Abstract
During genome replication, polymerase epsilon (Pol ε) acts as the major leading-strand DNA polymerase. Here we report the identification of biallelic mutations in POLE, encoding the Pol ε catalytic subunit POLE1, in 15 individuals from 12 families. Phenotypically, these individuals had clinical features closely resembling IMAGe syndrome (intrauterine growth restriction [IUGR], metaphyseal dysplasia, adrenal hypoplasia congenita, and genitourinary anomalies in males), a disorder previously associated with gain-of-function mutations in CDKN1C. POLE1-deficient individuals also exhibited distinctive facial features and variable immune dysfunction with evidence of lymphocyte deficiency. All subjects shared the same intronic variant (c.1686+32C>G) as part of a common haplotype, in combination with different loss-of-function variants in trans. The intronic variant alters splicing, and together the biallelic mutations lead to cellular deficiency of Pol ε and delayed S-phase progression. In summary, we establish POLE as a second gene in which mutations cause IMAGe syndrome. These findings add to a growing list of disorders due to mutations in DNA replication genes that manifest growth restriction alongside adrenal dysfunction and/or immunodeficiency, consolidating these as replisome phenotypes and highlighting a need for future studies to understand the tissue-specific development roles of the encoded proteins.
Key words: DNA replication, growth, adrenal failure, immunodeficiency, cell cycle, polymerase epsilon, IMAGe syndrome, microcephaly
Main Text
DNA replication is a fundamental cellular process necessary to ensure the faithful transmission of genetic information. In eukaryotes, three highly conserved DNA polymerases, polymerase epsilon, delta, and alpha, act in concert at the replication fork. Polymerase epsilon (Pol ε) is the major enzyme responsible for the synthesis of the leading strand1 and is consequently an essential gene.2 POLE encodes the catalytic subunit of Pol ε (POLE1), and somatic and germline missense mutations affecting the proofreading domain of POLE1 have been associated with colon and endometrial cancer.3, 4, 5, 6
Microcephalic primordial dwarfism comprises a group of prenatal-onset extreme growth disorders characterized by intrauterine growth retardation, short stature, and microcephaly. Genes involved in cell cycle progression, including multiple components of the replication licensing machinery, have been identified as monogenic causes of this disorder.7, 8, 9, 10, 11 As the molecular basis for many affected individuals remains to be determined, we performed whole-genome sequencing studies to identify further genes and facilitate more comprehensive diagnosis.
Whole-genome sequencing (WGS) of 48 individuals with microcephalic primordial dwarfism identified heterozygous POLE (GenBank: NM_006231.3) loss-of-function (LoF) variants in three subjects (P1, P3, P4; Table 1). These LoF variants were significantly enriched in our cohort compared to a control WGS dataset (GnomAD,12 p = 5.1 × 10−5, Fisher’s exact test, Table S1). As these variants were present in the unaffected parents, the WGS data were further evaluated and a second rare intronic variant in POLE identified, c.1686+32C>G (dbSNP: rs762985435). This was present in trans with the LoF mutation in all three probands (Table 1). Targeted sequencing of POLE and interrogation of existing whole-exome sequencing (WES) data in additional cases of primordial dwarfism identified five additional subjects compound heterozygous for LoF alleles and the c.1686+32C>G variant (P5–P9, Table 1). Notably, a clinical diagnosis of IMAGe syndrome (GeneReviews in Web Resources) (MIM: 614732) had been considered in individuals P1 and P3, with adrenal failure also reported in P5, P6, and P7. We therefore investigated cases of IMAGe syndrome drawn from other cohorts without an existing molecular diagnosis (i.e., CDKN1C mutation negative). These included three previously published IMAGe-affected case subjects.13, 14 Analysis of their WGS data identified additional POLE LoF variants inherited in trans with the intronic variant in individuals P11–P15 (Table 1). The c.1686+32C>G variant was part of a common haplotype in all individuals where WES/WGS performed, extending over 921 kbp (Figure S2, chr12:132341818–133263107, GRCh38). In P10 a missense variant (c.3019G>C) encoding a p.Ala1007Pro substitution was found, at a residue conserved to yeast (Figure S1) within the polymerase domain of the protein (Figure 1). All variants identified were sufficiently rare (MAF < 0.000112) and, where DNA available, segregation in families was consistent with an autosomal recessively inherited disorder (Table 1).
Table 1.
Biallelic POLE Mutations (GenBank: NM_006231.3)
| ID | Fam | Sex |
Allele 1 |
Allele 2 |
Mat Allele | Pat Allele | Country of Origin | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotide Change | Amino Acid Consequence | MAF | Nucleotide Change | Amino Acid Consequence | MAF | ||||||
| P1 | 1 | M | c.2091dupC | p.Phe699Valfs∗11 | 0 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 1 | 2 | UK |
| P2 | 1 | F | c.2091dupC | p.Phe699Valfs∗11 | 0 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 1 | 2 | UK |
| P3 | 2 | M | c.62+1G>A | Essential Splice Site Intron 1 | 0 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 2 | 1 | Ireland |
| P4 | 3 | F | c.5940G>A | p.Trp1980∗ | 0.000016 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 2 | 1 | Australia |
| P5 | 4 | M | c.4728+1G>T | Essential Splice Site Intron 36 | 0 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 2 | 1 | USA |
| P6 | 5 | F | c.3264_3275+13del | Essential Splice Site Intron 26 | 0.000016 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 1 | 2 | Canada |
| P7 | 6 | M | c.1A>T | p.? | 0.000081 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | n/a | n/a | USA |
| P8 | 7 | M | c.1A>T | p.? | 0.000081 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 2 | 1 | Ireland |
| P9 | 7 | F | c.1A>T | p.? | 0.000081 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 2 | 1 | Ireland |
| P10 | 8 | F | c.3019G>C | p.Ala1007Pro | 0.000009 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 1 | 2 | Ireland |
| P11 | 9 | F | c.5265delG | Ile1756Serfs∗5 | 0 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 2 | 1 | Australia |
| P12 | 9 | M | c.5265delG | Ile1756Serfs∗5 | 0 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 2 | 1 | Australia |
| P13 | 10 | F | c.2049C>G | p.Tyr683∗ | 0.000028 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 1 | 2 | Australia |
| P14 | 11 | M | c.6518_6519delCT | p.Ser2173Phefs∗130 | 0.000089 | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 2 | 1 | USA |
| P15 | 12 | M | c.801+2T>C | Essential Splice Site Intron 8 | – | c.1686+32C>G | p.Asn563Valfs∗16 | 0.000071 | 1 | 2 | USA |
Abbreviations: ID, individual number; Fam, family number; Mat, maternal; Pat, paternal; n/a, not available. All subjects harbored a loss-of-function mutation in combination with an intronic variant on the alternate allele identified as part of a shared haplotype and found to alter splicing in RNA studies. MAF indicates minor allele frequency in European (non-Finnish) population observed in gnomAD. None of the variants were present in any Non-European population in gnomAD.
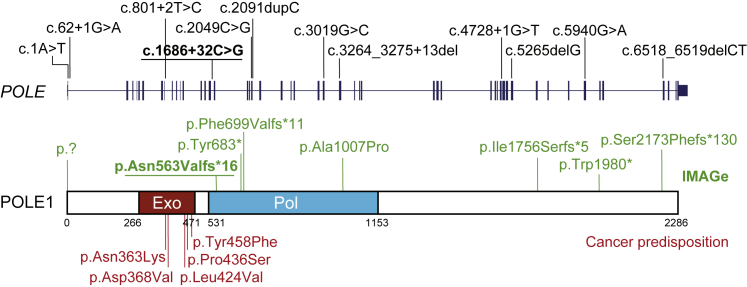
Figure 1.
Mutations Causing POLE-Associated IMAGe Syndrome Are Distinct from Mutations Conferring a Non-syndromic Susceptibility to Cancer
Schematic of the POLE gene, which encodes POLE1, the catalytic subunit of DNA polymerase epsilon. Domains: Pol, polymerase; Exo, exonuclease. Mutations identified in POLE subjects indicated above gene and protein (green). Recurrent intronic mutation underlined. For comparison, heterozygous germline missense mutations located in the exonuclease domain predisposing to colorectal cancer and other malignancies highlighted below (red).
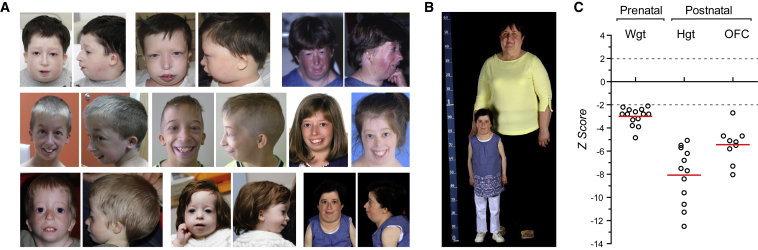
Phenotypically, affected individuals had severe growth failure of prenatal onset (Figure 2, Table S2). IUGR was present in all case subjects (birth weight was −3.0 ± 0.8 SD) with significant short stature evident postnatally (height −8.1 ± 2.4 SD). While head circumference was also significantly reduced (OFC −5.4 ± 1.5 SD), this was less severe, resulting in a relative macrocephaly. Those affected had a common facial appearance with micrognathia, crowded dentition, long thin nose, short wide neck, and small, low-set, posteriorly rotated ears (Figure 2). 12 individuals had adrenal insufficiency and all affected males had genitourinary abnormalities including bilateral cryptorchidism and/or hypospadias, with the majority of case subjects fulfilling clinical criteria for IMAGe syndrome (GeneReviews in Web Resources; Table 2, Table S3, Supplemental Note). Osteopenia and developmental dysplasia of the hip (DDH) were frequently observed and café-au-lait patches were notably present in a third of individuals.
Figure 2.
Individuals with Biallelic POLE Mutations Have Severely Impaired Pre- and Post-natal Growth and a Recognizable Facial Gestalt
(A) Photographs of POLE-deficient subjects demonstrating facial similarities. Written consent obtained from all families for photography.
(B and C) Severe pre-natal onset growth restriction occurs in POLE-deficient individuals.
(B) Adult POLE-deficient subject next to a control individual of average stature.
(C) Growth is severely impaired pre- and postnatally. Z-scores (standard deviations from population mean for age and sex) for birth weight and postnatal height and head circumference (OFC). Dashed lines 95% confidence interval for general population. Circles, individual subject data points; red bars, mean values.
Table 2.
Individuals with Biallelic Mutations in POLE Were Clinically Diagnosed with Primordial Dwarfism and Features of IMAGe Syndrome
| ID | Fam | Sex | Age | I | M+SI | A | Ge | −I | Other Features |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 1 | M | 18 | Y | Y | Y | Y | Y | scoliosis, osteopenia, small patella, seizures, gastrostomy, eczema |
| P2 | 1 | F | 1 | Y | Y | Y | – | Y | – |
| P3 | 2 | M | 7 | Y | Y | Y | Y | Y | midline accessory incisor, osteopenia, infant eczema |
| P4 | 3 | F | 50 | Y | Y | N | – | Y | IgM paraproteinaemia |
| P5 | 4 | M | 12 | Y | NA | Y | Y | Y | hypopituitarism, T cell lymphoma, gastrostomy, absent patella |
| P6 | 5 | F | 10 | Y | Y | Y | – | Y | bilat coxa valga, 11 ribs, 6 lumbar vertebrae, scoliosis, gastrostomy, infant eczema |
| P7 | 6 | M | 13 | Y | Y | Y | Y | N | hypopituitarism, atrial septal defect, brachydactyly, gastrostomy |
| P8 | 7 | M | 3 | Y | Y | N | Y | Y | DDH, gastrostomy |
| P9 | 7 | F | 2 | Y | Y | N | – | Y | DDH, gastrostomy |
| P10 | 8 | F | 39 | Y | Y | Y | – | N | DDH, 11 ribs, clinodactyly, osteopenia, café au lait patches |
| P11 | 9 | F | 0.2 | Y | NA | Y | – | Y | café au lait patch |
| P12 | 9 | F | 12 | Y | Y | Y | – | N | – |
| P13 | 10 | M | 22 | Y | Y | Y | Y | N | DDH, café au lait patch |
| P14 | 11 | F | 18 | Y | Y | Y | – | Y | gastrostomy, hypercalaemia in infancy, café au lait patches, DDH, kyphoscoliosis |
| P15 | 12 | M | 31 | Y | NA | Y | Y | Y | café au lait patches, seizures, osteopenia, osteoporosis, nodular sclerosis, Hodgkin’s lymphoma |
Abbreviations: ID, individual number; Fam, family number; I, intrauterine growth restriction; M+SI, skeletal involvement: metaphyseal dysplasia or other skeletal abnormalities reported in CDKN1C IMAGe-affected individuals (NA, not assessed); A, adrenal insufficiency; Ge, genitourinary abnormalities in males (– female, genitourinary anomalies not applicable); −I, immunodeficiency, either increased susceptibility to infections or documented lymphopenia/hypogammaglobinemia; DDH, developmental dysplasia of the hip; Y, yes; N, no. See Tables S1–S4 for extended clinical data and morphometrics.
A single homozygous intronic variant (c.4444+3A>G) in POLE has previously been reported to be associated with immunodeficiency, lymphopenia, and short stature (facial dysmorphism, immunodeficiency, livedo, and short stature, aka FILS syndrome [MIM: 615139]).15, 16 Five affected individuals identified in this study also had increased susceptibility to respiratory tract infections, with lymphocyte subset deficiencies and/or IgM hypogammaglobinemia identified in P1, P3, P4, P8, P9, P14, and P15 (Table 2, Table S4). Deficiency of natural killer cells was present in P1, P3, and P8. P1 had the most profound immunodeficiency, developing CMV pneumonitis and then subsequently developed EBV haemophagocytic lymphohistiocytosis, requiring an allogeneic bone marrow transplant. Notably, this subject’s sister (P2), who had the same compound heterozygous POLE mutations, died at 22 months from HSV infection. Therefore, our findings establish that the phenotype spectrum of biallelic POLE mutations extends from IMAGe syndrome to include immunodeficiency, in line with the phenotype and pathogenicity of the previously reported c.4444+3A>G mutation.15, 16
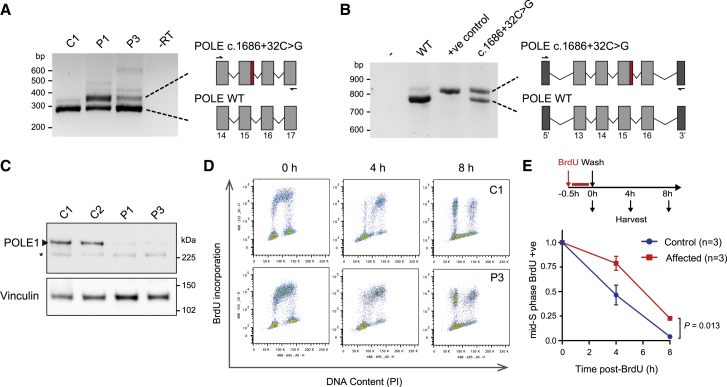
To establish whether the c.1686+32C>T variant affected the POLE transcript, RNA studies were performed on primary fibroblast lines derived from two subjects (P1, P3). RT-PCR using primers spanning POLE intron 15 demonstrated the presence of a larger PCR product (Figure 3), which capillary sequencing established to be due to retention of part of intron 15 within POLE transcripts (Figure S3). A minigene assay was then performed to assess splicing of this genomic segment and to directly confirm the contribution of the c.1686+32C>G variant. This demonstrated that the c.1686+32C>G variant markedly impaired splicing of the usual exon 15 splice donor site, leading to preferential use of a downstream alternate splice donor site in intron 15, although some canonical splicing also occurred (Figure 3). The inclusion of 47 bp of intronic DNA in the variant transcript results in a frameshift, which would lead to premature termination (p.Asn563Valfs∗16). While this transcript might be targeted for nonsense-mediated decay, any translated protein would also be non-functional given that this frameshift occurs at the start of the polymerase catalytic domain. Combined with a LoF mutation on the second allele, substantial reduction in POLE1 was therefore anticipated. Subsequent immunoblotting of total protein extracts from of primary fibroblasts from affected subjects confirmed that POLE1 levels were indeed markedly depleted (Figure 3; 5% ± 3% for P1 and 11% ± 4% P3, relative to the mean of both control subjects and normalized to vinculin loading control; mean ± SD for n = 2 independent experiments), with chromatin fractionation experiments demonstrating reduction of POLE1 in both soluble and chromatin-bound fractions (Figure S4). Taken together with the consistent clinical phenotype across case subjects, we concluded that the identified POLE variants were pathogenic, resulting in a phenotype spectrum substantially overlapping IMAGe syndrome.
Figure 3.
Common Intronic Variant Identified Causes Aberrant Splicing and POLE-Deficient Cells Show Deficiency of Polymerase Epsilon and Slowed S-phase Progression
(A) The c.1686+32C>G mutation causes aberrant splicing of intron 15 in subject cells. RT-PCR of POLE transcripts from primary fibroblasts. Primers indicated by arrows in schematic. P1, P3, POLE-deficient subjects; C1, C2, control subjects.
(B) Minigene assay demonstrating that aberrant splicing is a direct consequence of the c.1686+32C>G mutation. +ve control, point mutation in splice donor site, c.1686+1G>A. 5′ & 3′ indicate artificial vector-associated exons.
(C) POLE1 levels are markedly reduced in subject fibroblasts. Immunoblot of total cell extracts. POLE1 antibody raised against AA1-176. Vinculin, loading control. ∗ non-specific band.
(D and E) Fibroblast cells from affected individuals exhibit delayed S phase progression. Schematic, experimental set-up.
(D) Representative FACS plots.
(E) Quantification of n = 3 affected and n = 3 control cell lines from representative experiment (of n = 3 expts with n ≥ 2 biological replicates per group). Mid-S-phase mean (±SEM) BrdU-labeled cells, normalized to t = 0 time point are plotted for each group. p value, two-way ANOVA.
In keeping with an essential requirement for POLE in eukaryotes,2 the “leaky” c.1686+32C>G splice mutation permitted residual expression of functional POLE1 in all case subjects. This mutation in trans with truncating mutations would then be expected to lead to marked but partial loss of function. As POLE encodes POLE1, the catalytic subunit of the major leading-strand DNA polymerase Pol ε, reduced chromatin levels of POLE1 would therefore be expected to impact on the availability of Pol ε DNA polymerase activity during its canonical function in DNA replication. Consistent with this, time-course FACS analysis demonstrated delayed cell-cycle progression of BrdU-labeled primary fibroblasts from P1 and P3, indicative of impaired S-phase progression (Figure 3). While no viable model of POLE1 deficiency exists, a Pole4−/− mouse has been generated, which is similarly deficient for the Pol ε holoenzyme.17 This mouse also has significant prenatal onset growth failure, reduced brain size, and markedly reduced lymphocyte levels. Analysis of embryonic fibroblasts derived from this mouse alongside POLE primary human fibroblasts (derived from P1 and P3 in this study) established that in both cases Pol ε deficiency leads to reduced levels of chromatin-loaded Pol ε complexes, resulting in replication stress arising from reduced numbers of active replication origins.17
IMAGe syndrome has previously been found to be caused by dominant gain-of-function mutations in the imprinted gene, CDKN1C.18, 19 Here, we establish mutations of POLE as an autosomal-recessive cause of the IMAGe phenotype. These mutations contrast with heterozygous germline and somatic cancer-predisposing mutations that affect the exonuclease domain of POLE13, 4, 5, 6 (Figure 1). IMAGe and cancer mutations are likely to have differing functional outcomes, respectively leading to deficient DNA replication or to impaired proof-reading.20 Hence, a similar cancer predisposition in POLE1-deficient individuals or POLE heterozygous carriers cannot be assumed. However, P5 developed a T cell lymphoma at age 11 and P15 developed Hodgkin’s lymphoma at age 28. Given also the increased lymphoma rates in Pole4−/− mice,17 POLE1 deficiency may therefore confer an increased risk of lymphoma.
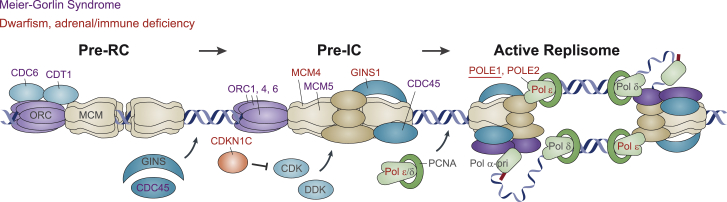
All CDKN1C IMAGe mutations cluster within its proliferating cell nuclear antigen (PCNA) binding domain,18, 19 targeting the PCNA binding PIP-box motif.21 As PCNA loads with Pol ε at replication initiation (Figures 4 and S5), the phenotypic overlap with POLE-associated IMAGe syndrome suggests a mechanistic link. Supporting this notion, biochemical studies of a Xenopus homolog suggests that CDKN ubiquitination and subsequent degradation is mediated by PCNA/polymerase loading23, 24 (Figure S5). Furthermore, single homozygous mutations in MCM411, 25 (MIM: 609981) and POLE226 have been associated with IUGR and short stature, alongside immunodeficiency, respectively with and without adrenal failure. Likewise, several families with GINS1 biallelic mutations have been reported to be associated with pre/postnatal growth restriction, chronic neutropenia, and NK cell deficiency (MIM: 610608).10 Hence, the identification of a cohort of individuals with POLE mutations that encompasses all these features consolidates this as a group of replisome-associated disorders (Figure 4, Table S5). Replication stress and p53-mediated cell death17 likely explain the immunodeficiency as well as global growth failure in POLE1-deficient individuals. However, why impaired replisome function should have a particularly strong impact on specific lymphoid lineages (T/B cells in POLE1/2-deficient subjects and NK cells in MCM4/GINS1-deficient individuals) or on adrenal cortical cells is unclear. Notably, another distinct form of primordial dwarfism, Meier-Gorlin syndrome (defined by the triad of short stature, patella hypoplasia, and microtia [MIM: 224690]) is also caused by biallelic (or de novo) mutations in genes involved in replication licensing and initiation7, 8, 9, 27, 28 (Figure 4). Further studies to understand the specific role(s) of the encoded replication proteins during development, along with the cellular and biochemical basis for the relationship between CDKN1C and Pol ε, will therefore be of interest.
Figure 4.
POLE1 Deficiency Links CDKN1C-IMAGe Syndrome18 with Other Replisome-Associated Disorders
Schematic of replication initiation (adapted by permission from Gaillard et al.22 copyright 2015 Macmillan Publishers), highlighting the sequential action of replisome-associated proteins, mutation of which causes MGS (blue text) and those that are associated with dwarfism with adrenal insufficiency and/or immune deficiency, including IMAGe syndrome (red text). During replication licensing, MCM helicases (MCM2-7) are loaded at replication origins by the ORC complex (ORC1-6) with CDC6 and CDT1 to form the pre-replicative complex (pre-RC). Subsequently, loading of additional replisome protein occurs, regulated by DDK and CDK kinases, to form the pre-initiation complex (pre-IC), that contains the CMG (CDC45, MCMs, GINS) complex. CDKN1C inhibits CDK activity. In the active replisome, Primase-Pol α initiates DNA synthesis with strands extended by the PCNA-associated DNA polymerases δ and ε. POLE1 and POLE2 are part of the Pol ε holoenzyme.
Consortia
Members of the Scottish Genome Partnership include Timothy J. Aitman, Andrew V. Biankin, Susanna L. Cooke, Wendy Inglis Humphrey, Sancha Martin, Lynne Mennie, Alison Meynert, Zosia Miedzybrodzka, Fiona Murphy, Craig Nourse, Javier Santoyo-Lopez, Colin A. Semple, and Nicola Williams.
Declaration of Interests
The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from the genetic testing services offered by Baylor Genetics.
Acknowledgments
We thank the families and clinicians for their involvement and participation; the Potentials Foundation and Walking with Giants Foundation; D. Fitzpatrick, N. Hastie, and W. Bickmore for discussions; E. Freyer for assistance with FACS analysis; IGMM core sequencing service; and Edinburgh Genomics (Clinical Division) for WGS sequencing. We thank Penny Jeggo for sharing cell lines. This work was supported by funding to the Jackson lab from European Research Council ERC Starter Grant HumGenSize, 281847; ERC Advanced Investigator Grant GrowCell, 788093; by a UK Medical Research Council Human Genetics Unit core grant (MRC, U127580972), and the Scottish Genomes Partnership. The Rios lab is supported by Texas Scottish Rite Hospital for Children and the Children’s Medical Center Foundation. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Boulton lab work is supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC0010048), the UK Medical Research Council (FC0010048), and the Wellcome Trust (FC0010048); a European Research Council (ERC) Advanced Investigator Grant (TelMetab); and Wellcome Trust Senior Investigator and Collaborative Grants. R.K.S. is funded by the Wellcome Trust (210752/Z/18/Z). The Scottish Genomes Partnership is funded by the Chief Scientist Office of the Scottish Government Health Directorates (SGP/1) and The Medical Research Council Whole Genome Sequencing for Health and Wealth Initiative.
Published: November 29, 2018
Footnotes
Supplemental Data include Supplemental Note, seven figures, five tables, and Supplemental Material and Methods and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.10.024.
Contributor Information
Jennie E. Murray, Email: jennie.murray@igmm.ed.ac.uk.
Andrew P. Jackson, Email: andrew.jackson@igmm.ed.ac.uk.
SGP Consortium:
Timothy J. Aitman, Andrew V. Biankin, Susanna L. Cooke, Wendy Inglis Humphrey, Sancha Martin, Lynne Mennie, Alison Meynert, Zosia Miedzybrodzka, Fiona Murphy, Craig Nourse, Javier Santoyo-Lopez, Colin A. Semple, and Nicola Williams
Web Resources
GeneReviews, Bennett, J., Schrier Vergano, S.A., and Deardorff, M.A. (1993). IMAGe syndrome, https://www.ncbi.nlm.nih.gov/books/NBK190103/
gnomAD Browser, http://gnomad.broadinstitute.org/
OMIM, http://www.omim.org/
Supplemental Data
References
- 1.Burgers P.M.J., Kunkel T.A. Eukaryotic DNA replication fork. Annu. Rev. Biochem. 2017;86:417–438. doi: 10.1146/annurev-biochem-061516-044709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg M., Johansson E. DNA polymerase ε. Subcell. Biochem. 2012;62:237–257. doi: 10.1007/978-94-007-4572-8_13. [DOI] [PubMed] [Google Scholar]
- 3.Palles C., Cazier J.B., Howarth K.M., Domingo E., Jones A.M., Broderick P., Kemp Z., Spain S.L., Guarino E., Salguero I., CORGI Consortium. WGS500 Consortium Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat. Genet. 2013;45:136–144. doi: 10.1038/ng.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Church D.N., Briggs S.E., Palles C., Domingo E., Kearsey S.J., Grimes J.M., Gorman M., Martin L., Howarth K.M., Hodgson S.V., NSECG Collaborators DNA polymerase ε and δ exonuclease domain mutations in endometrial cancer. Hum. Mol. Genet. 2013;22:2820–2828. doi: 10.1093/hmg/ddt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellido F., Pineda M., Aiza G., Valdés-Mas R., Navarro M., Puente D.A., Pons T., González S., Iglesias S., Darder E. POLE and POLD1 mutations in 529 kindred with familial colorectal cancer and/or polyposis: review of reported cases and recommendations for genetic testing and surveillance. Genet. Med. 2016;18:325–332. doi: 10.1038/gim.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas N., Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bicknell L.S., Bongers E.M., Leitch A., Brown S., Schoots J., Harley M.E., Aftimos S., Al-Aama J.Y., Bober M., Brown P.A. Mutations in the pre-replication complex cause Meier-Gorlin syndrome. Nat. Genet. 2011;43:356–359. doi: 10.1038/ng.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bicknell L.S., Walker S., Klingseisen A., Stiff T., Leitch A., Kerzendorfer C., Martin C.A., Yeyati P., Al Sanna N., Bober M. Mutations in ORC1, encoding the largest subunit of the origin recognition complex, cause microcephalic primordial dwarfism resembling Meier-Gorlin syndrome. Nat. Genet. 2011;43:350–355. doi: 10.1038/ng.776. [DOI] [PubMed] [Google Scholar]
- 9.Fenwick A.L., Kliszczak M., Cooper F., Murray J., Sanchez-Pulido L., Twigg S.R.F., Goriely A., McGowan S.J., Miller K.A., Taylor I.B., WGS500 Consortium Mutations in CDC45, Encoding an Essential Component of the Pre-initiation Complex, Cause Meier-Gorlin Syndrome and Craniosynostosis. Am. J. Hum. Genet. 2016;99:125–138. doi: 10.1016/j.ajhg.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cottineau J., Kottemann M.C., Lach F.P., Kang Y.H., Vély F., Deenick E.K., Lazarov T., Gineau L., Wang Y., Farina A. Inherited GINS1 deficiency underlies growth retardation along with neutropenia and NK cell deficiency. J. Clin. Invest. 2017;127:1991–2006. doi: 10.1172/JCI90727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gineau L., Cognet C., Kara N., Lach F.P., Dunne J., Veturi U., Picard C., Trouillet C., Eidenschenk C., Aoufouchi S. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J. Clin. Invest. 2012;122:821–832. doi: 10.1172/JCI61014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan T.Y., Jameson J.L., Campbell P.E., Ekert P.G., Zacharin M., Savarirayan R. Two sisters with IMAGe syndrome: cytomegalic adrenal histopathology, support for autosomal recessive inheritance and literature review. Am. J. Med. Genet. A. 2006;140:1778–1784. doi: 10.1002/ajmg.a.31365. [DOI] [PubMed] [Google Scholar]
- 14.Pedreira C.C., Savarirayan R., Zacharin M.R. IMAGe syndrome: a complex disorder affecting growth, adrenal and gonadal function, and skeletal development. J. Pediatr. 2004;144:274–277. doi: 10.1016/j.jpeds.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 15.Pachlopnik Schmid J., Lemoine R., Nehme N., Cormier-Daire V., Revy P., Debeurme F., Debré M., Nitschke P., Bole-Feysot C., Legeai-Mallet L. Polymerase ε1 mutation in a human syndrome with facial dysmorphism, immunodeficiency, livedo, and short stature (“FILS syndrome”) J. Exp. Med. 2012;209:2323–2330. doi: 10.1084/jem.20121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiffault I., Saunders C., Jenkins J., Raje N., Canty K., Sharma M., Grote L., Welsh H.I., Farrow E., Twist G. A patient with polymerase E1 deficiency (POLE1): clinical features and overlap with DNA breakage/instability syndromes. BMC Med. Genet. 2015;16:31. doi: 10.1186/s12881-015-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellelli R., Borel V., Logan C., Svendsen J., Cox D.E., Nye E., Metcalfe K., O’Connell S.M., Stamp G., Flynn H.R. Polε Instability Drives Replication Stress, Abnormal Development, and Tumorigenesis. Mol. Cell. 2018;70:707–721.e7. doi: 10.1016/j.molcel.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arboleda V.A., Lee H., Parnaik R., Fleming A., Banerjee A., Ferraz-de-Souza B., Délot E.C., Rodriguez-Fernandez I.A., Braslavsky D., Bergadá I. Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome. Nat. Genet. 2012;44:788–792. doi: 10.1038/ng.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamajima N., Johmura Y., Suzuki S., Nakanishi M., Saitoh S. Increased protein stability of CDKN1C causes a gain-of-function phenotype in patients with IMAGe syndrome. PLoS ONE. 2013;8:e75137. doi: 10.1371/journal.pone.0075137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shcherbakova P.V., Pavlov Y.I., Chilkova O., Rogozin I.B., Johansson E., Kunkel T.A. Unique error signature of the four-subunit yeast DNA polymerase epsilon. J. Biol. Chem. 2003;278:43770–43780. doi: 10.1074/jbc.M306893200. [DOI] [PubMed] [Google Scholar]
- 21.Borges K.S., Arboleda V.A., Vilain E. Mutations in the PCNA-binding site of CDKN1C inhibit cell proliferation by impairing the entry into S phase. Cell Div. 2015;10:2. doi: 10.1186/s13008-015-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaillard H., García-Muse T., Aguilera A. Replication stress and cancer. Nat. Rev. Cancer. 2015;15:276–289. doi: 10.1038/nrc3916. [DOI] [PubMed] [Google Scholar]
- 23.Furstenthal L., Swanson C., Kaiser B.K., Eldridge A.G., Jackson P.K. Triggering ubiquitination of a CDK inhibitor at origins of DNA replication. Nat. Cell Biol. 2001;3:715–722. doi: 10.1038/35087026. [DOI] [PubMed] [Google Scholar]
- 24.Chuang L.C., Yew P.R. Proliferating cell nuclear antigen recruits cyclin-dependent kinase inhibitor Xic1 to DNA and couples its proteolysis to DNA polymerase switching. J. Biol. Chem. 2005;280:35299–35309. doi: 10.1074/jbc.M506429200. [DOI] [PubMed] [Google Scholar]
- 25.Hughes C.R., Guasti L., Meimaridou E., Chuang C.H., Schimenti J.C., King P.J., Costigan C., Clark A.J., Metherell L.A. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J. Clin. Invest. 2012;122:814–820. doi: 10.1172/JCI60224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frugoni F., Dobbs K., Felgentreff K., Aldhekri H., Al Saud B.K., Arnaout R., Ali A.A., Abhyankar A., Alroqi F., Giliani S. A novel mutation in the POLE2 gene causing combined immunodeficiency. J. Allergy Clin. Immunol. 2016;137:635–638.e1. doi: 10.1016/j.jaci.2015.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guernsey D.L., Matsuoka M., Jiang H., Evans S., Macgillivray C., Nightingale M., Perry S., Ferguson M., LeBlanc M., Paquette J. Mutations in origin recognition complex gene ORC4 cause Meier-Gorlin syndrome. Nat. Genet. 2011;43:360–364. doi: 10.1038/ng.777. [DOI] [PubMed] [Google Scholar]
- 28.Burrage L.C., Charng W.L., Eldomery M.K., Willer J.R., Davis E.E., Lugtenberg D., Zhu W., Leduc M.S., Akdemir Z.C., Azamian M. De Novo GMNN Mutations Cause Autosomal-Dominant Primordial Dwarfism Associated with Meier-Gorlin Syndrome. Am. J. Hum. Genet. 2015;97:904–913. doi: 10.1016/j.ajhg.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.