Abstract
Sympathomimetic amine compounds are often pooled together and incorrectly assumed to be interchangeable with respect to potential adverse effects. A brief and specific review of sympathomimetic compounds and one instance (i.e., hepatotoxicity) where these compounds have been improperly grouped together is covered. A review of the proposed mechanisms through which known hepatotoxic sympathomimetic agents (e.g., 3,4-methylenedioxymethamphetamine or MDMA, methamphetamine and amphetamine) cause liver injury, along with a corresponding review of in vitro data, interventional data, animal model studies and observational data allow for a comparison/contrast of different agents and reveals a lack of potential toxicity for some agents (e.g., pseudoephedrine, phenylephrine, ephedrine, 1,3-dimethylamylamine, phentermine) in this broad category. Data show that compounds within the broad group of sympathomimetics display divergent pharmacological and toxicological profiles and can be clearly distinguished with respect to liver injury. These data serve as a reminder to clinicians and others, that even small structural differences between molecules can lead to drastically different pharmacological/toxicological profiles and that one should not assume that all sympathomimetic agents are hepatotoxic. Such assumptions could lead to diagnostic errors and incorrect or insufficient treatment.
Keywords: Sympathomimetic, Liver, Hepatotoxicity, Amphetamines, Mechanism
1. Introduction
Compounds that mimic the actions of epinephrine and norepinephrine are traditionally referred to as sympathomimetic agents [1]. The compounds that fit this generally broad category are further defined by their mechanism, whether it is indirect (e.g., amphetamine or tyramine), working to enhance the actions of catecholamines (i.e., epinephrine, norepinephrine and dopamine) or direct (e.g., epinephrine and phenylephrine), via binding directly to and activating a given adrenergic receptor [1]. Some compounds (e.g., ephedrine) may be considered mixed, consisting of both direct and indirect mechanisms. Finally, direct acting agents may be further subdivided based upon their selectivity for a given receptor (e.g., phenylephrine as a selective α-1 adrenergic receptor agonist) or lack thereof (e.g., epinephrine and norepinephrine as non-selective adrenergic receptor agonists).
Individuals may encounter sympathomimetic agents in the form of diet (e.g., tyramine in fermented foods and p-synephrine in varieties of oranges), prescription pharmaceuticals (e.g., phentermine and sibutramine), over the counter medications (e.g., pseudoephedrine and phenylephrine) and dietary supplements (e.g., p-synephrine and beta-phenethylamine). While these compounds are generally thought to be fairly safe, some sympathomimetic compounds can produce cardiovascular and neurological side effects, especially in cases of abuse or intentional overdose, while some but not all, may also cause liver injury.
The past inclusion of one sympathomimetic compound (1,3-dimethylpentylamine or 1,3-dimethylamylamine, formerly marketed as a decongestant, Forthane) in dietary supplements has led to speculation that due to its sympathomimetic effects upon cardiovascular and neurological systems, and superficial chemical similarity to amphetamines, it may be hepatotoxic, effectively arguing that any sympathomimetic compound has the potential for liver injury [2,3]. Similarly, speculation has also indicated that the alkaloid, ephedrine may be hepatotoxic based upon case reports where Ephedra species (i.e., Ephedra sinica or ma-huang), which contain several alkaloids including pseudoephedrine and ephedrine, were associated with liver injury [4]. This speculation is based upon the noted hepatotoxic effects seen in cases of overdose with amphetamine and the more frequently encountered cases of liver injury seen with amphetamine derivatives such as 3,4-methylenedioxymethamphetamine or MDMA (ecstasy) and methamphetamine, and their superficial chemical similarity to ephedrine with some shared physiological effects (e.g., cardiovascular effects such as increased heart rate and blood pressure). However, the lack of any confirmed reports with other sympathomimetics, which also possess chemical similarities and some physiological effects (e.g., phenylephrine, phentermine, pseudoephedrine, tuaminoheptane) indicates these arguments should be evaluated more critically and considered alongside available evidence to determine their validity. The key mechanisms through which established hepatotoxic sympathomimetic amines (e.g., MDMA, amphetamine and methamphetamine) are proposed to cause liver injury, including the production of reactive metabolites, hyperthermia, neurotransmitter efflux, oxidation of biogenic amines, mitochondrial impairment and apoptosis are reviewed allowing for a comparison/contrast with commonly consumed sympathomimetic amines (e.g., phenylephrine, pseudoephedrine, ephedrine, 1,3-dimethylamylamine) in order to show key distinctions using in vitro data, animal models, interventional (i.e., randomized, controlled trials) and observational data (e.g., case-control studies and case reports) in humans. These data show that some sympathomimetic agents (e.g., phenylephrine, phentermine, pseudoephedrine, ephedrine, 1,3-dimethylamylamine) lack evidence for hepatotoxic potential as compared to agents such as MDMA, amphetamine and methamphetamine, demonstrating that such speculation and extrapolation is unsupported.
2. Mechanism of amphetamine-induced hepatotoxicity
While several hypotheses have been put forth regarding the possible mechanisms involved in cases of amphetamine-induced liver injury, those mainly considered are production of reactive metabolites, hyperthermia, increased neurotransmitter efflux, oxidation of biogenic amines, mitochondrial impairment and apoptosis [5,6]. While the aforementioned are considered the direct mechanisms, genetic polymorphisms, particularly in Cytochrome P450 2D6 (CYP2D6), environmental factors and other drug abuse may also play a role in susceptibility to these effects. In the following subsections, each proposed mechanism will be addressed while comparing/contrasting known hepatotoxic sympathomimetic agents such as MDMA, amphetamine and methamphetamine, with those not known to cause liver injury (e.g., pseudoephedrine, phenylephrine, ephedrine, 1,3-dimethylamylamine, tuaminoheptane) in order to show key distinctions.
2.1. Production of reactive metabolites
At least some of the hepatotoxicity seen with amphetamine is believed to be due to the formation of a reactive intermediate (presumably an epoxide) prior to formation of glutathione conjugate, (glutathione-S-yl)-p-hydroxyamphetamine. This could potentially lead to glutathione depletion and increased oxidative stress and damage [5,6]. MDMA (i.e., due to formation of catechol-containing metabolites which are further oxidized to reactive o-quinone metabolites) also produces glutathione conjugated metabolites that may cause glutathione depletion [5,6].
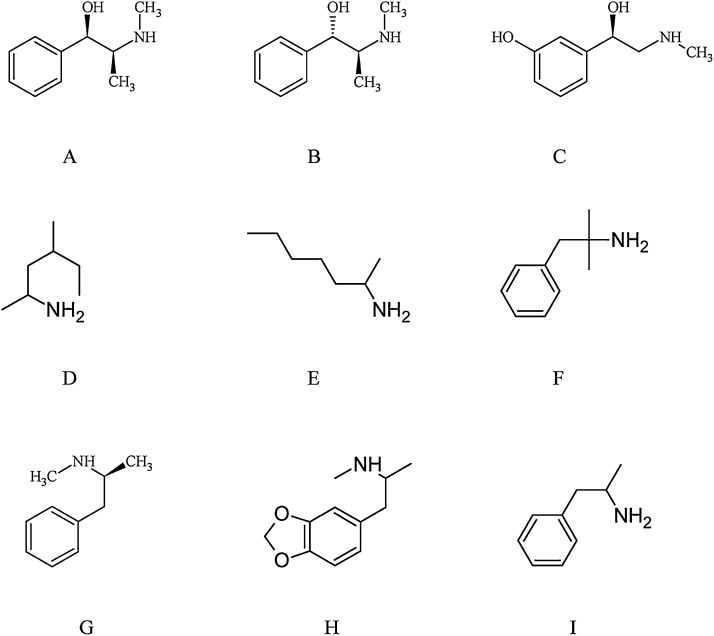
In contrast to amphetamine, compounds such as tuaminoheptane and 1,3-dimethylamylamine obviously lack the necessary aromatic ring for aromatic hydroxylation to occur (Fig. 1), while also lacking the aromatic ring necessary for catechol formation as demonstrated in the case of MDMA [[6], [7], [8]]. Pseudoephedrine, while possessing an aromatic ring and basic phenethylamine skeleton undergoes N-demethylation to form norpseudoephedrine as a minor (i.e. less than 1%) metabolite [9], while it does not appear to undergo aromatic hydroxylation in man, calling into question the potential for increased oxidative stress (Fig. 1). Similarly, ephedrine also undergoes N-demethylation to form norephedrine but does not appear to undergo aromatic hydroxylation in man [10,11].
Fig. 1.
Structures of ephedrine (A), pseudoephedrine (B), phenylephrine (C), 1,3-dimethylamylamine (D), tuaminoheptane (E), phentermine (F), Methamphetamine (G), MDMA (H), Amphetamine (I).
2.2. Hyperthermia
While hyperthermia is a well-known symptom experienced after administration of amphetamines and in cases of intoxication [[12], [13], [14], [15], [16], [17], [18]], these effects have not been noted for various compounds such as tuaminoheptane, ephedrine, 1,3-dimethylamylamine, pseudoephedrine, oxymetazoline and phenylephrine [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]]. Rather, adverse effects seem confined to neurological and cardiovascular systems. Ephedrine has been noted to cause hyperthermia in rats [30]. However, the selective beta 2-adrenergic receptor agonist, salbutamol as well as the non-selective adrenergic agonist, epinephrine also caused hyperthermia in rats [30]. In humans however, these compounds have not been noted to cause hyperthermia even in cases of overdose [[31], [32], [33], [34]] and are not known to cause liver injury. The lack of hyperthermia from beta 2-adrenergic agonists in humans versus rodents may be explained by the interspecies differences between humans and rodents, in which the latter may be more prone to hyperthermia from sympathomimetic agents [35,36]. A double-blind, placebo-controlled trial in healthy men administered ephedrine and caffeine prior to exercise in a hot environment, failed to find an increase in internal body temperature as compared to placebo [37]. Whether the inclusion of caffeine could have altered the thermoregulatory response to ephedrine is unknown; however, if anything, the combination would be expected to enhance any potential hyperthermic effect of ephedrine considering the known additive or synergistic effects when the two are combined [38]. Others have estimated that ephedrine possesses only 6% of the body temperature-raising potency of amphetamine in humans [39] Interestingly, the selective beta 3-adrenergic agonist, mirabegron has been shown to increase energy expenditure in humans [40,41], and is not known to cause hyperthermia or liver injury [42].
Of other interest is a recent case series involving young children presenting after accidental ingestion of amphetamine based stimulants (i.e., those typically prescribed to treat attention deficit hyperactivity disorder (ADHD)—lisdexamfetamine and amphetamine-dextroamphetamine) [43]. Interestingly, while agitation, hypertension and tachycardia were frequently noted, hyperthermia was not demonstrated [43]. Others have reported similar findings (i.e., frequent presentation with agitation and tachycardia without hyperthermia) in young children exposed to methamphetamine [44]. The lack of hyperthermia in young children as compared to adults presenting after ingestion of amphetamines is difficult to explain but it has been suggested that this discrepancy may be due to a lower ingested dose by children [44]. This suggestion seems reasonable considering the relative lack of liver injury noted with therapeutic use of amphetamine-dextroamphetamine as compared to cases of intoxication [45,46], also indicating potential dose-dependency [6,39,[47], [48], [49], [50], [51], [52], [53], [54], [55]].
2.2.1. Distinguishing increased metabolic rate from hyperthermia
With respect to the previous discussion, an important distinction should be made between effects upon metabolic rate or thermogenesis and hyperthermia, in response to sympathomimetic agents. An increase in metabolic rate or thermogenesis may be demonstrated with sympathomimetic agents such as ephedrine as well as spicy food, caffeine and caffeinated beverages but does not result in thermoregulatory dysfunction, allowing normal body temperature to be maintained via compensatory mechanisms [37,[56], [57], [58], [59], [60], [61], [62], [63]]. In contrast, drug-induced hyperthermia, as seen with agents such as methamphetamine and MDMA, results from dysfunction or failure of central hypothalamic body temperature control [15,59,64], although other regions of the body may also play a role [15]. Others have previously suggested that MDMA and the broad category of sympathomimetics induce thermogenesis via distinct mechanisms [12]. The differential effects upon body temperature regulation as seen with agents such as methamphetamine and MDMA as compared to ephedrine and 1,3-dimethylamylamine, for example, can be attributed to differences in monoamine activity, especially dopaminergic and serotonergic activity—See Increased Neurotransmitter Efflux. While norepinephrine and more generally, adrenergic activity are known to play a role in drug-induced hyperthermia experienced with methamphetamine and MDMA, dopamine and serotonin are also thought to play critical roles [15,18,[65], [66], [67]] and can explain the lack of hyperthermia from sympathomimetics which have only adrenergic effects via direct or indirect (i.e., increased norepinephrine and epinephrine) mechanisms.
2.3. Increased neurotransmitter efflux
Amphetamines are known to increase efflux of neurotransmitters such as dopamine, serotonin and norepinephrine. At least some of the hypothesized mechanism regarding hepatotoxicity and increased efflux of these neurotransmitters likely overlaps with other mechanisms (e.g., oxidation of biogenic amines and hyperthermia). Once again, clear distinctions can be made between compounds such as pseudoephedrine, ephedrine and 1,3-dimethylamylamine as compared with amphetamine, methamphetamine and MDMA. For example, in vitro data analyzing activity at human cloned transporters demonstrated that 1,3-dimethylamylamine was essentially inactive (Ki > 10,000 nmol) for the dopamine transporter (DAT) and serotonin transporter (SERT), while having modest activity at the norepinephrine transporter (NET) (Ki of 649 nmol), suggesting it is a selective modulator of norepinephrine activity [68]. Human pharmacokinetic data confirm that NET is a plausible target [29], although data showing no change in serum norepinephrine undermine this notion [69]. Amphetamine and the more potent enantiomer of MDMA (i.e., S(+)-MDMA) on the other hand, possessed Ki values of 109 and 897 nmol; 5728 and 948 nmol; and 101 and 398 nmol, for DAT, SERT and NET, respectively. These data show that 1,3-dimethylamylamine is distinct pharmacologically from amphetamine and MDMA, with the former showing selective activity at the NET, while the latter show activity at multiple monoamine transporters. Others have demonstrated that the structurally similar compound, tuaminoheptane also has activity at the NET [70]. Similarly, pseudoephedrine and ephedrine also show a pharmacological profile which indicates a mechanism of selective modulation of norepinephrine [72,71]. Such distinctions at least in the case of 1,3-dimethylamylamine and tuaminoheptane may be at least partially due to the lack of the aromatic ring [73,74] (Fig.1). As neurotransmitter efflux is related to locomotor stimulating effects in animal models, it is also worth noting that 1,3-dimethylamylamine demonstrates distinct (i.e., no stimulation of locomotor activity) effects as compared to amphetamine and methamphetamine, which significantly increase locomotor activity [75,76]. Finally, ephedrine has been shown to possess far less potency as compared to amphetamine and methamphetamine with respect to the release and uptake inhibition of norepinephrine and dopamine in vitro, while essentially being inactive regarding the release and uptake inhibition of serotonin, as opposed to MDMA which is rather potent in this regard [72]. Such differences may explain ephedrine’s relatively weak (i.e., 6% potency) effect upon body temperature as compared to amphetamine in humans [39].
2.4. Oxidation of biogenic amines
While the increase in catecholamine levels seen with amphetamines is not believed to result in hepatic damage through an alteration in liver blood flow [45], the oxidation of biogenic amines (i.e., the catecholamines, dopamine, epinephrine and norepinephrine) produces reactive quinone metabolites which can lead to oxidative damage [5]. Dopamine and dopamine-like metabolites seem to have the greatest potential for oxidative damage. Aside from the notion that compounds such as pseudoephedrine and 1,3-dimethylamylamine only seem to affect norepinephrine, other data show a lack of increased oxidative stress. For example, a randomized, double-blind, placebo-controlled, 12-week study in healthy men administered 1,3-dimethylamylamine alone or in combination with caffeine, found no change in markers of oxidative stress, including malondialdehyde (MDA), advanced oxidation protein products (AOPP) and trolox equivalent antioxidant capacity (TEAC) [77]. Wistar rats given a dietary supplement containing 1,3-dimethylamylamine in fact showed evidence of an anti-oxidative effect including decreases in thiobarbituric acid reactive substances (TBARS) and AOPP [78]. This can be contrasted with the increase in markers of oxidative stress (e.g., TBARS) in rat liver cells and in humans (e.g., MDA) who are chronic users of methamphetamine [[79], [80], [81], [82]]. Others have shown using a model of lipopolysaccharide-induced acute liver failure in d-galactosamine sensitized male Wistar rats, that orally administered pseudoephedrine and ephedrine are able to decrease serum MDA [83].
2.5. Mitochondrial impairment and apoptosis
Mitochondrial impairment and apoptosis leading to injury have been shown with compounds such as methamphetamine and MDMA [5,6,53,[84], [85], [86], [87]]. As noted previously however, in an acute liver injury model in male Wistar rats, pseudoephedrine and ephedrine inhibited hepatocellular apoptosis presumably via an anti-inflammatory effect [83]. Incidentally, anti-inflammatory effects were previously reported for pseudoephedrine [88]. Few data are available which have evaluated mitochondrial function, although an in vitro study evaluating ephedrine in human hepatic stellate cells found evidence of mitochondrial damage via oxidative stress [89]. However, the concentrations used (e.g., 120 and 240 μg/mL) were extremely high and unlikely to be relevant to in vivo administration. For example, a 50 mg oral dose of ephedrine in humans has been shown to produce an average peak plasma concentration of approximately 138 ng/mL, which is consistent with others [90,91]. Furthermore, even in cases of severe overdose in cases of attempted suicide, concentrations have only reached 23 μg/mL after a 7500 mg dose. While selective accumulation in the liver can occur, the liver to plasma ratio in cases of overdose seem to be consistently around 2:1 to 3:1. For example, a fatality after ingestion of 2100 mg of ephedrine demonstrated blood and liver concentrations of 5 μg/mL and 15 μg/g, respectively. Another case with an unknown dose demonstrated blood and liver concentrations of 11 μg/mL and 24 μg/g, respectively. Thus, reaching such concentrations in vivo without lethality is unlikely. Considering ephedrine’s pharmacokinetics and pharmacological profile with partial effects upon dopaminergic activity, it may still be possible for hepatotoxicity to occur [91,72]. However, considering the similar (i.e., equally or far less potent at releasing or inhibiting uptake of norepinephrine, dopamine and serotonin) profile as compared to phentermine, which lacks findings of liver injury [92,93], it would be difficult to explain such a discrepancy at least based upon effects on monoamine levels [72].
2.5.1. Ephedra versus ephedrine
It is important to note that Ephedra species (i.e., Ephedra sinica or ma-huang) have been implicated in causing liver injury in case reports [83]. However, aside from the limitations inherent to case reports [[94], [95], [96], [97], [98], [99]] it is important to distinguish the natural product, Ephedra species, which contain several alkaloids (including pseudoephedrine and ephedrine) and other constituents, from the single, synthetically produced alkaloids, ephedrine and pseudoephedrine, as the latter have not been implicated in case reports. Whether the case reports involving Ephedra are due to contaminants, misidentification or the combination of alkaloids and other constituents is unknown [100]. Furthermore, at least some cases presumed to be due to the herb consisted of multi-ingredient formulas where known hepatotoxic substances such as green tea extract and other potentially hepatotoxic herbs were also included [83,[101], [102], [103], [104]]. A recent study in F344 rats administered up to 1000 mg/kg of Ephedra extract containing ephedrine and pseudoephedrine failed to find adverse effects upon the liver [105], while Ephedra species demonstrated hepatoprotective effects in models of induced acute and chronic liver failure in mice with noted anti-inflammatory effects and inhibition of hepatocellular apoptosis [106,107]. Interestingly, an in vitro assay using human hepatoblastoma cells (HepG2) demonstrated that when Ephedra (ma-huang) extracts were normalized for their ephedrine content, they displayed greater cytotoxicity relative to ephedrine itself, indicating that there may be other constituents responsible for toxicity [108]. While ephedrine and pseudoephedrine showed cytotoxicity in the HepG2 cell line, the concentrations required (i.e., > 300 μg/mL) again indicate that such data are likely irrelevant to in vivo administration [108]. While Ephedra species have been banned for consumption as dietary supplements in the United States, ephedrine and pseudoephedrine are still generally available over the counter in most states [109].
2.5.2. Liver injury
2.5.2.1. Animal models
With the end of result of apoptotic effects expected to yield liver injury, one may also evaluate controlled studies in humans and animal models for evidence of potential hepatotoxicity. For example, ephedrine and pseudoephedrine decreased liver injury markers (e.g, alanine aminotransferase or ALT and bilirubin) in experimentally induced acute liver injury in Wistar rats [83], while methamphetamine and MDMA are known to increase liver injury markers and demonstrate histological changes indicative of injury in rats [87,110,111]. In Wistar rats given a formulation containing 1,3-dimethylamylamine, no changes were found in ALT, aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT).
2.5.2.2. Humans
With respect to human studies, randomized, double-blind, placebo-controlled trials conducted with various sympathomimetic agents (i.e., pseudoephedrine, ephedrine, 1,3-dimethylamylamine, p-synephrine and phentermine) in different populations failed to show any evidence of liver injury (Table 1). In addition to these interventional studies, a multicenter prospective study also failed to identify pseudoephedrine or ephedrine as a cause of liver injury [112], while MDMA conversely is a well-known cause of liver injury [113]. Finally, a case-control study failed to find any increased risk for liver injury in those consuming 1,3-dimethylamylamine-containing dietary supplements [25]. While some case reports have implicated 1,3-dimethylamylamine-containing supplements in liver injury, major shortcomings such as a lack of exclusion of alternative causes, missed diagnoses, retrospective case-seeking in response to publicity and a lack of objective causality assessment have been noted [3].
Table 1.
Human Trials Evaluating Liver Injury Markers with Sympathomimetic Agents (SA), Pseudoephedrine (PSE), Ephedrine (EPH), 1,3-Dimethylamylamine (DMAA), p-Synephrine (SYN) and Phentermine (PHE).
| Agent | Duration | Subjects (n) Assigned to SA | Liver Injury Markers Evaluated | Change Relative to Placebo | Note | Reference |
|---|---|---|---|---|---|---|
| PSE alone or in combination with cetirizine. | 2 weeks. | 456 | AST, ALT, bilirubin. | Not statistically significant. | Males and females. | [114] |
| EPH in combination with caffeine. | 20 weeks. | 32 | AST, alkaline phosphatase (ALP), bilirubin. | Not statistically significant. | Obese males and females. | [115] |
| EPH in combination with caffeine. | Minimum of 12 weeks. | 152 | AST, ALP, bilirubin. | Not statistically significant. | Males and females attempting smoking cessation. | [116] |
| EPH in combination with caffeine. | 4 weeks. | 6 | AST, ALT. | Not statistically significant. | Morbidly obese females. | [117] |
| DMAA alone or in combination with caffeine. | 12 weeks. | 25 | AST, ALT, GGT and bilirubin. | Not statistically significant. | Young healthy males. | [77] |
| SYN alone or in combination with naringin and hesperidin. | 8 weeks. | 50 | AST, ALT, ALP, bilirubin. | Not statistically significant. | Healthy males and females. | [118] |
| PHE | 12 weeks. | 35 | AST, ALT, bilirubin. | Not statistically significant. | Obese Korean males and females. | [92] |
3. Incorrect attributions of causality and treatment errors
The simple assumption that some structural similarities between two molecules will unequivocally cause them to possess similar or identical pharmacological/toxicological effects is deemed a futile approach or a “waste of time” by medicinal chemists [119], who know that even small structural differences may result in divergent activity. However, a reminder to healthcare practitioners and other scientists seems appropriate [2,4]. In cases where such cognitive errors (i.e., assuming there is a causal relationship between an adverse effect and a given agent based only upon the structure of an agent or a shared physiological effect, despite evidence to the contrary) are present, diagnostic errors may occur which can result in harm to patients due to inappropriate or insufficient treatment [120,121].
3.1. An example: the unopposed alpha effect controversy
An example where serious issues may arise from the assumption that all sympathomimetic agents produce similar adverse effects can be seen in the case of the “unopposed alpha effect”, which is the hypothesis that in cases of sympathomimetic intoxications, the use of a beta-adrenergic receptor antagonist could result in the alpha-adrenergic receptor agonism being unopposed, potentially leading to vasoconstriction and hypertension [122]. Due to the assumption that all sympathomimetic agents are similar (i.e., the original hypothesis was based upon cocaine intoxication), this led to the practice of avoiding the use of beta-adrenergic receptor antagonists in cases of overdose with sympathomimetic agents [123,124]. However, some clinicians have discovered that this view is unwarranted [[122], [123], [124], [125], [126], [127], [128], [129], [130], [131]], demonstrating clear benefit with the use of beta-adrenergic receptor antagonists in cases of sympathomimetic agent intoxications (including ephedrine and pseudoephedrine), while even proponents of the unopposed alpha effect hypothesis have acknowledged that sympathomimetic agents may have divergent toxicological effects, allowing for deviation from the hypothesized contraindication [132].
3.2. Difficulties in diagnosis aided by use of an objective causality assessment method
In cases of liver injury, specifically, drug induced liver injury (DILI); the diagnosis itself may be difficult due to the need for exclusion of a multitude of other causes [133]; some have found up to 34% of suspected DILI cases were probably not actual DILI [134]. In cases of actual DILI, if medications or herbs and dietary supplements are used concomitantly or sequentially, identifying the causal agent objectively is also extremely difficult, if not impossible [135]. Indeed, as some have shown, up to 50% of cases may be based upon unconvincing evidence or fail to identify alternative causes [136,137]. The use of a causality assessment method such as the Roussel Uclaf Causality Assessment Method (RUCAM) can be helpful to identify agents that may be a cause and does not include “structural similarity” or “shared physiological effects” as a method for identifying potential hepatotoxic agents [133]. However, in cases where clinicians are either unaware of the RUCAM or neglect to use it appropriately, the use of structural similarities or a shared physiological effect as an indicator of potential causality may occur [2,4]. The present review demonstrates that such speculation is unsupported by available data.
4. Conclusion
Available data demonstrate that sympathomimetic compounds have diverse effects with respect to molecular targets, physiological effects and toxicological mechanisms. Sympathomimetic agents such as pseudoephedrine, ephedrine, phenylephrine, and 1,3-dimethylamylamine fail to show evidence of production of reactive metabolites, hyperthermia, increased neurotransmitter efflux, oxidation of biogenic amines, mitochondrial impairment and apoptosis as compared to known hepatotoxic agents such as MDMA, amphetamine and methamphetamine. The broad assumption that superficially similar molecules will produce similar toxicological effects is again disconfirmed and should serve as a cautionary warning to clinicians and researchers to avoid drawing unsupported parallels between compounds based only upon structure or some shared physiological effects. Such speculation can lead to diagnostic errors or inadequate treatment.
Conflict of interest
The author has served as a consultant to companies in the dietary supplement industry who have manufactured products containing sympathomimetics. However, these companies would not be expected to gain financially by the work and have no knowledge of or influence over this work at the time of submission.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Transparency document
References
- 1.Biaggioni I., Robertson D. Adrenoceptor agonists & sympathomimetic drugs. Basic & clinical pharmacology. 2009;12:129–149. [Google Scholar]
- 2.Roytman M.M., Poerzgen P., Navarro V. Botanicals and hepatotoxicity. Clin. Pharmacol. Ther. 2018 doi: 10.1002/cpt.1097. [DOI] [PubMed] [Google Scholar]
- 3.Willson C. An evaluation of case report studies concerning dietary supplements containing aegeline and DMAA (1, 3-Dimethylamylamine) Int. J. Med. Res. Health Sci. 2018;7(7):162–178. [Google Scholar]
- 4.Zheng E., Navarro V. Liver injury due to herbal and dietary supplements: a review of individual ingredients. Clin. Liver Dis. (Hoboken) 2016;7(4):80–83. doi: 10.1002/cld.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho M., Pontes H., Remiao F., L Bastos M., Carvalho F. Mechanisms underlying the hepatotoxic effects of ecstasy. Curr. Pharm. Biotechnol. 2010;11(5):476–495. doi: 10.2174/138920110791591535. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho M., Carmo H., Costa V.M., Capela J.P., Pontes H., Remião F. Toxicity of amphetamines: an update. Arch. Toxicol. 2012;86(8):1167–1231. doi: 10.1007/s00204-012-0815-5. [DOI] [PubMed] [Google Scholar]
- 7.Gopisankar M.G. CYP2D6 pharmacogenomics. Egypt. J. Med. Hum. Genet. 2017;18(4):309–313. [Google Scholar]
- 8.Lussenburg B.M., Keizers P.H., de Graaf C., Hidestrand M., Ingelman-Sundberg M., Vermeulen N.P., Commandeur J.N. The role of phenylalanine 483 in cytochrome P450 2D6 is strongly substrate dependent. Biochem. Pharmacol. 2005;70(8):1253–1261. doi: 10.1016/j.bcp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Tseng Y.L., Shieh M.H., Kuo F.H. Metabolites of ephedrines in human urine after administration of a single therapeutic dose. Forensic Sci. Int. 2006;157(2-3):149–155. doi: 10.1016/j.forsciint.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Kawai K., Baba S. Studies on drug metabolism by use of isotopes. XVII. Mass spectrometric quantification of urinary metabolites of deuterated l-ephedrine in man. Chem. Pharm. Bull. 1976;24(11):2728–2732. doi: 10.1248/cpb.24.2728. [DOI] [PubMed] [Google Scholar]
- 11.Sever P.S., Dring L.G., Williams R.T. The metabolism of (−)-ephedrine in man. Eur. J. Clin. Pharmacol. 1975;9(2-3):193–198. doi: 10.1007/BF00614017. [DOI] [PubMed] [Google Scholar]
- 12.Freedman R.R., Johanson C.E., Tancer M.E. Thermoregulatory effects of 3, 4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2005;183(2):248–256. doi: 10.1007/s00213-005-0149-6. [DOI] [PubMed] [Google Scholar]
- 13.Jordan S.C., Hampson F. Amphetamine poisoning associated with hyperpyrexia. Br. Med. J. 1960;2(5202):844. doi: 10.1136/bmj.2.5202.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalant H., Kalant O.J. Death in amphetamine users: causes and rates. Can. Med. Assoc. J. 1975;112(3):299. [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto R.R., Seminerio M.J., Turner R.C., Robson M.J., Nguyen L., Miller D.B., O’callaghan J.P. Methamphetamine-induced toxicity: an updated review on issues related to hyperthermia. Pharmacol. Ther. 2014;144(1):28–40. doi: 10.1016/j.pharmthera.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orrenius S., Maehly A.C. Lethal amphetamine intoxication. Z. Fã¼r Rechtsmedizin. 1970;67(3):184–189. doi: 10.1007/BF00200358. [DOI] [PubMed] [Google Scholar]
- 17.Parrott A.C. MDMA and temperature: a review of the thermal effects of ‘Ecstasy’in humans. Drug Alcohol Depend. 2012;121(1-2):1–9. doi: 10.1016/j.drugalcdep.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Spiller H.A., Hays H.L., Aleguas A. Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management. CNS Drugs. 2013;27(7):531–543. doi: 10.1007/s40263-013-0084-8. [DOI] [PubMed] [Google Scholar]
- 19.Akovaz . Avadel Legacy Pharmaceuticals, LLC; Chesterfield, Missouri: 2017. Akovaz (ephedrine Sulfate Injection) Full Prescribing Information. [Google Scholar]
- 20.Brown J.A., Buckley N.A. Toxicity from bodybuilding supplements and recreational use of products containing 1, 3-dimethylamylamine. Med. J. Aust. 2013;198(8):414–415. doi: 10.5694/mja12.11167. [DOI] [PubMed] [Google Scholar]
- 21.Dokuyucu R., Gokce H., Sahan M., Sefil F., Tas Z.A., Tutuk O. Systemic side effects of locally used oxymetazoline. Int. J. Clin. Exp. Med. 2015;8(2):2674. [PMC free article] [PubMed] [Google Scholar]
- 22.Forrester M.B. Exposures to 1, 3-dimethylamylamine-containing products reported to Texas poison centers. Hum. Exp. Toxicol. 2013;32(1):18–23. doi: 10.1177/0960327112454895. [DOI] [PubMed] [Google Scholar]
- 23.Gosselin S. In: Goldfrank’S Toxicologic Emergencies. Hoffman R.S., Howland M., Lewin N.A., Nelson L.S., Goldfrank L.R., editors. McGraw-Hill Medical; New York: 2015. [Google Scholar]
- 24.Laccourreye O., Werner A., Giroud J.P., Couloigner V., Bonfils P., Bondon-Guitton E. Benefits, limits and danger of ephedrine and pseudoephedrine as nasal decongestants. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2015;132(1):31–34. doi: 10.1016/j.anorl.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Lammie C.J. 2013. Report of the Department of Defense 1, 3 Dimethylamylamine (DMAA) Safety Review Panel. [Google Scholar]
- 26.Milgrom H., Bender B. Adverse effects of medications for rhinitis. Ann. Allergy Asthma Immunol. 1997;78(5):439–446. doi: 10.1016/S1081-1206(10)63230-9. [DOI] [PubMed] [Google Scholar]
- 27.Mortuaire G., De Gabory L., Francois M., Massé G., Bloch F., Brion N. Rebound congestion and rhinitis medicamentosa: nasal decongestants in clinical practice. Critical review of the literature by a medical panel. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2013;130(3):137–144. doi: 10.1016/j.anorl.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Mouatt J.R. Massey University; Palmerston North, New Zealand: 2008. The Physiological Effects of Pseudoephedrine on Endurance Cycling: a Thesis Submitted in the Partial Fulfilment of the Requirements for the Degree of Master of Science in Sport and Exercise Science. [Google Scholar]
- 29.Schilling B.K., Hammond K.G., Bloomer R.J., Presley C.S., Yates C.R. Physiological and pharmacokinetic effects of oral 1, 3-dimethylamylamine administration in men. BMC Pharmacol. Toxicol. 2013;14(1):52. doi: 10.1186/2050-6511-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamahara J., Kimata M., Sawada T., Fujimura H. Possible involvement of β2-adrenoceptors in hyperthermic effect of l-ephedrine in rats. J. Pharmacobio-dyn. 1985;8(8):591–596. doi: 10.1248/bpb1978.8.591. [DOI] [PubMed] [Google Scholar]
- 31.Dey Pharma L.P. 2012. Prescribing Information: EpiPen (epinephrine injection) [Google Scholar]
- 32.Hoffman R.S. In: Goldfrank’S Toxicologic Emergencies. Hoffman R.S., Howland M., Lewin N.A., Nelson L.S., Goldfrank L.R., editors. McGraw-Hill Medical; New York: 2015. [Google Scholar]
- 33.Raj A.S., Dhileepan S. Prefilled ephedrine syringes. Anaesthesia. 2004;59(6):621–622. doi: 10.1111/j.1365-2044.2004.03818.x. [DOI] [PubMed] [Google Scholar]
- 34.Simons F.E.R., Ardusso L.R., Bilò M.B., El-Gamal Y.M., Ledford D.K., Ring J. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ. J. 2011;4(2):13. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Easton N., Marsden C.A. Ecstasy: are animal data consistent between species and can they translate to humans? J. Psychopharmacol. 2006;20(2):194–210. doi: 10.1177/0269881106061153. [DOI] [PubMed] [Google Scholar]
- 36.Gordon C.J. Thermophysiological responses to hyperthermic drugs: extrapolating from rodent to human. Prog. Brain Res. 2007;162:63–79. doi: 10.1016/S0079-6123(06)62005-0. [DOI] [PubMed] [Google Scholar]
- 37.Bell D.G., Jacobs I., McLellan T.M., Miyazaki M., Sabiston C.M. Thermal regulation in the heat during exercise after caffeine and ephedrine ingestion. Aviat. Space Environ. Med. 1999;70(6):583–588. [PubMed] [Google Scholar]
- 38.Magkos F., Kavouras S.A. Caffeine and ephedrine. Sport. Med. 2004;34(13):871–889. doi: 10.2165/00007256-200434130-00002. [DOI] [PubMed] [Google Scholar]
- 39.Martin W.R., Sloan J.W., Sapira J.D., Jasinski D.R. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin. Pharmacol. Ther. 1971;12(2part1):245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- 40.Cypess A.M., Weiner L.S., Roberts-Toler C., Elía E.F., Kessler S.H., Kahn P.A. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21(1):33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loh R.K., Formosa M.F., La Gerche A., Reutens A.T., Kingwell B.A., Carey A.L. Acute metabolic and cardiovascular effects of mirabegron in healthy individuals. Diabetes Obes. Metab. 2018 doi: 10.1111/dom.13516. [DOI] [PubMed] [Google Scholar]
- 42.LiverTox . National Institutes of Health; 2018. Drug Record: Mirabegron.https://livertox.nih.gov/Mirabegron.htm (Accessed 2 September 2018) [Google Scholar]
- 43.Wood K.E., McCarthy P.J., Krasowski M.D. A case series involving young children presenting with accidental ingestion of amphetamine based stimulants. Toxicol. Rep. 2018 doi: 10.1016/j.toxrep.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matteucci M.J., Auten J.D., Crowley B., Combs D., Clark R.F. Methamphetamine exposures in young children. Pediatr. Emerg. Care. 2007;23(9):638–640. doi: 10.1097/PEC.0b013e31814a6a79. [DOI] [PubMed] [Google Scholar]
- 45.Jones A.L., Simpson K.J. Ecstasy (MDMA) and amphetamine intoxications. Aliment. Pharmacol. Ther. 1999;13 doi: 10.1046/j.1365-2036.1999.00454.x. 129ą133. [DOI] [PubMed] [Google Scholar]
- 46.Vanga R.R., Bal B., Olden K.W. Adderall induced acute liver injury: a rare case and review of the literature. Case Rep. Gastrointest. Med. 2013;2013 doi: 10.1155/2013/902892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carvalho M., Carvalho F., Bastos M.L. Is hyperthermia the triggering factor for hepatotoxicity induced by 3, 4-methylenedioxymethamphetamine (ecstasy)? An in vitro study using freshly isolated mouse hepatocytes. Arch. Toxicol. 2001;74(12):789–793. doi: 10.1007/s002040000200. [DOI] [PubMed] [Google Scholar]
- 48.de Wit H., Clark M., Brauer L.H. Effects of d-amphetamine in grouped versus isolated humans. Pharmacol. Biochem. Behav. 1997;57(1-2):333–340. doi: 10.1016/s0091-3057(96)00316-4. [DOI] [PubMed] [Google Scholar]
- 49.Green A.R., O’shea E., Colado M.I. A review of the mechanisms involved in the acute MDMA (ecstasy)-induced hyperthermic response. Eur. J. Pharmacol. 2004;500(1-3):3–13. doi: 10.1016/j.ejphar.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Halpin L.E., Gunning W.T., Yamamoto B.K. Methamphetamine causes acute hyperthermia‐dependent liver damage. Pharmacol. Res. Perspect. 2013;1(1) doi: 10.1002/prp2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levi M.S., Divine B., Hanig J.P., Doerge D.R., Vanlandingham M.M., George N.I. A comparison of methylphenidate-, amphetamine-, and methamphetamine-induced hyperthermia and neurotoxicity in male Sprague–Dawley rats during the waking (lights off) cycle. Neurotoxicol. Teratol. 2012;34(2):253–262. doi: 10.1016/j.ntt.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Miller L., Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacology. 1983;80(3):199–205. doi: 10.1007/BF00436152. [DOI] [PubMed] [Google Scholar]
- 53.Valente M.J., Araújo A.M., Bastos M.D.L., Fernandes E., Carvalho F., Guedes de Pinho P., Carvalho M. Editor’s highlight: characterization of hepatotoxicity mechanisms triggered by designer cathinone drugs (β-Keto amphetamines) Toxicol. Sci. 2016;153(1):89–102. doi: 10.1093/toxsci/kfw105. [DOI] [PubMed] [Google Scholar]
- 54.Vizeli P., Liechti M.E. Safety pharmacology of acute MDMA administration in healthy subjects. J. Psychopharmacol. 2017;31(5):576–588. doi: 10.1177/0269881117691569. [DOI] [PubMed] [Google Scholar]
- 55.Yehuda S., Wurtman R.J. The effects of D-amphetamine and related drugs on colonic temperatures of rats kept at various ambient temperatures. Life Sci. 1972;11(18):851–859. doi: 10.1016/0024-3205(72)90101-4. [DOI] [PubMed] [Google Scholar]
- 56.Acheson K.J., Zahorska-Markiewicz B., Pittet P., Anantharaman K., Jéquier E. Caffeine and coffee: their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am. J. Clin. Nutr. 1980;33(5):989–997. doi: 10.1093/ajcn/33.5.989. [DOI] [PubMed] [Google Scholar]
- 57.Armstrong L.E., Casa D.J., Maresh C.M., Ganio M.S. Caffeine, fluid-electrolyte balance, temperature regulation, and exercise-heat tolerance. Exerc. Sport Sci. Rev. 2007;35(3):135–140. doi: 10.1097/jes.0b013e3180a02cc1. [DOI] [PubMed] [Google Scholar]
- 58.Chaiyata P., Puttadechakum S. Effect of chili pepper (Capsicum frutescens) ingestion on plasma glucose response and metabolic rate in thai. J. Med. Assoc. Thai. 2003;86:854–860. [PubMed] [Google Scholar]
- 59.Dao C.K., Nowinski S.M., Mills E.M. The heat is on: molecular mechanisms of drug-induced hyperthermia. Temperature. 2014;1(3):183–191. doi: 10.4161/23328940.2014.985953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ely B.R., Ely M.R., Cheuvront S.N. Marginal effects of a large caffeine dose on heat balance during exercise-heat stress. Int. J. Sport Nutr. Exerc. Metab. 2011;21(1):65–70. doi: 10.1123/ijsnem.21.1.65. [DOI] [PubMed] [Google Scholar]
- 61.Jaedig S., Henningsen N.C. Increased metabolic rate in obese women after ingestion of potassium, magnesium-and phosphate-enriched orange juice or injection of ephedrine. Int. J. Obes. 1991;15(6):429–436. [PubMed] [Google Scholar]
- 62.Persky A.M., Ng C., Song M.H., Lancaster M.E., Balderson D.E., Paulik M.A., Brouwer K.L. Comparison of the acute pharmacodynamic responses after single doses of ephedrine or sibutramine in healthy, overweight volunteers. Int. J. Clin. Pharmacol. Ther. 2004;42(8):442–448. doi: 10.5414/cpp42442. [DOI] [PubMed] [Google Scholar]
- 63.Shannon J.R., Gottesdiener K., Jordan J., Kong C.H.E.N., Flattery S., Larson P.J. Acute effect of ephedrine on 24-h energy balance. Clin. Sci. 1999;96(5):483–491. [PubMed] [Google Scholar]
- 64.Cox B., Lomax P. Pharmacologic control of temperature regulation. Annu. Rev. Pharmacol. Toxicol. 1977;17(1):341–353. doi: 10.1146/annurev.pa.17.040177.002013. [DOI] [PubMed] [Google Scholar]
- 65.Liechti M.E., Saur M.R., Gamma A., Hell D., Vollenweider F.X. Psychological and physiological effects of MDMA (“Ecstasy”) after pretreatment with the 5-HT2 antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000;23(4):396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- 66.Numachi Y., Ohara A., Yamashita M., Fukushima S., Kobayashi H., Hata H. Methamphetamine-induced hyperthermia and lethal toxicity: role of the dopamine and serotonin transporters. Eur. J. Pharmacol. 2007;572(2-3):120–128. doi: 10.1016/j.ejphar.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 67.Shioda K., Nisijima K., Yoshino T., Kuboshima K., Iwamura T., Yui K., Kato S. Risperidone attenuates and reverses hyperthermia induced by 3, 4-methylenedioxymethamphetamine (MDMA) in rats. Neurotoxicology. 2008;29(6):1030–1036. doi: 10.1016/j.neuro.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Iversen L., Gibbons S., Treble R., Setola V., Huang X.P., Roth B.L. Neurochemical profiles of some novel psychoactive substances. Eur. J. Pharmacol. 2013;700(1-3):147–151. doi: 10.1016/j.ejphar.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bloomer R.J., Harvey I.C., Farney T.M., Bell Z.W., Canale R.E. Effects of 1, 3-dimethylamylamine and caffeine alone or in combination on heart rate and blood pressure in healthy men and women. Phys. Sportsmed. 2011;39(3):111–120. doi: 10.3810/psm.2011.09.1927. [DOI] [PubMed] [Google Scholar]
- 70.Schlessinger A., Geier E., Fan H., Irwin J.J., Shoichet B.K., Giacomini K.M., Sali A. Structure-based discovery of prescription drugs that interact with the norepinephrine transporter, NET. Proc. Natl. Acad. Sci. 2011 doi: 10.1073/pnas.1106030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rothman R.B., Vu N., Partilla J.S., Roth B.L., Hufeisen S.J., Compton-Toth B.A. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J. Pharmacol. Exp. Ther. 2003;307(1):138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- 72.Rothman R.B., Baumann M.H., Dersch C.M., Romero D.V., Rice K.C., Carroll F.I., Partilla J.S. Amphetamine‐type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39(1):32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 73.Heal D.J., Smith S.L., Gosden J., Nutt D.J. Amphetamine, past and present–a pharmacological and clinical perspective. J. Psychopharmacol. 2013;27(6):479–496. doi: 10.1177/0269881113482532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodricks, J. V., & Lumpkin, M. H. (2013). DMAA as a dietary ingredient. JAMA internal medicine, 173(7), 594-595.Rothman, R. B., Baumann, M. H., Dersch, C. M., Romero, D. V. [DOI] [PubMed]
- 75.Dolan S.B., Gatch M.B. Abuse liability of the dietary supplement dimethylamylamine. Drug Alcohol Depend. 2015;146:97–102. doi: 10.1016/j.drugalcdep.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van der Schoot J., Ariëns E.J., van Rossum J., Hurkmans J. Phenylisopropylamine derivatives, structure and action. ArzneimittelForschung. 1962;12:902–907. [PubMed] [Google Scholar]
- 77.Bloomer R.J., Farney T.M., Harvey I.C., Alleman R.J. Safety profile of caffeine and 1, 3-dimethylamylamine supplementation in healthy men. Hum. Exp. Toxicol. 2013;32(11):1126–1136. doi: 10.1177/0960327113475680. [DOI] [PubMed] [Google Scholar]
- 78.Zovico P.V.C., Curty V.M., Leal M.A.S., Meira E.F., Dias D.V., de Melo Rodrigues L.C. Effects of controlled doses of Oxyelite Pro on physical performance in rats. Nutr. Metab. (Lond) 2016;13(1):90. doi: 10.1186/s12986-016-0152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carvalho F., Remião F., Soares M.E., Catarino R., Queiroz G., Bastos M.L. d-Amphetamine-induced hepatotoxicity: possible contribution of catecholamines and hyperthermia to the effect studied in isolated rat hepatocytes. Arch. Toxicol. 1997;71(7):429–436. doi: 10.1007/s002040050407. [DOI] [PubMed] [Google Scholar]
- 80.Lourenço T.C., Bósio G.C., Cassiano N.M., Cass Q.B., Moreau R.L. Chiral separation of 3, 4-methylenedioxymethamphetamine (MDMA) enantiomers using batch chromatography with peak shaving recycling and its effects on oxidative stress status in rat liver. J. Pharm. Biomed. Anal. 2013;73:13–17. doi: 10.1016/j.jpba.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 81.Solhi H., Malekirad A., Mohammad Kazemifar A., Sharifi F. Oxidative stress and lipid peroxidation in prolonged users of methamphetamine. Drug Metab. Lett. 2013;7(2):79–82. doi: 10.2174/187231280702140520191324. [DOI] [PubMed] [Google Scholar]
- 82.Suriyaprom K., Tanateerabunjong R., Tungtrongchitr A., Tungtrongchitr R. Alterations in malondialdehyde levels and laboratory parameters among methamphetamine abusers. Journal of the Medical Association of Thailand= Chotmaihet thangphaet. 2011;94(12):1533–1539. [PubMed] [Google Scholar]
- 83.Wu Z., Kong X., Zhang T., Ye J., Fang Z., Yang X. Pseudoephedrine/ephedrine shows potent anti-inflammatory activity against TNF-α-mediated acute liver failure induced by lipopolysaccharide/d-galactosamine. Eur. J. Pharmacol. 2014;724:112–121. doi: 10.1016/j.ejphar.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 84.Behroozaghdam M., Hashemi M., Javadi G., Mahdian R., Soleimani M. Expression of bax and bcl2 genes in MDMA-induced hepatotoxicity on rat liver using quantitative real-time PCR method through triggering programmed cell death. Iran. Red Crescent Med. J. 2015;(11):17. doi: 10.5812/ircmj.24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eskandari M.R., Rahmati M., Khajeamiri A.R., Kobarfard F., Noubarani M., Heidari H. A new approach on methamphetamine-induced hepatotoxicity: involvement of mitochondrial dysfunction. Xenobiotica. 2014;44(1):70–76. doi: 10.3109/00498254.2013.807958. [DOI] [PubMed] [Google Scholar]
- 86.Moon K.H., Upreti V.V., Yu L.R., Lee I.J., Ye X., Eddington N.D. Mechanism of 3, 4‐methylenedioxymethamphetamine (MDMA, ecstasy)‐mediated mitochondrial dysfunction in rat liver. Proteomics. 2008;8(18):3906–3918. doi: 10.1002/pmic.200800215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Q., Wei L.W., Xiao H.Q., Xue Y., Du S.H., Liu Y.G., Xie X.L. Methamphetamine induces hepatotoxicity via inhibiting cell division, arresting cell cycle and activating apoptosis: in vivo and in vitro studies. Food Chem. Toxicol. 2017;105:61–72. doi: 10.1016/j.fct.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 88.Hikino H., Konno C., Takata H., Tamada M. Antiinflammatory principle of Ephedra herbs. Chem. Pharm. Bull. 1980;28(10):2900–2904. doi: 10.1248/cpb.28.2900. [DOI] [PubMed] [Google Scholar]
- 89.Lee A.Y., Jang Y., Hong S.H., Chang S.H., Park S., Kim S. Ephedrine-induced mitophagy via oxidative stress in human hepatic stellate cells. J. Toxicol. Sci. 2017;42(4):461–473. doi: 10.2131/jts.42.461. [DOI] [PubMed] [Google Scholar]
- 90.Backer R., Tautman D., Lowry S., Harvey C.M., Poklis A. Fatal ephedrine intoxication. Journal of Forensic Science. 1997;42(1):157–159. [PubMed] [Google Scholar]
- 91.Berlin I., Warot D., Aymard G., Acquaviva E., Legrand M., Labarthe B. Pharmacodynamics and pharmacokinetics of single nasal (5 mg and 10 mg) and oral (50 mg) doses of ephedrine in healthy subjects. Eur. J. Clin. Pharmacol. 2001;57(6-7):447–455. doi: 10.1007/s002280100317. [DOI] [PubMed] [Google Scholar]
- 92.Kim K.K., Cho H.J., Kang H.C., Youn B.B., Lee K.R. Effects on weight reduction and safety of short-term phentermine administration in Korean obese people. Yonsei Med. J. 2006;47(5):614–625. doi: 10.3349/ymj.2006.47.5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.LiverTox . National Institutes of Health; 2018. Drug Record: Phetermine.https://livertox.nih.gov/Phentermine.htm (Accessed 2 September 2018) [Google Scholar]
- 94.Brighton B., Bhandari M., Tornetta P., Felson D.T. Hierarchy of evidence: from case reports to randomized controlled trials. Clin. Orthop. Relat. Res. 2003;413:19–24. doi: 10.1097/01.blo.0000079323.41006.12. [DOI] [PubMed] [Google Scholar]
- 95.Hutchinson T.A., Lane D.A. Assessing methods for causality assessment of suspected adverse drug reactions. J. Clin. Epidemiol. 1989;42(1):5–16. doi: 10.1016/0895-4356(89)90020-6. [DOI] [PubMed] [Google Scholar]
- 96.Impicciatore P., Mucci M. Completeness of published case reports on suspected adverse drug reactions. Drug Saf. 2010;33(9):765–773. doi: 10.2165/11537500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 97.Karch S.B. Peer review and the process of publishing of adverse drug event reports. J. Forensic Leg. Med. 2007;14(2):79–84. doi: 10.1016/j.jcfm.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 98.Nissen T., Wynn R. The clinical case report: a review of its merits and limitations. BMC Res. Notes. 2014;7(1):264. doi: 10.1186/1756-0500-7-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sica G.T. Bias in research studies. Radiology. 2006;238(3):780–789. doi: 10.1148/radiol.2383041109. [DOI] [PubMed] [Google Scholar]
- 100.Nadir A., Agrawal S., King P.D., Marshall J.B. Acute hepatitis associated with the use of a Chinese herbal product, ma-huang. Am. J. Gastroenterol. 1996;(7):91. [PubMed] [Google Scholar]
- 101.Bajaj J., Knox J.F., Komorowski R., Saeian K. Case report: the irony of herbal hepatitis: ma-huang-Induced hepatotoxicity associated with compound heterozygosity for hereditary hemochromatosis. Dig. Dis. Sci. 2003;48(10):1925–1928. doi: 10.1023/a:1026105917735. [DOI] [PubMed] [Google Scholar]
- 102.Melchart D., Hager S., Albrecht S., Dai J., Weidenhammer W., Teschke R. Herbal Traditional Chinese Medicine and suspected liver injury: a prospective study. World J. Hepatol. 2017;9(29):1141. doi: 10.4254/wjh.v9.i29.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Neff G.W., Reddy K.R., Durazo F.A., Meyer D., Marrero R., Kaplowitz N. Severe hepatotoxicity associated with the use of weight loss diet supplements containing ma huang or usnic acid. J. Hepatol. 2004;41(6):1062–1064. doi: 10.1016/j.jhep.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 104.Skoulidis F., Alexander G.J., Davies S.E. Ma huang associated acute liver failure requiring liver transplantation. Eur. J. Gastroenterol. Hepatol. 2005;17(5):581–584. doi: 10.1097/00042737-200505000-00017. [DOI] [PubMed] [Google Scholar]
- 105.Han H.Y., Huh J.I., Han S.R., Kang M.G., Yoon S., Han J.S. Assessing the safety of an Ephedrae Herba aqueous extract in rats: a repeat dose toxicity study. Regul. Toxicol. Pharmacol. 2018;94:144–151. doi: 10.1016/j.yrtph.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 106.Ghasemi M., Azarnia M., Jamali M., Mirabolghasemi G., Nazarian S., Naghizadeh M.M. Protective effects of Ephedra pachyclada extract on mouse models of carbon tetrachloride-induced chronic and acute liver failure. Tissue Cell. 2014;46(1):78–85. doi: 10.1016/j.tice.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 107.Yamada I., Goto T., Takeuchi S., Ohshima S., Yoneyama K., Shibuya T. Mao (Ephedra sinica Stapf) protects against D-galactosamine and lipopolysaccharide-induced hepatic failure. Cytokine. 2008;41(3):293–301. doi: 10.1016/j.cyto.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 108.Lee M.K., Cheng B.W.H., Che C.T., Hsieh D.P.H. Cytotoxicity assessment of Ma-huang (Ephedra) under different conditions of preparation. Toxicol. Sci. 2000;56(2):424–430. doi: 10.1093/toxsci/56.2.424. [DOI] [PubMed] [Google Scholar]
- 109.NAMSDL—National Alliance for Model State Drug Laws . 2015. Ephedrine and Pseudodephedrine: Summary of State Retail Sales Laws.http://www.namsdl.org/library/1A09503A-F9D0-6A20-9BD7F72C4717F2F4/ Charlottesville, VA, USA. Available at: [Google Scholar]
- 110.Golchoobian R., Nabavizadeh F., Roghani M., Foroumadi A., Mohammadian M. Ghrelin alleviates MDMA-Induced disturbance of serum glucose and lipids levels in the rat. Acta Med. Iran. 2018;55(12):736–743. [PubMed] [Google Scholar]
- 111.Shahraki M.R., Irani M. The effects of ecstasy on liver function tests, blood glucose, and lipids profile of male rats. Int. J. High Risk Behav. Addict. 2014;3(4) doi: 10.5812/ijhrba.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reuben A., Koch D.G., Lee W.M. Drug‐induced acute liver failure: results of a US multicenter, prospective study. Hepatology. 2010;52(6):2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Andreu V., Mas A., Bruguera M., Salmerón J.M., Moreno V., Nogué S., Rodés J. Ecstasy: a common cause of severe acute hepatotoxicity. J. Hepatol. 1998;29(3):394–397. doi: 10.1016/s0168-8278(98)80056-1. [DOI] [PubMed] [Google Scholar]
- 114.Groseclaude M., Mees K., Pinelli M.E., Lucas M., Van de Venne H. Cetrizine and pseudoephedrine retard, given alone or in combination, in patients with seasonal allergic rhinitis. Rhinology. 1997;35:67–73. [PubMed] [Google Scholar]
- 115.Molnar D., Török K., Erhardt E., Jeges S. Safety and efficacy of treatment with an ephedrine/caffeine mixture. The first double-blind placebo-controlled pilot study in adolescents. Int. J. Obes. 2000;24(12):1573. doi: 10.1038/sj.ijo.0801433. [DOI] [PubMed] [Google Scholar]
- 116.Nørregaard J., Jørgensen S., Mikkelsen K.L., Tønnesen P., Iversen E., Sørensen T. The effect of ephedrine plus caffeine on smoking cessation and post cessation weight gain. Clin. Pharmacol. Ther. 1996;60(6):679–686. doi: 10.1016/S0009-9236(96)90217-9. [DOI] [PubMed] [Google Scholar]
- 117.Bracale R., Petroni M.L., Davinelli S., Bracale U., Scapagnini G., Carruba M.O., Nisoli E. Muscle uncoupling protein 3 expression is unchanged by chronic ephedrine/caffeine treatment: results of a double blind, randomised clinical trial in morbidly obese females. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0098244. e98244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaats G.R., Miller H., Preuss H.G., Stohs S.J. A 60 day double-blind, placebo-controlled safety study involving Citrus aurantium (bitter orange) extract. Food Chem. Toxicol. 2013;55:358–362. doi: 10.1016/j.fct.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 119.Kubinyi H. Chemical similarity and biological activities. J. Braz. Chem. Soc. 2002;13(6):717–726. [Google Scholar]
- 120.Berner E.S., Graber M.L. Overconfidence as a cause of diagnostic error in medicine. Am. J. Med. 2008;121(5):S2–S23. doi: 10.1016/j.amjmed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 121.van den Berge K., Mamede S. Cognitive diagnostic error in internal medicine. Eur. J. Intern. Med. 2013;24(6):525–529. doi: 10.1016/j.ejim.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 122.King A., Dimovska M., Bisoski L. Sympathomimetic toxidromes and other pharmacological causes of acute hypertension. Curr. Hypertens. Rep. 2018;20(1):8. doi: 10.1007/s11906-018-0807-9. [DOI] [PubMed] [Google Scholar]
- 123.Richards J.R., Hollander J.E., Ramoska E.A., Fareed F.N., Sand I.C., Izquierdo Gómez M.M., Lange R.A. β-blockers, cocaine, and the unopposed α-stimulation phenomenon. J. Cardiovasc. Pharmacol. Ther. 2017;22(3):239–249. doi: 10.1177/1074248416681644. [DOI] [PubMed] [Google Scholar]
- 124.Richards J.R., Horowitz B.Z. Coronary vasospasm precipitated by pseudoephedrine, not metoprolol. Can. J. Cardiol. 2017;33(12):1737–e9. doi: 10.1016/j.cjca.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 125.Akay S., Ozdemir M. Acute coronary syndrome presenting after pseudoephedrine use and regression with beta-blocker therapy. Can. J. Cardiol. 2008;24(11):e86–e88. doi: 10.1016/s0828-282x(08)70200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burkhart K.K. Intravenous propranolol reverses hypertension after sympathomimetic overdose: two case reports. J. Toxicol. Clin. Toxicol. 1992;30(1):109–114. doi: 10.3109/15563659208994450. [DOI] [PubMed] [Google Scholar]
- 127.Freeman K., Feldman J.A. Cocaine, myocardial infarction, and β-blockers: time to rethink the equation? Ann. Emerg. Med. 2008;51(2):130–134. doi: 10.1016/j.annemergmed.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 128.Mores N., Campia U., Navarra P., Cardillo C., Preziosi P. No cardiovascular effects of single-dose pseudoephedrine in patients with essential hypertension treated with β-blockers. Eur. J. Clin. Pharmacol. 1999;55(4):251–254. doi: 10.1007/s002280050624. [DOI] [PubMed] [Google Scholar]
- 129.Richards J.R., Ramoska E.A., Sand I.C. Beta-blocker treatment of caffeine-induced tachydysrhythmias. Clin. Toxicol. 2016;54(5):466. doi: 10.3109/15563650.2016.1159311. (Philadelphia, Pa.) [DOI] [PubMed] [Google Scholar]
- 130.Richards J.R., Gould J.B., Laurin E.G., Albertson T.E. Metoprolol treatment of dual cocaine and bupropion cardiovascular and central nervous system toxicity. Clin. Exp. Emerg. Med. 2018 doi: 10.15441/ceem.17.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schurr J.W., Gitman B., Belchikov Y. Controversial Therapeutics: The β‐Adrenergic Antagonist and Cocaine‐Associated Cardiovascular Complications Dilemma. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2014;34(12):1269–1281. doi: 10.1002/phar.1486. [DOI] [PubMed] [Google Scholar]
- 132.Laskowski L.K., Nelson L.S., Smith S.W., Hoffman R.S. Authors’ response to:“Beta-blocker treatment of caffeine-induced tachydysrhythmias”. Clin. Toxicol. 2016;54(5) doi: 10.3109/15563650.2016.1159313. 467-467. [DOI] [PubMed] [Google Scholar]
- 133.Danan G., Teschke R. RUCAM in drug and herb induced liver injury: the update. Int. J. Mol. Sci. 2015;17(1):14. doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Teschke R., Danan G. Drug induced liver injury with analysis of alternative causes as confounding variables. Br. J. Clin. Pharmacol. 2018 doi: 10.1111/bcp.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lim R., Choudry H., Conner K., Karnsakul W. A challenge for diagnosing acute liver injury with concomitant/sequential exposure to multiple drugs: can causality assessment scales be utilized to identify the offending drug? Case Rep. Pediatr. 2014;2014 doi: 10.1155/2014/156389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Björnsson E.S., Hoofnagle J.H. Categorization of drugs implicated in causing liver injury: critical assessment based on published case reports. Hepatology. 2016;63(2):590–603. doi: 10.1002/hep.28323. [DOI] [PubMed] [Google Scholar]
- 137.Teschke R., Schulze J., Schwarzenboeck A., Eickhoff A., Frenzel C. Herbal hepatotoxicity: suspected cases assessed for alternative causes. Eur. J. Gastroenterol. Hepatol. 2013;25(9):1093–1098. doi: 10.1097/MEG.0b013e3283603e89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.