Abstract
To fully unravel the ixodid ticks’ role as vectors of viral pathogens, their susceptibility to new control measures, and their ability to develop acaricide resistance, acclimatization of ticks under laboratory conditions is greatly needed. However, the unique and complicated feeding behavior of these ticks compared to that of other hematophagous arthropods requires efficient and effective techniques to infect them with tick-borne viruses (TBVs). In addition, relatively expensive maintenance of animals for blood feeding and associated concerns about animal welfare critically limit our understanding of TBVs. This mini review aims to summarize the current knowledge about the artificial infection of hard ticks with viral pathogens, which is currently used to elucidate virus transmission and vector competence and to discover immune modulators related to tick–virus interactions. This review will also present the advantages and limitations of the current techniques for tick infection. Fortunately, new artificial techniques arise, and the limitations of current protocols are greatly reduced as researchers continuously improve, streamline, and standardize the laboratory procedures to lower cost and produce better adoptability. In summary, convenient and low-cost techniques to study the interactions between ticks and TBVs provide a great opportunity to identify new targets for the future control of TBVs.
Keywords: tick-borne viruses, ixodid ticks, virus infection, blood feeding, tick–virus interactions
Introduction
Ticks are the most economically important vectors of livestock diseases (Arthur, 1962) and are considered second to mosquitoes in transmitting human diseases (de la Fuente et al., 2008; Socolovschi et al., 2009). Among the pathogens transmitted by these bloodsucking ectoparasites, tick-borne viruses (TBVs) present a severe health risk to both humans and domestic animals (Hoogstraal, 1973). TBVs comprise a wide range of viruses classified into eight virus families: Asfarviridae, Nairoviridae, Peribunyaviridae, Phenuiviridae, Flaviviridae, Orthomyxoviridae, Rhabdoviridae, and Reoviridae (Brackney and Armstrong, 2016; Kazimírová et al., 2017). Among these viral families, Nairoviridae and Flaviviridae are considered to have the TBVs of most importance to public health, including the tick-borne encephalitis virus (TBEV) and the Crimean–Congo hemorrhagic fever virus (CCHFV), which are known to cause severe clinical symptoms in humans (Nuttall et al., 1994; Labuda and Nuttall, 2004; Brackney and Armstrong, 2016; Kazimírová et al., 2017).
Of the 900 currently known tick species, less than 10% are implicated as virus vectors, and these include the Ornithodoros and Argas genera for the argasid ticks and Ixodes, Haemaphysalis, Hyalomma, Amblyomma, Dermacentor, and Rhipicephalus genera in ixodid ticks (Labuda and Nuttall, 2004; de la Fuente et al., 2017).
Although the role of ticks in the transmission of viruses has been known for over a century (Mansfield et al., 2017), the understanding of tick–virus interactions important for tick antiviral immunity, pathogen replication, and transmission of the virus to an animal host remains limited and at an early stage (Mitzel et al., 2007; Kopacek et al., 2010; Liu et al., 2012). Moreover, the diversity of tick-borne viruses has been less thoroughly studied than that of mosquito-borne viruses (Yoshii et al., 2015).
In addition, ixodid ticks differ essentially from other blood-feeding insects in terms of their digestive physiology, feeding behavior (Obenchain and Galun, 1982), and the long duration of the blood meal, which can take up to several weeks (Waladde and Rice, 1982). Moreover, tick attachment at feeding sites on the host requires correct physical and chemical stimuli for a successful engorgement (Guerin et al., 2000).
Since it is estimated that TBVs spend more than 95% of their life cycle within the tick vector (de la Fuente et al., 2017), a very intimate and highly specific association between tick vector species and the transmitted TBVs is normally maintained (Brites-Neto et al., 2015). With this in mind, artificial viral infection of ticks using experimental laboratory techniques can greatly improve our understanding of tick–virus interaction, particularly transmission pathways and vector competence. A comprehensive review of artificial tick infections using pathogens other than TBVs and the ixodid (hard) tick life cycle has already been made by Bonnet and Liu (2012). In this mini review, different techniques for the viral infection of hard ticks were presented, indicating their advantages and limitations with respect to their application to viral transmission and vector competency studies (summarized in Table 1).
Table 1.
Summary of the techniques used to artificially infect ticks with representative ticks and viruses, their major advantages/disadvantages and associated references.
| Tick-infection methods | Tick species | Virus studied | Main advantages | Main disadvantages |
|---|---|---|---|---|
| Direct feeding on infected host |
D. andersoni H. longicornis R. appendiculatus |
Powassan virus1 SFTS virus2 Thogoto virus3 |
Can infect a greater number of ticks; resembles the normal acquisition | Requires animal host; lacks quantification of acquired viral load |
| Co-feeding infection |
I. ricinus D. marginatus R. appendiculatus H. truncatum A. americanum H. longicornis |
TBEV4-8 Louping ill virus9 Bhanja virus5 Palma virus5 Thogoto virus9,10 CCHFV11 Heartland virus12 Thogoto virus13 |
An established natural viral infection of ticks | Requires animal host; greatly depends on the distance among feeding ticks |
| Membrane-feeding method |
I. ricinus I. ricinus D. reticulatus |
Bluetongue virus14 African swine fever virus15 |
Reduces variation within a given treatment group | Requires chemical and physical stimuli to enhance tick attachment; depends on the length of the hypostome; long attachment time |
| Capillary feeding |
A. variegatum R. appendiculatus I. ricinus D. reticulatus |
Dugbe virus16 Bluetongue virus14 |
Mimics the natural route of infection; can estimate the amount of introduced pathogen | Complicated maintenance of the integrity of the mouthparts of the ticks after removal |
| Percoxal injection |
H. longicornis A. variegatum |
Langat virus17,18 Thogoto virus19 |
Can estimate the amount of pathogen to be introduced | Requires a microinjector; may produce higher tick mortality due to injury |
| I. ricinus | TBEV4-6,8,20 | |||
| Louping ill virus9 | ||||
| Anal pore injection |
H. truncatum I. ricinus |
CCHFV21 TBEV19 |
||
| H. longicornis | Langat virus17 | |||
| Infection by immersion |
I. scapularis A. americanum |
LGTV22,23 Heartland virus12 |
Low cost; relatively simple artificial method; can synchronously infect ticks with a defined virus stock | May not generate cohorts of infected ticks with equal pathogen burden |
1Chernesky (1969), 2Luo et al. (2015), 3Booth et al. (1989), 4Labuda et al. (1996), 5Labuda et al. (1997a), 6Khasnatinov et al. (2009), 7Slovák et al. (2014), 8Labuda et al. (1993), 9Jones et al. (1997), 10Jones et al. (1987), 11Gordon et al. (1993), 12Godsey et al. (2016), 13Talactac et al. (2018), 14Bouwknegt et al. (2010), 15De carvalho Ferreira et al. (2014), 16Steele and Nuttall (1989), 17Talactac et al. (2016), 18Talactac et al. (2017b), 19Kaufman and Nuttall (1996), 20Belova et al. (2012), 21Gonzalez et al. (1989), 22McNally et al. (2012), and 23Tumban et al. (2011).
Methods for Infecting Ticks
Direct Feeding on Infected Host
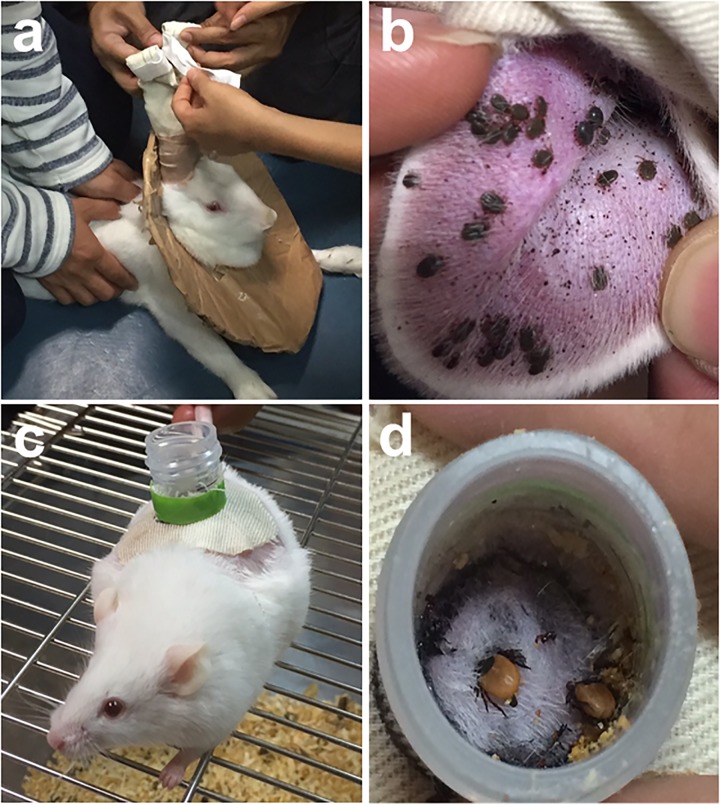
Infesting ticks on infected natural hosts remains the method most closely resembling the normal acquisition of a virus in the wild. Direct feeding on infected host can be facilitated by using feeding bags (Figures 1a,b) or feeding chambers (Figures 1c,d). However, the maintenance and handling of animal hosts can be expensive and difficult, particularly for wild animals (Bonnet and Liu, 2012). The direct feeding technique also lacks the capacity to quantify the pathogen dose acquired by the tick during or post feeding. The technique may also not be appropriate for virus strains not suited for replication in the vertebrate hosts (Mitzel et al., 2007). In addition, it remains a challenge to synchronize viremia with tick feeding, and for ethical reasons, the use of alternative artificial methods in infecting ticks without the use of laboratory animals is still preferred (Bonnet and Liu, 2012).
FIGURE 1.
Feedings bags are glued or taped to clean-shaven ears of long-eared rabbit to allow tick engorgement (a). Haemaphysalis longicornis nymphs infested on a rabbit’s ear (b). A mouse with a feeding chamber attached on its back (c). Ixodes persulcatus adult ticks infested on a mouse via chamber feeding (d). Tick infestation using feeding bags or chamber feeders can be readily utilized to infect ticks with viruses via direct feeding on infected hosts.
Various hosts, mostly small laboratory animals, have already been infected for direct tick acquisition of the virus. Dermacentor andersoni ticks were previously infected by infesting rabbits injected intravenously with large doses of the Powassan virus (Chernesky, 1969). Laboratory mice were also previously used to study severe fever with thrombocytopenia syndrome virus (SFTSV) transmission by Haemaphysalis longicornis (Luo et al., 2015), while Rhipicephalus appendiculatus specimens were infected with the Thogoto virus (THOV) by allowing them to feed on THOV-infected Syrian hamsters (Booth et al., 1989). Transmission of West Nile virus from infected mice to naïve I. ricinus nymphs through direct blood feeding was also previously observed (Lawrie et al., 2004).
Co-feeding Infection
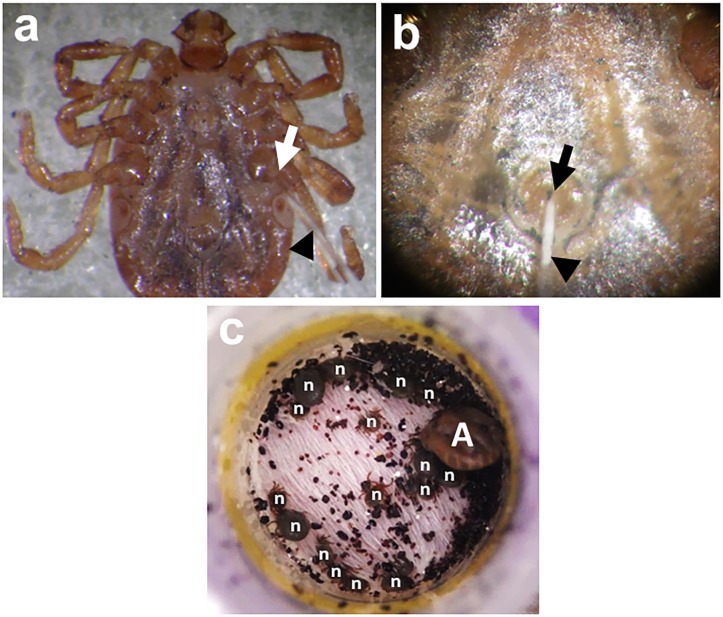
Non-viremic transmission, or co-feeding transmission (Figure 2c), is an important transmission mechanism for TBVs established by Jones et al. (1987). It occurs between infected and uninfected ticks when they co-feed in close proximity on susceptible hosts, even when these hosts do not develop viremia (Alekseev and Chunikhin, 1990; Labuda et al., 1993; Jones et al., 1997; Labuda et al., 1997a,b). Though co-feeding is an established natural tick infection method, it requires an animal host for feeding and may not produce high infection rates, as transmission of the virus from infected and uninfected ticks greatly depends on the proximity or distance among feeding ticks.
FIGURE 2.
Percoxal (a) and anal pore/rectal (b) injections to H. longicornis adult ticks. Infection is accomplished by injecting the virus inoculum containing an estimated virus titer using glass microneedles (black arrowheads) at the joint of the tick coxa and trochanter of the 4th pair of legs (white arrow) or into the tick’s anal aperture (black arrow). After injection, ticks will be kept for 24 h in a 25°C incubator to observe for any mortality arising from possible injury due to the injection. Co-feeding (c) between a THOV-infected H. longicornis adult (A) and smaller uninfected/naïve nymphs (n). The ticks were infested on mice using feeding chamber/capsule method. All engorged nymphs post infestation will be collected and allowed to molt. Twenty-one days after molting, newly emerged adult ticks will be examined for either the presence of infectious virions or viral RNA using a focus formation assay or real-time PCR, respectively.
Co-feeding experiments were mostly conducted in small laboratory or wild animals. Virus transmission experiments using yellow-necked mice (Apodemus flavicollis) and bank voles (Clethrionomys glareolus) (Labuda et al., 1996, 1997b), BALB/c mice (Khasnatinov et al., 2009; Slovák et al., 2014), European hedgehog (Erinaceus europaeus), striped field mouse (A. agrarius) European pine vole (Pitymys subterraneus), and common pheasant (Phaseanus colchicus) (Labuda et al., 1993) were used to study TBEV transmission by I. ricinus.
Co-feeding transmission of the Louping ill virus on I. ricinus was also evaluated in mountain hares (Lepus timidus), New Zealand white rabbits (Oryctolagus cuniculus) and red deer (Cervus elaphus) (Jones et al., 1997). Non-viremic transmission was also established for Thogoto virus (THOV) on R. appendiculatus (Jones et al., 1987, 1997) and CCHFV on Hyalomma truncatum, H. impeltatum (Gordon et al., 1993) and Amblyomma variegatum (Gonzalez et al., 1991) using guinea pigs (Cavia porcellus). Co-feeding transmission was also observed for Bhanja virus and Palma virus in D. marginatus, D. reticulatus, and I. ricinus ticks infested on mice (Labuda et al., 1997a) and Heartland virus in Amblyomma americanum infested on rabbits (Godsey et al., 2016). Lastly, co-feeding transmission of THOV was recently demonstrated in H. longicornis ticks infested on BALB/c mice (Talactac et al., 2018).
Membrane-Feeding Methods
Another alternative to tick infestation is through membrane feeding. Membranes from animal and non-animal origin (e.g., silicone membranes) are usually utilized, with variable success, to feed ticks. This method could also be used for studies on the dynamics of pathogen transmission, since it can reduce the variation within a given treatment group because the blood meal from the same donor reduces the variation that may arise from individual tick–host relationships (Krober and Guerin, 2007).
However, this method requires chemical and physical stimuli to enhance attachment by hard ticks to membranes (Kuhnert, 1996). Its use may also depend on the length of the hypostome in all life stages of the hard ticks to be studied (Krober and Guerin, 2007). In addition, this type of artificial feeding is more challenging for ixodid ticks, since they require longer time for attachment (de Moura et al., 1997. This method was previously used in infecting I. ricinus, I. hexagonus, D. reticulatus, and R. bursa with the Bluetongue virus (Bouwknegt et al., 2010). The unlikely involvement of I. ricinus and D. reticulatus as biological vectors of African swine fever virus was also shown using membrane feeding (De carvalho Ferreira et al., 2014).
Infection Through Capillary Feeding
The introduction of pathogens to ixodid ticks via capillary feeding was first attempted by Chabaud (1950). In this technique, the ticks are normally pre-fed on animals, followed by a careful mechanical removal of ticks from the host. Eventually, a capillary tube containing the pathogen is placed over the tick’s mouthparts, and the tick is immobilized on a slide (Burgdorfer, 1957; Bouwknegt et al., 2010). Capillary feeding provides a number of advantages, especially that it mimics the natural route of infection of ticks, and it can estimate the amount of pathogen to be introduced. However, maintaining the integrity of the mouthparts of the ticks after removal is crucial for a successful capillary feeding (Bonnet and Liu, 2012). This technique was previously used in infecting A. variegatum and R. appendiculatus with the Dugbe virus (Steele and Nuttall, 1989) and I. ricinus, I. hexagonus, D. reticulatus, and R. bursa with the Bluetongue virus (Bouwknegt et al., 2010).
Infection Through Injection
Direct injection of the virus inoculum through the cuticle (between the coxa and trochanter) has the advantage of estimating the viral dose received by the ticks (Figure 2a). However, this method bypasses the midgut barrier of ticks during feeding, making it unrepresentative of the natural route of infection for ticks (Mitzel et al., 2007). This technique also requires a microinjector to efficiently introduce the inoculum into the tick and may produce higher tick mortality due to injection injury (Rechav et al., 1999). Previous studies using this technique include the infection of H. longicornis with the Langat virus (Talactac et al., 2016, 2017a), A. variegatum with the Thogoto virus (Kaufman and Nuttall, 1996), I. ricinus with TBEV (Labuda et al., 1993, 1996, 1997b; Khasnatinov et al., 2009; Belova et al., 2012), and the Louping ill virus (Jones et al., 1997). D. marginatus, D. reticulatus, and I. ricinus ticks also previously received percoxal injections with the Bhanja and Palma viruses (Labuda et al., 1997a). Alternatively, anal pore or rectal injection of the virus directly into the gut of the tick can be used (Figure 2b), though it also requires skill to avoid puncturing the gut upon injection. This method has been used to infect H. truncatum with CCHFV (Gonzalez et al., 1989), I. ricinus with TBEV (Belova et al., 2012), and H. longicornis with Langat virus (Talactac et al., 2017b) and THOV (Talactac et al., 2018).
Infection Through Immersion
Infection of ticks through immersion provides a low cost and relatively simple artificial method, since it can synchronously infect a large number of ticks with a defined virus stock. The ticks are believed to be infected when they successfully swallowed the immersion medium containing the virus; with the ingested virus ultimately reaching the midgut (Mitzel et al., 2007). The virus can also possibly penetrate the tick’s exoskeletons, especially the immature ones. Larvae and nymphs have less sturdy exoskeleton, since arthropods must be able to hydrolyze the chitin for cuticle degradation and development during the immature stages (You et al., 2003). However, its major limitation is the generation of cohorts of infected ticks with an equal pathogen burden (Kariu et al., 2011). Infection of ticks using this method was previously reported for I. scapularis infected with Langat virus (Tumban et al., 2011; McNally et al., 2012), the dengue virus (Tumban et al., 2011), and TBEV (Mitzel et al., 2007) and for A. americanum infected with the Heartland virus (Godsey et al., 2016).
Conclusion
To fully understand the interaction of ticks with TBVs, efficient techniques for the artificial infection and maintenance of tick colonies under laboratory conditions are crucial. As emphasized in this mini review, it is the unique but complicated feeding behavior of ixodid ticks that makes studies related to virus transmission, vector competence, and other aspects of tick–virus interaction a challenging endeavor. However, with the availability of these alternative feeding methods and techniques to infect ticks with different viruses of public health importance, the potential for studies on TBVs to catch up with the advances in mosquito-borne viral disease research is no longer a far-fetched scenario. In addition, the limitations of current techniques do not outweigh importance of studying TBVs. Understanding the interactions between ticks and the TBVs they transmit offers a great opportunity to identify new targets for the future control of TBVs.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to K. Kusakisako of the Laboratory of Infectious Diseases, Joint Faculty of Veterinary Medicine, Kagoshima University, for his helpful comments and suggestions regarding this manuscript.
Footnotes
Funding. This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI grant No. 15H05264, 16H05028, and 17K19328, the Takeda Science Foundation, and the Japanese Government Ministry of Education, Culture, Sports, Science and Technology Scholarship (Monbukagakusho: MEXT) for doctoral fellowship.
References
- Alekseev A. N., Chunikhin S. P. (1990). Exchange of tick-borne encephalitis virusbetween Ixodidae simultaneously feeding on animals with subthreshold levels of viraemia. Med. Parazitol. 2 48–50. [PubMed] [Google Scholar]
- Arthur D. G. (1962). Ticks and Disease. Oxford: Pergamon Press. [Google Scholar]
- Belova O. A., Burenkova L. A., Karganova G. G. (2012). Different tick-borne encephalitis virus (TBEV) prevalences in unfed versus partially engorged ixodid ticks evidence of virus replication and changes in tick behavior. Ticks Tick Borne Dis. 3 240–246. 10.1016/j.ttbdis.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Bonnet S., Liu X. Y. (2012). Laboratory artificial infection of hard ticks: a tool for the analysis of tick-borne pathogen transmission. Acarologia. 52 453–464. 10.1051/acarologia/20122068 [DOI] [Google Scholar]
- Booth T. F., Davies C. R., Jones L. D., Staunton D., Nuttall P. A. (1989). Anatomical basis of thogoto virus infection in BHK cell culture and in the ixodid tick vector, Rhipicephalus appendiculatus. J. Gen. Virol. 70 1093–1104. 10.1099/0022-1317-70-5-1093 [DOI] [PubMed] [Google Scholar]
- Bouwknegt C., van Rijn P. A., Schipper J. J. M., Hölzel D., Boonstra J., Nijhof A. M., et al. (2010). Potential role of ticks as vectors of bluetongue virus. Exp. Appl. Acarol. 52 183–192. 10.1007/s10493-010-9359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackney D. E., Armstrong P. M. (2016). Transmission and evolution of tick-borne viruses. Curr. Opin. Virol. 21 67–74. 10.1016/j.coviro.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Brites-Neto J., Duarte K. M. R., Martins T. F. (2015). Tick-borne infections in human and animal population worldwide. Vet. World 8 301–315. 10.14202/vetworld.2015.301-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W. (1957). Artificial feeding of ixodid ticks for studies on the transmission of disease agents. J. Infect. Dis. 100 212–214. 10.1093/infdis/100.3.212 [DOI] [PubMed] [Google Scholar]
- Chabaud A. G. (1950). Sur la nutrition artificielle destiques. Ann. Parasitol. Hum. Comp. 25 142–147. 10.1051/parasite/1950251042 [DOI] [Google Scholar]
- Chernesky M. A. (1969). Powassan virus transmission by ixodid ticks infected after feeding on viremic rabbits injected intravenously. Can. J. Microbiol. 6 521–526. 10.1139/m69-090 [DOI] [PubMed] [Google Scholar]
- De carvalho Ferreira H. C., Tudela Zúquete S., Wijnveld M., Weesendorp E., Jongejan F., Stegeman A., et al. (2014). No evidence of African swine fever virus replication in hard ticks. Ticks Tick Borne Dis. 5 582–589. 10.1016/j.ttbdis.2013.12.012 [DOI] [PubMed] [Google Scholar]
- de la Fuente J., Antunes S., Bonnet S., Cabezas-Cruz A., Domingos A. G., Estrada-Peña A. (2017). Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 7:114. 10.3389/fcimb.2017.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J., Estrada-Pena A., Venzal J. M., Kocan K. M., Sonenshine D. E. (2008). Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 13:6938–6946. 10.2741/3200 [DOI] [PubMed] [Google Scholar]
- de Moura S. T., da Fonseca A. H., Fernandes C. G., Butler J. F. (1997). Artificial feeding of Amblyomma cajennense (Fabricius, 1787) (Acari:Ixodidae) through silicone membrane. Mem. Inst. Oswaldo Cruz. 92 545–548. 10.1590/S0074-02761997000400019 [DOI] [PubMed] [Google Scholar]
- Godsey M. S., Savage H. M., Burkhalter K. L., Bosco-Lauth A. M., Delorey M. J. (2016). Transmission of heartland virus (Bunyaviridae: Phlebovirus) by experimentally infected Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 53 1226–1233. 10.1093/jme/tjw080 [DOI] [PubMed] [Google Scholar]
- Gonzalez J. P., Cornet J. P., Wilson M. L., Camicas J. L. (1991). Crimean-Congo haemorrhagic fever virus replication in adult Hyalomma truncatum and Amblyomma variegatum ticks. Res. Virol. 142 483–488. 10.1016/0923-2516(91)90071-A [DOI] [PubMed] [Google Scholar]
- Gonzalez J. P., NDiaye M., Diop A., Wilson M. L. (1989). “Laboratoire d’Ecologie Virale,” in Rapport Annuel De l’Institut Pasteur De Dakar, ed. Digoutte J. P. (Dakar: Institut Pasteur; ), 100–110. [Google Scholar]
- Gordon S. W., Linthicum K. J., Moulton J. R. (1993). Transmission of crimean-congo hemorrhagic fever virus in two species of hyalomma ticks from infected adults to cofeeding immature forms. Am. J. Trop. Med. Hyg. 48 576–580. 10.4269/ajtmh.1993.48.576 [DOI] [PubMed] [Google Scholar]
- Guerin P. M., Kröber T., McMahon C., Guerenstein P., Grenacher S., Vlimant M., et al. (2000). Chemosensory and behavioural adaptations of ectoparasitic arthropods. Nova Acta Leopold. 83 213–229. [Google Scholar]
- Hoogstraal H. (1973). “Viruses and ticks,” in Viruses and Invertebrates, ed. Gibbs A. J. (The Hague: North-Holland Publishings; ),351–390. [Google Scholar]
- Jones L. D., Davies C. R., Steele G. M., Nuttall P. A. (1987). A novel mode of arbovirus transmission involving a nonviremic host. Science 237 775–777. 10.1126/science.3616608 [DOI] [PubMed] [Google Scholar]
- Jones L. D., Gaunt M., Hails R. S., Laurenson K., Hudson P. J., Reid H., et al. (1997). Transmission of louping ill virus between infected and uninfected ticks co-feeding on mountain hares. Med. Vet. Entomol. 11 172–176. 10.1111/j.1365-2915.1997.tb00309.x [DOI] [PubMed] [Google Scholar]
- Kariu T., Coleman A. S., Anderson J. F., Pal U. (2011). Methods for rapid transfer and localization of lyme disease pathogens within the tick gut. J. Vis. Exp. 48:2544. 10.3791/2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman W. R., Nuttall P. A. (1996). Amblyomma variegatum (Acari: Ixodidae): mechanism and control of arbovirus secretion in tick saliva. Exp. Parasitol. 82 316–323. 10.1006/expr.1996.0039 [DOI] [PubMed] [Google Scholar]
- Kazimírová M., Thangamani S., Bartíková P., Hermance M., Holíková V., Štibrániová I., et al. (2017). Tick-Borne Viruses and Biological Processes at the Tick-Host-Virus Interface. Front. Cell. Infect. Microbiol. 7:339. 10.3389/fcimb.2017.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasnatinov M. A., Ustanikova K., Frolova T. V., Pogodina V. V., Bochkova N. G., Levina L. S. (2009). Non-hemagglutinating flaviviruses: molecular mechanisms for the emergence of new strains via adaptation to European ticks. PLoS One 4:e7295. 10.1371/journal.pone.0007295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopacek P., Hajdusek O., Buresova V., Daffre S. (2010). Tick innate immunity. Adv. Exp. Med. Biol. 708 137–162. 10.1007/978-1-4419-80595_8 [DOI] [PubMed] [Google Scholar]
- Krober T., Guerin P. M. (2007). In vitro feeding assays for hard ticks. Trends Parasitol. 23 445–449. 10.1016/j.pt.2007.07.010 [DOI] [PubMed] [Google Scholar]
- Kuhnert F. (1996). Feeding of hard ticks in vitro: new perspectives for rearing and for the identification of systemic acaricides. ALTEX. 13 76–87. [PubMed] [Google Scholar]
- Labuda M., Alves M. J., Elečková E., Kožuch O., Filipe A. R. (1997a). Transmission of tick-borne bunyaviruses by cofeeding ixodid ticks. Acta Virol. 41 325–328. [PubMed] [Google Scholar]
- Labuda M., Kozuch O., Zuffová E., Elecková E., Hails R. S., Nuttall P. A. (1997b). Tick-borne encephalitis virus transmission between ticks cofeeding on specific immune natural rodent hosts. Virology 235 138–143. [DOI] [PubMed] [Google Scholar]
- Labuda M., Austyn J. M., Zuffova E., Kozuch O., Fuchsberger N., Lysy J., et al. (1996). Importance of localized skin infection in tick-borne encephalitis virus transmission. Virology 219 357–366. 10.1006/viro.1996.0261 [DOI] [PubMed] [Google Scholar]
- Labuda M., Nuttall P. A. (2004). Tick-borne viruses. Parasitology 129 S221–S245. 10.1017/S0031182004005220 [DOI] [PubMed] [Google Scholar]
- Labuda M., Nuttall P. A., Kozuch O., Elecková E., Williams T., Zuffová E. (1993). Nonviremic transmission of tick-borne encephalitis virus: a mechanism for arbovirus survival in nature. Experientia 49 802–805. 10.1007/BF01923553 [DOI] [PubMed] [Google Scholar]
- Lawrie C. H., Uzcátegui N. Y., Gould E. A., Nuttall P. A. (2004). Ixodid and argasid tick species and west nile virus. Emerg. Infect. Dis. 10 653–657. 10.3201/eid1004.030517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Dai J., Zhao Y. O., Narasimhan S., Yang Y., Zhang L., et al. (2012). Ixodes scapularis JAK-STAT pathway regulates tick antimicrobial peptides, thereby controlling the agent of human granulocytic anaplasmosis. J. Infect. Dis. 206 1233–1241. 10.1093/infdis/jis484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Zhao L., Wen H., Zhang Z., Liu J., Fang L., et al. (2015). Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg. Infect. Dis. 21 1770–1776. 10.3201/eid2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield K. L., Jizhou L., Phipps L. P., Johnson N. (2017). Emerging tick-borne viruses in the twenty-first century. Front. Cell. Infect. Microbiol. 7:298. 10.3389/fcimb.2017.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally K. L., Mitzel D. N., Anderson J. M., Ribeiro J. M. C., Valenzuela J. G., Myers T. G., et al. (2012). Differential salivary gland transcript expression profile in ixodes scapularis nymphs upon feeding or flavivirus infection. Ticks Tick Borne Dis. 3 18–26. 10.1016/j.ttbdis.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzel D. N., Wolfinbarger J. B., Long R. D., Masnick M., Best S. M., Bloom M. E. (2007). Tick-borne flavivirus infection in ixodes scapularis larvae: development of a novel method for synchronous viral infection of ticks. Virology 365 410–418. 10.1016/j.virol.2007.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall P. A., Jones L. D., Labuda M., Kaufman W. R. (1994). Adaptations of arboviruses to ticks. J. Med. Entomol. 31 1–9. 10.1093/jmedent/31.1.1 [DOI] [PubMed] [Google Scholar]
- Obenchain F. D., Galun R. (1982). The Physiology of Ticks. Oxford: Pergamon Press. [Google Scholar]
- Rechav Y., Zyzak M., Fielden L. J., Childs J. E. (1999). Comparison of methods for introducing and producing artificial infection of ixodid ticks (Acari: Ixodidae) with Ehrlichia chaffeensis. J. Med. Entomol. 36 414–419. 10.1093/jmedent/36.4.414 [DOI] [PubMed] [Google Scholar]
- Slovák M., Kazimírová M., Siebenstichová M., Ustaníková K., Klempa B., Gritsun T., et al. (2014). Survival dynamics of tick-borne encephalitis virus in ixodes ricinus ticks. Ticks Tick Borne Dis. 5 962–969. 10.1016/j.ttbdis.2014.07.019 [DOI] [PubMed] [Google Scholar]
- Socolovschi C., Mediannikov O., Raoult D., Parola P. (2009). The relationship between spotted fever group rickettsiae and ixodid ticks. Vet. Res. 40:34. 10.1051/vetres/2009017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele G. M., Nuttall P. A. (1989). Difference in vector competence of two species of sympatric ticks, Amblyomma variegatum and Rhipicephalus appendiculatus, for Dugbe virus (Nairovirus, Bunyaviridae). Virus Res. 14 73–84. 10.1016/0168-1702(89)90071-3 [DOI] [PubMed] [Google Scholar]
- Talactac M. R., Yada Y., Yoshii K., Hernandez E. P., Kusakisako K., Maeda H., et al. (2017a). Characterization and antiviral activity of a newly identified defensin-like peptide, HEdefensin, in the hard tick Haemaphysalis longicornis. Dev. Comp. Immunol. 68 98–107. 10.1016/j.dci.2016.11.013 [DOI] [PubMed] [Google Scholar]
- Talactac M. R., Yoshii K., Hernandez E. P., Kusakisako K., Galay R. L., Fujisaki K., et al. (2017b). Synchronous langat virus infection of Haemaphysalis longicornis using anal pore picroinjection. Viruses. 9:189. 10.3390/v9070189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talactac M. R., Yoshii K., Hernandez E. P., Kusakisako K., Galay R. L., Fujisaki K., et al. (2018). Vector competence of Haemaphysalis longicornis ticks for a Japanese isolate of the thogoto virus. Sci. Rep. 8:9300. 10.1038/s41598-018-27483-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talactac M. R., Yoshii K., Maeda H., Kusakisako K., Hernandez E. P., Tsuji N., et al. (2016). Virucidal activity of Haemaphysalis longicornis longicin P4 peptide against tick-borne encephalitis virus surrogate langat virus. Parasit. Vectors. 9:59. 10.1186/s13071-016-1344-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumban E., Mitzel D. N., Maes N. E., Hanson C. T., Whitehead S. S., Hanley K. A. (2011). Replacement of the 3’ untranslated variable region of mosquito-borne dengue virus with that of tick-borne langat virus does not alter vector specificity. J. Gen. Virol. 92 841–848. 10.1099/vir.0.026997-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waladde S. M., Rice M. J. (1982). “The sensory basis of tick feeding behaviour,” in Physiology of Ticks, eds Obenchain F. D., Galun R. (Oxford: Pergamon Press; ), 71–118. [Google Scholar]
- Yoshii K., Okamoto N., Nakao R., Klaus-Hofstetter R., Yabu T., Masumoto H., et al. (2015). Isolation of the thogoto virus from a Haemaphysalis longicornis in Kyoto City, Japan. J. Gen. Virol. 96 2099–2103. 10.1099/vir.0.000177 [DOI] [PubMed] [Google Scholar]
- You M., Xuan X., Tsuji N., Kamio T., Taylor D., Suzuki N., et al. (2003). Identification and molecular characterization of a chitinase from the hard tick Haemaphysalis longicornis. J. Biol. Chem. 278 8556–8563. 10.1074/jbc.M206831200 [DOI] [PubMed] [Google Scholar]