.00928-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H, Xiang ZQ, Li Y, Kurupati RK, Jia B, Bian A, Zhou DM, Hutnick N, Yuan S, Gray C, Serwanga J, Auma B, Kaleebu P, Zhou X, Betts MR, Ertl HCJ. 2010. Adenovirus-based vaccines: comparison of vectors from three species of Adenoviridae. J Virol 84:10522–10532. doi: 10.1128/JVI.00450-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CY, Senac JS, Weaver EA, May SM, Jelinek DF, Greipp P, Witzig T, Barry MA. 2011. Species D adenoviruses as oncolytics against B-cell cancers. Clin Cancer Res 17:6712–6722. doi: 10.1158/1078-0432.CCR-11-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stichling N, Suomalainen M, Flatt JW, Schmid M, Pacesa M, Hemmi S, Jungraithmayr W, Maler MD, Freudenberg MA, Pluckthun A, May T, Koster M, Fejer G, Greber UF. 2018. Lung macrophage scavenger receptor SR-A6 (MARCO) is an adenovirus type-specific virus entry receptor. PLoS Pathog 14:e1006914. doi: 10.1371/journal.ppat.1006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ambriovic-Ristov A, Gabrilovac J, Cimbora-Zovko T, Osmak M. 2004. Increased adenoviral transduction efficacy in human laryngeal carcinoma cells resistant to cisplatin is associated with increased expression of integrin alphavbeta3 and coxsackie adenovirus receptor. Int J Cancer 110:660–667. doi: 10.1002/ijc.20176. [DOI] [PubMed] [Google Scholar]
- 30.Huang KC, Yasruel Z, Guerin C, Holland PC, Nalbantoglu J. 2007. Interaction of the coxsackie and adenovirus receptor (CAR) with the cytoskeleton: binding to actin. FEBS Lett 581:2702–2708. doi: 10.1016/j.febslet.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Kalin S, Amstutz B, Gastaldelli M, Wolfrum N, Boucke K, Havenga M, DiGennaro F, Liska N, Hemmi S, Greber UF. 2010. Macropinocytotic uptake and infection of human epithelial cells with species B2 adenovirus type 35. J Virol 84:5336–5350. doi: 10.1128/JVI.02494-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Havenga MJ, Lemckert AA, Ophorst OJ, van Meijer M, Germeraad WT, Grimbergen J, van Den Doel MA, Vogels R, van Deutekom J, Janson AA, de Bruijn JD, Uytdehaag F, Quax PH, Logtenberg T, Mehtali M, Bout A. 2002. Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J Virol 76:4612–4620. doi: 10.1128/JVI.76.9.4612-4620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman SL, Grote HJ, Wilm C. 2012. Matched rabbit monoclonal antibodies against alpha v-series integrins reveal a novel alpha v beta 3-LIBS epitope, and permit routine staining of archival paraffin samples of human tumors. Biol Open 1:329–340. doi: 10.1242/bio.2012364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durrant TN, van den Bosch MT, Hers I. 2017. Integrin alphaIIbbeta3 outside-in signaling. Blood 130:1607–1619. doi: 10.1182/blood-2017-03-773614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koistinen P, Heino J. 2002. The selective regulation of alpha(v)beta(1) integrin expression is based on the hierarchical formation of alpha(v)-containing heterodimers. J Biol Chem 277:24835–24841. doi: 10.1074/jbc.M203149200. [DOI] [PubMed] [Google Scholar]
- 36.Veesler D, Cupelli K, Burger M, Graber P, Stehle T, Johnson JE. 2014. Single-particle EM reveals plasticity of interactions between the adenovirus penton base and integrin alpha(V)beta(3). Proc Natl Acad Sci U S A 111:8815–8819. doi: 10.1073/pnas.1404575111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu E, Pache L, Von Seggern DJ, Mullen TM, Mikyas Y, Stewart PL, Nemerow GR. 2003. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J Virol 77:7225–7235. doi: 10.1128/JVI.77.13.7225-7235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takagi J, Petre BM, Walz T, Springer TA. 2002. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110:599–611. doi: 10.1016/S0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 39.Shayakhmetov DM, Lieber A. 2000. Dependence of adenovirus infectivity on length of the fiber shaft domain. J Virol 74:10274–10286. doi: 10.1128/JVI.74.22.10274-10286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luisoni S, Greber UF. 2016. Biology of adenovirus cell entry: receptors, pathways, mechanisms A2, p 27–58. In Curiel DT. (ed), Adenoviral vectors for gene therapy, 2nd ed Academic Press, San Diego, CA. [Google Scholar]
- 41.Bailey CJ, Crystal RG, Leopold PL. 2003. Association of adenovirus with the microtubule organizing center. J Virol 77:13275–13287. doi: 10.1128/JVI.77.24.13275-13287.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casiraghi C, Gianni T, Campadelli-Fiume G. 2016. Alpha v beta 3 integrin boosts the innate immune response elicited in epithelial cells through plasma membrane and endosomal Toll-like receptors. J Virol 90:4243–4248. doi: 10.1128/JVI.03175-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang SA, Endo RI, Nemerow GR. 1995. Up-regulation of integrins alpha-v-beta-3 and alpha-v-beta-5 on human monocytes and t-lymphocytes facilitates adenovirus-mediated gene delivery. J Virol 69:2257–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trinh HV, Lesage G, Chennamparampil V, Vollenweider B, Burckhardt CJ, Schauer S, Havenga M, Greber UF, Hemmi S. 2012. Avidity binding of human adenovirus serotypes 3 and 7 to the membrane cofactor CD46 triggers infection. J Virol 86:1623–1637. doi: 10.1128/JVI.06181-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petitclerc E, Stromblad S, von Schalscha TL, Mitjans F, Piulats J, Montgomery AM, Cheresh DA, Brooks PC. 1999. Integrin alpha(v)beta3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res 59:2724–2730. [PubMed] [Google Scholar]
- 46.Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, Kostense S, Penders G, Helmus N, Koudstaal W, Cecchini M, Wetterwald A, Sprangers M, Lemckert A, Ophorst O, Koel B, van Meerendonk M, Quax P, Panitti L, Grimbergen J, Bout A, Goudsmit J, Havenga M. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol 77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]