ABSTRACT
The transition from skotomorphogenesis to photomorphogenesis is regulated in part by the COP1/SPA complex and phytochrome-interacting factors (PIFs) in Arabidopsis. The constitutive photomorphogenic (cop) phenotypes of cop1 and spaQ mutants have been shown to result from a high abundance of positively acting transcription factors. Here, we show that the four major PIF proteins are unstable in cop1 mutants and that overexpression of PIF1, PIF3, PIF4 and PIF5 suppresses cop1 phenotypes in the dark. A comparison of the transcriptome data among cop1, spaQ and pifQ reveals remarkably overlapping gene expression profiles with preferential regulation of PIF direct target genes. Additionally, HFR1 strongly inhibits the in vivo binding and transcriptional activation activity of PIF1 in the dark. Taken together, these data suggest that the cop phenotypes of the cop1 and spaQ mutants are due to a combination of the reduced level of PIFs, increased levels of positively acting transcription factors (e.g. HY5/HFR1) and the HFR1-mediated inhibition of PIF-targeted gene expression in the dark.
This article has an associated ‘The people behind the papers’ interview.
KEY WORDS: E3 ubiquitin ligase, Phytochrome-interacting factors, Photomorphogenesis, 26S proteasome degradation, Skotomorphogenesis, Arabidopsis
Highlighted Article: A revised Arabidopsis model suggests that the cop phenotype is due to reduced levels of PIFs, increased levels of positively acting transcription factors and HFR1-mediated inhibition of PIF activity in the dark.
INTRODUCTION
Plants have evolved contrasting developmental programs for the successful establishment of young postgermination seedlings early in their life cycle. In darkness, plants undergo skotomorphogenesis, which is defined by elongated hypocotyls, an apical hook and closed cotyledons. This developmental program is suited for protection of the apical region during rapid emergence of seedlings through the soil surface. Once the seedlings are exposed to ambient light, they undergo photomorphogenesis, defined by short hypocotyls, absence of an apical hook, and open, expanded, green cotyledons. This growth pattern allows seedling body plan formation for maximal light capture and autotrophic growth (Gommers and Monte, 2018). Photomorphogenesis has been proposed to be the default pathway for plant development because a series of mutants displaying constitutive photomorphogenic (cop) phenotypes in the dark has been described (Xu et al., 2015). These include 11 loci encoding the CONSTITUTIVE PHOTOMORPHOGENIC1/DE-ETIOLATED1/FUSCA (COP/DET/FUS) genes (Lau and Deng, 2012), four loci encoding SUPPRESSOR OF PHYA-105 (SPA1-SPA4) (Laubinger et al., 2004) and a small family of basic helix-loop-helix (bHLH) transcription factor genes called PHYTOCHROME-INTERACTING FACTORs (PIF1-PIF8) (Leivar and Quail, 2011; Pham et al., 2018b). These genes encode proteins that act additively and/or synergistically to prevent precocious germination and seedling establishment in the dark.
Among these repressors of photomorphogenesis, COP1 functions as an E3 ubiquitin ligase in association with SPA1-SPA4, targeting a variety of substrates, including the positively acting transcription factors (e.g. HY5/HFR1/LAF1 and others) in light-signaling pathways for Ubiquitin/26S proteasome-mediated degradation (Hardtke et al., 2000; Hoecker, 2017; Jang et al., 2005; Osterlund et al., 2000; Seo et al., 2003; Yang et al., 2005b). COP1, SPAs and CUL4 form CUL4COP1-SPA E3 ubiquitin ligase complexes that target positively acting factors in the dark (Chen et al., 2010). Consistently, cop1, spaQ and cul4cs (co-suppressed) lines display constitutive photomorphogenic (cop) phenotypes in the dark. In addition, another complex, called the COP9 signalosome (CSN), comprises eight distinct subunits (CSN1-CSN8) and is involved in deconjugation of NEDD8/RUB1 to CULLIN RING ligases (CRLs) (Lau and Deng, 2012; Serino and Deng, 2003). Plants with mutations in any of these subunits also display cop phenotypes in the dark.
DET1 is a nuclear protein that binds to the N-terminal tail of histone H2B and regulates cell type-specific expression of light-regulated genes (Benvenuto et al., 2002; Pepper et al., 1994). It also promotes skotomorphogenesis, in part by stabilizing PIFs in the dark (Dong et al., 2014). In addition, DET1 suppresses seed germination by destabilizing HFR1 and stabilizing PIF1 (Shi et al., 2015). It also interacts with COP10 and DAMAGED DNA-BINDING PROTEIN 1 (DDB1) to form the CUL4CDD complex, which represses photomorphogenesis in the dark, in part by degrading positively acting transcription factors (Chen et al., 2006; Schroeder et al., 2002).
PIFs belong to the bHLH family of transcription factors that repress photomorphogenesis in the dark by promoting skotomorphogenic development. There are eight PIFs (PIF1-PIF8) in Arabidopsis, with a high degree of sequence similarity (Pham et al., 2018b). However, individual pif mutants display distinct phenotypes, which are especially pronounced in the four major pif mutants [pif1, pif3, pif4 and pif5, collectively called the ‘PIF quartet’ (pifQ)]. For example, pif1 seeds germinate under red and far-red light as well as in darkness (Oh et al., 2004; Shen et al., 2005), suggesting that PIF1 is a repressor of light-induced seed germination. Both pif1 and pif3 mutants have more chlorophyll and carotenoids compared with wild type during the transition from dark to light (Huq et al., 2004; Moon et al., 2008; Stephenson et al., 2009; Toledo-Ortíz et al., 2010), suggesting that PIF1 and PIF3 suppress the biosynthesis of these pigments. pif3, pif4 and pif5 mutants display hypersensitive phenotypes in response to red light, in part by inducing co-degradation of these PIFs with phyB (Huq and Quail, 2002; Khanna et al., 2007; Monte et al., 2004; Zhu and Huq, 2014). In this process, multiple kinases (e.g. PPKs) and E3 ubiquitin ligases (e.g. CUL3LRB) participate in inducing the co-degradation of PIFs and phyB in response to light (Ni et al., 2017, 2014; Pham et al., 2018b). Thus, phyB is more abundant in these mutants, resulting in hypersensitive phenotypes under red light. In addition, other kinases (e.g. PPKs, BIN2 and CK2) and E3 ubiquitin ligases (e.g. CUL1EBF1/2, CUL1CTG10, CUL3BOP and CUL4COP1-SPA) induce the degradation of PIFs in response to light in a phytochrome-dependent manner to promote photomorphogenesis (Bernardo-García et al., 2014; Bu et al., 2011; Dong et al., 2017; Majee et al., 2018; Ni et al., 2017; Pham et al., 2018b; Zhang et al., 2017). Strikingly, the quadruple mutant of the PIF quartet, pifQ, displays constitutive photomorphogenesis in the dark (Leivar et al., 2008; Shin et al., 2009), suggesting that these PIFs repress photomorphogenesis in the dark. They do so by regulating gene expression directly and indirectly in an individual to a shared manner (Pfeiffer et al., 2014; Pham et al., 2018b).
The cop phenotypes of the cop1 and spaQ mutants were thought to be due primarily to a high abundance of the positively acting transcription factors (e.g. HY5/HFR1/LAF1 and others) in the dark (Hoecker, 2017). However, a few reports showed that PIFs are less abundant in cop1 mutants (Bauer et al., 2004; Pham et al., 2018a; Shen et al., 2008; Xu et al., 2017; Zhu et al., 2015) and also to a lesser extent in spaQ mutants (Leivar et al., 2008; Ni et al., 2014; Pham et al., 2018a), suggesting that the instability of PIFs contributes to the cop phenotypes of these mutants. Here, we show that the gene expression signature of cop1 and spaQ overlaps with pifQ in the dark, with preferential targeting of PIF direct target genes, suggesting that the cop phenotype of cop1 and spaQ is partly due to a reduced level of PIFs in these backgrounds. In addition, we also show that the positively acting transcription factor HFR1 strongly inhibits the DNA-binding activity of PIF1 by sequestration; thereby promoting the cop phenotypes of cop1 and spaQ in the dark.
RESULTS
COP1 and SPA positively regulate PIF protein level in darkness
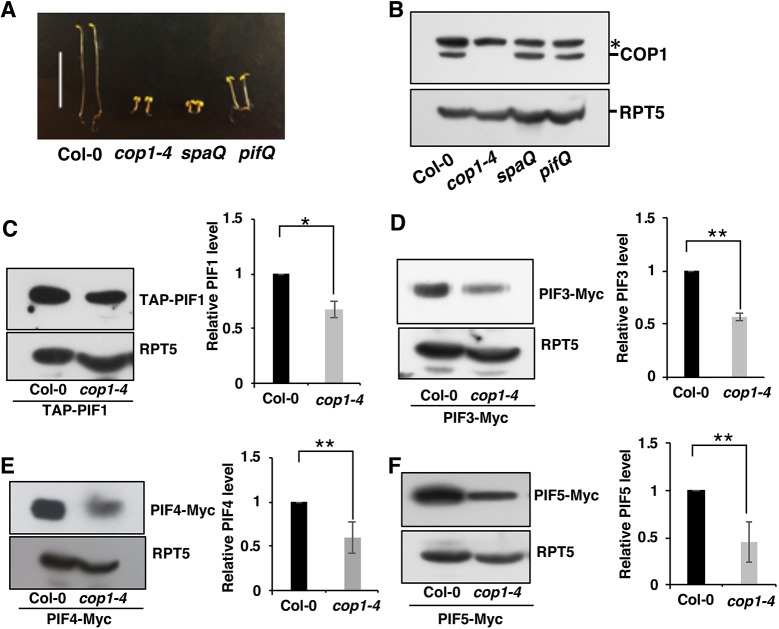
The cop phenotypes of cop1-4, spaQ and pifQ have been previously described (Fig. 1A) (Deng et al., 1992; Laubinger et al., 2004; Leivar et al., 2008; Shin et al., 2009). To examine whether the cop phenotype of pifQ is due to a reduction in the COP1 level, we performed immunoblots using an anti-COP1 antibody for 4-day-old dark-grown seedlings of wild type, cop1-4, spaQ and pifQ. Results showed that the COP1 level in pifQ and spaQ was similar to that in wild-type seedlings (Fig. 1B), suggesting that the pifQ phenotype is not due to a reduction in the COP1 level.
Fig. 1.
COP1 positively regulates PIF protein level in darkness. (A) Visible constitutive photomorphogenic phenotypes of 4-day-old dark-grown seedlings. (B) Immunoblot showing COP1 endogenous protein levels in wild-type (Col-0), cop1-4, spaQ and pifQ dark-grown seedlings. Total protein was extracted from 4-day-old dark-grown seedlings, separated on 8% SDS-PAGE gel, and probed with anti-COP1 and anti-RPT5 antibodies. Asterisk indicates a cross-reacting band. (C-F) Immunoblots and graphs showing PIF protein levels. The plants overexpressing TAP-PIF1, PIF3-Myc, PIF4-Myc or PIF5-Myc in a Col-0 or cop1-4 background were grown under the conditions described in the Materials and Methods. Total protein was separated on a 6.5% SDS-PAGE gel and probed with anti-Myc and anti-RPT5 antibodies. PIF protein levels were quantified from three biological replicates (n=3) and normalized with RPT5 levels. The PIF protein level in Col-0 was set as 1. *P<0.05, **P<0.01. Scale bar: 10 mm in A.
Previously, we and others showed that the PIF levels in cop1 and spaQ mutants are reduced compared with wild type in the dark (Bauer et al., 2004; Leivar et al., 2008; Pham et al., 2018a; Zhu et al., 2015). To systematically analyze PIF levels without the transcriptional regulation in these mutants, we crossed the overexpression lines of tagged PIF1, PIF3, PIF4 and PIF5 using the constitutively active 35S promoter in cop1-4, TAP-PIF1 and PIF5-Myc in spaQ mutant backgrounds, and performed immunoblots for protein levels. Although, the overexpression data might be quantitatively different compared with the native PIF levels in these backgrounds because of high expression, the results showed that all four PIF levels were reduced in cop1-4, as previously reported (Fig. 1C-F) (Bauer et al., 2004; Pham et al., 2018a; Xu et al., 2017; Zhu et al., 2015). Both TAP-PIF1 and PIF5-Myc levels were reduced in spaQ compared with wild type (Fig. S1). Thus, the cop phenotype of cop1-4 and spaQ might be due, in part, to a reduction in the PIF levels in these backgrounds.
We also examined the transcript levels of the native PIFs in the cop1-4 and spaQ mutants using quantitative RT-PCR assays (RT-qPCR; Fig. S2). The transcript levels of PIF1, PIF3 and PIF4 were similar between Columbia-0 (Col-0) plants and cop1-4 mutants, whereas the transcript level of PIF5 was strongly increased in cop1-4 mutants. In spaQ mutants, the transcript levels of PIF1, PIF3 and PIF4 were slightly lower, whereas the level of the PIF5 transcript was slightly higher compared with wild-type seedlings. These data illustrate that COP1 and SPA proteins positively regulate PIF protein levels in darkness, possibly by destabilizing HFR1, given that the latter has been shown to induce degradation of PIF1 by heterodimerization (Xu et al., 2017).
PIFs are degraded in cop1-4 and spaQ mutants through the 26S proteasome
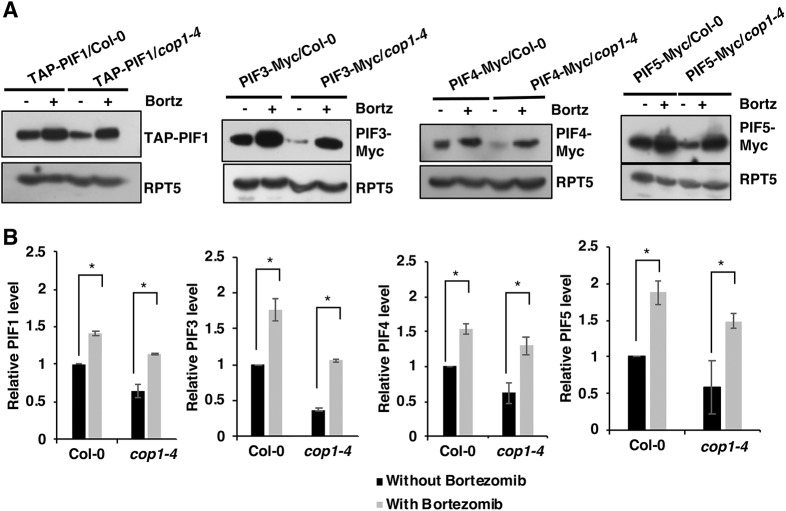
PIFs are stable in the dark and undergo degradation in light through the 26S proteasome pathway (Pham et al., 2018b). However, a recent study showed that PIF1 is also degraded in the dark by direct heterodimerization with HFR1 (Xu et al., 2017). To test whether the degradation of other PIFs in the cop1-4 and spaQ backgrounds in the dark is also through the 26S proteasome pathway, we treated dark-grown seedlings with a proteasome inhibitor (bortezomib) for 4 h and then extracted total protein for immunoblots. Results showed that the proteasome inhibitor prevented the degradation of all four PIFs in the cop1-4 background (Fig. 2). Both TAP-PIF1 and PIF5-Myc were also stabilized in the spaQ background upon bortezomib treatment (Fig. S1). These data suggest that the COP1/SPA complex stabilizes PIFs in the dark, probably by destabilizing HFR1.
Fig. 2.
Instability of PIFs in the dark in cop1 backgrounds is 26S-proteasome dependent. (A) PIF protein levels in 4-day-old dark-grown wild-type and cop1-4 seedlings with and without treatment with a 26S protease inhibitor (bortezomib or Bortz). Total proteins were extracted and separated on 6.5% SDS-PAGE gels and probed with anti-Myc and anti-RPT5 antibodies. RPT5 was used as a loading control. (B) Bar graphs showing the quantitative PIF protein levels in those backgrounds from three biological replicates (n=3). In each graph, the PIF protein level in the Col-0 background without bortezomib treatment was set as 1. Data are mean±s.d. *P<0.05.
PIF overexpression partially suppresses the cop phenotypes of cop1-4 and spaQ
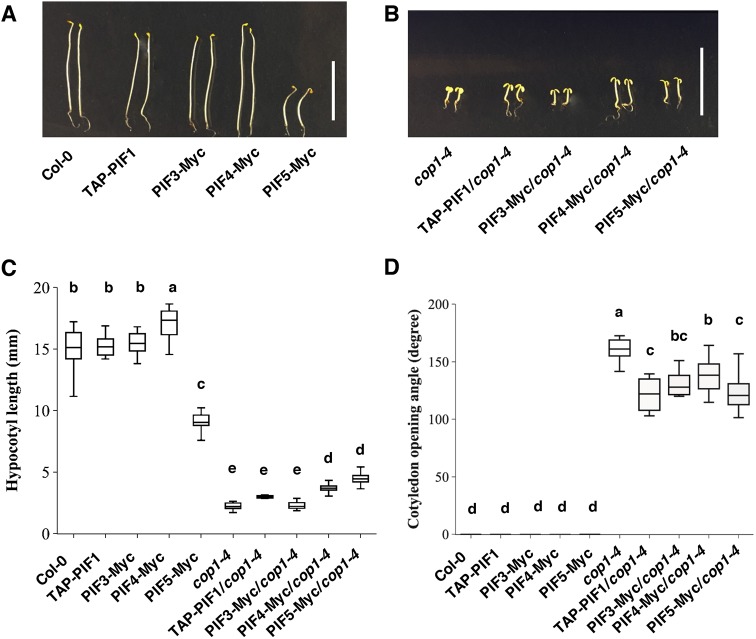
If the reduced level of PIFs in the cop1-4 and spaQ backgrounds contributes to the cop phenotypes of these mutants, we hypothesized that an overexpression of these PIFs in the cop1 and spaQ mutants is expected to suppress the cop phenotypes. To test this hypothesis, we overexpressed four PIFs (TAP-PIF1, PIF3-Myc, PIF4-Myc and PIF5-Myc) in the cop1-4 background and two PIFs (TAP-PIF1 and PIF5-Myc) in the spaQ background and examined their phenotypes in the dark. Results showed that, whereas the hypocotyl lengths of TAP-PIF1/cop1-4 and PIF3-Myc/cop1-4 were comparable to those of cop1-4, the hypocotyl lengths of PIF4-Myc/cop1-4 and PIF5-Myc/cop1-4 were significantly longer compared with cop1-4 (Fig. 3A-C). Moreover, all four PIF overexpression lines in the cop1-4 mutant displayed a significantly smaller cotyledon opening angle compared with that of cop1-4 (Fig. 3B,D), suggesting that PIFs suppress the cop phenotypes of cop1-4. Similarly, an overexpression of PIF5-Myc in the spaQ background suppressed both the hypocotyl lengths and cotyledon angle phenotypes of the spaQ mutant compared with overexpression of spaQ only, whereas overexpression of TAP-PIF1 in spaQ only suppressed the cotyledon angle phenotype (Fig. S3A-C). These data also suggested that the cop phenotype of cop1-4 and spaQ is partially due to a reduced level of PIFs.
Fig. 3.
Overexpression of PIFs partially suppresses the constitutive photomorphogenetic phenotypes of cop1-4. (A,B) Visible phenotypes of 4-day-old dark-grown seedlings with PIF overexpression in Col-0 and cop1-4 backgrounds. (C,D) Box plots representing the hypocotyl lengths and cotyledon opening angle measurements. Three independent biological replicates were performed with an average of 30 Col-0 or cop1-4 seedlings with PIF overexpression grown under the same conditions as described in the Materials and Methods. Significant differences between the different genotypes were determined using one-way ANOVA and Tukey's HSD tests, indicated by different letters. Scale bar: 10 mm.
cop1-4, spaQ and pifQ display a large overlapping set of co-regulated genes
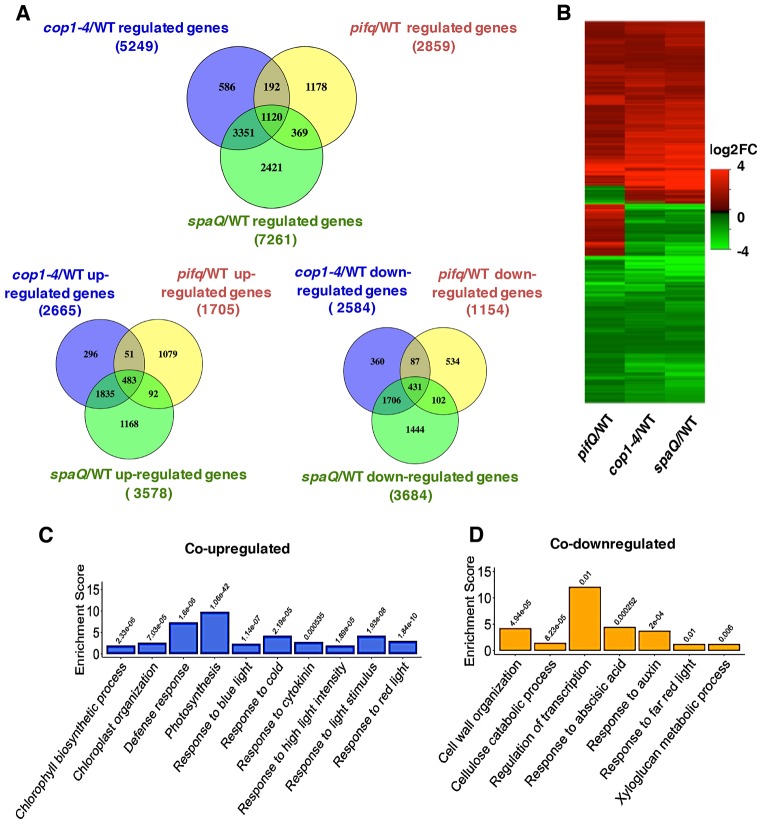
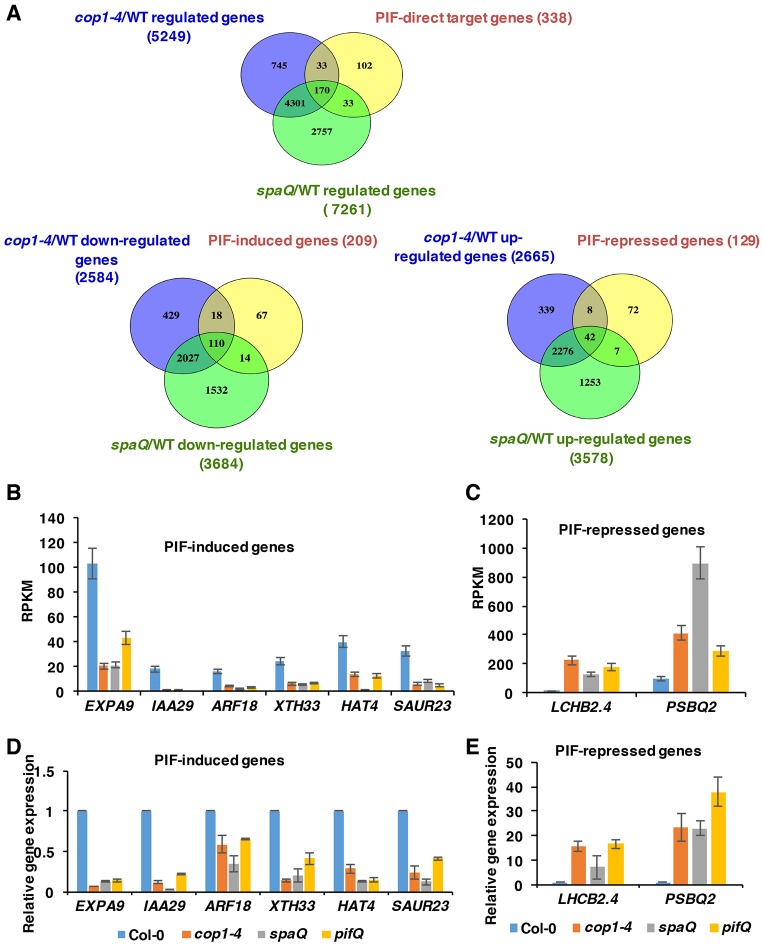
Previously, the cop phenotypes of the cop1 mutant were demonstrated to mainly result from the high abundance of positively acting transcription factors (e.g. HY5/HFR1/LAF1 and others) in the dark (Hoecker, 2017). Given that PIFs were unstable in cop1 and spaQ mutants in the dark (Fig. 1) (Bauer et al., 2004; Leivar et al., 2008; Pham et al., 2018a; Zhu et al., 2015), we hypothesized that COP1-, SPA- and PIF-regulated genes might overlap in genome-wide expression analyses. To test this hypothesis, we analyzed the results from previously published data (Zhang et al., 2013), and our own recent RNA-sequencing (RNA-Seq) experiments using cop1-4, spaQ and pifQ mutant seedlings grown in darkness. Although these experiments were performed in two different laboratories and the growth conditions were slightly different, the results showed that a large proportion of the differentially expressed genes (1120) overlapped among cop1-4, spaQ and pifQ (Fig. 4A,B; Data set S1). Approximately 39% of the PIF-regulated genes displayed overlapping expression patterns with COP1- and SPA-regulated genes. Among these 1120 genes, 483 genes were upregulated and 431 genes were downregulated in all three backgrounds compared with wild type (Fig. 5A,B). Interestingly, 206 of the PIF-regulated genes displayed opposite regulation to the COP1- and SPA-regulated genes (Fig. 4A,B; Data set S3).
Fig. 4.
COP1 and SPA mediate light-regulated transcriptomic changes partly through PIFs. (A) Venn diagram showing 1120 co-regulated, 483 co-upregulated and 431 co-downregulated differentially expressed genes in three different pairwise comparisons (cop1-4/WT, spaQ/WT and pifQ/WT). (B) Hierarchical clustering displaying 1120 differentially expressed genes in comparisons indicated. The data show co-regulated genes identified as having at least a 2-fold difference in gene expression (FDR<0.05). The color represents the log2 of the -fold change in expression. cop1-4/WT and spaQ/WT: comparison of the expression profiles of dark-grown cop1-4 and spaQ with Col-0, respectively. (C,D) Bar graphs showing enrichment analysis of GO biological processes significantly co-upregulated (C) and co-downregulated (D) in cop1-4/WT, spaQ/WT, and pifQ/WT seedlings. Enrichment scores indicate the percentages of involved genes/total genes. Fisher exact P-values are presented on the top of each bar.
Fig. 5.
A significant number of PIF-direct target genes is co-regulated in cop1-4 and spaQ mutants. (A) Venn diagram showing that, among 338 PIF direct target genes, 170 genes are co-regulated by COP1 and SPA, 110 genes are downregulated and 42 genes are upregulated in cop1-4 and spaQ mutants. (B,C) RNA-Seq expression patterns of various PIF-induced genes (B) and PIF-repressed genes (C) in cop1-4, spaQ and pifQ seedlings grown in the dark. (D,E) qRT-PCR shows the similar expression patterns of various PIF-induced genes (D) and PIF-repressed genes (E) in cop1-4, spaQ and pifQ seedlings grown in the dark. Gene expression levels in mutants were normalized to PP2A and the expression level in Col-0 was set as 1.
To identify the biological processes controlled by these co-regulated genes, we performed Gene Ontology (GO) analyses of the co-regulated genes and divided them into two classes: upregulated versus downregulated genes (Fig. 4C,D; Data set S2). A total of 94 enriched GO terms were identified for these co-regulated genes (Fig. S4). The co-upregulated genes were enriched in chlorophyll biosynthetic processes, defense responses, photosynthesis, response to light stimulus (including red light and blue light), response to cold, and cytokinin. The co-downregulated genes were involved in the regulation of transcription, cell wall organization, response to hormones (abscisic acid and auxin), response to red light, and also metabolic processes. These results were consistent with the cop phenotypes of these mutants.
We performed further analysis on the PIF-regulated 206 genes that displayed opposite regulation by COP1 and SPA (Fig. 4A,B; Data set S3). GO analyses of these 206 genes oppositely regulated between pifQ and cop1-4/spaQ using Database for Annotation, Visualization and Integrated Discovery (DAVID) (Data set S3) and GO Analysis Toolkit and Database for Agricultural Community (AgriGo) (Figs S5 and S6) showed that PIFs function oppositely compared with COP1 and SPA proteins in a few biological processes. For example, many genes involved in responses to UV-B and flavonoid biosynthesis were downregulated in pifQ, but upregulated in cop1-4 and spaQ (Fig. S5; Data set S3). Similarly, many other genes involved in defense responses, salicylic acid (SA) metabolism and signaling were upregulated in pifQ, but downregulated in cop1-4 and spaQ (Fig. S6; Data set S3. Thus, although PIFs and the COP/SPA complex repress photomorphogenesis coordinately, they also function antagonistically in a few biological processes.
Direct target genes of PIFs are co-regulated in cop1-4 and spaQ
If COP1 and SPA repress photomorphogenesis in the dark, in part by stabilizing PIFs, then the PIF direct target gene expression is expected to be affected in cop1-4 and spaQ. Interestingly, among 338 PIF direct target genes (Pfeiffer et al., 2014), 170 (>50%) genes were co-regulated by cop1-4 and spaQ (Fig. 5A; Data set S4). Furthermore, among the 209 PIF-induced genes, 110 genes were downregulated in cop1-4 and spaQ. In addition, among 129 PIF-repressed genes, 42 genes were upregulated in the cop1-4 and spaQ backgrounds (Fig. 5A; Data set S4). GO analyses revealed that most of these genes function in response to red and far-red light signaling, auxin responses, and the regulation of transcription. Strikingly, the degree and direction of expression of these PIF direct genes were similar among all three cop mutant groups.
To verify the RNA-Seq data by an independent method, we selected a subset of PIF direct target genes involved in auxin responses, cell wall organization and photosynthesis, and performed RT-qPCR analyses to determine the relative expression patterns in the cop1-4, spaQ and pifQ mutants compared with wild type. Results showed a strikingly similar pattern among cop1-4, spaQ and pifQ for both PIF-induced and -repressed genes (Fig. 5D,E), consistent with the RNA-Seq data (Fig. 5B,C). These data also suggested that the cop phenotype of the cop1 and spaQ mutants is partly due to the reduced level of PIFs and their target gene expression.
HFR1 represses the transcriptional activity of PIF1
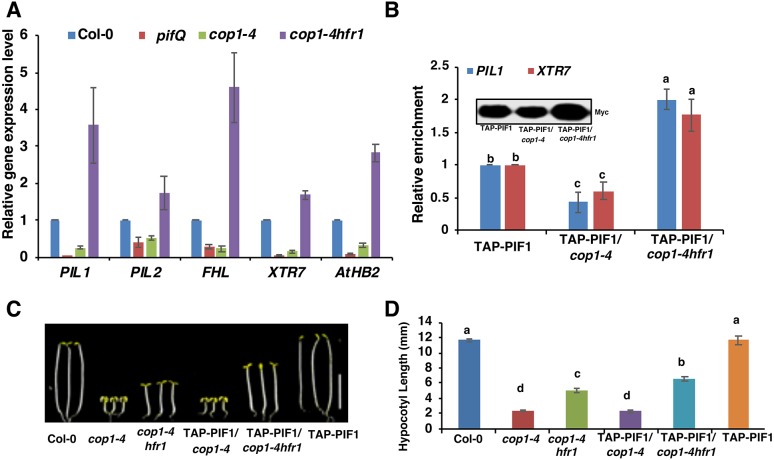
Previously, it was shown that HFR1, a HLH transcription factor, is more abundant in the cop1-4 and spaQ backgrounds compared with wild type (Hoecker, 2017). Given that HFR1 inhibits the DNA-binding activity of PIFs (Shi et al., 2013) and that the PIF levels were reduced in the cop1-4 and spaQ mutants (Fig. 1B-F; Fig. S1A), we hypothesized that the high abundance of HFR1 in the cop1-4 and spaQ backgrounds might contribute to the cop phenotypes of these mutants. To test this hypothesis, we selected a subset of the PIF1 direct target genes that are also regulated by HFR1 and performed RT-qPCR analyses using dark-grown wild-type Col-0, pifQ, cop1-4 and cop1-4hfr1 seedlings. Results showed that the selected genes were expressed at a reduced level in both pifQ and cop1-4 mutant backgrounds, similar to the RNA-Seq data (Fig. 6A). Strikingly, the expression level of these genes was higher in the cop1-4hfr1 double-mutant background compared with cop1-4 (Fig. 6A). However, the increased expression of the PIF1 target genes in the cop1-4hfr1 mutant might be due to either the high PIF1 protein level and/or the loss of suppression by HFR1 of the DNA-binding activity of PIF1. To distinguish between these possibilities, we performed chromatin immunoprecipitation followed by qPCR (ChIP-qPCR) for dark-grown seedlings expressing the TAP-PIF1 fusion protein in the cop1-4 and cop1-4hfr1 backgrounds. We also examined the immunoprecipitated TAP-PIF1 protein level in all these backgrounds during the ChIP assay (Fig. 6B, inset), and divided the promoter enrichments by the protein levels for each genotype to calculate the relative promoter occupancy of PIF1 independent of PIF1 protein level. Results showed that the immunoprecipitated TAP-PIF1 protein level was lower in the cop1-4 background, but higher in the cop1-4hfr1 double-mutant background compared with the TAP-PIF1-only background, as previously reported (Fig. 6B, inset) (Zhu et al., 2015). The relative promoter occupancy of PIF1 showed that the DNA-binding activity of TAP-PIF1 was also reduced in the cop1-4 background and increased in the cop1-4hfr1 background (Fig. 6B). These data further extend the recent report that HFR1 suppresses the DNA-binding activity of PIF1 not only in imbibed seeds (Shi et al., 2013), but also in seedlings. Thus, HFR1 not only regulates the protein abundance, but also the DNA-binding activity of PIF1 in etiolated seedlings.
Fig. 6.
The transcriptional activation activity of PIFs is higher in the cop1-4hfr1 background compared with the cop1-4 background. (A) The expression levels of PIF target genes are lower in the pifQ and cop1-4 backgrounds, but higher in the cop1-4 hfr1 background. Total seedling RNA was extracted from 3-day-old dark-grown wild-type Col-0, pifQ, cop1-4 and cop1-4hfr1 seedlings. PP2A was used as an internal control. Wild-type Col-0 was set as 1. Error bars indicate standard deviation (n=3 independent biological repeats). (B) The PIL1 and XTR7 promoter occupancies of TAP-PIF1 were reduced in the cop1-4 background but increased in the cop1-4hfr1 background. ChIP-qPCR assays were performed on 3-day-old dark-grown seedlings expressing TAP-PIF1 fusion protein on cop1-4 and cop1-4hfr1 backgrounds. (C) Photographs of wild-type, cop1-4, cop1-4hfr1, cop1-4/TAP-PIF1 and cop1-4hfr1/TAP-PIF1 seedlings. Seedlings were grown in the dark for 5 days. (D) Bar graph showing hypocotyl lengths of various genotypes as described in C. Error bars indicate standard deviation. Significant differences between different genotypes were determined using one-way ANOVA and Tukey's HSD tests, indicated by different letters. Scale bar: 5 mm.
To examine the significance of regulation of PIF1 by HFR1, we measured the hypocotyl lengths of dark-grown of wild-type, cop1-4, cop1-4hfr1, cop1-4/TAP-PIF1, cop1-4hfr1/TAP-PIF1 and TAP-PIF1 seedlings. Results showed that the hypocotyl lengths of cop1-4hfr1/TAP-PIF1 seedlings were longer than that of cop1-4hfr1 seedlings (Fig. 6C,D). The hypocotyl length of cop1-4 seedlings was similar to that of cop1-4/TAP-PIF1 seedlings, possibly because of the reduced TAP-PIF1 protein level and/or increased sequestration of TAP-PIF1 by HFR1 in the cop1-4 background. Thus, TAP-PIF1 has an increased function in regulating hypocotyl lengths in the cop1-4hfr1 background compared with the cop1-4-only background.
DISCUSSION
Analyses of cop mutants have had an important role in our understanding of light-signaling pathways in plants. The prevailing view of the molecular basis of the cop phenotype is that the increased abundance of the positively acting transcription factors (e.g. HY5/HFR1/LAF1 and others) in cop1-4 and spaQ mutants in the dark results in cop phenotypes under darkness (Jang et al., 2005; Osterlund et al., 2000; Saijo et al., 2003; Seo et al., 2003; Yang et al., 2005a,b). Although several studies have reported a reduced abundance of PIFs in various cop mutants compared with wild type (Bauer et al., 2004; Leivar et al., 2008; Ni et al., 2014; Pham et al., 2018a; Shen et al., 2008; Zhu et al., 2015), the mechanism of this reduction and its contribution to the cop phenotype remain unknown. Here, we provide biochemical, molecular and genomic evidence supporting the hypothesis that the reduced PIF levels in cop1 and spaQ mutants contribute to their cop phenotypes. First, we showed that PIFs are actively degraded in the dark in the cop1-4 and spaQ backgrounds through the 26S proteasome pathway (Fig. 1, Fig. S1). Second, the genome-wide gene expression patterns largely overlapped among COP1-, SPA- and PIF-regulated genes, with an altered expression of a set of PIF direct target genes (Figs 4 and 5). Third, PIF1 was sequestered in the cop1-4 background by an increased abundance of HFR1 and possibly other HLH proteins, resulting in reduced PIF activity in the cop1-4 background (Fig. 6). Fourth, overexpression of PIF1 in the cop1-4hfr1 background promoted hypocotyl elongation in the dark. Fifth, overexpression of major PIFs in the cop1-4 and spaQ backgrounds suppressed the cop phenotypes of the cop1-4 and spaQ mutants (Fig. 3, Fig. S3). Overall, these data suggest that the reduction in PIF levels and PIF activity in the cop1-4 and spaQ backgrounds contributes to their cop phenotypes.
Despite similar morphological and molecular phenotypes among cop1-4, spaQ and pifQ, the GO analyses of the differentially expressed genes oppositely regulated between pifQ and cop1-4/spaQ revealed that these genes also have distinct roles in plant signaling pathways. One of the striking differences is in the enrichment of the genes involved in SA metabolism and signaling in pifQ compared with cop1-4 and spaQ, suggesting that PIFs suppress defense responses, as previously discussed (Paik et al., 2017). In fact, PIFs are known to promote growth possibly by suppressing defense responses, given that a trade-off between growth versus defense is a well-known phenomenon in plant growth and development (Paik et al., 2017). By contrast, the genes involved in UV-B responses and flavonoid biosynthesis were downregulated in pifQ, but upregulated in cop1-4 and spaQ. Although, a role for PIFs in UV-B signaling has not yet been examined in detail, a recent study suggested that PIFs are involved in UV-B-induced leaf hyponasty (Fierro et al., 2015). However, the COP1 and SPA proteins function positively in UV-B signaling pathways (Huang et al., 2013; Tilbrook et al., 2013). Overall, these analyses highlight both common and distinct functions of PIFs, COP1 and SPA proteins in regulating biological processes in plants.
COP1/SPA proteins might regulate the abundance and activity of PIFs in multiple ways. For example, a recent study showed a noncanonical function of the COP1/SPA complex in inhibiting BIN2 kinase from phosphorylating PIF3 and regulating PIF3 abundance (Ling et al., 2017). Another source of the opposing functions between PIFs and the COP1/SPA complex is the increased abundance of the positively acting transcription factors, especially HFR1, in the cop1-4 and spaQ backgrounds. HFR1 is an atypical bHLH protein that sequestered PIFs from binding to DNA as well as reducing PIF abundance (Fig. 6) (Hornitschek et al., 2009; Shi et al., 2013; Xu et al., 2017). Similar to HFR1, the HECATE family of bHLH proteins also inhibits PIF activity, and is degraded in the dark, possibly by the COP1/SPA complex (Zhu et al., 2016). Thus, the COP1/SPA complex might negatively regulate the abundance of factors that function antagonistically to PIFs.
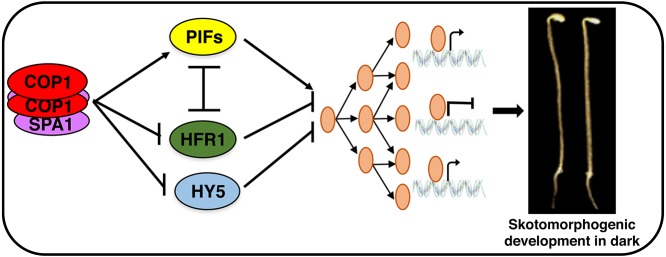
In summary, we propose a revised model for the molecular bases of cop phenotypes in plants (Fig. 7). First, as previously hypothesized, an increased abundance of the positively acting transcription factors (e.g. HY5, LAF1, HFR1 and others) in the cop1 and spaQ mutants promotes photomorphogenesis in the dark. Second, a reduced level of PIFs in the cop1 and spaQ mutants contributes to the cop phenotype in the dark. Finally, a reduction in PIF activity because of the increased abundance of atypical bHLH proteins (e.g. HFR1, HECATE and possibly others) in cop1 and spaQ mutants additively promotes the cop phenotypes. It is notable that all three activities are tightly linked to each other, contributing in concert to the skotomorphogenic and photomorphogenic development of plants.
Fig. 7.
Model showing how COP1 and SPA proteins regulate various transcription factors to promote skotomorphogenesis in the dark. Mutations in COP1, SPA and PIFs result in cop phenotypes in the dark. PIFs and HFR1 reciprocally regulate their abundance, whereas HFR1 inhibits PIF activity by sequestration. Regulation of the abundance and activity of these transcription factors by the COP1-SPA complex promotes skotomorphogenic development.
MATERIALS AND METHODS
Plant materials, growth conditions and measurements
Seeds of the Col-0 ecotype of Arabidopsis thaliana were used for all experiments. Seeds were surface sterilized and then plated on Murashige and Skoog (MS) medium without sucrose. After stratification at 4°C for 3 days, seeds were exposed to white light for 3 h at room temperature to trigger germination before placing them back in the dark for an additional 4 days. These 4-day-old seedlings were then used for protein extraction for western blots and measurements of hypocotyl lengths and cotyledon opening angle phenotypes, using the ImageJ software (rsb.info.nih.gov/ij/). A total of 90 seedlings from three biological replicates were measured. Significant differences between different genotypes were determined using one-way ANOVA and Tukey's HSD tests, indicated by different letters in the figures accompanying this report.
Generation of transgenic lines
The pif1, pifQ, cop1-4, cop1-4hfr1, cop1-4/TAP-PIF1 (Castillon et al., 2009; Xu et al., 2014; Zhu et al., 2015), TAP-PIF1 (Bu et al., 2011), PIF3-Myc (Park et al., 2004), PIF4-Myc (Shor et al., 2017) and PIF5-Myc (Sakuraba et al., 2014) plants were as previously published. PIF overexpression lines were crossed with cop1-4 and spaQ mutants. The crossed homozygous lines were selected from the F3 population using antibiotic selection. The cop1-4 mutants were selected by sequencing. The spaQ homozygous lines were selected by genotyping spa mutants. Primers used for sequencing and genotyping are listed in Table S1. For the generation of cop1-4hfr1/TAP-PIF1, cop-4hfr1 was crossed into TAP-PIF1 to obtain the F1 generation. cop1-4hfr1/TAP-PIF1 was obtained by genotyping and antibiotic selection (gentamycin) of the F2 and F3 generations. The primers used for sequencing and genotyping are listed in Table S1.
Transcriptomic analyses
RNA-Seq was performed using 3-day-old dark-grown seedlings. Seeds were kept in the dark for 3 days at 4°C and exposed to 3 h of white light. After 21 h in the dark, plates were then treated with 2000 μmol m−2 far-red light for the true-dark condition, as previously described (Leivar et al., 2008). Total RNA was extracted after 2 days in darkness. Raw data and processed data for the total read counts of sequencing reads in Col-0, cop1-4 and spaQ can be accessed from the Gene Expression Omnibus database under accession number GSE112662.
For the RNA-seq analysis, raw read quality was accessed using FastQC (www.bioinformatics.babraham.ac.uk/projects/fastqc/). Raw reads were then aligned to the Arabidopsis genome using Bowtie2 (Langmead and Salzberg, 2012) and TopHat (Trapnell et al., 2012). The annotation of the Arabidopsis genome was obtained from TAIR10 (www.arabidopsis.org/). Read count data were performed by HTseq (Anders et al., 2015) (htseq.readthedocs.io/en/master/index.html).
Differentially expressed genes in cop1-4/WT and spaQ/WT were identified using the DESeq2 package (Love et al., 2014). The differential gene expression was defined as those differing by ≥2-fold with adjusted P value (FDR) ≤0.05.
Differentially expressed genes in pifQ and the PIF differential direct target genes list were obtained from RNA-Seq and ChIP-Seq data, respectively, under the accession number GSE43286 (Pfeiffer et al., 2014). Venn diagrams were generated using Venny 2.1.0 (bioinfogp.cnb.csic.es/tools/venny/). Heatmaps were generated using DESeq2 and the ComplexHeatmap package (Gu et al., 2016) in the R statistical program. GO enrichment analyses were performed using DAVID v6.8 (david.ncifcrf.gov/). GO bar graphs were generated based on the significant enriched terms with the lowest P value and FDR (≤0.05) for GO terms. Hieratical graph results for the GO term analysis of cop1-4-, spaQ- and pifQ-regulated genes were also performed by AgriGo (bioinfo.cau.edu.cn/agriGO/index.php).
RT-qPCR assay
For determining the transcript levels of PIFs and PIF direct target genes by RT-qPCR assays, total RNA was extracted from seedlings grown under the same conditions used for the RNA-Seq experiments. M-MLV reverse transcriptase (Thermo Fisher Scientific) was used to reverse transcribe 1 μg of total RNA treated with on-column DNase I (Sigma Aldrich). A RT-qPCR assay was performed using Power SYBR green (Applied Biosystems). Gene-specific primers are listed in Table S1. PP2A (At1g13320) was used as the internal control to normalize the expression of different genes. The calculation of the levels of expression of different genes relative to PP2A was as follows: 2ΔCt, where ΔCt=Ct (PP2A)−Ct (specific gene) and Ct indicates the cycle threshold values. Relative expression was quantified from three biological replicates. Error bars indicate mean±s.d. Student's t-test assuming unequal variances was performed, and the P values are indicated in each figure.
Protein extraction and immunoblot analyses
For examination of the COP1 protein level, 0.2 g tissue from 4-day-old dark-grown seedlings was extracted in extraction buffer as previously described (Zhu et al., 2015). Total protein was separated on 8% SDS-PAGE gels. Proteins were transferred to a PVDF membrane and western blots were detected with anti-COP1 or anti-RPT5 (Enzo Life Sciences) antibodies for endogenous COP1 and RPT5, respectively.
For PIF protein levels in the Col-0 and mutant backgrounds, total protein from 50 seedlings was extracted using 50 μl urea extraction buffer [48% urea (w/v), 0.1 M phosphate buffer pH 6.8, 10 mM Tris-Cl pH 6.8, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1× protease inhibitor cocktail]. Samples were centrifuged at 16,000 g for 10 min and heated at 65°C for 10 min. Supernatants were analyzed on 6.5% SDS-PAGE gels and detected using anti-Myc (dilution 1/1000, OP10-200UG, EMD Millipore) and anti-RPT5 antibodies (dilution 1/3000, BML-PW8245-0100, Enzo Life Sciences).
For treatment with a 26S proteasome inhibitor (bortezomib, B-1408, LC Laboratories), 4-day-old dark-grown seedlings were transferred to 5 ml liquid MS media containing 40 μM bortezomib and incubated in the dark for 4 h. Total protein was then extracted using the urea extraction buffer as described earlier. For quantitation of protein levels, ImageJ software was used to measure the band intensities from three independent biological replicates, and normalized to RPT5 protein levels. Error bars indicate mean±s.d. Student's t-test assuming unequal variances was performed, and P values are indicated in each figure.
ChIP assay
ChIP-qPCR assays were performed on 3-day-old dark-grown seedlings expressing TAP-PIF1 fusion protein in cop1-4 and cop1-4hfr1 backgrounds. Anti-Myc (71D10, Cell Signaling Technologies) antibody was used to immunoprecipitate TAP-PIF1 and associated DNA. DNA was amplified using primers specific to the G-box fragment or control regions. Anti-Myc (OP10-200UG, EMD Millipore) antibody was used to determine the immunoprecipitated TAP-PIF1 protein level in each background. Both the TAP-PIF1 promoter enrichment from the ChIP-qPCR and TAP-PIF1 protein level quantified by ImageJ were set as 1. The relative enrichment of the -fold change in cop1-4/TAP-PIF1 and cop1-4hfr1/TAP-PIF1 were first normalized compared with the TAP-PIF1 only for their promoter enrichment and protein levels, respectively, and the promoter enrichment levels were then divided by the protein levels for each repeat. Final averages of three independent biological repeats for each genotype were calculated and shown as a bar graph (Fig. 6). One biological repeat of the TAP-PIF1 protein level was shown as an example (Fig. 6B, inset). Error bars indicate standard deviation (n=3 independent biological repeats).
Supplementary Material
Acknowledgements
We thank Drs Xing Wang Deng for sharing the cop1 mutant, Ute Hoecker for sharing the spaQ mutant, Giltsu Choi for sharing the PIF3-Myc and PIF5-Myc seeds, and Woe Yeon Kim for the COP1 antibody. We thank the Huq lab members for the technical support and critical reading of the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: E.H., V.N.P.; Methodology: V.N.P., X.X.; Validation: V.N.P.; Formal analysis: E.H., V.N.P., X.X.; Investigation: V.N.P., X.X.; Writing - original draft: E.H., V.N.P.; Writing - review & editing: E.H., V.N.P., X.X.; Supervision: E.H.; Project administration: E.H.; Funding acquisition: E.H.
Funding
This work was supported by grants from the National Institutes of Health (GM-114297) and the National Science Foundation (MCB-1543813) to E.H. Deposited in PMC for release after 12 months.
Data availability
Raw data for the RNA-Seq in this study have been deposited in Gene Expression Omnibus (GEO) under accession number GSE112662. RNA-Seq data for PIF-regulated genes and ChIP-Seq data for PIF direct target genes are available in GEO under accession numbers GSE39217 and GSE43286, respectively.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.169870.supplemental
References
- Anders S., Pyl P. T. and Huber W. (2015). HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166-169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D., Viczian A., Kircher S., Nobis T., Nitschke R., Kunkel T., Panigrahi K. C., Adam E., Fejes E., Schafer E. et al. (2004). Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16, 1433-1445. 10.1105/tpc.021568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuto G., Formiggini F., Laflamme P., Malakhov M. and Bowler C. (2002). The photomorphogenesis regulator DET1 binds the amino-terminal tail of histone H2B in a nucleosome context. Curr. Biol. 12, 1529-1534. 10.1016/S0960-9822(02)01105-3 [DOI] [PubMed] [Google Scholar]
- Bernardo-García S., de Lucas M., Martínez C., Espinosa-Ruiz A., Davière J.-M. and Prat S. (2014). BR-dependent phosphorylation modulates PIF4 transcriptional activity and shapes diurnal hypocotyl growth. Genes Dev. 28, 1681-1694. 10.1101/gad.243675.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Q., Zhu L., Yu L., Dennis M., Lu X., Person M., Tobin E., Browning K. and Huq E. (2011). Phosphorylation by CK2 enhances the rapid light-induced degradation of PIF1. J. Biol. Chem. 286, 12066-12074. 10.1074/jbc.M110.186882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillon A., Shen H. and Huq E. (2009). Blue light induces degradation of the negative regulator Phytochrome Interacting Factor 1 to promote photomorphogenic development of Arabidopsis seedlings. Genetics 182, 161-171. 10.1534/genetics.108.099887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Shen Y., Tang X., Yu L., Wang J., Guo L., Zhang Y., Zhang H., Feng S., Strickland E. et al. (2006). Arabidopsis CULLIN4 forms an E3 ubiquitin ligase with RBX1 and the CDD complex in mediating light control of development. Plant Cell 18, 1991-2004. 10.1105/tpc.106.043224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. D., Huang X., Gusmaroli G., Terzaghi W., Lau O. S., Yanagawa Y., Zhang Y., Li J. G., Lee J.-H., Zhu D. M. et al. (2010). Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with constitutively photomorphogenic1-suppressor of PHYA complexes to regulate photomorphogenesis and flowering time. Plant Cell 22, 108-123. 10.1105/tpc.109.065490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X.-W., Matsui M., Wei N., Wagner D., Chu A. M., Feldman K. A. and Quail P. H. (1992). COP1, an arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a Gβ homologous domain. Cell 71, 791-801. [DOI] [PubMed] [Google Scholar]
- Dong J., Tang D., Gao Z., Yu R., Li K., He H., Terzaghi W., Deng X. W. and Chen H. (2014). Arabidopsis DE-ETIOLATED1 represses photomorphogenesis by positively regulating Phytochrome-Interacting Factors in the dark. Plant Cell 26, 3630-3645. 10.1105/tpc.114.130666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Ni W., Yu R., Deng X. W., Chen H. and Wei N. (2017). Light-dependent degradation of PIF3 by SCFEBF1/2 promotes a photomorphogenic response in Arabidopsis. Curr. Biol. 27, 2420-2430. 10.1016/j.cub.2017.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro A. C., Leroux O., De Coninck B., Cammue B. P. A., Marchal K., Prinsen E., Van Der Straeten D. and Vandenbussche F. (2015). Ultraviolet-B radiation stimulates downward leaf curling in Arabidopsis thaliana. Plant Physiol. Biochem. 93, 9-17. 10.1016/j.plaphy.2014.12.012 [DOI] [PubMed] [Google Scholar]
- Gommers C. M. M. and Monte E. (2018). Seedling establishment: a dimmer switch-regulated process between dark and light signaling. Plant Physiol. 176, 1061-1074. 10.1104/pp.17.01460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Eils R. and Schlesner M. (2016). Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847-2849. 10.1093/bioinformatics/btw313 [DOI] [PubMed] [Google Scholar]
- Hardtke C. S., Gohda K., Osterlund M. T., Oyama T., Okada K. and Deng X. W. (2000). HY5 stability and activity in arabidopsis is regulated by phosphorylation in its COP1 binding domain. EMBO J. 19, 4997-5006. 10.1093/emboj/19.18.4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker U. (2017). The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr. Opin. Plant Biol. 37, 63-69. 10.1016/j.pbi.2017.03.015 [DOI] [PubMed] [Google Scholar]
- Hornitschek P., Lorrain S., Zoete V., Michielin O. and Fankhauser C. (2009). Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J. 28, 3893-3902. 10.1038/emboj.2009.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Ouyang X., Yang P., Lau O. S., Chen L., Wei N. and Deng X. W. (2013). Conversion from CUL4-based COP1–SPA E3 apparatus to UVR8–COP1–SPA complexes underlies a distinct biochemical function of COP1 under UV-B. Proc. Natl Acad. Sci. USA 110, 16669-16674. 10.1073/pnas.1316622110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E. and Quail P. H. (2002). PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 21, 2441-2450. 10.1093/emboj/21.10.2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E., Al-Sady B., Hudson M., Kim C., Apel K. and Quail P. H. (2004). Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305, 1937-1941. 10.1126/science.1099728 [DOI] [PubMed] [Google Scholar]
- Jang I.-C., Yang J. Y., Seo H. S. and Chua N. H. (2005). HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev. 19, 593-602. 10.1101/gad.1247205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R., Shen Y., Marion C. M., Tsuchisaka A., Theologis A., Schäfer E. and Quail P. H. (2007). The basic helix-loop-helix transcription factor pif5 acts on ethylene biosynthesis and phytochrome signaling by distinct mechanisms. Plant Cell 19, 3915-3929. 10.1105/tpc.107.051508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B. and Salzberg S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. S. and Deng X. W. (2012). The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 17, 584-593. 10.1016/j.tplants.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Laubinger S., Fittinghoff K. and Hoecker U. (2004). The SPA quartet: a family of WD-repeat proteins with a central role in suppression of photomorphogenesis in arabidopsis. Plant Cell 16, 2293-2306. 10.1105/tpc.104.024216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P. and Quail P. H. (2011). PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 16, 19-28. 10.1016/j.tplants.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E., Oka Y., Liu T., Carle C., Castillon A., Huq E. and Quail P. H. (2008). Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 18, 1815-1823. 10.1016/j.cub.2008.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J.-J., Li J., Zhu D. and Deng X. W. (2017). Noncanonical role of Arabidopsis COP1/SPA complex in repressing BIN2-mediated PIF3 phosphorylation and degradation in darkness. Proc. Natl Acad. Sci. USA 114, 3539-3544. 10.1073/pnas.1700850114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M. I., Huber W. and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majee M., Kumar S., Kathare P., Wu S., Gingerich D., Nayak N., Salaita L., Dinkins R., Martin K., Goodin M. et al. (2018). A kelch F-box protein positively influences seed germination by targeting phytochrome-interacting factor1. Proc. Natl. Acad. Sci. USA 115, E4120-E4129. 10.1073/pnas.1711919115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte E., Tepperman J. M., Al-Sady B., Kaczorowski K. A., Alonso J. M., Ecker J. R., Li X., Zhang Y. and Quail P. H. (2004). The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc. Natl. Acad. Sci. USA 101, 16091-16098. 10.1073/pnas.0407107101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J., Zhu L., Shen H. and Huq E. (2008). PIF1 directly and indirectly regulates chlorophyll biosynthesis to optimize the greening process in Arabidopsis. Proc. Natl. Acad. Sci. USA 105, 9433-9438. 10.1073/pnas.0803611105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W., Xu S.-L., Tepperman J. M., Stanley D. J., Maltby D. A., Gross J. D., Burlingame A. L., Wang Z.-Y. and Quail P. H. (2014). A mutually assured destruction mechanism attenuates light signaling in Arabidopsis. Science 344, 1160-1164. 10.1126/science.1250778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W., Xu S.-L., González-Grandío E., Chalkley R. J., Huhmer A. F. R., Burlingame A. L., Wang Z.-Y. and Quail P. H. (2017). PPKs mediate direct signal transfer from phytochrome photoreceptors to transcription factor PIF3. Nat. Commun. 8, 15236 10.1038/ncomms15236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Kim J., Park E., Kim J. I., Kang C. and Choi G. (2004). PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16, 3045-3058. 10.1105/tpc.104.025163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund M. T., Hardtke C. S., Wei N. and Deng X. W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405, 462-466. 10.1038/35013076 [DOI] [PubMed] [Google Scholar]
- Paik I., Kathare P. K., Kim J.-I. and Huq E. (2017). Expanding roles of PIFs in signal integration from multiple processes. Mol. Plant 10, 1035-1046. 10.1016/j.molp.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Kim J., Lee Y., Shin J., Oh E., Chung W.-I., Liu J. R. and Choi G. (2004). Degradation of phytochrome interacting factor 3 in phytochrome-mediated light signaling. Plant Cell Physiol. 45, 968-975. 10.1093/pcp/pch125 [DOI] [PubMed] [Google Scholar]
- Pepper A., Delaney T., Washburnt T., Poole D. and Chory J. (1994). DET1, a negative regulator of light-mediated development and gene expression in arabidopsis, encodes a novel nuclear-localized protein. Cell 78, 109-116. 10.1016/0092-8674(94)90577-0 [DOI] [PubMed] [Google Scholar]
- Pfeiffer A., Shi H., Tepperman J. M., Zhang Y. and Quail P. H. (2014). Combinatorial complexity in a transcriptionally-centered signaling hub in arabidopsis. Mol. Plant 7, 1598-1618. 10.1093/mp/ssu087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham V. N., Kathare P. K. and Huq E. (2018a). Dynamic regulation of PIF5 by COP1-SPA complex to optimize photomorphogenesis in Arabidopsis. Plant J. (in press) 96, 260-273. 10.1111/tpj.14074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham V. N., Kathare P. K. and Huq E. (2018b). Phytochromes and phytochrome interacting factors. Plant Physiol. 176, 1025-1038. 10.1104/pp.17.01384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y., Sullivan J. A., Wang H., Yang J., Shen Y., Rubio V., Ma L., Hoecker U. and Deng X. W. (2003). The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 17, 2642-2647. 10.1101/gad.1122903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba Y., Jeong J., Kang M.-Y., Kim J., Paek N.-C. and Choi G. (2014). Phytochrome-interacting transcription factors PIF4 and PIF5 induce leaf senescence in Arabidopsis. Nat. Commun. 5, 4636 10.1038/ncomms5636 [DOI] [PubMed] [Google Scholar]
- Schroeder D. F., Gahrtz M., Maxwell B. B., Cook R. K., Kan J. M., Alonso J. M., Ecker J. R. and Chory J. (2002). De-Etiolated 1 and damaged DNA binding protein 1 interact to regulate arabidopsis photomorphogenesis. Curr. Biol. 12, 1462-1472. 10.1016/S0960-9822(02)01106-5 [DOI] [PubMed] [Google Scholar]
- Seo H. S., Yang J.-Y., Ishikawa M., Bolle C., Ballesteros M. L. and Chua N.-H. (2003). LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 423, 995-999. 10.1038/nature01696 [DOI] [PubMed] [Google Scholar]
- Serino G. and Deng X.-W. (2003). The COP9 signalosome: regulating plant development through the control of proteolysis. Annu. Rev. Plant Biol. 54, 165-182. 10.1146/annurev.arplant.54.031902.134847 [DOI] [PubMed] [Google Scholar]
- Shen H., Moon J. and Huq E. (2005). PIF1 is regulated by light-mediated degradation through the ubiquitin-26S proteasome pathway to optimize seedling photomorphogenesis in Arabidopsis. Plant J. 44, 1023-1035. 10.1111/j.1365-313X.2005.02606.x [DOI] [PubMed] [Google Scholar]
- Shen H., Ling Z., Castillon A., Majee M., Downie B. and Huq E. (2008). Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME INTERACTING FACTOR 1 depends upon its direct physical interactions with photoactivated phytochromes. Plant Cell 20, 1586-1602. 10.1105/tpc.108.060020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Zhong S., Mo X., Liu N., Nezames C. D. and Deng X. W. (2013). HFR1 sequesters PIF1 to govern the transcriptional network underlying light-initiated seed germination in arabidopsis. Plant Cell 25, 3770-3784. 10.1105/tpc.113.117424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Wang X., Mo X., Tang C., Zhong S. and Deng X. W. (2015). Arabidopsis DET1 degrades HFR1 but stabilizes PIF1 to precisely regulate seed germination. Proc. Natl Acad. Sci. USA 112, 3817-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Kim K., Kang H., Zulfugarov I. S., Bae G., Lee C.-H., Lee D. and Choi G. (2009). Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc. Nat. Acad. Sci. USA 106, 7660-7665. 10.1073/pnas.0812219106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor E., Paik I., Kangisser S., Green R. and Huq E. (2017). PHYTOCHROME INTERACTING FACTORS mediate metabolic control of the circadian system in Arabidopsis. New Phytol. 215, 217-228. 10.1111/nph.14579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson P. G., Fankhauser C. and Terry M. J. (2009). PIF3 is a repressor of chloroplast development. Proc. Natl. Acad. Sci. USA 106, 7654-7659. 10.1073/pnas.0811684106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilbrook K., Arongaus A. B., Binkert M., Heijde M., Yin R. and Ulm R. (2013). The UVR8 UV-B photoreceptor: perception, signaling and response. Arabidopsis Book 11, e0164 10.1199/tab.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Ortíz G., Huq E. and Rodríguez-Concepción M. (2010). Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 107, 11626-11631. 10.1073/pnas.0914428107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D. R., Pimentel H., Salzberg S. L., Rinn J. L. and Pachter L. (2012). Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562-578. 10.1038/nprot.2012.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Paik I., Zhu L. and Huq E. (2015). Illuminating progress in phytochrome-mediated light signaling pathways. Trends Plant Sci. 20, 641-650. 10.1016/j.tplants.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Xu X., Paik I., Zhu L., Bu Q., Huang X., Deng X. W. and Huq E. (2014). phytochrome interacting factor1 enhances the E3 ligase activity of constitutive photomorphogenic1 to synergistically repress photomorphogenesis in arabidopsis. Plant Cell 26, 1992-2006. 10.1105/tpc.114.125591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Kathare P. K., Pham V. N., Bu Q., Nguyen A. and Huq E. (2017). Reciprocal proteasome-mediated degradation of PIFs and HFR1 underlies photomorphogenic development in Arabidopsis. Development 144, 1831-1840. 10.1242/dev.146936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Lin R., Hoecker U., Liu B., Xu L. and Wang H. (2005a). Repression of light signaling by Arabidopsis SPA1 involves post-translational regulation of HFR1 protein accumulation. Plant J 43, 131-141. 10.1111/j.1365-313X.2005.02433.x [DOI] [PubMed] [Google Scholar]
- Yang J., Lin R., Sullivan J., Hoecker U., Liu B., Xu L., Deng X. W. and Wang H. (2005b). Light regulates COP1-mediated degradation of HFR1, a transcription factor essential for light signaling in arabidopsis. Plant Cell 17, 804-821. 10.1105/tpc.104.030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Mayba O., Pfeiffer A., Shi H., Tepperman J. M., Speed T. P. and Quail P. H. (2013). A quartet of PIF bHLH Factors provides a transcriptionally centered signaling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in arabidopsis. PLoS Genet. 9, e1003244 10.1371/journal.pgen.1003244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Holmlund M., Lorrain S., Norberg M., Bakó L., Fankhauser C. and Nilsson O. (2017). BLADE-ON-PETIOLE proteins act in an E3 ubiquitin ligase complex to regulate PHYTOCHROME INTERACTING FACTOR 4 abundance. eLife 6, e26759 10.7554/eLife.26759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L. and Huq E. (2014). Suicidal co-degradation of the phytochrome interacting factor 3 and phytochrome B in response to light. Mol. Plant 7, 1709-1711. 10.1093/mp/ssu108 [DOI] [PubMed] [Google Scholar]
- Zhu L., Bu Q., Xu X., Paik I., Huang X., Hoecker U., Deng X. W. and Huq E. (2015). CUL4 forms an E3 ligase with COP1 and SPA to promote light-induced degradation of PIF1. Nat. Commun. 6, 7245 10.1038/ncomms8245 [DOI] [PubMed] [Google Scholar]
- Zhu L., Xin R., Bu Q., Shen H., Dang J. and Huq E. (2016). A negative feedback loop between PHYTOCHROME INTERACTING FACTORs and HECATE proteins fine tunes photomorphogenesis in Arabidopsis. Plant Cell 28, 855-874. 10.1105/tpc.16.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.