Abstract
Amyloidosis is a heterogeneous group of disorders characterized by misfolding of extracellular proteins. Pulmonary amyloidosis secondary to Sjogren's syndrome (SS) is rare and to the best our knowledge has not been described in indigenous population. There is also minimal information on its clinical and radiological features. We' describe here 52-year-old Australian Indigenous women with underlying Sjogren's syndrome who was initially suspected to have a metastatic lung cancer with FGD avid lung nodule on PET scan. However, wedge resection of the nodule demonstrated eosinophilic homogenous material that demonstrated apple-green birefringence under polarized light after staining with Congo red with immunohistochemistry pattern in keeping with AL amyloidosis.
Keywords: Sjogren's syndrome, Amyloidosis, Lung nodule, Pulmonary cysts, Indigenous
1. Introduction
Patients presenting with Pulmonary Nodular and cystic lesion can represent wide differential diagnosis. Similarly, variety of pulmonary manifestations have been described in literature in association with Sjogren's syndrome, the most common of which are interstitial lung disease (ILD) and tracheobronchial disease [1]. Amyloidosis secondary to Sjogren's syndrome manifesting as nodule-cystic lung disease is infrequently reported, more so in Australian indigenous population [[2], [3], [4], [5]]. We describe herewith a case of 52-year-old Australian Indigenous woman with a known history of primary Sjogren's syndrome, who presented with chronic cough and was found to have Pulmonary AL Amyloidosis after extensive investigation of the lung lesions which were initially masquerading as lung malignancy.
1.1. Case
A 52-year-old Australian Indigenous women presented with 6 months history of dry cough. There were no other respiratory symptoms. She did not have any constitutional symptoms, in particular no history of fever, anorexia or unintentional weight loss. She denied any history or known contact for tuberculosis.
Her past medical history included Sjogren's syndrome, initially diagnosed at the age of 42 years and had clinical manifestation of sicca syndrome, xeropthalmia and xerostomia (dry eyes and dry oral cavity). She had a family history for breast cancer, Sjogren's syndrome and Systemic Lupus Erythematosus. She had a very insignificant history of smoking as a teenager. She was not on any regular medications other than NSAID for nonspecific back pain.
Physical examination was un-remarkable other than for an enlarged thyroid gland. There was no palpable lymphadenopathy, clubbing or other signs of connective tissue disease. Respiratory examination was normal, in particular there were no crackles to suggest presence of interstitial lung disease.
Laboratory examination demonstrated normal white cell/differential count. Serum electrolytes and liver function testes were also normal. Her ESR was elevated at 38mm/h, anti-nuclear antibody (ANA) titre was raised with a titre of 1:1280 demonstrating a speckled and nucleolar pattern. Extractable nuclear antigen (ENA) was positive for SS-A (Ro) and SS-B (La) and other ENA such as RNP, Sm, Scl – 70 and Jo-1 including ds-DNA antibody were negative. Thyroid function test was also normal, HIV and hepatitis serology were negative. Serum immunoglobulin showed slightly elevated IgG level at 17.7 (6.5–16.0) and IgA and IgM were normal. Pulmonary function test was normal with a Forced Vital Capacity (FVC) of 3.00L (88%), Forced Expiratory Volume in 1 second (FEV1) was 2.55L (94%) with FEV1/FVC Ratio of 0.85 (107%). The Gas transfer (DLCO) was normal at 86% and the lung volumes were preserved with Total lung Capacity (TLC) at 80% predicted respectively.
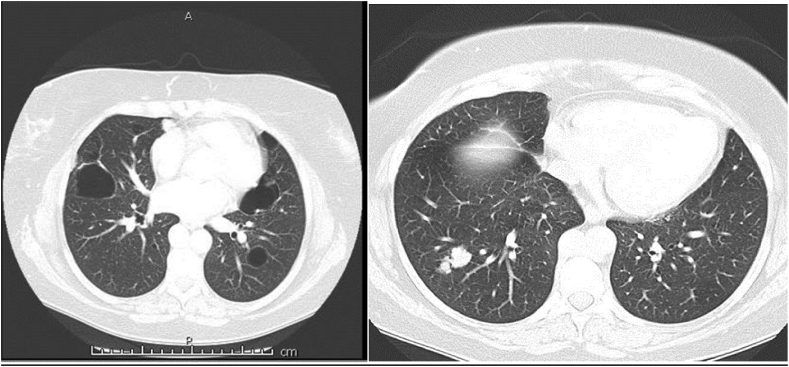
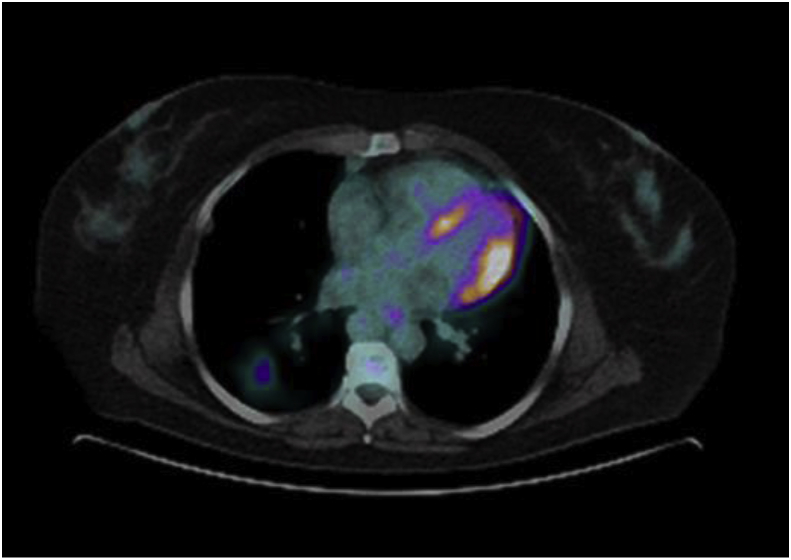
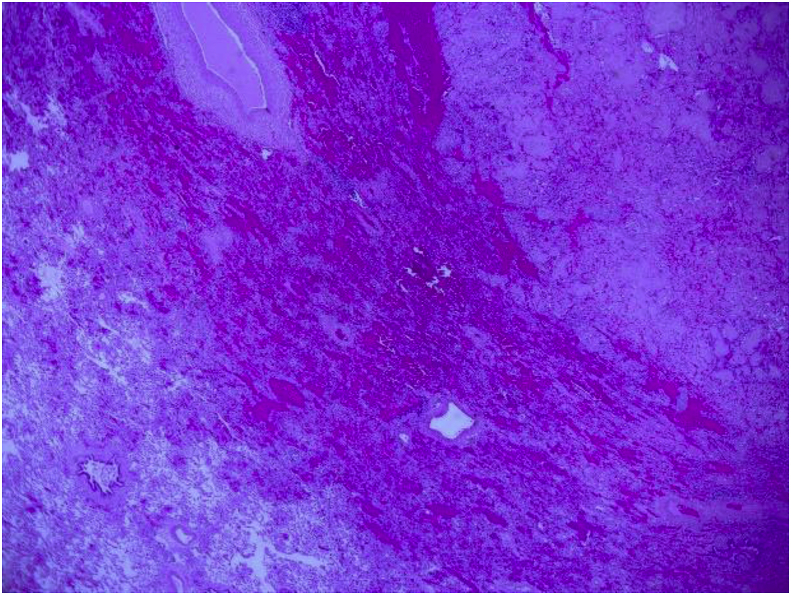
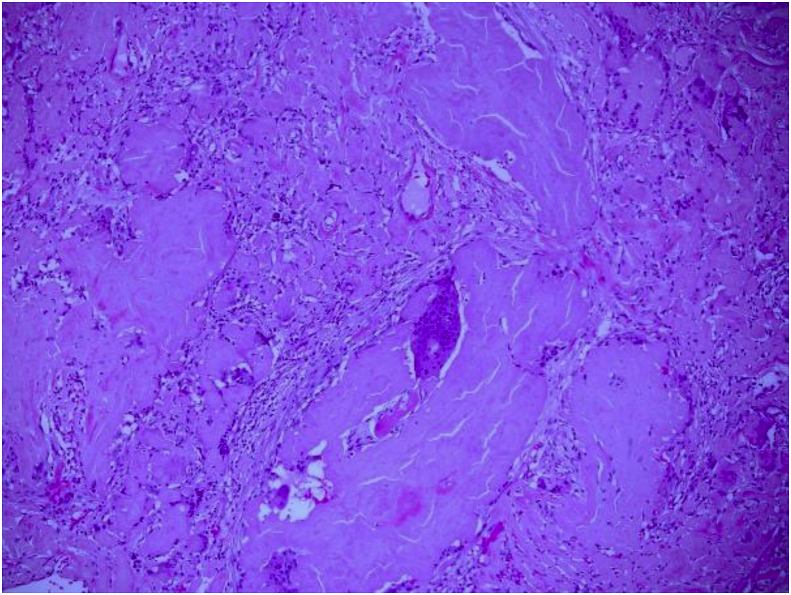
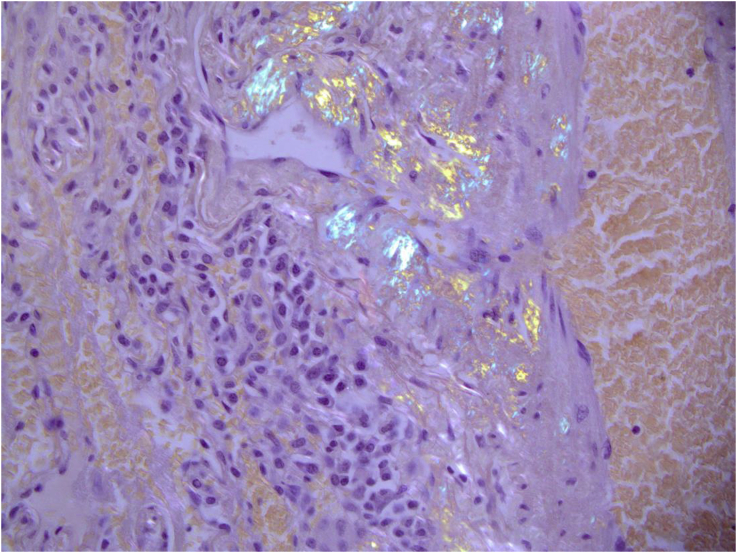
A CT of the chest performed demonstrated multiple pulmonary nodules bilaterally, including a solitary non-calcified multilobulated opacity in the right lower lobe (RLL) measuring 28 × 17 mm with slight speculation (Fig. 1). The CT scan also demonstrated multiple bilateral pulmonary cysts. (Fig. 1). There was no evidence of Interstitial Lung Disease (ILD) or hilar or mediastinal lymphadenopathy. Incidental retrosternal extension of the enlarged thyroid gland was noted. Ultra sound of the thyroid gland demonstrated benign cystic lesion. A high index for malignancy with multiple metastasis was considered in the differential diagnosis. Hence, she underwent a bronchoscopy, which did not demonstrate endobronchial abnormalities and washings were negative for malignant cells and acid-fast bacilli. Cultures were negative for Acid fast bacilli (AFB), bacterial or fungal elements. She underwent a PET scan which showed positive uptake in the right lower lobe lesion with an SUV of 4.2, suspicious for malignancy and included in the differential of possible inflammatory process (Fig. 2). A percutaneous CT guided biopsy of the right lower lobe lesion was attempted, which was unfortunately complicated by an iatrogenic pneumothorax, and the results were inconclusive. In order to make a definitive diagnosis she underwent a video assisted thoracotomy with a wedge excision of the RLL nodule. Surprisingly, the biopsy showed evidence of amorphous material and demonstrated apple-green birefringence under polarised light after staining with Congo-Red in keeping with pulmonary AL amyloidosis (Fig. 3, Fig. 4, Fig. 5). In-situ hybridisation with kappa and lambda stains showed preferential kappa staining (Fig. 3, Fig. 4, Fig. 5). Biopsy results also identified Immunoglobulin heavy chain, with rearrangement studies demonstrating polyclonal lymphocyte population in pulmonary infiltrate. Serum electrophoresis did not identify paraprotein with free light chains showing Kappa 29 mg/L (3–19), Lambda 26 mg/L (6–26) and K/L Ratio of 1.11 (0.25–1.65). In order to exclude systemic AL amyloid, patient underwent bone marrow biopsy and skeletal survey, which were normal. Her cough improved with symptomatic management. As localized AL amyloidosis has a very low likelihood of systemic dissemination she remains on surveillance and was not offered systemic treatment as she had interval symptom resolution.
Fig. 1.
CT chest showing multiple cystic lesions and right lower lobe nodule.
Fig. 2.
PET-CT scan showing positive uptake in the right lower lobe.
Fig. 3.
Low power image showing normal lung vs amyloid.
Fig. 4.
High power image showing lung amyloid with multinucleated giant cells.
Fig. 5.
Biopsy showing evidence of birefringence under polarised light after staining with Congo-Red.
1.2. Discussion
Our case represents a rare presentation of nodular and cystic pulmonary lesions demonstrated on CT chest diagnosed to have pulmonary amyloidosis in an Australian Indigenous female in association with Sjogren's syndrome. The patient initial presentation resembled metastatic lung cancer and our case highlights the importance of keeping a broad-minded approach as amyloidosis may mimic and affect prognostication and management. It also reflects the role of surgical lung biopsy in making accurate diagnosis.
Amyloidosis is a large group of rare disorders caused by mis-folding of autologous proteins and their subsequent deposition resulting in dysfunction of organs. The two most common forms are light chain (AL) and reactive (AA) amyloidosis [3,6]. Involvement of the lung in Amyloidosis is relatively common. It can occur as isolated pulmonary amyloidosis or more commonly systemic amyloidosis with another organ involvement [5,6]. Lung diseases associated with chronic inflammation (such as bronchiectasis) can lead to systemic AA (apolipoprotein serum amyloid A) amyloidosis. Similarly, respiratory manifestations are common in systemic amyloidosis. Finally, amyloidosis can involve lung parenchyma or the respiratory tract. From the clinician's perspective, amyloidosis-related lung disease mainly manifests in three different forms: Nodular pulmonary amyloidosis, Diffuse alveolar-septal amyloidosis and tracheobronchial amyloidosis [3]. These have been described in Table 1. Nodular Amyloidosis vary in distribution and can be solitary or multiple [7,8]. Tracheobronchial amyloidosis present endoscopically as Amyloid deposits are confirmed with tissue biopsy and by using Congo red staining on polarized light microscopy [1], further analysis to improve amyloid characterization include laser microdissection and mass spectrometry [9]. Further, a link between amyloidosis and cancer has been reported frequently. There have been previous reports of localized low-grade B Non-Hodgkin lymphoma with localized amyloid deposition in tissues [10,11,13]. Amyloidosis patients are also at increased risk of myeloma and squamous cell cancer [12]. The mechanism is thought to be increased production of amyloid precursor proteins or peptides by tumours in AL amyloidosis with malignant transformation caused by chronic stimulation or inflammation [13,14]. It is difficult to reliably differentiate nodular amyloidosis from lung cancer as they mimic in appearance. Amyloid lung nodules can have positron emission tomography (PET) uptake. This is though less common with only seven previously published cases in English literature which causes deception for lung cancer. It is difficult to reliably differentiate clinico-radiologically the two despite noted associations of amyloidosis with Sjogren syndrome, Lymphoma or Myeloma and histological examination is necessary. In most reported cases as well as ours, surgical lung biopsy is required to reach a definitive diagnosis [4] and rule out lung primary or metastasis or lymphoma. This affects treatment decisions and prognostication.
Table 1.
Pulmonary manifestations of amyloidosis.
| Pulmonary Involvement in Amyloidosis | |||
|---|---|---|---|
| Pulmonary Interstitial Amyloidosis or diffuse alveolo-septal amyloidosis | Nodular Amyloidosis | Tracheobronchial Amyloidosis | |
| Amyloidosis subtype | Systemic AL amyloidosis (Upto 90% cases), Sometimes, Systemic AA, ATTRwt, hereditary ATTR. Rarely Localized |
Usually Localized AL (Light chain) or AL/AH type (mixed light chain/heavy chain) | Localized AL amyloidosis, Co-localization tracheal and laryngeal |
| Age/Demographics | Mean Age 60 years | Mean age 67 years, Male: Female = 3: 2 |
Mean age: 50 years No sex predilection |
| Clinical Presentation | Usually identified post-mortem as lung involvement rarely dominates clinical picture Else, Interstitial Lung disease Usually have pulmonary hypertension, heart failure with restrictive cardiomyopathy |
Asymptomatic Incidental Diagnosis |
Symptomatic Cough, haemoptysis, Stridor, Dyspnea, Wheezing, Recurrent pneumonia, atelectasis BRONCHOSOPY: Irregular whitish deposits, diffuse, fragile typically posterior trachea wall narrowing airway multifocal submucosal plaques |
| Pathophysiology and Radiology | Reticular opacities, interlobular septal thickening, micronodules, ground-glass opacification, traction bronchiectasis and honeycombing, Mediastinal Lymphadenopathy can be present | Nodules peripheral subpleural distribution, can be bilateral, variable size, unusual cystic changes have been described. Slow growth | Tracheal and bronchial wall thickening with possible calcification |
| Pulmonary Function Test | Restrictive pattern with hypoxemia on exertion | May be normal or slight restrictive with preserved gas transfer |
|
| Association |
|
|
Rare association with multiple myeloma |
| Differential Diagnosis |
|
|
|
| Histopathology |
|
|
|
| Treatment | Treatment of systemic amyloidosis, Chemotherapy | Conservative Surgical Excision, May be left alone once diagnosis conformed, Occasional cases may need chemotherapy if progression. | Laser or forceps debridement or external beam radiation. Repeated bronchoscopic intervention, stents |
| Prognosis | Poor | Excellent Prognosis | Depends on the extent and distribution |
Pulmonary involvement in patients with Sjogren's syndrome is varied and becoming more widely understood and is estimated to occur in between 9 and 20% of patients with Primary Sjogren's Syndrome [1,3], though cough can be present in upto 60% cases. The presentations described include multiple cysts or nodules, bronchiectasis and lymphoproliferative Disease [[1], [2], [3], [4], [5], [6]]. These are described in Table 2. Pulmonary amyloidosis secondary to Sjogren's syndrome has been reported infrequently in the literature [3]. Our patient's CT chest findings demonstrated both multiple pulmonary nodules and multiple cysts. In concordance with most other reports in the literature, our case identified the AL form of amyloidosis in a woman.
Table 2.
Respiratory involvement in Sjogren's syndrome.
| Respiratory involvement in Sjogren's Syndrome |
|---|
| Common manifestations: |
| 1. Cough due to dry airways |
| 2. Bronchiolitis |
| 3. Bronchiectasis |
| 4. Interstitial Lung Disease |
| most common Non specific Interstitial Pneumonitis (NSIP), other patterns include UIP (Usual Interstitial Pneumonitis), LIP (Lymphocytic Interstitial Pneumonitis), Organising Pneumonia |
| 5. Recurrent Chest Infections |
| Rare manifestations |
| 1. Pulmonary Lymphoma |
| 2. Pulmonary Amyloidosis |
| 3. Pulmonary Hypertension |
In conclusion, we describe here a rare presentation of pulmonary amyloidosis secondary to Sjogren's syndrome (SS) that has not been yet been described in indigenous population to the best of our knowledge. Our case and review also adds up to the literature on the pulmonary manifestations of Amyloidosis and Sjogren's Syndrome. The case represented here also highlights the role of surgical lung biopsy in the diagnosis of the condition.
References
- 1.Flament T., Bigot A., Chaigne B., Henique H., Diot E., Marchand-Adam S. Pulmonary manifestations of Sjögren's syndrome. Eur. Respir. Rev. 2016;25:110–123. doi: 10.1183/16000617.0011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baqir M., Kluka E.M., Aubry M.C., Hartman T.E., Yi E.S., Bauer P.R., Ryu J.H. Amyloid-associated cystic lung disease in primary Sjögren's syndrome. Respir. Med. 2013;107:616–621. doi: 10.1016/j.rmed.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Rajagopala S., Singh N., Gupta K., Gupta D. Pulmonary amyloidosis in Sjogren's syndrome: a case report and systematic review of the literature. Respirology. 2010;15:860–868. doi: 10.1111/j.1440-1843.2010.01772.x. [DOI] [PubMed] [Google Scholar]
- 4.Sowa T., Komatsu T., Fujinaga T., Kato T. A case of solitary pulmonary nodular amyloidosis with Sjögren’s syndrome. Ann. Thorac. Cardiovasc. Surg. 2013;19:247–249. doi: 10.5761/atcs.cr.12.01890. [DOI] [PubMed] [Google Scholar]
- 5.Hara K. Amyloidosis and cysts of the Lung in Sjogren's syndrome. Jpn. J. Chest Dis. 2009;68:311–322. [Google Scholar]
- 6.Milani P., Basset M., Russo F., Foli A., Palladini G., Merlini G. The lung in amyloidosis. Eur. Respir. Rev. 2017;26 doi: 10.1183/16000617.0046-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickford H.A., Swensen S.J., Utz J.P. Thoracic cross-sectional imaging of amyloidosis. AJR. 1997;168:351–355. doi: 10.2214/ajr.168.2.9016204. [DOI] [PubMed] [Google Scholar]
- 8.Hui A.N., Koss M.N., Hochholzer L. Amyloidosis presenting in the lower respiratory tract. Clinicopathologic, radiologic, immunohistochemical, and histochemical studies on 48 cases. Arch. Pathol. Lab Med. 1986;110:212–218. [PubMed] [Google Scholar]
- 9.Vrana J.A., Gamez J.D., Madden B.J., Theis J.D., Bergen H.R., Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114:4957–4959. doi: 10.1182/blood-2009-07-230722. [DOI] [PubMed] [Google Scholar]
- 10.Grogg K.L., Aubry M.C., Vrana J.A., Theis J.D., Dogan A. Nodular pulmonary amyloidosis is characterized by localized immunoglobulin deposition and is frequently associated with an indolent B-cell lymphoproliferative disorder. Am. J. Surg. Pathol. 2013;37:406–412. doi: 10.1097/PAS.0b013e318272fe19. [DOI] [PubMed] [Google Scholar]
- 11.Jeong Yeon Joo, Kyung Soo L., Chung M., Han J., Chung M., Kim K., Seo J., Franquet T. Amyloidosis and lymphoproliferative disease in Sjogren syndrome: thin-section computed tomography findings and histopathologic comparisons. J. Comput. Assist. Tomogr. 2004;28:776–781. doi: 10.1097/00004728-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Hemminki K., Li X., Fosti A. Cancer risk in amyloidosis patients in Sweden with novel findings on non-Hodgkin lymphoma and skin cancer. Ann. Oncol. 2014;25:511–518. doi: 10.1093/annonc/mdt544. [DOI] [PubMed] [Google Scholar]
- 13.Dacic S., Colby T.V., Yousem S.A. Nodular amyloidoma and primary pulmonary lymphoma with amyloid production: a differential diagnostic problem. Mod. Pathol. 2000;13:934–940. doi: 10.1038/modpathol.3880170. [DOI] [PubMed] [Google Scholar]
- 14.Xu L., Frazier A., Burke A. Isolated pulmonary amyloidomas: report of 3 cases with histologic and imaging findings. Pathol. Res. Pract. 2013;209:62–66. doi: 10.1016/j.prp.2012.10.009. [DOI] [PubMed] [Google Scholar]