Patients who are chronically infected with HIV-1 experience sequelae related to chronic inflammation. The mechanisms of this inflammation have not been elucidated. Here, we describe a class of drugs that target the P2X proinflammatory signaling receptors in a human tonsil explant model. This model highlights differences in HIV-1 stimulation of lymphoid tissue inflammation and peripheral blood. These drugs serve to block both HIV-1 infection and production of IL-10 and IL-1β in lymphoid tissue, suggesting a novel approach to HIV-1 therapeutics in which both HIV-1 replication and inflammatory signaling are simultaneously targeted.
KEYWORDS: IL-1β, IL-10, P2X, cytokines, human immunodeficiency virus, infectious disease, inflammasome, inflammation, purinergic, receptors
ABSTRACT
HIV-1 causes a persistent infection of the immune system that is associated with chronic comorbidities. The mechanisms that underlie this inflammation are poorly understood. Emerging literature has implicated proinflammatory purinergic receptors and downstream signaling mediators in HIV-1 infection. This study probed whether inhibitors of purinergic receptors would reduce HIV-1 infection and HIV-1-stimulated inflammation. An ex vivo human tonsil histoculture infection model was developed to support HIV-1 productive infection and stimulated the inflammatory cytokine interleukin-1 beta (IL-1β) and the immunosuppressive cytokine interleukin-10 (IL-10). This study tests whether inhibitors of purinergic receptors would reduce HIV-1 infection and HIV-1-stimulated inflammation. The purinergic P2X1 receptor antagonist NF449, the purinergic P2X7 receptor antagonist A438079, and azidothymidine (AZT) were tested in HIV-1-infected human tonsil explants to compare levels of inhibition of HIV-1 infection and HIV-stimulated inflammatory cytokine production. All drugs limited HIV-1 productive infection, but P2X-selective antagonists (NF449 and A438079) significantly lowered HIV-stimulated IL-10 and IL-1β. We further observed that P2X1- and P2X7-selective antagonists can act differentially as inhibitors of both HIV-1 infection and HIV-1-stimulated inflammation. Our findings highlight the differential effects of HIV-1 on inflammation in peripheral blood compared to those in lymphoid tissue. For the first time, we demonstrate that P2X-selective antagonists act differentially as inhibitors of both HIV-1 infection and HIV-1-stimulated inflammation. Drugs that block these pathways can have independent inhibitory activities against HIV-1 infection and HIV-induced inflammation.
IMPORTANCE Patients who are chronically infected with HIV-1 experience sequelae related to chronic inflammation. The mechanisms of this inflammation have not been elucidated. Here, we describe a class of drugs that target the P2X proinflammatory signaling receptors in a human tonsil explant model. This model highlights differences in HIV-1 stimulation of lymphoid tissue inflammation and peripheral blood. These drugs serve to block both HIV-1 infection and production of IL-10 and IL-1β in lymphoid tissue, suggesting a novel approach to HIV-1 therapeutics in which both HIV-1 replication and inflammatory signaling are simultaneously targeted.
INTRODUCTION
HIV-1 infection remains a major global health concern, despite the development of effective antiviral therapies to control the virus. An estimated 36.7 million people live with HIV-1, with 1.1 million people infected in the United States (1). Individuals on antiretroviral therapy (ART) can live long and healthy lives with suppressed viremia; however, infected individuals experience chronic inflammation associated with comorbidities and increased risk of mortality (2–5). Despite undetectable viremia levels, long-term-treated HIV-1 patients experience significantly higher rates of age-associated noncommunicable comorbidities (AANCCs), such as cardiovascular disease, frailty, and cognitive decline (6). The accelerated aging phenomenon has introduced new considerations in the care of HIV-1-infected patients (7–9).
The mechanisms underlying this chronic inflammation in HIV-1 infection are multifactorial. Depletion of CD4+ T cells at mucosal surfaces during HIV-1 infection can lead to reduced integrity of the mucosal epithelium and increased bacterial translocation (10). The subsequent elevated levels of plasma bacterial cell wall lipopolysaccharide (LPS) are associated with inflammatory biomarkers in HIV-1 patients, such as chronic monocyte activation, increased soluble CD14 (sCD14), and production of proinflammatory cytokines (11). Low-level viremia continues to stimulate systemic inflammation (12, 13). Despite numerous lines of evidence, no unifying mechanism has defined a connection between factors mediating early HIV-1 infection and cellular mechanisms of innate immune signaling.
Emerging literature has implicated the proinflammatory purinergic receptors in HIV-1 pathogenesis (14–30). Purinergic receptors mediate inflammation in many disease states (14, 31–35) in response to extracellular nucleotides that are released from inflamed or dying cells (15, 16). P2X receptor subtypes are nonselective cation channels that can be found on a wide variety of tissue types, notably lymphocytes, monocyte/macrophages, and dendritic cells (DCs) (17–20). They are critical mediators of the innate immune response in a variety of different disease states, including rheumatoid arthritis, transplant rejection, and inflammatory bowel disease (21–24). The P2X7 subtype is most highly expressed in immune cells (20, 25). P2X7 receptors, in concert with Toll-like receptors (TLRs), activate the NLRP3 (NACHT, LRR, and PYD domain-containing protein 3) inflammasome complex. The NLRP3 inflammasome is a highly conserved innate immune mechanism responsible for responding to pathogens by signaling cells to undergo proinflammatory cytokine production. This signaling mediates caspase-1-dependent release of interleukin-1β (IL-1β) (18, 25), which can be secreted to promote inflammation or can stimulate the proinflammatory lymphocyte programmed cell death known as pyroptosis, which has been proposed to be an important cause of CD4+ T cell depletion (26, 27).
P2X1 and P2X7 subtypes are predominantly expressed on CD4+ T cells, the primary target of HIV-1 infection (36, 37). Recent studies by our group and others demonstrate that nonselective P2 antagonists block HIV-1 infection (28, 29). Nonselective P2X antagonists can reduce neurotoxic effects in murine neuron-microglial cocultures exposed to the HIV-1 transactivator of transcription (Tat) (30). These inhibitors can block HIV-1 infection in a dose-dependent manner during cell-to-cell and cell-free HIV-1 infection (29). Selective inhibitors of P2X receptors reduced HIV-1 replication in macrophages (38). Graziano et al. corroborated the importance of P2X7 in HIV-1 infection of macrophages by showing that P2X7 inhibition blocked release of HIV-1 virions (39). Expression of P2X7 on human astrocytes is increased in the presence of HIV-1 Tat, and P2X7 inhibitors have been demonstrated to reduce HIV-1-induced neuronal and microglial damage (40–42). Recently, Menkova-Garnier et al. demonstrated that P2X7 inhibitors restore T cell differentiation in CD34+ cells derived from HIV-infected immunological nonresponders (43). Additionally, P2X1-selective inhibitors were shown to inhibit HIV-1 fusion by blocking virus interactions with the coreceptors C-C chemokine receptor 5 (CCR5) and CXC chemokine receptor 4 (CXCR4) (28, 36). As P2X receptors are also known mediators of inflammation and inflammatory signaling, it was of interest to understand whether HIV-1-stimulated inflammatory cytokine production would be abrogated by P2X inhibition.
Here, we examined the role of P2X-selective antagonists on HIV-1 productive infection and investigated whether these inhibitors block inflammatory cytokine production in response to HIV-1 stimulation. We demonstrate through an ex vivo tonsil model that P2X-selective antagonists can reduce HIV-1-stimulated IL-10 and IL-1β production, suggesting an important role for P2X inhibition in HIV-1 infection and HIV-stimulated inflammatory cytokine production.
(This article was submitted to an online preprint archive [44]).
RESULTS
P2X inhibitors NF449 and A438079 can reduce HIV-1 productive infection in PBMCs.
Prior studies have reported that antagonists of proinflammatory purinergic receptors that detect extracellular ATP, here referred to as P2X inhibitors, can inhibit productive HIV-1 infection in T cell lines (28, 29, 37). We tested the role of a P2X1 inhibitor, NF449, and a P2X7 inhibitor, A438079, in blocking HIV-1 productive infection in peripheral blood mononuclear cells (PBMCs). Activated PBMCs were infected with HIV-1 NL-CI, an X4-tropic virus with an mCherry reporter, as previously described (45, 46), in the presence of NF449 and A438079. The reverse transcriptase inhibitor azidothymidine (AZT) was tested as a positive control (Fig. 1A). NF449 significantly reduced HIV-1 infection in PBMCs down to 25% while A438079 was less effective and reduced HIV-1 infection down to 60%. AZT inhibited infection by nearly 90%. None of the drugs tested exhibited toxicity on PBMCs (Fig. 1B).
FIG 1.
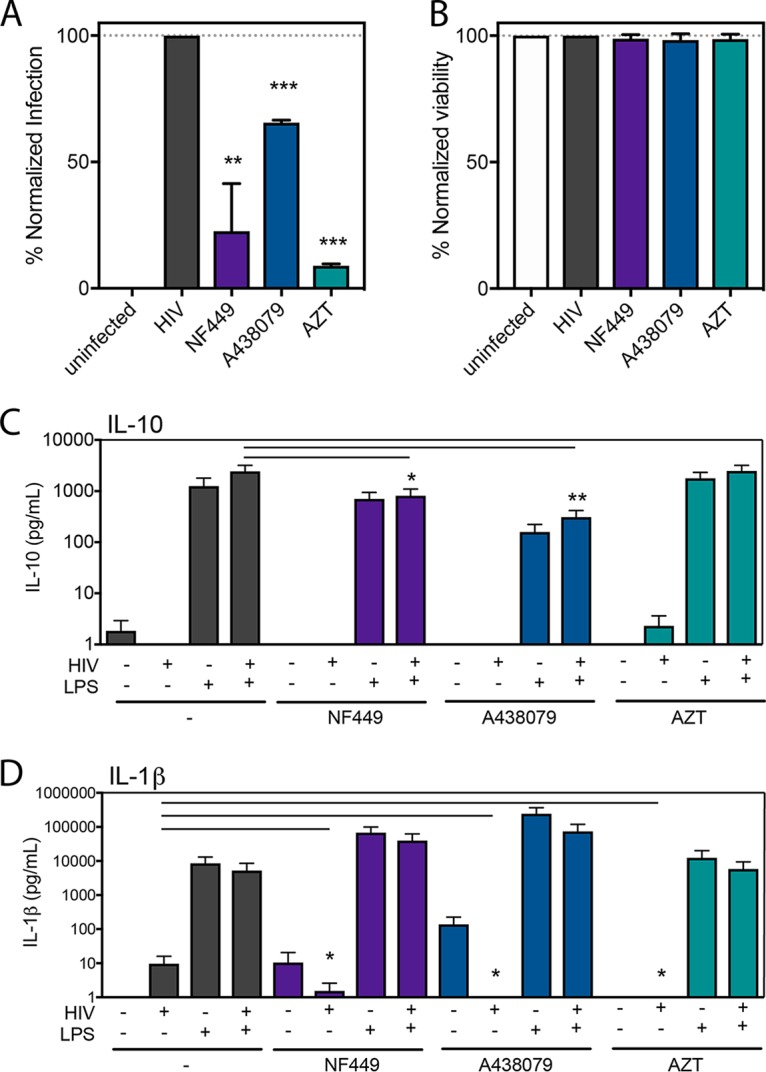
NF449 and A438079 inhibit HIV-1 productive infection in PBMCs with minimal inhibition of inflammatory cytokines. PBMCs were isolated, activated, and infected with HIV-1 NL-CI (X4 tropic) for 48 h. Cells were fixed and analyzed by flow cytometry and then normalized to the HIV-1-infected condition for productive infection (A) and viability (B). (C) PBMCs were isolated and immediately exposed to HIV-1MN (X4-tropic) for 12 h in the presence or absence of LPS (1 pg/ml). Supernatants were collected and subjected to a cytokine bead array (CBA) (BD Biosciences) for analysis of production of IL-10 and IL-1β. Mean values ± standard errors of the means are presented for IL-10 and IL-1β from three donors. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
Next, we tested the ability of these P2X-selective inhibitors to block HIV-1-stimulated inflammatory cytokine production. PBMCs were isolated and exposed to HIV-1MN and tested for stimulation of inflammatory cytokines IL-10, IL-1β, tumor necrosis factor (TNF), interleukin-12p70 (IL-12p70), interleukin-8 (IL-8), and interleukin-6 (IL-6) by a multiplex bead immunoassay (Cytometric Bead Array [CBA]; (BD Biosciences). Our initial findings indicated that minimal IL-10 and IL-1β cytokine elevation was observed with HIV-1 infection of PBMCs (Fig. 1C and D). As stated before, it is known that inflammasome activation requires two signals; the first is a TLR agonist, and the second is a P2X agonist that we propose is stimulated by HIV-1 infection (47, 48). Therefore, we tested the effect of the addition of a physiological level of LPS (1 pg/ml), a TLR4 agonist that has been reported to be circulating in the blood of HIV-infected individuals (10, 49, 50). Addition of LPS resulted in stimulation of IL-10 (Fig. 1C) and IL-1β (Fig. 1D) levels, but with only a small and not significant increase in HIV-1-dependent IL-10 production. We determined that the PBMC model was not sufficient to test the interaction between HIV-1 infection and stimulation of inflammatory cytokine production. We therefore pursued the establishment of a system that was more physiologically relevant.
An ex vivo HLAC supports HIV-1 productive infection.
Ex vivo infection of human lymphoid aggregate cultures (HLACs) with HIV-1 is a well-studied model system in which HIV-1-induced inflammasome activation has been characterized (51–56) and is an appropriate system to study inflammatory signaling that results from HIV-1 infection. Unlike blood-derived CD4-T cells, lymphoid-derived cells are not naturally resistant to pyroptosis in culture and do not require activation or addition of exogenous TLR agonists for HIV-1 infection (52). Human tonsil explants were obtained from healthy tonsillectomy patients, homogenized as previously described (57), and cultivated in HLACs. HLACs were infected with HIV-1 NL-CI and harvested at 0, 2, 5, 8, and 12 days postinfection (dpi) (Fig. 2A). Infection was quantified by flow cytometric detection of mCherry-positive viable cells as indicative of HIV-1 NL-CI infection (Fig. 2B). Peak infection was noted on day 8, with a decline by day 12. Infection was statistically significant on days 2 to 12. Viability of these cells was quantified by flow cytometric detection of live cells (Fig. 2C). Viability under the infected condition fell to below 20% by 12 dpi. At 8 dpi, there was a statistically significant difference between viability of infected cells and that of uninfected cells that corresponded to the timing of peak infection. Figure 2D shows representative flow cytometry plots of the viability of cells over the course of infection, indicating waning viability over the course of infection. Figure 2E shows representative flow cytometry plots of the infection of a subset of live cells at 0, 2, 5, 8, and 12 dpi. Overall, we observed that this HLAC system was able to support HIV-1 productive infection using a reporter virus.
FIG 2.
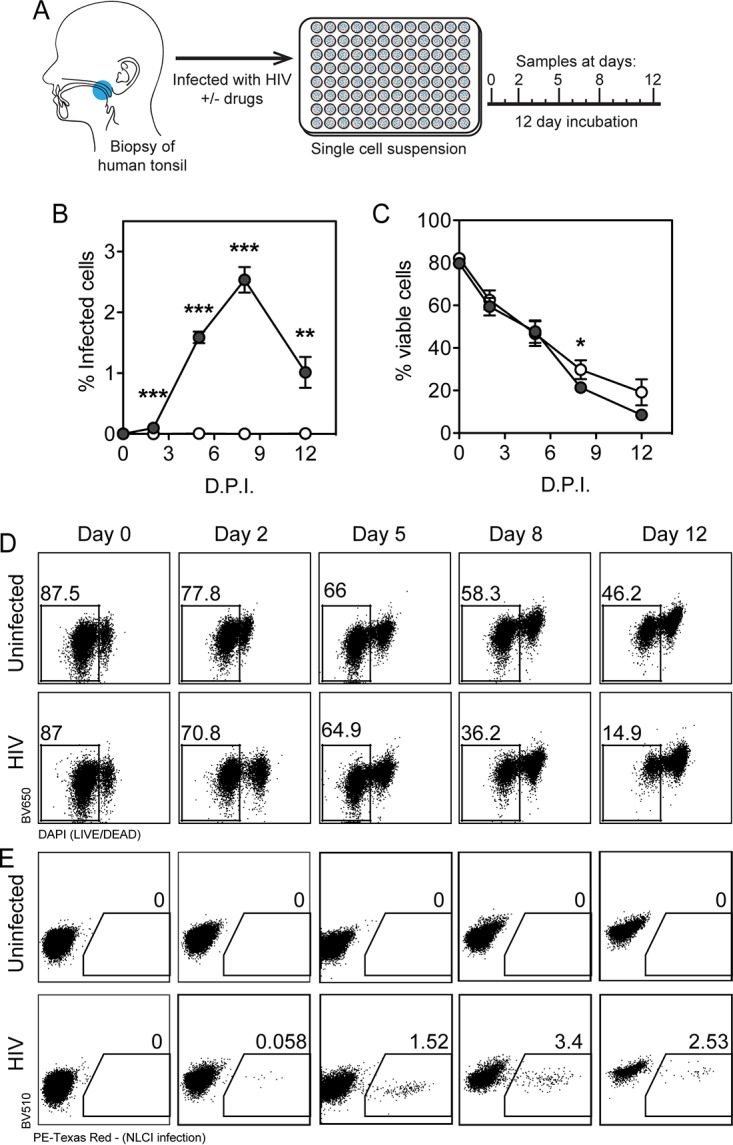
Human lymphoid aggregate culture (HLAC) of tonsil explant model supports HIV-1 infection. (A) Human tonsil explants were collected, dissected, homogenized, and passed through a cell strainer. Cells were subjected to Ficoll fractionation, and human lymphoid aggregate cells (HLACs) were plated and then infected with HIV-1 NL-CI. Cells were collected at 0, 2, 5, 8, and 12 dpi. (Printed with permission from Mount Sinai Health System.) (B) HLACs were collected on the indicated days and analyzed by flow cytometry for productive infection by NL-CI mCherry fluorescence. Infected cells were quantified by the percentage of positive PE-Texas Red events. (C) HLACs were analyzed by flow cytometry to quantify viable cells. Viable cells were quantified by the percentage of negative DAPI events. (D) Representative flow cytometry plots of uninfected and infected cells are shown for Live/Dead Fixable Dead Cell staining (Thermo Fisher) by flow cytometry, indicating viability of the 12-day infection time course. (D) Representative flow cytometry plots of uninfected and infected cells are shown for mCherry signal as indicative of HIV-1 productive infection. Mean values ± standard errors of the means are presented from three donors. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
NF449 and A438079 reduce HIV-1 productive infection in HLACs.
Using this tonsil system that can support HIV-1 infection, we tested whether two P2X antagonists, NF449 (a P2X1 to P2X7 inhibitor) and A438079 (a P2X7 inhibitor), would reduce HIV-1 productive infection in comparison to treatment with AZT. HLACs were prepared as described in the legend of Fig. 2, and cells were harvested at 0, 2, 5, 8, and 12 dpi. Viability of these cells was quantified by flow cytometric detection of live cells (Fig. 3A). As shown in Fig. 2B, viability of cells declined over the infection course by 12 dpi. Interestingly, NF449 and AZT resulted in statistically significant increased cell survival compared to the level with the infected control that was most pronounced between 5 and 12 dpi. Data in Fig. 3B represent infection as measured by quantification of cells with mCherry signal as indicative of HIV-1 NL-CI productive infection. Both NF449 and A438079 at 100 μM reduced HIV-1 productive infection at all time points from 2 to 12 dpi, comparable to inhibition seen with AZT. Surprisingly, A438079 (100 μM) effectively inhibited productive HIV-1 infection in human tonsil cells at 8 and 12 dpi although it incompletely inhibited productive infection of PBMCs (Fig. 1A). Titration of these three drugs was performed during peak infection at 8 dpi (Fig. 3C), indicating dose-dependent inhibition of HIV-1 productive infection with NF449, A438079, and AZT, with 50% inhibitory concentration (IC50) values of 12.4 μM, 36.3 μM, and 12.0 μM, respectively.
FIG 3.
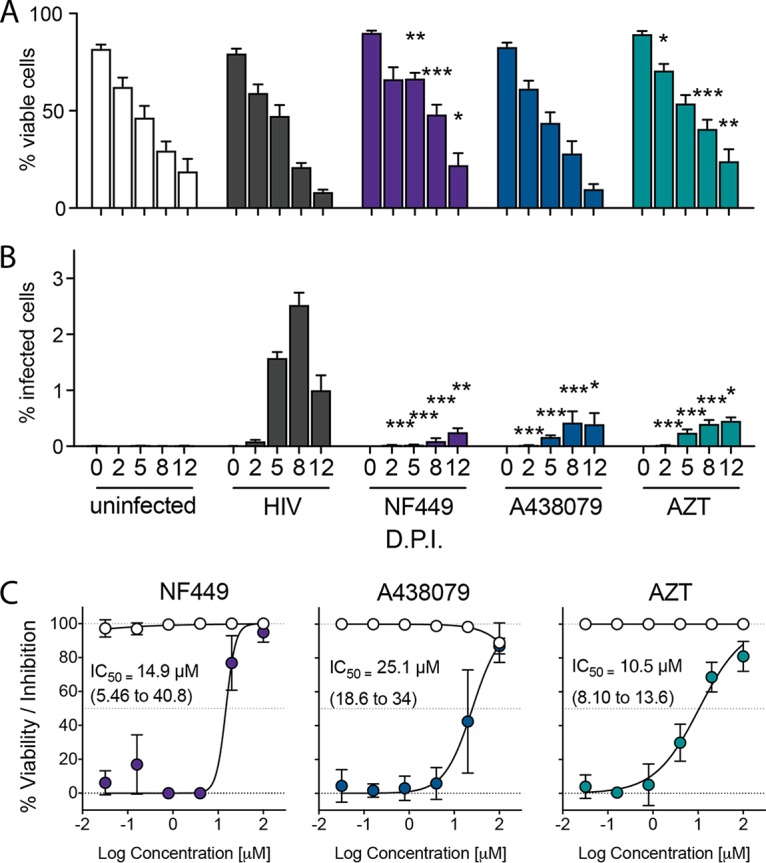
NF449, A438079, and AZT block HIV-1 replication in HLACs. HLACs were collected at 0, 2, 5, 8, and 12 dpi. (A) HLACs were analyzed by flow cytometry to quantify viable cells. (B) HLACs were analyzed by flow cytometry for productive infection by NL-CI mCherry fluorescence. (C) NF449, A438079, and AZT were tested for dose-dependent inhibition in infected HLACs at 8 dpi by a 1:5 titration down from 100 μM. Open circles indicate viability, and filled circles indicate percent inhibition of productive infection. Mean values ± standard errors of the means are presented from three donors. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
HIV-1 infection is inhibited by NF449 and A438079 in human tonsil explant tissue blocks.
We next tested the supernatants of human tonsil explant tissue blocks to determine how infection levels are associated with secreted cytokines. While HLACs allow for more cellular analysis of viability and infection via flow cytometry, the human tonsil explant tissue block model allows for preservation of the tonsil tissue cytoarchitecture. Given prior evidence that a lymphoid tissue microenvironment can support the course of HIV-1 infection and pyroptosis in both HLACs and tissue blocks (51, 54, 56, 58–60), we pursued the development of a tonsil model that could recapitulate the observations of HIV-1 infection and stimulation of inflammatory cytokine production. To do this, tonsils were dissected and cut into small blocks and suspended at the liquid-air interface on collagen rafts. Supernatants were collected on days 2, 5, 8, and 12 postinfection (Fig. 4A) and saved for analysis. Medium with drug was changed completely on each indicated day postinfection, and therefore quantification represents cumulative accumulation of viral production.
FIG 4.
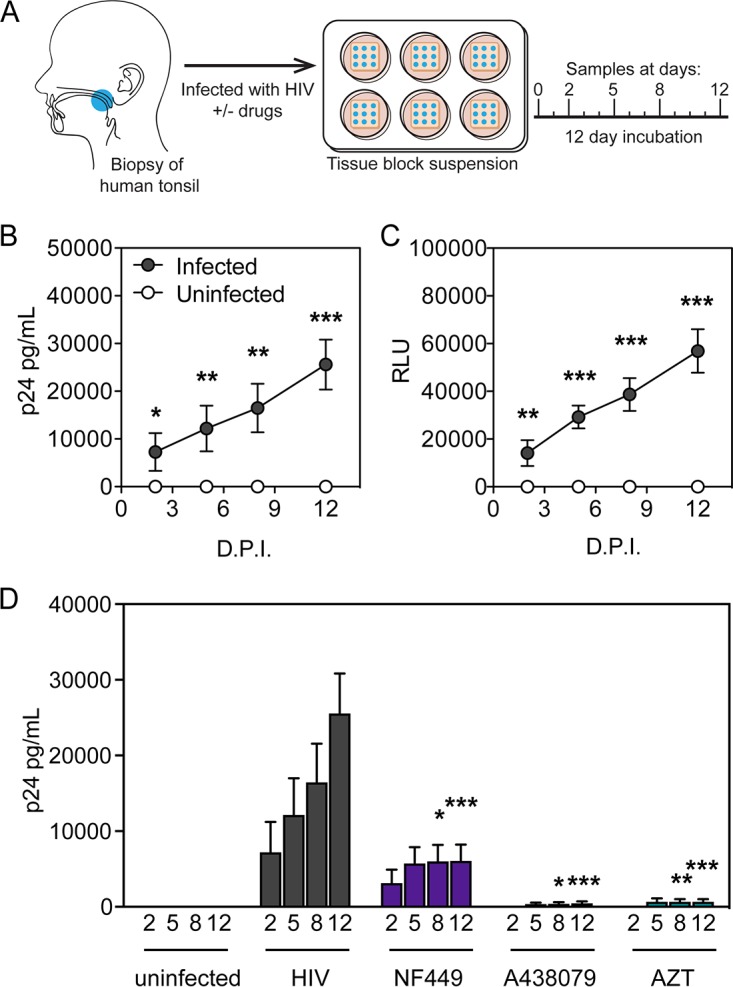
HIV-1 infection and inhibition in human tonsil explant tissue blocks. (A) Human tonsil explant tissue blocks were collected, dissected, and plated in blocks on GelFoam for 24 h prior to infection. Blocks were infected with HIV-1 NL-CI. Supernatants were collected at 2, 5, 8, and 12 dpi. (Printed with permission from Mount Sinai Health System.) (B) Supernatants collected from human tonsil explant tissue blocks at the indicated times were measured for HIV-1 p24 by ELISA. Unfilled circles indicate uninfected samples, and filled circles indicate infected samples. (C) Supernatants from human tonsil explant tissue blocks on each day were tested for HIV-1 infectivity as quantified by TZM-bl assay. (D) Human tonsil explants were infected with HIV-1 NL-CI in the presence or absence of the indicated inhibitors (100 μM). Supernatants were collected at 2, 5, 8, and 12 dpi after infection. HIV-1 p24 antigen levels were measured by ELISA in samples in which infected tonsils were incubated in the presence or absence of the inhibitors NF449, A438079, and AZT at 100 μM. Data represent cumulative HIV-1 p24 production by adding the measurements at each successive time point. Mean values ± standard errors of the means are presented from six donors. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P < 0.0001.
First, we attempted to determine if HIV-1 infection and inhibition in the human tonsil explant tissue block model were comparable to that with the HLAC model. Infection was monitored by measurement of viral antigen by HIV-1 p24 enzyme-linked immunosorbent assay (ELISA) (Fig. 4B) and by measuring infectivity in the supernatants by exposure to the HIV-1 indicator TZM-bl cell line and quantification of relative luminescence units (RLU) (Fig. 4C). HIV-1 infection resulted in significant p24 antigen accumulation from 2 to 12 dpi accompanied by infectivity of the TZM-bl cell lines.
We next tested the effect of these drugs on reducing HIV-1 p24 antigen accumulation in the ex vivo tonsil tissue model over a 12-day infection (Fig. 4D). A significant reduction of HIV-1 p24 antigen accumulation was observed with NF449 (100 μM) treatment at 8 and 12 dpi. As in the HLAC system used in the experiments shown in Fig. 3, A438079 (100 μM) inhibited HIV-1 p24 antigen accumulation at 8 and 12 dpi. In histoculture, A438079 inhibited HIV-1 productive infection to an extent similar to that of the positive control, AZT (100 μM), that fully blocked productive infection.
HIV-1 infection is associated with inflammatory cytokine production in an ex vivo lymphoid model.
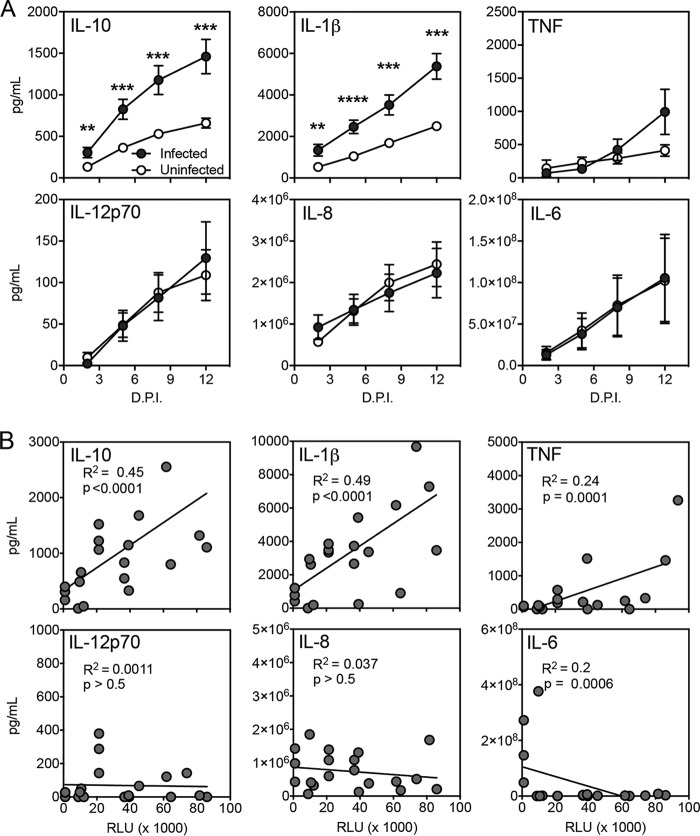
With confirmation of the establishment of productive infection by HIV-1 in this ex vivo lymphoid model, we next examined if HIV-1 infection stimulated inflammatory cytokine production. Supernatants were harvested from uninfected and infected human tonsil explant tissue blocks and were analyzed for proinflammatory cytokines production. Supernatants were harvested over the 12-day infection time course and subjected to measurement by CBA (BD Biosciences) (Fig. 5A). Cumulative measurements indicated that HIV-1 exposure stimulated a significant increase in IL-10 and IL-1β from 2 to 12 dpi. HIV-1 exposure stimulated a modest but not significant increase of TNF at 12 dpi and did not stimulate an increase in IL-12p70, IL-8, or IL-6.
FIG 5.
HIV-1 stimulates production of IL-10 and IL-1β in a human tonsil explant model. Human tonsil explant tissue blocks were infected with HIV-1 NL-CI. Supernatants were collected at 2, 5, 8, and 12 dpi. (A) Cytokines IL-10, IL-1β, TNF, IL-12p70, IL-8, and IL-6 were measured in the harvested supernatants, quantified by CBA (BD Biosciences), and analyzed by flow cytometry. Data represent cumulative cytokine production by adding the measurements at each successive time point. Mean values ± standard errors of the means are presented from six donors. (B) Quantification of TZM-bl infectivity by RLU and cytokine levels were compared by regression analysis for IL-10, IL-1β, TNF, IL-12p70, IL-8, and IL-6 for values at days 2, 5, 8, and 12 dpi. A positive correlation was identified between RLU and IL-10 and IL-1β values. Mean values ± standard errors of the means are presented from five donors. **, P ≤ 0.01; ***, P ≤ 0.001; ****, P < 0.0001.
We further sought to test whether the stimulation of these cytokines was associated with the magnitude of infection. Figure 5B demonstrates cytokine production plotted as a function of TZM-bl infectivity. Production of both IL-10 and IL-1β was positively correlated with HIV-1 infection, with R2 values of 0.45 and 0.49, respectively. In contrast, HIV-1 infectivity was not associated with significant increases in TNF, IL-12p70, IL-8, and IL-6. These observations suggest that this model can support the stimulation of inflammatory cytokine production that is proportional to the level of HIV-1 infection.
NF449 and A438079 reduce HIV-1-stimulated IL-10 and IL-1β production in human tonsil cells.
We next examined the role of purinergic signaling pathways in the induction of cytokines by HIV-1 infection. Based on our prior observations that P2X antagonists reduced HIV-1 infection and fusion (29, 61), it was of interest to test selective P2X antagonists to identify whether these drugs would interfere with HIV-1 stimulation of cytokines in the tonsil system. We tested whether NF449 and A438079 would reduce HIV-1-stimulated inflammatory cytokine production. Human tonsil explant tissue blocks were infected with HIV-1 NL-CI as described in the legend of Fig. 4. Supernatants from infected human tonsil explant tissue blocks were harvested at 2, 5, 8, and 12 dpi and analyzed for inflammatory cytokines by CBA (BD Biosciences). Cumulative IL-10 and IL-1β production over the 12-day infection course was measured in the presence or absence of indicated antagonists. HIV-1 infection stimulated IL-10 production, which was significantly reduced by NF449 and A438079 at 5, 8, and 12 dpi (Fig. 6A). Additionally, HIV-1 infection stimulated IL-1β production, which was significantly reduced by A438079 at 8 and 12 dpi (Fig. 6B). NF449 did not significantly reduce IL-1β production until 12 dpi. AZT was not expected to inhibit production of either cytokine but did inhibit IL-10 production to a lesser extent than NF449 or A438079. AZT did not reduce IL-1β levels. These observations support the notion that P2X-selective antagonists act on HIV-associated inflammation differently than conventional ART. Overall, we conclude that NF449 and A438079 inhibit HIV-1 replication and IL-10 and IL-1β release in human tonsil explants. This suggests that P2X-selective antagonists are active in reducing both HIV-1 infection and associated inflammation.
FIG 6.
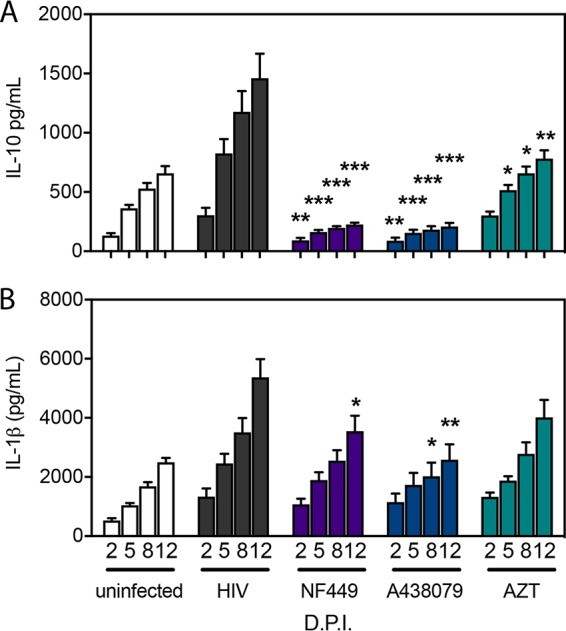
NF449 and A438079 reduce HIV-1-induced elevated cytokines IL-10 and IL-1β in ex vivo human tonsils. Human tonsil explants were infected with HIV-1 NL-CI in the presence or absence of the indicated inhibitors (100 μM). IL-10 (A) and IL-1β (B) were measured from supernatants harvested at 2, 5, 8, and 12 dpi in the presence or absence of the inhibitors NF449, A438079, and AZT. Cytokines were measured in the supernatants of harvested samples, quantified by CBA (BD Biosciences), and analyzed by flow cytometry. Data represent cumulative cytokine production by summing the measurements at each successive time point. Mean values ± standard errors of the means are presented from six donors. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
DISCUSSION
Here, we demonstrate a human ex vivo tonsil model that can support HIV-1 infection over a 12-day incubation. This model represents an important experimental system to test the signaling that mediates HIV-1 infection and HIV-1-stimulated inflammation and illustrates an important distinction between HIV-1 inflammation in peripheral blood and that in lymphoid tissues. As soluble cytokine production is challenging to measure in PBMCs, it has been necessary for investigators to probe lymphoid tissues for evidence of HIV-1-stimulated immune activation and pyroptosis, which cannot be demonstrated in peripheral blood (51–56). We demonstrate HIV-1 productive infection of human tonsils in HLACs and in human tonsil explant tissue blocks. The advantage to the HLAC system is that cell viability can be measured alongside productive infection using a fluorescent reporter virus. The human tonsil explant tissue blocks allow for measurement of soluble cytokine production and the measurement of p24 antigen as a measure of HIV-1 spreading infection. These two models together were used to demonstrate HIV-1 replication and HIV-1-stimulated IL-10 and IL-1β production.
In the HLAC model, we demonstrated that HIV-1 productive infection occurred with a peak at 8 dpi and a corresponding decline in cell viability. All three drugs tested, NF449, A438079, and AZT, reduced HIV-1 productive infection, while NF449 and AZT resulted in statistically significant increased cell survival, most notably at 8 to 12 dpi, which likely relates to inhibition of HIV-1 productive infection. Dose-dependent inhibition of HIV-1 productive infection was noted for all three drugs, with IC50 values all in the 10 to 100 μM range. Interestingly, A438079 did not enhance cell survival, as did NF449 and AZT, even though all three reduced HIV-1 productive infection. This suggests that the mechanism of A438079 inhibition is different from that of NF449 and may relate more to the inhibition of infection through cytokine production than through direct action on HIV-1 viral entry.
In the human tonsil explant tissue blocks, HIV-1 p24 antigen and TZM-bl infectivity steadily increased over the 12-day infection. NF449, A438079, and AZT inhibited HIV-1 p24 and HIV-1 productive infection to statistically a significant extent by 8 and 12 dpi. At these same time points, IL-10 and IL-1β cytokine stimulation steadily increased, and these levels positively correlated with TZM-bl infectivity, suggesting a correlative relationship between the extent of HIV-1 exposure or infection and IL-10 and IL-1β cytokine stimulation.
The P2X-selective antagonists tested, NF449 and A438079, reduced HIV-1-stimulated levels of IL-10 with statistical significance between 5 and 12 dpi. Treatment with AZT at the corresponding time points resulted in less inhibition of IL-10 than under the infected condition. These observations highlight the unique properties of NF449 and A438079 as novel agents that reduce inflammatory changes independent of their antiviral properties.
Additionally, the drugs were tested for effect on HIV-stimulated IL-1β production. NF449 reduced HIV-1-stimulated levels modestly at 12 dpi whereas A438079 reduced HIV-1-stimulated levels of IL-1β at 8 to 12 dpi. AZT did not reduce IL-1β levels. Of note, A438079 did not achieve full inhibition of HIV-1 productive infection in PBMCs but unexpectedly demonstrated strong inhibition of HIV-1 p24 accumulation in human tonsil explants and reduced IL-10 and IL-1β secretion. This surprising observation suggests that the nature of A438079 inhibition of productive infection in tonsils may not be ascribed to a direct link between A438079 and HIV-1 entry but, rather, to cytokine-dependent signaling. There are several possible explanations for this phenomenon. The immunomodulatory role of IL-10 may serve to enhance HIV-1 permissivity (62–69), but when levels of IL-10 are reduced by A438079, cells may be more susceptible to A438079 inhibition of HIV-1 infection. Tonsil tissue represents mixed cellular populations with stromal compartments having altered sensitivity to P2X inhibition compared to that of PBMCs (70–72). It will be of interest to explore the cell type heterogeneity of HIV-1 infection in tonsils to determine the cell type and signaling mechanisms that drive IL-10 and IL-1β production. These directions may lead to the development of novel therapeutic agents that retain inhibition of HIV-1 infection spread and can reduce HIV-1-stimulated levels of IL-10 and IL-1β.
The role of P2X receptors in HIV-1 pathogenesis likely relates to downstream activation of the NLRP3 inflammasome. The NLRP3 activates immature caspase-1 to activate caspase-1, which cleaves and activates pro-IL-1β. IL-1β plays a pivotal role in signaling of other inflammatory cytokines. Emerging evidence suggests a key role for the inflammasome in atherosclerotic disease progression (73–83) and in HIV-1 disease (47, 84–89). Inflammasome activation requires two signals, one for priming (i.e., TLR signaling which results in transcriptional regulation) and then one for activation of inflammasome complex assembly. Together with TLR signaling, P2X7 can signal inflammasome activation and subsequent IL-1β release (90). As the tonsil tissue contains soluble factors that likely include TLR agonists, inflammasome activation is readily measurable with second signal stimulation, i.e., P2X7 activation by HIV-1 infection. HIV-1-infected patients have elevated circulating levels of LPS, which can serve to increase transcriptional activation of proinflammatory cytokines (11, 50). Elevated levels of IL-1β are associated with many of the AANCCs seen in HIV-1-infected patients (91–94). Currently, there are multiple clinical trials assessing the safety and efficacy of anti-IL-1β antibodies in cardiovascular disease (95, 96). Canakinumab, a human monoclonal IL-1β antibody, has been shown to significantly decrease arterial inflammation in HIV-1-infected individuals (97). The role of P2X receptors in the secretion of IL-1β may represent a key mechanism for HIV-1-associated inflammation. Elevated IL-1β is observed in HIV-1-infected patients (88, 98–100), and an emerging body of literature implicates the role of IL-1β in atherosclerotic cardiovascular disease in both HIV-1-infected and uninfected patients (95–97). Intriguing studies in CD4+ T cells find that pathogen sensor interferon-gamma-inducible protein 16 (IFI-16) recognition of HIV-1 DNA can activate the NLRP3 inflammasome that induces pyroptosis and may represent a mechanism for CD4+ T cell depletion in HIV-1 disease and progression to advanced disease (54–56).
The fact that both IL-10 and IL-1β were together stimulated by HIV-1 infection and reduced by NF449 and A438079 is surprising, given that they have opposing inflammatory effects. IL-10 is an immunomodulatory cytokine with immunosuppressive activities and has been implicated in immune exhaustion and cell death through inhibition of NF-κB activity (62–66). IL-10 activation has been linked to P2X7 signaling, with the observation of down-modulation of IL-10 receptor expression with P2X7 activation (101). IL-10 has the potential to impact many areas of HIV-1 infection, including CD4 function, chemokine receptor expression, and modulation of replication (67–69). IL-10 gene polymorphisms and epigenetics have been shown to be associated with variations in HIV-1 transmission and disease progression as long-term nonprogressors (LTNP) have low IL-10 levels compared with those of HIV-1 progressors (102–107). The known impact of IL-10 to reduce HIV-1 infection suggests that IL-10-stimulation by HIV-1 may be important to modulate the immune response to HIV-1 infection and may therefore account for the relatively low levels of induction of other proinflammatory cytokines such as IL-6 and TNF. Further studies are needed to understand the role of IL-10 in HIV-1 disease progression and inflammation, and P2X-selective antagonists may play a role in developing novel therapeutics that target both HIV-1 infection and inhibition of IL-10 signaling.
Figure 7A summarizes the observations of HIV-1 infection and inflammatory cytokine production in both PBMCs and tonsils. While all drugs inhibit HIV-1 infection, A438079 had the least effect on inhibition of HIV-1 infection. It should be noted that IL-10 and IL-1β stimulation was not observed in PBMCs. Since it was not possible to demonstrate HIV-1-specific stimulation of inflammatory cytokines in this model, it was necessary to establish a lymphoid model that would more accurately recapitulate inflammatory cytokine signaling. In the tonsil model, all three drugs inhibited HIV-1 infection to comparable magnitudes in HLACs, while A438079 and AZT inhibited p24 accumulation more than NF449. By comparison, NF449 and A438079 inhibited IL-10 production strongly while AZT only modestly reduced IL-10 production. Finally, NF449 and A438079 inhibited IL-1β production modestly while AZT did not inhibit IL-1β production.
FIG 7.
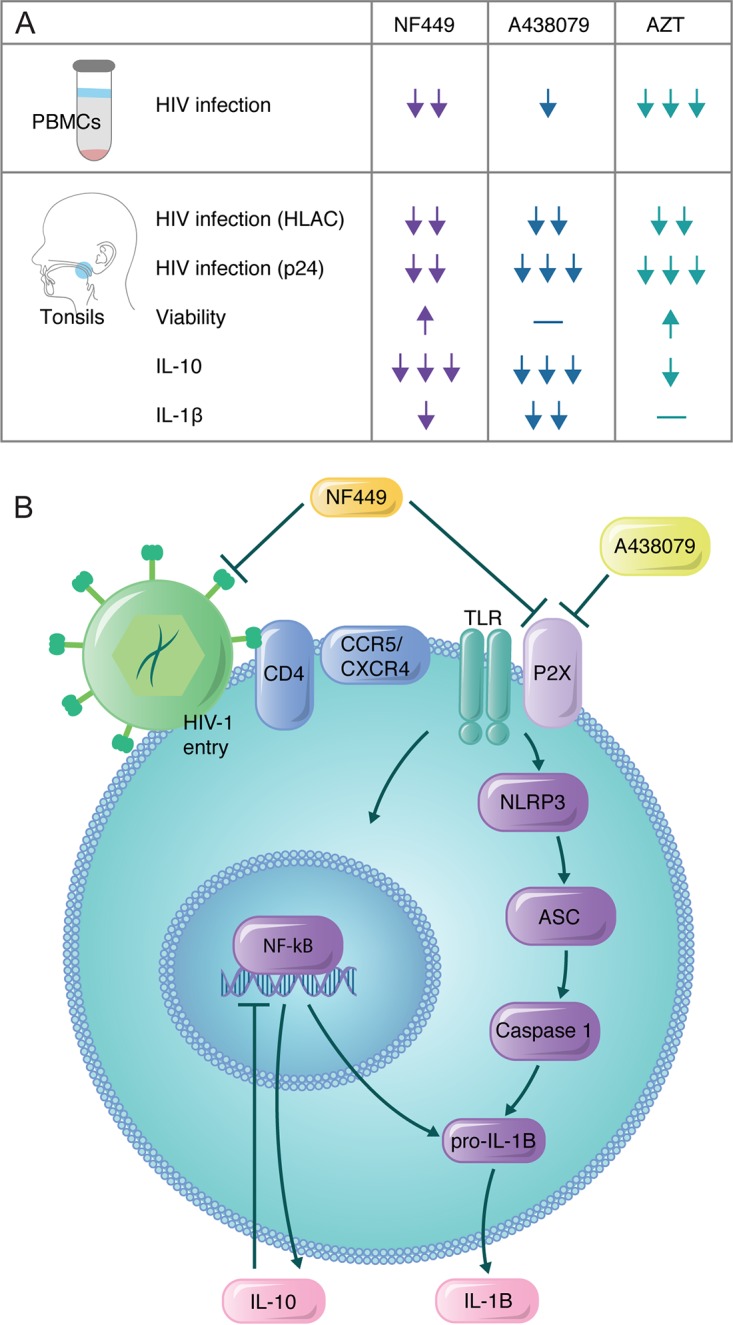
Model for signaling in HIV-1-mediated inflammation. (A) Table summarizing observations from drug activity across PBMC and tonsil models. Arrows indicate relative magnitudes of the effects of inhibition in each of the models depicted. (B) NF449 inhibits HIV-1 productive infection and downstream signaling of the NLRP3 inflammasome. This pathway is activated in concert with TLR4 receptor activation, which can drive caspase-1 to cleave pro-IL-1β to mature IL-1β. Mature IL-1β can be secreted or induce pyroptosis in CD4+ T cells. This signaling can also activate NF-κB-dependent transcriptional regulation of IL-10. Inhibition of this mechanism by NF449 may explain enhanced cell survival in tonsil cells. A438079, in contrast, may act directly on P2X7 to inhibit receptor signaling that is required for HIV-1 entry. P2X7 inhibition results in the inability to activate the NLRP3 inflammasome and pyroptosis in tonsil cells, which does not occur in PBMCs. Therefore, A438079 depends on intact inflammasome signaling to exert inhibition on HIV-1 productive infection. (Printed with permission from Mount Sinai Health System.)
Taking these findings together, we propose the model shown in Fig. 7B, in which P2X-selective antagonists play a role in inhibiting HIV-1-stimulated inflammation. The model indicates that NF449 and A438079 may have different mechanisms of action. Stimulation of inflammatory signaling by P2X7 and TLR4 results in NLRP3-dependent production of IL-1β as well as NF-κB-dependent regulation of IL-10. NF449 inhibits this inflammation and HIV-1 productive infection in both PBMCs and tonsil cells, suggesting that the inhibition is not dependent on intact inflammasome signaling. In contrast, A438079 inhibits HIV-1 productive infection that is limited to the tonsil system, indicating that this inhibition is dependent on intact NLRP3 signaling mechanisms that are not activated in PBMCs. The role of cytokine signaling in permissivity to HIV-1 infection in mixed cell populations is an important area of future investigation as the tonsil tissue model system is important in understanding the interplay between HIV-1 pathogenesis and the immune response.
Here, we demonstrate that P2X-selective antagonists have the potential to reduce HIV-1 infection and HIV-1-stimulated inflammatory cytokine production. We conclude from these studies that P2X-selective antagonists may represent potential HIV-1 therapeutic options that serve to inhibit HIV-1 replication and innate immune sensing. Further studies will be necessary to identify selective inhibitors that are amenable to pharmacologic development and the precise mechanism of their inhibition, but these observations introduce important prospects for dually active therapeutic options that would reduce the burden of morbidity and mortality of chronic inflammation in HIV-1-infected individuals.
MATERIALS AND METHODS
Virus production.
HIV-1 NL-CI contains mCherry in place of nef, and nef expression is directed by a downstream internal ribosome entry site (IRES) (45). Pseudoviruses were produced by cotransfecting 293T/17 cells with HIV-1 rev- and env-expressing plasmids and the pNL4-3Δenv R-E- plasmid using jetPEI transfection reagent (Polyplus-transfection SA). Supernatants were harvested after 48 h and clarified by high-speed centrifugation (Sorvall ST 40R centrifuge; Thermo Fisher Scientific) at 100,000 × g at 4°C for 2 h and by 0.45-μm-pore-size filtration. Single-use aliquots were stored at −80°C. Viral stocks were quantified via enzyme-linked immunosorbent assay (ELISA), as described below. HIV-1MN (X4-tropic) was produced at the AIDS Vaccine Program, National Cancer Institute, as previously described (108–110).
Cells and cell lines.
PBMCs were obtained from deidentified HIV-1-negative blood donors (New York Blood Center), purified by Ficoll (HyClone) density gradient centrifugation, and maintained in RPMI 1640 medium (Sigma) containing 10% fetal bovine serum (FBS) (Sigma), 100 U/ml penicillin (Gibco), 10 U/ml streptomycin (Gibco), and 2 mM glutamine (Gibco) (complete RPMI medium). The 293T/17 cell line was used to produce pseudoviruses and was purchased from the American Type Culture Collection. The TZM-bl cell line was obtained from John C. Kappes, Xiaoyun Wu, and Tranzyme, Inc., through the NIH AIDS Reagent Program (ARP). The 293T/17 and TZM-bl cells were maintained in Dulbecco’s modified Eagle Medium (DMEM) (Sigma) containing 10% cosmic calf serum (CCS) (HyClone) and 100 U/ml penicillin, and 10 U/ml streptomycin, and 2 mM glutamine (Gibco) (complete DMEM).
Establishment of human explant tonsil model and processing of HLACs.
Human tonsils were collected from routine tonsillectomy performed at the Mount Sinai Health System in New York by B. Tweel under an Institutional Review Board-approved protocol. Tonsils were collected within several hours of surgery and dissected into 2-mm tissue blocks. Human tonsil explant tissue blocks were plated at 9 per well atop a collagen sponge (GelFoam; Pfizer) in a six-well plate in ∼3 ml of medium (Costar), as previously described (57). Medium was completely changed every 2 to 3 days with or without the indicated inhibitor (see the figures and figure legends) and saved in aliquots at −80°C for further experiments. For HLAC experiments, dissected tissue was passed through a 40-μm-pore-size cell strainer and purified by Ficoll density gradient centrifugation, as previously described (52). Cells were plated at 2.5 × 105 cells/well in a round-bottom 96-well plate (Costar) and spun down at 500 × g for 3 min every 2 to 3 days to replace medium, with or without the indicated inhibitor. Human tonsil explant tissue blocks and HLACs were maintained in RPMI 1640 medium (Life Technologies) containing 15% FBS, 2 mM GlutaMAX (Life Technologies), 2 mM l-glutamine (Corning), 1 mM sodium pyruvate (Corning), 1% minimal essential medium (MEM) nonessential amino acids (Corning), 2.5 μg/ml amphotericin B (HyClone), 50 mg/ml gentamicin sulfate (Corning), and 0.3 mg/ml Timentin (bioWORLD).
Antagonists.
Inhibitors were tested for the ability to block HIV-1 infection and HIV-1-associated inflammation at 100 μM, unless otherwise stated. These included NF449 (Tocris), a P2X1-selective antagonist, A438079 (Tocris), a P2X7-selective antagonist, and the reverse transcriptase inhibitor azidothymidine (AZT) (Sigma). NF449 and A438079 were diluted from 10 mM stocks reconstituted in dimethyl sulfoxide (DMSO) while AZT was diluted from 10 mM stocks reconstituted in water.
Flow cytometry and gating strategy.
An LSR II flow cytometer (BD Biosciences) was used to detect infection and viability in PBMCs and HLACs. Viable cells were detected with Live/Dead Fixable Dead Cell Stain (Life Technologies), an amine-reactive fluorescent dye that can penetrate the membranes of dead cells but not live cell membranes. Samples were stained with Live/Dead Fixable Blue Dead Cell Stain or Live/Dead Fixable Violet Dead Cell Stain at a concentration of 1:1,000 in wash buffer (phosphate-buffered saline [PBS] supplemented with 2 mM EDTA and 0.5% bovine serum albumin). Stained cells were incubated at 4°C for 30 min and then washed and fixed in 2% paraformaldehyde for flow cytometry. All cells were initially discriminated by side scatter (SSC) area versus forward scatter (FSC) area (SSC-A/FSC-A); doublets were excluded using FSC height (FSC-H) versus FSC-A. Viability was determined by gating on negative populations for Live/Dead Fixable Dead Cell Stain. Infection was detected by the presence of mCherry in cells infected with HIV-1 NL-CI. mCherry was detected using the phycoerythrin-Texas Red (PE-Texas Red) channel, Live/Dead Fixable Violet Dead Cell Stain was detected with the 3-carboxy-6,8-difluoro-7-hydroxycoumarin (Pacific Blue) channel, and Live/Dead Fixable Blue Dead Cell Stain was detected with the 4′,6-diamidino-2-phenylindole (DAPI) channel. All cells within a single experiment were analyzed using the same instrument settings. Flow cytometry data were exported and analyzed using FlowJo software, version 9.3.2 (Tree Star, Ashland, OR).
Productive infection in PBMCs.
PBMCs were activated with phytohemagglutinin (PHA) (4 μg/ml) and IL-2 (50 U/ml) for 3 days and infected by spinoculation, as previously described (111, 112). Briefly, 2.5 × 105 cells were incubated in the presence or absence of indicated inhibitors in a 96-well flat bottom plate for 30 min at 37°C and then spun at 1,200 × g for 99 min with 47.7 ng of HIV-1 NL-CI. After overnight incubation at 37°C, the culture medium was replaced with complete RPMI medium containing IL-2 (50 U/ml) and 10 μM AZT. At 48 h after spinoculation, cells were stained and fixed in 2% paraformaldehyde for flow cytometry, as described above.
Ex vivo infection of human tonsil explant tissue blocks and HLACs.
Human tonsil explant tissue blocks from each donor were individually inoculated with 5 µl of HIV-1 NL-CI (equivalent to 3.24 ng of p24) or left uninfected in the presence or absence of the indicated inhibitors. We measured HIV-1 p24 antigen in harvested supernatants and infectivity in RLU by TZM-bl assay as described below, as the NL-CI fluorophore can be detected only in cell-based systems. Data are expressed as a cumulative value to account for total successive medium changes. For HLAC experiments, cells were incubated in the presence or absence of the indicated inhibitors for 30 min at 37°C before infection with 25 ng of HIV-1 NL-CI p24 per well. Cells were stained and fixed for flow cytometry, as described above.
p24 ELISA.
Viral stocks and tonsil tissue supernatants were quantified via ELISA with coating antibody D7320 (sheep anti-HIV-1-p24 gag; Aalto Bio Reagents), as previously described (61, 113). Briefly, anti-p24 capture antibody was coated on high-binding plates (Costar) at 1:200 in 0.1 M NaHCO3. After overnight incubation at room temperature, plates were blocked with 2% nonfat dry milk for 1 h. HIV-1 samples were treated with 1% Empigen and added to wells, along with titration of p24 standard, at room temperature for 3 h. Alkaline phosphatase-conjugated mouse anti-HIV-1 p24 (Cliniqa) was added (1:8,000 in Tris-buffered saline with Tween 20 [TBST] and 20% sheep serum) and incubated for 1 h. Plates were developed with Sapphire substrate (Tropix) and measured on a FluoStar Optima plate reader.
TZM-bl HIV-1 infectivity assay.
Virus infectivity of supernatants collected from tonsil was measured using a β-galactosidase-based luciferase assay (Promega) with TZM-bl target cells, as previously described (114). Briefly, TZM-bl cells were plated at 1.5 × 104 cells/well in a flat-bottom 96-well plate (Costar). Harvested supernatants (containing 0.1 ng of HIV-1 p24) were added to each well and then incubated at 37°C. Medium was exchanged 24 h after incubation, and a luciferin-galactoside substrate (6-O-β-galactopyranosyl-luciferin) was added after 48 h. The cleavage of the substrate by β-galactosidase generates luminescent signals measured in RLU. Each test and control condition were tested in duplicate or triplicate. Assay controls included replicate wells of TZM-bl cells alone (cell control). The virus inputs were the diluted virus stocks yielding equivalent numbers of RLU (typically ∼100,000 RLU) under the different assay conditions. The number of RLU present in uninfected samples was subtracted as background for all samples for each time point.
Cytokine measurements.
PBMCs were isolated from patient samples, and 2.5 × 105 cells per well in a 96-well plate were incubated in the presence or absence of dilutions of inhibitor for 30 min. HIV-1MN (X4-tropic) was added at 300 ng/ml in the presence or absence of 1 pg/ml LPS (Sigma) and incubated for 12 h. Supernatants were collected from PBMCs or samples of tonsil tissue and were analyzed for IL-10, IL-1β, TNF, IL-12p70, IL-8, and IL-6 using a BDT CBA Human Inflammatory Cytokines kit (BD Biosciences). Standard curves were generated, and cytokine concentrations were extrapolated using FCAP (Flow Cytometric Analysis Program) Array software (BD Biosciences). The measurements indicated are representative of three separate PBMC donors and six separate tonsil donors.
Statistical analysis and calculations.
Comparisons were performed using GraphPad Prism, version 7.0d (GraphPad Software). DMSO-treated controls were set to 100%, and drug-treated conditions were expressed as a percentage of the control value. Statistical analyses were performed on inhibition data that reached ≥50% with a one-tailed Student’s t test. A P value of less than 0.05 was considered statistically significant.
Study approval.
Icahn School of Medicine at Mount Sinai (ISMMS) IRB protocol number 06-0980 was approved by the Program for the Protection of Human Subjects Institutional Review Board of the Mount Sinai Health System (New York, NY, USA). All patients participating in tonsil analysis gave written informed consent.
ACKNOWLEDGMENTS
We thank the members of the Chen laboratory for meaningful discussions. We thank Maxwell Allison and Elizabeth Osota for their help in preparing samples for tonsil tissue.
T.H.S. was funded by NIH grant K08AI20806 and by the Schneider-Lesser Foundation. This work was supported by grants to B.K.C. from NIH (NIAID R01AI074420 and NIDA Avant-Garde award DP1DA028866).
A.Y.S. and N.D.D. designed and performed experiments. A.Y.S. and N.D.D. carried out productive infection assays; R.G., M.O., and N.B. provided reagents and guidance on cytokine measurements. B.T. contributed the tonsils. K.W.H., J.A.B., and J.K.L. assisted A.Y.S. in protocol development and tonsil processing. A.Y.S., N.D.D., R.G., K.W.H., and T.H.S. assisted in data analysis, and B.T., M.O., N.B., J.K.L., B.K.C., and T.H.S. participated in data interpretation. A.Y.S. and T.H.S. wrote the paper. T.H.S. and B.K.C. conceived the approach.
We declare that no conflicts of interest exist.
REFERENCES
- 1.Yoshimura K. 2017. Current status of HIV/AIDS in the ART era. J Infect Chemother 23:12–16. doi: 10.1016/j.jiac.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Raffetti E, Donato F, Casari S, Castelnuovo F, Sighinolfi L, Bandera A, Maggiolo F, Ladisa N, di Pietro M, Fornabaio C, Digiambenedetto S, Quiros-Roldan E. 2017. Systemic inflammation-based scores and mortality for all causes in HIV-infected patients: a MASTER cohort study. BMC Infect Dis 17:193. doi: 10.1186/s12879-017-2280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, Bacchetti P, Shlipak M, Grunfeld C. 2010. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr 55:316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eastburn A, Scherzer R, Zolopa AR, Benson C, Tracy R, Do T, Bacchetti P, Shlipak M, Grunfeld C, Tien PC. 2011. Association of low level viremia with inflammation and mortality in HIV-infected adults. PLoS One 6:e26320. doi: 10.1371/journal.pone.0026320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, Fiellin DA, Vanasse GJ, Butt AA, Rodriguez-Barradas MC, Gibert C, Oursler KA, Deeks SG, Bryant K, Team VP. 2012. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis 54:984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, Prins M, Reiss P, AGEhIV Cohort Study Group . 2014. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 59:1787–1797. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- 7.Deeks SG. 2011. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aberg JA. 2006. Management of dyslipidemia and other cardiovascular risk factors in HIV-infected patients: case-based review. Top HIV Med 14:134–139. [PubMed] [Google Scholar]
- 9.Aberg JA. 2006. The changing face of HIV care: common things really are common. Ann Intern Med 145:463–465. doi: 10.7326/0003-4819-145-6-200609190-00011. [DOI] [PubMed] [Google Scholar]
- 10.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 11.Vassallo M, Mercie P, Cottalorda J, Ticchioni M, Dellamonica P. 2012. The role of lipopolysaccharide as a marker of immune activation in HIV-1 infected patients: a systematic literature review. Virol J 9:174. doi: 10.1186/1743-422X-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt PW, Landay AL, Sinclair E, Martinson JA, Hatano H, Emu B, Norris PJ, Busch MP, Martin JN, Brooks C, McCune JM, Deeks SG. 2011. A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS One 6:e15924. doi: 10.1371/journal.pone.0015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt PW, Hatano H, Sinclair E, Lee TH, Busch MP, Martin JN, McCune JM, Deeks SG. 2011. HIV-specific CD4+ T cells may contribute to viral persistence in HIV controllers. Clin Infect Dis 52:681–687. doi: 10.1093/cid/ciq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillai S, Bikle DD. 1992. Adenosine triphosphate stimulates phosphoinositide metabolism, mobilizes intracellular calcium, and inhibits terminal differentiation of human epidermal keratinocytes. J Clin Invest 90:42–51. doi: 10.1172/JCI115854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, Couillin I. 2010. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 16.Trautmann A. 2009. Extracellular ATP in the immune system: more than just a “danger signal". Sci Signal 2:pe6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 17.Burnstock G, Knight GE. 2004. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 18.Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. 1997. Tissue distribution of the P2X7 receptor. Neuropharmacology 36:1277–1283. doi: 10.1016/S0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 19.Di Virgilio F. 2007. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci 28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. 2006. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 21.Eltzschig HK, Sitkovsky MV, Robson SC. 2012. Purinergic signaling during inflammation. N Engl J Med 367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keystone EC, Wang MM, Layton M, Hollis S, McInnes IB, Team DCS. 2012. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann Rheum Dis 71:1630–1635. doi: 10.1136/annrheumdis-2011-143578. [DOI] [PubMed] [Google Scholar]
- 23.Stock TC, Bloom BJ, Wei N, Ishaq S, Park W, Wang X, Gupta P, Mebus CA. 2012. Efficacy and safety of CE-224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J Rheumatol 39:720–727. doi: 10.3899/jrheum.110874. [DOI] [PubMed] [Google Scholar]
- 24.Vergani A, Tezza S, D'Addio F, Fotino C, Liu K, Niewczas M, Bassi R, Molano RD, Kleffel S, Petrelli A, Soleti A, Ammirati E, Frigerio M, Visner G, Grassi F, Ferrero ME, Corradi D, Abdi R, Ricordi C, Sayegh MH, Pileggi A, Fiorina P. 2013. Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation 127:463–475. doi: 10.1161/CIRCULATIONAHA.112.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubyak GR. 2012. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol 14:1697–1706. doi: 10.1111/cmi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo H, Callaway JB, Ting JP. 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Place DE, Kanneganti TD. 2018. Recent advances in inflammasome biology. Curr Opin Immunol 50:32–38. doi: 10.1016/j.coi.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giroud C, Marin M, Hammonds J, Spearman P, Melikyan GB. 2015. P2X1 receptor antagonists inhibit HIV-1 fusion by blocking virus-coreceptor interactions. J Virol 89:9368–9382. doi: 10.1128/JVI.01178-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swartz TH, Esposito AM, Durham ND, Hartmann BM, Chen BK. 2014. P2X-selective purinergic antagonists are strong inhibitors of HIV-1 fusion during both cell-to-cell and cell-free infection. J Virol 88:11504–11515. doi: 10.1128/JVI.01158-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorrell ME, Hauser KF. 2014. Ligand-gated purinergic receptors regulate HIV-1 Tat and morphine related neurotoxicity in primary mouse striatal neuron-glia co-cultures. J Neuroimmune Pharmacol 9:233–244. doi: 10.1007/s11481-013-9507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belete HA, Hubmayr RD, Wang S, Singh RD. 2011. The role of purinergic signaling on deformation induced injury and repair responses of alveolar epithelial cells. PLoS One 6:e27469. doi: 10.1371/journal.pone.0027469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busillo JM, Azzam KM, Cidlowski JA. 2011. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem 286:38703–38713. doi: 10.1074/jbc.M111.275370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deli T, Csernoch L. 2008. Extracellular ATP and cancer: an overview with special reference to P2 purinergic receptors. Pathol Oncol Res 14:219–231. doi: 10.1007/s12253-008-9071-7. [DOI] [PubMed] [Google Scholar]
- 34.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. 2007. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem 282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 35.McIlvain HB, Ma L, Ludwig B, Manners MT, Martone RL, Dunlop J, Kaftan EJ, Kennedy JD, Whiteside GT. 2010. Purinergic receptor-mediated morphological changes in microglia are transient and independent from inflammatory cytokine release. Eur J Pharmacol 643:202–210. doi: 10.1016/j.ejphar.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 36.Marin M, Du Y, Giroud C, Kim JH, Qui M, Fu H, Melikyan GB. 2015. High-throughput HIV-cell fusion assay for discovery of virus entry inhibitors. Assay Drug Dev Technol 13:155–166. doi: 10.1089/adt.2015.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giroud C, Du Y, Marin M, Min Q, Jui NT, Fu H, Melikyan GB. 2017. Screening and functional profiling of small-molecule HIV-1 entry and fusion inhibitors. Assay Drug Dev Technol 15:53–63. doi: 10.1089/adt.2017.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hazleton JE, Berman JW, Eugenin EA. 2012. Purinergic receptors are required for HIV-1 infection of primary human macrophages. J Immunol 188:4488–4495. doi: 10.4049/jimmunol.1102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graziano F, Desdouits M, Garzetti L, Podini P, Alfano M, Rubartelli A, Furlan R, Benaroch P, Poli G. 2015. Extracellular ATP induces the rapid release of HIV-1 from virus containing compartments of human macrophages. Proc Natl Acad Sci U S A 112:E3265–E3273. doi: 10.1073/pnas.1500656112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Q, Wu H, Tao J, Liu C, Deng Z, Liu Y, Chen G, Liu B, Xu C. 2017. Effect of naringin on gp120-induced injury mediated by P2X7 receptors in rat primary cultured microglia. PLoS One 12:e0183688. doi: 10.1371/journal.pone.0183688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Q, Wu H, Qin S, Liu C, Chen Y, Yang Y, Xu C. 2016. The P2X7 receptor involved in gp120-induced cell injury in BV2 microglia. Inflammation 39:1814–1826. doi: 10.1007/s10753-016-0417-0. [DOI] [PubMed] [Google Scholar]
- 42.Tewari M, Seth P. 2015. Emerging role of P2X7 receptors in CNS health and disease. Ageing Res Rev 24:328–342. doi: 10.1016/j.arr.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Menkova-Garnier I, Hocini H, Foucat E, Tisserand P, Bourdery L, Delaugerre C, Benne C, Lévy Y, Lelièvre J-D. 2016. P2X7 receptor inhibition improves CD34 T-cell differentiation in HIV-infected immunological nonresponders on c-ART. PLoS Pathog 12:e1005571. doi: 10.1371/journal.ppat.1005571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soare AY, Durham ND, Gopal R, Tweel B, Hoffman KW, Brown JA, O’Brien M, Bhardwaj N, Lim JK, Chen BK, Swartz TH. 2018. P2X antagonists inhibit HIV-1 productive infection and inflammatory cytokines interleukin-10 (IL-10) and IL-1β in a human tonsil explant model. bioRxiv 10.1101/366179. [DOI] [PMC free article] [PubMed]
- 45.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661–671. doi: 10.1016/S1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 46.Li H, Zony C, Chen P, Chen BK. 2017. Reduced potency and incomplete neutralization of broadly neutralizing antibodies against cell-to-cell transmission of HIV-1 with transmitted founder Envs. J Virol 91:e0245-16. doi: 10.1128/JVI.02425-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez JC, Latz E, Urcuqui-Inchima S. 2014. HIV-1 induces the first signal to activate the NLRP3 inflammasome in monocyte-derived macrophages. Intervirology 57:36–42. doi: 10.1159/000353902. [DOI] [PubMed] [Google Scholar]
- 48.He Y, Hara H, Nunez G. 2016. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bukh AR, Melchjorsen J, Offersen R, Jensen JM, Toft L, Stovring H, Ostergaard L, Tolstrup M, Sogaard OS. 2011. Endotoxemia is associated with altered innate and adaptive immune responses in untreated HIV-1 infected individuals. PLoS One 6:e21275. doi: 10.1371/journal.pone.0021275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marchetti G, Tincati C, Silvestri G. 2013. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev 26:2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doitsh G, Greene WC. 2016. Dissecting how CD4 T cells are lost during HIV infection. Cell Host Microbe 19:280–291. doi: 10.1016/j.chom.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munoz-Arias I, Doitsh G, Yang Z, Sowinski S, Ruelas D, Greene WC. 2015. Blood-derived CD4 T cells naturally resist pyroptosis during abortive HIV-1 infection. Cell Host Microbe 18:463–470. doi: 10.1016/j.chom.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galloway NL, Doitsh G, Monroe KM, Yang Z, Munoz-Arias I, Levy DN, Greene WC. 2015. Cell-to-cell transmission of HIV-1 is required to trigger pyroptotic death of lymphoid-tissue-derived CD4 T cells. Cell Rep 12:1555–1563. doi: 10.1016/j.celrep.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC. 2014. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC. 2014. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343:428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC. 2010. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell 143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glushakova S, Baibakov B, Margolis LB, Zimmerberg J. 1995. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat Med 1:1320–1322. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- 58.Brocca-Cofano E, Xu C, Wetzel KS, Cottrell ML, Policicchio BB, Raehtz KD, Ma D, Dunsmore T, Haret-Richter GS, Musaitif K, Keele BF, Kashuba AD, Collman RG, Pandrea I, Apetrei C. 2018. Marginal effects of systemic CCR5 blockade with maraviroc on oral simian immunodeficiency virus transmission to infant macaques. J Virol 92:e00576-16. doi: 10.1128/JVI.00576-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deleage C, Turkbey B, Estes JD. 2016. Imaging lymphoid tissues in nonhuman primates to understand SIV pathogenesis and persistence. Curr Opin Virol 19:77–84. doi: 10.1016/j.coviro.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Introini A, Fitzgerald W, Vanpouille C, Margolis L. 2018. Histoculture and infection with HIV of functional human lymphoid tissue on Gelfoam. Methods Mol Biol 1760:187–197. doi: 10.1007/978-1-4939-7745-1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Esposito AM, Cheung P, Swartz TH, Li H, Tsibane T, Durham ND, Basler CF, Felsenfeld DP, Chen BK. 2016. A high throughput Cre-lox activated viral membrane fusion assay identifies pharmacological inhibitors of HIV entry. Virology 490:6–16. doi: 10.1016/j.virol.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwon DS, Angin M, Hongo T, Law KM, Johnson J, Porichis F, Hart MG, Pavlik DF, Tighe DP, Kavanagh DG, Streeck H, Addo MM, Kaufmann DE. 2012. CD4+ CD25+ regulatory T cells impair HIV-1-specific CD4 T cell responses by upregulating interleukin-10 production in monocytes. J Virol 86:6586–6594. doi: 10.1128/JVI.06251-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y, Fonseca SG, Van Grevenynghe J, Boulassel MR, Bruneau J, Shoukry NH, Routy JP, Douek DC, Haddad EK, Sekaly RP. 2010. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med 16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Porichis F, Hart MG, Zupkosky J, Barblu L, Kwon DS, McMullen A, Brennan T, Ahmed R, Freeman GJ, Kavanagh DG, Kaufmann DE. 2014. Differential impact of PD-1 and/or interleukin-10 blockade on HIV-1-specific CD4 T cell and antigen-presenting cell functions. J Virol 88:2508–2518. doi: 10.1128/JVI.02034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosser DM, Zhang X. 2008. Interleukin-10: new perspectives on an old cytokine. Immunol Rev 226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. 2011. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 67.Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, Jessen H, Pereyra F, Kavanagh DG, Walker BD, Kaufmann DE. 2009. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114:346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwon DS, Kaufmann DE. 2010. Protective and detrimental roles of IL-10 in HIV pathogenesis. Eur Cytokine Netw 21:208–214. doi: 10.1684/ecn.2010.0201. [DOI] [PubMed] [Google Scholar]
- 69.Liu J, Zhan W, Kim CJ, Clayton K, Zhao H, Lee E, Cao JC, Ziegler B, Gregor A, Yue FY, Huibner S, MacParland S, Schwartz J, Song HH, Benko E, Gyenes G, Kovacs C, Kaul R, Ostrowski M. 2014. IL-10-producing B cells are induced early in HIV-1 infection and suppress HIV-1-specific T cell responses. PLoS One 9:e89236. doi: 10.1371/journal.pone.0089236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Unutmaz D, KewalRamani VN, Marmon S, Littman DR. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med 189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kedzierska K, Crowe SM. 2001. Cytokines and HIV-1: interactions and clinical implications. Antivir Chem Chemother 12:133–150. doi: 10.1177/095632020101200301. [DOI] [PubMed] [Google Scholar]
- 72.Reuter MA, Pombo C, Betts MR. 2012. Cytokine production and dysregulation in HIV pathogenesis: lessons for development of therapeutics and vaccines. Cytokine Growth Factor Rev 23:181–191. doi: 10.1016/j.cytogfr.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, Ting JP, Virgin HW, Kastan MB, Semenkovich CF. 2012. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab 15:534–544. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink PJ, Slütter B, Foks AC, Bot I, Kuiper J. 2017. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice−brief report. Arterioscler Thromb Vasc Biol 37:1457–1461. doi: 10.1161/ATVBAHA.117.309575. [DOI] [PubMed] [Google Scholar]
- 75.Li Y, Xu S, Jiang B, Cohen RA, Zang M. 2013. Activation of sterol regulatory element binding protein and NLRP3 inflammasome in atherosclerotic lesion development in diabetic pigs. PLoS One 8:e67532. doi: 10.1371/journal.pone.0067532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baldrighi M, Mallat Z, Li X. 2017. NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis 267:127–138. doi: 10.1016/j.atherosclerosis.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 77.Wang R, Wang Y, Mu N, Lou X, Li W, Chen Y, Fan D, Tan H. 2017. Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab Invest 97:922–934. doi: 10.1038/labinvest.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoseini Z, Sepahvand F, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H. 2018. NLRP3 inflammasome: its regulation and involvement in atherosclerosis. J Cell Physiol 233:2116–2132. doi: 10.1002/jcp.25930. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Han Z, Fan Y, Zhang J, Chen K, Gao L, Zeng H, Cao J, Wang C. 2017. MicroRNA-9 inhibits NLRP3 inflammasome activation in human atherosclerosis inflammation cell models through the JAK1/STAT signaling pathway. Cell Physiol Biochem 41:1555–1571. doi: 10.1159/000470822. [DOI] [PubMed] [Google Scholar]
- 80.Karasawa T, Takahashi M. 2017. Role of NLRP3 inflammasomes in atherosclerosis. J Atheroscler Thromb 24:443–451. doi: 10.5551/jat.RV17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li WL, Hua LG, Qu P, Yan WH, Ming C, Jun YD, Yuan LD, Nan N. 2016. NLRP3 inflammasome: a novel link between lipoproteins and atherosclerosis. Arch Med Sci 12:950–958. doi: 10.5114/aoms.2016.61356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paramel Varghese G, Folkersen L, Strawbridge RJ, Halvorsen B, Yndestad A, Ranheim T, Krohg-Sorensen K, Skjelland M, Espevik T, Aukrust P, Lengquist M, Hedin U, Jansson JH, Fransen K, Hansson GK, Eriksson P, Sirsjo A. 2016. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc 5:e003031. doi: 10.1161/JAHA.115.003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi X, Xie WL, Kong WW, Chen D, Qu P. 2015. Expression of the NLRP3 inflammasome in carotid atherosclerosis. J Stroke Cerebrovasc Dis 24:2455–2466. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 84.Chivero ET, Guo ML, Periyasamy P, Liao K, Callen SE, Buch S. 2017. HIV-1 Tat primes and activates microglial NLRP3 inflammasome-mediated neuroinflammation. J Neurosci 37:3599–3609. doi: 10.1523/JNEUROSCI.3045-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toksoy A, Sennefelder H, Adam C, Hofmann S, Trautmann A, Goebeler M, Schmidt M. 2017. Potent NLRP3 inflammasome activation by the HIV reverse transcriptase inhibitor abacavir. J Biol Chem 292:2805–2814. doi: 10.1074/jbc.M116.749473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mamik MK, Hui E, Branton WG, McKenzie BA, Chisholm J, Cohen EA, Power C. 2017. HIV-1 viral protein R activates NLRP3 inflammasome in microglia: implications for HIV-1 associated neuroinflammation. J Neuroimmune Pharmacol 12:233–248. doi: 10.1007/s11481-016-9708-3. [DOI] [PubMed] [Google Scholar]
- 87.Haque S, Lan X, Wen H, Lederman R, Chawla A, Attia M, Bongu RP, Husain M, Mikulak J, Saleem MA, Popik W, Malhotra A, Chander PN, Singhal PC. 2016. HIV promotes NLRP3 inflammasome complex activation in murine HIV-associated nephropathy. Am J Pathol 186:347–358. doi: 10.1016/j.ajpath.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo H, Gao J, Taxman DJ, Ting JP, Su L. 2014. HIV-1 infection induces interleukin-1beta production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J Biol Chem 289:21716–21726. doi: 10.1074/jbc.M114.566620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pontillo A, Brandao LA, Guimaraes RL, Segat L, Athanasakis E, Crovella S. 2010. A 3'UTR SNP in NLRP3 gene is associated with susceptibility to HIV-1 infection. J Acquir Immune Defic Syndr 54:236–240. doi: 10.1097/QAI.0b013e3181dd17d4. [DOI] [PubMed] [Google Scholar]
- 90.Mariathasan S, Monack DM. 2007. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 91.Schechter ME, Andrade BB, He T, Richter GH, Tosh KW, Policicchio BB, Singh A, Raehtz KD, Sheikh V, Ma D, Brocca-Cofano E, Apetrei C, Tracy R, Ribeiro RM, Sher A, Francischetti IMB, Pandrea I, Sereti I. 2017. Inflammatory monocytes expressing tissue factor drive SIV and HIV coagulopathy. Sci Transl Med 9:eaam5441. doi: 10.1126/scitranslmed.aam5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim CJ, Rousseau R, Huibner S, Kovacs C, Benko E, Shahabi K, Kandel G, Ostrowski M, Kaul R. 2017. Impact of intensified antiretroviral therapy during early HIV infection on gut immunology and inflammatory blood biomarkers. AIDS 31:1529–1534. doi: 10.1097/QAD.0000000000001515. [DOI] [PubMed] [Google Scholar]
- 93.Merlin JS, Westfall AO, Heath SL, Goodin BR, Stewart JC, Sorge RE, Younger J. 2017. Brief report: IL-1beta levels are associated with chronic multisite pain in people living With HIV. J Acquir Immune Defic Syndr 75:e99–e103. doi: 10.1097/QAI.0000000000001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tufa DM, Ahmad F, Chatterjee D, Ahrenstorf G, Schmidt RE, Jacobs R. 2016. Brief report: HIV-1 infection results in increased frequency of active and inflammatory SlanDCs that produce high level of IL-1beta. J Acquir Immune Defic Syndr 73:34–38. doi: 10.1097/QAI.0000000000001082. [DOI] [PubMed] [Google Scholar]
- 95.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT. 2017. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 96.Pashun RA, Frishman WH. 2015. Therapeutic role of innovative anti-inflammatory medications in the prevention of acute coronary syndrome. Cardiol Rev 23:252–260. doi: 10.1097/CRD.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 97.Hsue P, Deeks S, Ishai AE, Hur S, Li D, Sterman F, Lalezari J, Rupert A, Ganz P, Tawakol A. 2017. IL-1β inhibition significantly reduces atheroscleoritc inflammation in treated HIV, abstr 126. Abstr Conf Retroviruses Opportunistic Infect, Seattle, WA. [Google Scholar]
- 98.Allers K, Fehr M, Conrad K, Epple HJ, Schurmann D, Geelhaar-Karsch A, Schinnerling K, Moos V, Schneider T. 2014. Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. J Infect Dis 209:739–748. doi: 10.1093/infdis/jit547. [DOI] [PubMed] [Google Scholar]
- 99.Shive CL, Mudd JC, Funderburg NT, Sieg SF, Kyi B, Bazdar DA, Mangioni D, Gori A, Jacobson JM, Brooks AD, Hardacre J, Ammori J, Estes JD, Schacker TW, Rodriguez B, Lederman MM. 2014. Inflammatory cytokines drive CD4+ T-cell cycling and impaired responsiveness to interleukin 7: implications for immune failure in HIV disease. J Infect Dis 210:619–629. doi: 10.1093/infdis/jiu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu CM, Osborne BJ, Hungate BA, Shahabi K, Huibner S, Lester R, Dwan MG, Kovacs C, Contente-Cuomo TL, Benko E, Aziz M, Price LB, Kaul R. 2014. The semen microbiome and its relationship with local immunology and viral load in HIV infection. PLoS Pathog 10:e1004262. doi: 10.1371/journal.ppat.1004262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rizzo R, Ferrari D, Melchiorri L, Stignani M, Gulinelli S, Baricordi OR, Di Virgilio F. 2009. Extracellular ATP acting at the P2X7 receptor inhibits secretion of soluble HLA-G from human monocytes. J Immunol 183:4302–4311. doi: 10.4049/jimmunol.0804265. [DOI] [PubMed] [Google Scholar]
- 102.de Medeiros RM, Valverde-Villegas JM, Junqueira DM, Graf T, Lindenau JD, de Mello MG, Vianna P, Almeida SE, Chies JA. 2016. Rapid and slow progressors show increased IL-6 and IL-10 levels in the pre-AIDS stage of HIV infection. PLoS One 11:e0156163. doi: 10.1371/journal.pone.0156163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chatterjee A, Rathore A, Sivarama P, Yamamoto N, Dhole TN. 2009. Genetic association of IL-10 gene promoter polymorphism and HIV-1 infection in North Indians. J Clin Immunol 29:71–77. doi: 10.1007/s10875-008-9220-5. [DOI] [PubMed] [Google Scholar]
- 104.Singh S, Sharma A, Arora SK. 2016. Combination of low producer AA-genotypes in IFN-gamma and IL-10 genes makes a high risk genetic variant for HIV disease progression. Cytokine 77:135–144. doi: 10.1016/j.cyto.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 105.Freitas FB, Lima SS, Feitosa RNM, Azevedo VN, Ishak M. d O G, Ishak R, Vallinoto ACR. 2015. Polymorphisms in the IFNgamma, IL-10, and TGFbeta genes may be associated with HIV-1 infection. Dis Markers 2015:248571. doi: 10.1155/2015/248571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Naicker DD, Wang B, Losina E, Zupkosky J, Bryan S, Reddy S, Jaggernath M, Mokgoro M, Goulder PJ, Kaufmann DE, Ndung'u T. 2012. Association of IL-10-promoter genetic variants with the rate of CD4 T-cell loss, IL-10 plasma levels, and breadth of cytotoxic T-cell lymphocyte response during chronic HIV-1 infection. Clin Infect Dis 54:294–302. doi: 10.1093/cid/cir811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shrestha S, Wiener HW, Aissani B, Song W, Shendre A, Wilson CM, Kaslow RA, Tang J. 2010. Interleukin-10 (IL-10) pathway: genetic variants and outcomes of HIV-1 infection in African American adolescents. PLoS One 5:e13384. doi: 10.1371/journal.pone.0013384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O'Brien M, Manches O, Wilen C, Gopal R, Huq R, Wu V, Sunseri N, Bhardwaj N. 2016. CD4 receptor is a key determinant of divergent HIV-1 sensing by plasmacytoid dendritic cells. PLoS Pathog 12:e1005553. doi: 10.1371/journal.ppat.1005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest 115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manches O, Munn D, Fallahi A, Lifson J, Chaperot L, Plumas J, Bhardwaj N. 2008. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest 118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Durham ND, Yewdall AW, Chen P, Lee R, Zony C, Robinson JE, Chen BK. 2012. Neutralization resistance of virological synapse-mediated HIV-1 Infection is regulated by the gp41 cytoplasmic tail. J Virol 86:7484–7495. doi: 10.1128/JVI.00230-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Durham ND, Chen BK. 2015. HIV-1 cell-free and cell-to-cell infections are differentially regulated by distinct determinants in the Env gp41 cytoplasmic tail. J Virol 89:9324–9337. doi: 10.1128/JVI.00655-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moore JP, McKeating JA, Weiss RA, Sattentau QJ. 1990. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 250:1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- 114.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]