Abstract
Colorectal cancer (CRC) is a major cause of cancer death worldwide. CRC has poor prognosis and there is a crucial need for new diagnostic and prognostic biomarkers to avoid CRC-related deaths. CRC can be considered a sporadic disease in most cases (75%-80%), but it has been suggested that crosstalk between gene mutations (i.e., mutations of BRAF, KRAS, and p53 as well as microsatellite instability) and epigenetic alterations (i.e., DNA methylation of CpG island promoter regions) could play a pivotal role in cancer development. A number of studies have focused on molecular testing to guide targeted and conventional treatments for patients with CRC, sometimes with contrasting results. Some of the most useful innovations in the management of CRC include the possibility to detect the absence of KRAS, BRAF, NRAS and PIK3CA gene mutations with the subsequent choice to administer targeted adjuvant therapy with anti-epidermal growth factor receptor antibodies. Moreover, CRC patients can benefit from tests for microsatellite instability and for the detection of loss of heterozygosity of chromosome 18q that can be helpful in guiding therapeutic decisions as regards the administration of 5-FU. The aim of this review was to summarize the most recent evidence on the possible use of genetic or epigenetic biomarkers for diagnosis, prognosis and response to therapy in CRC patients.
Keywords: Biomarkers, Colorectal cancer, Epigenetics, Tumor markers, DNA methylation
Core tip: Colorectal cancer (CRC) is one of the leading causes of cancer death in the world today. Therefore, any improvement in early diagnosis, selection of appropriate treatment regimen, and effective follow up can be crucial in decreasing related mortalities. This review discusses the most useful and promising genetic and epigenetic biomarkers for CRC. There is growing evidence that these biomarkers could help future development of more personalized treatment approaches.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cause of cancer death worldwide, with an estimated 2.2 million new cases and 1.1 million deaths in the next ten years[1]. Therapeutic strategies for stage I, II, and III disease includes surgery, adjuvant chemotherapy only for selected patients with stage II and most patients with stage III CRC, and radiotherapy for patients with stage II and III rectal cancers[2,3]. Palliative therapies are used for patients with metastasis or stage IV colorectal cancers that are not resectable; in these patients, the objective is to control symptoms and increase survival[4]. CRC has poor prognosis and there is a crucial need for new diagnostic and prognostic biomarkers to avoid CRC-related deaths[5]. Many recent studies have focused on molecular testing to guide targeted and conventional treatments for patients with CRC[6]. The molecular analysis of biomarkers for CRC is making great progress, but the inclusion of novel molecular tests into routine clinical practice faces huge challenges such as a better comprehension of genetic mutations in CRC, the need for laboratory techniques able to measure the resulting phenotypes and genotypes, and the achievement of regulatory qualification for clinical use. In 2011, a National Comprehensive Cancer Network (NCCN) Task Force aimed to assess the clinical utility of tumor markers for different cancer types (including CRC), and underlined common difficulties that clinicians may experience in the management of oncological patients, and then suggested recommendations for the community interested in developing tumor markers[6]. Many of the published findings on molecular biomarkers are controversial, and currently the most reliable molecular marker in clinical practice is the KRAS gene for patients receiving epidermal growth factor receptor (EGFR) - targeted therapy for CRC metastatic disease[7]. In 2017, an Expert Panel of The American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology developed guidelines that aimed to determine standard molecular biomarker testing of CRC tissues in order to direct EGFR therapies and standard chemotherapy regimens. The Expert Panel carried out a literature search that included more than 4000 scientific papers and concluded that mutations in EGFR signaling pathway genes may predict negative response to EGFR-directed therapies for CRC[8]. The process of carcinogenesis in CRC is related to different mechanisms that include, among others, chromosomal instability (CIN), CpG island methylator phenotype (CIMP), and microsatellite instability (MSI)[9]. In 1990, Fearon and Vogelstein described a classical genetic model for colorectal cancerogenesis characterized by the accumulation of mutations in the adenomas, the subsequent mutational activation of the KRAS oncogene , and the inactivation of the genes encoding p53[10]. Recent studies suggested crosstalk between gene mutations (i.e., mutations of BRAF, KRAS, and p53 and microsatellite instability) and epigenetic alterations (i.e., DNA methylation of CpG island promoter regions) in cancer development; in fact, gene mutations could potentially affect epigenetic patterns and epigenetic changes could guide mutation processes and genome instability[11]. CRC can be considered a sporadic disease in most cases (75%-80%), as a consequence of the accumulation of both mutations and epigenetic alterations of numerous genes[12]. A number of studies on DNA methylation concluded that there are no less than three subtypes of CRC in relation to the rate of DNA methylation and mutations in key genes for CRC[13]. The interaction of both gene mutations and epigenetic changes could be responsible for the development of malignant adenocarcinomas as a result of the interference on signaling pathways that control cell growth and tumor progression[14]. Currently, research has moved towards the identification of mutations in key genes involved in the progression of cancer (i.e., APC, CTNNB1, BRAF and KRAS), which are implicated in the WNT and the RAS-RAF-mitogen-activated protein kinase (MAPK) signaling cascades, and, eventually, in the classical adenoma-carcinoma sequence pathway (Table 1). The aim of this review was to summarize the most recent evidence on the possible use of genetic or epigenetic biomarkers for diagnosis, prognosis and response to therapy in CRC patients.
Table 1.
Examples of biomarkers for colorectal cancer diagnosis, progression, prognosis and treatment
| Biomarker | Prognostic factor | Predictive factor | |
| BRAF mutations | Specific phenotype and metastasis; resistance to anti-EGFR mAb | Yes[6,110] | Yes[111], Potentially[6,110] |
| KRAS mutations | Heterogeneity of CRC; resistance to anti-EGFR mAb | Yes potentially[110] | Yes[6,110,111] |
| MSI | Resistance to 5-FU | Yes[6,110], No[111] | - |
| APC mutations | Poorer overall survival | Yes[66] | Yes[65] |
| Micro-RNA | Early detection of CRC, prognostic stratification and therapy-response prediction | Yes[72] | Yes[73] |
| PIK3CA mutations | Poor prognosis and particular clinico-pathological characteristics; resistance to anti-EGFR mAb | Yes[82] | Yes[110] |
| Loss of PTEN | High tendency to develop metastasis; Resistance to anti-EGFR mAb | - | Yes potentially[110,111] |
| TP53 expression | Poor prognosis | Yes potentially[110], No[111] | - |
| Loss of NDST4 | Adverse prognosis; molecular predictor of metastasis | Yes[95] | Yes[95] |
| Loss of 18qLOH | Poor prognosis | Yes[111], Potentially[110] | - |
| IGFR-1R | High levels in metastastic CRC; poor overall survival | Yes[104] | Yes[104] |
TISSUE- BASED BIOMARKERS
BRAF
BRAF is a gene that encodes a serine-threonine protein kinase and is a regulator of the MAPK pathway that is located downstream of KRAS. BRAF represents a prognostic biomarker and a possible target for therapies in patients with CRC[15]. Activating mutations of BRAF occur most frequently in codon 600, and are demonstrable in different types of cancers, for example CRC (10%), melanoma (50%)[16], and lung tumors (1%–2%)[17]. The conversion of valine 600 to glutamic acid (V600E) accounts for 80% of the BRAF mutations in CRC. There is evidence that KRAS and BRAF mutations are mutually exclusive events in cancer progression and development[18]. Many studies highlighted different responses to anti-EGFR treatment according to BRAF status, with a failing rate of anti-EGFR up to 12%-15% in BRAF (V600E) mutation carriers[19]. Some studies showed a high methylation rate (CIMP-high) in BRAF mutation carriers compared to BRAF wild-type (WT) cancer; furthermore, it has been demonstrated a marked association between BRAF mutation and MSI[20]. BRAF mutant cancers are characterized by high prevalence in women and in elderly patients (> 70 years)[21], four or more positive lymph nodes, high-grade histology, defective mismatch repair status, and are mainly sited in the right side of the colon, while wild type tumors can generally affect any part of the colon and rectum[22]. Many retrospective studies underlined the poor prognosis in patients with BRAF mutations. Roth et al[23] evaluated the prognostic role of KRAS and BRAF in 3278 patients with stage II and III colon cancer patients receiving irinotecan added to fluorouracil (FU)/leucovorin (FA) as adjuvant treatment. The results confirmed that the KRAS mutation status does not have significant prognostic value, while BRAF is prognostic for overall survival in MSI low and stable tumors, especially in stage II patients[23]. Similar results were observed in a study by Yokota et al[24] carried out in 229 patients on the prognostic impact of KRAS/BRAF mutations in advanced and recurrent CRC patients receiving chemotherapy treatment. KRAS and BRAF mutations were observed in 34.5% and 6.5% of patients, respectively. The overall survival in patients with KRAS and BRAF mutations (27.7 and 11.0 months respectively) was significantly poorer than that observed in patients with wild type forms of these genes. The results confirmed that BRAF mutations can be considered a strong prognostic factor for poor survival in advanced and recurrent CRC[24]. Nowadays, there is growing interest in the understanding of treatment implications of BRAF mutations. The MRC FOCUS trial evaluated the effects of FU, FU/irinotecan or FU/oxaliplatin administration in in 711 patients with advanced CRC and showed, as previously reported, that patients with BRAF mutations had a lower overall survival compared to patients with BRAF-WT. It is noteworthy that the response to chemotherapy treatment was not influenced by BRAF status, suggesting that BRAF mutations should not be considered as predictive biomarkers for irinotecan or oxaliplatin[25]. A number of studies highlighted that BRAF mutations in CRC can predict the lack of response to anti-EGFR treatment. Bokemeyer et al[26] analyzed pooled individual patient data from the CRYSTAL and OPUS randomized clinical trials (RCTs). The results of these RCTs showed that when cetuximab was added to first line chemotherapy treatment in patients with KRAS-WT CRC, there was a significant improvement in overall survival, progression-free survival, and best overall response rate. No significant differences were observed in the outcome between BRAF mutation carriers and BRAF-WT receiving EGFR-targeting therapies. Patients with BRAF mutations had a poorer prognosis compared to those with BRAF-WT[26]. A meta-analysis by Mao et al [27] carried out on 11 studies (7 studies for unselected mCRC patients and 4 studies for patients with KRAS-WT metastatic CRC),demonstrated that the BRAF(V600E) mutation is related to a lack of response in KRAS-WT metastatic CRC patients receiving anti-EGFR monoclonal antibodies. Another meta-analysis that included 463 patients with RAS-WT/BRAF mutant status CRC reported similar results. The analysis included 9 phase III trials and 1 phase II trial (6 first-line and 2 second-line trials, plus 2 trials involving chemorefractory patients). The addition of anti-EGFR monoclonal antibodies cetuximab and panitumumab in the BRAF mutant subgroup did not lead to any improvement in outcome compared to standard therapy or best supportive care. These results underlined the importance of BRAF mutation evaluation before starting anti-EGFR monoclonal antibody therapies[28]. Because of their growing importance, the NCCN guidelines strongly recommend BRAF and RAS (KRAS exon 2 and non-exon 2; NRAS) mutation testing for diagnosis of stage IV CRC[6]. Based on this evidence, BRAF mutations may be used as a biomarker to screen metastatic CRC patients who could benefit from therapy with anti-EGFR antibodies.
KRAS
The KRAS gene encodes a small GTPase transductor protein that regulates cellular growth and differentiation[29]. The KRAS-WT protein is transitorily activated during signal transduction, but mutations in the KRAS gene could lead to the continuous activation of this signal transduction pathway and, as a result, to cell transformation and inefficacy of therapy with anti-EGFR antibodies[14]. Most activating mutations of KRAS involve codons 12 (82%-87%) and 13 (13%-18%), and only rarely codons 61, 63 and 146. Mutations in codon 12 are linked to mucinous CRC, while mutations in codon 13 are predominantly non-mucinous, showing more aggressive behavior and a tendency to develop metastasis[30]. A number of studies pointed out the key role of KRAS mutations as predictive markers for anti-EGFR therapy. An open-label, randomized, multicenter, phase III study by Van Cutsem et al[31] showed that the administration of cetuximab in patients with metastatic KRAS-WT CRC receiving irinotecan, FU, and leucovorin (FOLFIRI) resulted in significant benefits as regards overall survival, progression-free survival and response compared with FOLFIRI alone. These results confirmed the importance of KRAS mutation status as a strong predictive biomarker for the efficacy of cetuximab plus FOLFIRI[31]. The benefits from cetuximab in advanced CRC patients with KRAS-WT, but not in those with KRAS mutation, were also reported in a RCT by Karapetis et al[32]. The Authors analyzed tumor samples from 394 patients with CRC, who were given cetuximab plus best supportive care (BSC) or BSC alone. Of these patients 42.3% showed at least one mutation in exon 2 of the KRAS gene. In CRC patients with KRAS-WT, cetuximab significantly improved overall survival and progression-free survival when compared with BSC alone. These differences were not observed in CRC patients with KRAS mutations. The presence of KRAS mutations represents a negative predictive factor, and plays a crucial role in the decision about the use of anti-EGFR therapy (Figure 1).
Figure 1.
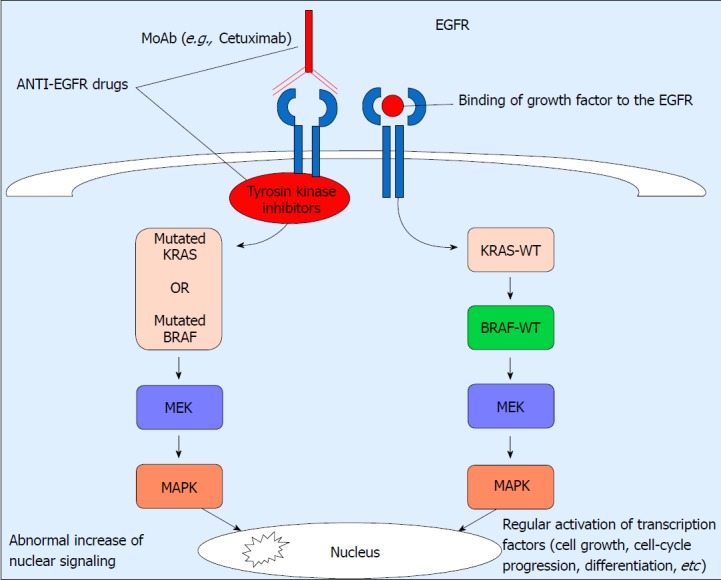
Epidermal growth factor receptor pathway in patients with wild-type and mutant BRAF and KRAS. On the right side of the figure, the normal epidermal growth factor receptor (EGFR) pathway is characterized by the binding of growth factor to the EGFR that leads to regular activation of transcription factors and cell-cycle progression; On the left side, mutations in BRAF or KRAS, which are mutually exclusive, cause the activation of the EGFR pathway and therefore an abnormal increase of nuclear signaling and no response to monoclonal antibodies. EGFR: Epidermal growth factor receptor; MEK: Mitogen-activated protein kinase kinase; MAPK: Mitogen-activated protein kinase; MoAb: monoclonal antibodies; WT: Wild type.
Microsatellite instability
Microsatellites are short tandem repeats of DNA sequences positioned throughout the human genome. MSI is a hypermutable phenotype caused by a deficient DNA mismatch repair (MMR) system, mainly due to the inactivation of the four MMR genes (MSH2, MLH1, MSH6 and PMS2) that leads to a failure in the correction of the insertion or the deletion of repeating units during DNA replication [33]. MSI is observed in about 15% of all CRCs; 3% of these are associated with Lynch syndrome (Hereditary non polyposis colorectal cancer or HNPCC), and the other 12% are due to sporadic, hypermethylation of the promoter of the MLH1 gene, in patients with the CpG island methylator phenotype[34]. CRCs with microsatellite instability are more frequent in the right colon, are mucinous with signet ring cell morphology, show poor differentiation and strong lymphocyte infiltration. Overall, CRC patients with MSI have a better prognosis that those without MSI and show a different response to chemotherapy treatment[35]. It has been observed that stage II or stage III CRC patients with stable or low MSI could benefit from adjuvant chemotherapy with 5-fluorouracil, while patients with stage II CRC and high MSI show a 3-fold increase in mortality, probably due to the immunosuppressive effects of the therapy[36]. On the contrary, a retrospective study carried out by Fallik et al[37] on a small number of patients (n = 72) with metastatic CRC showed that the administration of irinotecan could be useful in MSI tumors even if these results need further clarification and are not yet applicable in routine clinical practice. A meta-analysis of eight independent studies conducted by Des Guetz et al[38] included a total of 287 patients who received 5FU-based chemotherapy, and 678 patients who were treated with other chemotherapy regimens (5FU or capecitabine with oxaliplatin and/or irinotecan). The data were analysed with a random-effect model due to heterogeneity between studies. The authors concluded that MSI status is not a predictive factor for the effect of chemotherapy, with comparable results in both MSI-High and MSI-stable metastatic CRC tumors[38]. MSI can be considered a promising prognostic marker for CRC patients and MSI status can be assessed through a panel of five markers (BAT25, BAT26, D2S123, D5S346, and D17S2720) and the use of polymerase chain reaction (PCR). MSI-high is characterized by instability at two loci or more, and MSI-Low by instability at one locus[39]. Currently, the main clinical use of MSI testing is to detect patients with Lynch syndrome. About 15% of all CRCs show MSI, and among these 75%–80% are characterized by acquired methylation of MLH1; 2%–3% of all CRCs show germ-line mutations in one of the MMR genes[40]. There is growing interest in MSI testing as regards the adjuvant setting to guide therapeutic choices; however, the implication of MSI in the metastatic setting is not well recognized.
EPIGENETIC MARKERS IN CRC
CpG island methylator phenotype
The term “epigenetics” refers to modifications in the phenotype or gene expression that do not implicate DNA sequence changes. Among these, DNA methylation is one of the most studied CRC biomarkers and plays a pivotal role in the alteration of gene expression observed in cancerogenesis[41] (Table 2). Hypermethylation of CpG islands sited in the promoter regions of tumor suppressor genes is a well-recognized mechanism for gene inactivation[42]. The inactivation of gene transcription is due to changes in the chromatin structure of a gene promoter that becomes inaccessible to transcription factors[42]. This epigenetic alteration is able to inactivate a number of cellular pathways that include, for example, DNA repair system (hMLH1, MGMT), apoptosis (DAPK), angiogenesis inhibition (THBS1), metastasis suppression (TIMP3), cell cycle regulation (p14 ARF, p15 INK 4b, p16 INK4a), and cell adherence (CDH1, CDH13)[43,44]. There is evidence of a strong correlation between CIMP-high and right colon cancer, microsatellite instability and a high rate of BRAF mutation[44]. Some studies showed that abnormal methylations of DNA repair genes, for example, MGMT and MLH1, may lead to the progression from adenoma to cancer. The mechanisms involved are the creation of a more prone state for G->A mutations, as frequently observed in KRAS in the case of methylated MGMT and a favorable condition for MSI in the case of methylated MLH1[45,46]. Lee et al[47] suggested that the CIMP could be used as a predictive marker for anti-EGFR therapy. However further prospective studies are needed to confirm this hypothesis. Methylated genes such as MLH1, VIM and SEPT9, could be used as biomarkers for colorectal cancer and as DNA-based colon cancer screening tests. Methylated Vimentin (mVim) is a validated stool-based biomarker for early detection of colorectal cancer available in the US (ColoGuard assay; LabCorp)[48]. The methylated VIM gene is observed in most CRC (53%–84%). The test is PCR based and is able to measure methylated VIM and DNA integrity with high sensitivity and specificity (83% and 82%, respectively)[49]. A recent meta-analysis of 25 studies by Nian et al[50] pointed out that methylated SEPT9 (Epi proColon; Epigenomics AG) can be considered as an effective blood-based assay in CRC detection, mostly for advanced tumors. The proportion of heterogeneity due to threshold effect was 0.02, which indicated the absence of significant thread hold effect among the included studies. Meta-regression demonstrated that study types, country (Asian population or not), sample size (less or greater than 300) kits used (Epipro colon or not), and risk of bias of included studies were all sources of heterogeneity of sensitivity and specificity[50]. Perez-Carbonell et al[51] carried out a systematic analysis of a panel of methylated CRC-specific genes (SEPT9, TWIST1, IGFBP3, GAS7, ALX4 and miR137) and observed that methylation levels of all these genes were significantly higher in CRC compared to normal subjects (P < 0.0001), mainly as regards miR137 and IGFBP3 (86.7% and 83%, respectively). The combination of these two genes showed a sensitivity of 95.5% and a specificity of 90.5% for the detection of CRC, thus representing a promising diagnostic biomarker. Moreover, the results of this study underlined that hypomethylation of IGFBP3 could represent an independent risk factor for poor prognosis in patients with stage II and III CRC. Interestingly, in stage II and III CRC patients who showed hypermethylation of IGFBP3, adjuvant chemotherapy with 5FU did not improve overall survival or disease free progression[51]. Methylated IGFBP-3 could be used as a potential target for the development of novel anticancer drugs, for example demethylating agents. Further studies are needed to clarify the association between methylated IGFBP-3 and low recurrence-free survival, and to report the efficacy of demethylating agent alone or combined with adjuvant therapy in CRC patients[52]. A study by Tang et al[53] underlined the importance of methylated secreted frizzled-related protein 2 (SFRP2) as a possible marker for CRC detection and staging. SFRP2 can be isolated from CRC tissues, serum and fecal DNA, with sensitivity for CRC that ranges from 66.9% to 88.2%. A higher specificity of SFRP2 methylation levels for CRC was observed in serum compared to tissue and stool DNA. Furthermore, there was a significant association of serum SFRP2 with low differentiation grade, serosal or subserosal infiltration, lymph node metastasis and TNM stage of CRC[53]. Other promising blood biomarkers include methylated thrombomodulin (THBD) that detected 71% of all CRCs at a specificity of 80%[54], and methylated syndecan 2 (SDC2) that showed a sensitivity of 92% for CRC at stage I[55]. There is emerging evidence that epigenetic mechanisms could affect the response to chemotherapy[56]. Increased thymidylate synthetase (TYMS) expression, which is regulated by histone acetylation and deacetylation, seems to be the main mechanism involved in the development of resistance to 5-FU. A study by Watson et al[57] showed that CRC patients with TYMS amplification receiving post-resection 5-FU-based chemotherapy, showed shorter median survival. Other genes involved in pyrimidine metabolism that could determine resistance to 5-FU, thus guiding the chemotherapy choice for CRC, include thymidine phosphorylase (TYMP), uridine monophosphate/cytidine monophosphate kinase (UMPK), and dehydrogenase (DYPD) genes[53]. A clinical trial by Ebert et al[58] examined an initial cohort of 74 patients, followed by four cohorts of patients (total n = 220) and showed that CRC patients with high levels of methylation of the gene encoding transcription factor AP-2 epsilon (TFAP2E) did not benefit from chemotherapy treatment with 5-FU, irinotecan or oxaliplatin (P < 0.001). TFAP2E resistance is mediated through its downstream target gene DKK4, encoding dickkopf homolog 4 protein. In CRC patients with TFAP2E hypermethylation, targeting of DKK4 could represent a possible option to bypass the resistance to chemotherapy mediated by TFAP2E[58]. Some studies showed a possible association between methylation of the SPARC gene (coding for the matricellular protein osteonectin)[59], and methylation of the UGT1A1 gene (coding for the UDP glucuronosyltransferase-1A1 enzyme)[56] to a reduction of chemosensitivity to 5-FU or irinotecan. Amatu et al[60] carried out a phase II study with dacarbazine in CRC patients who did not respond to standard chemotherapy (oxaliplatin, irinotecan, fluoropyrimidines, and cetuximab or panitumumab if KRAS-WT). Dacarbazine is an antineoplastic alkylating agent that acts by DNA methylation and causes base pair mismatch. Considering all CRC patients, 40% present hypermethylation of the MGMT gene and dacarbazine is effective only in tumors that lack MGMT[60].
Table 2.
Examples of epigenetic biomarkers for colorectal cancer
| Epigenetic markers | |
| Methylated genes/loci | |
| p14 ARF, p15 INK 4b, p16 INK4a | Cell cycle regulation |
| hMLH1, MGMT | DNA repair system; progression from adenoma to cancer |
| DAPK | Apoptosis |
| THBS1 | Angiogenesis inhibition |
| SPARC | Lymphovascular invasion, metastasis |
| TIMP3 | Metastasis suppression |
| CDH1, CDH13 | Cell adherence |
| Methylation biomarkers | |
| VIM, SEPT9, SFRP2 | Biomarkers for CRC and as DNA-based colon cancer screening tests |
| TWIST1, IGFBP3, GAS7, ALX4, SDC2 | Higher methylation levels in CRC compared to normal subjects (promising diagnostic biomarkers) |
| Candidate biomarkers | |
| Methylated UGT1A1 | Affects irinotecan treatment (in vitro) |
| Methylated DYPD, UMPK, and SPARC | Affect 5-FU treatment (in vitro) |
| TFAP2E | No responsiveness to oxaliplatin, irinotecan, and 5-FU |
CRC: Colorectal cancer; FU: Fluorouracil.
APC
Adenomatous polyposis coli (APC) is a suppressor gene that was detected by genetic linkage analysis in familial adenomatous polyposis (FAP). Mutated APC is also responsible for most sporadic CRCs[61]. APC acts as an antagonist of the WNT signaling pathway and regulates many cell activities such as migration and adhesion, transcriptional activation, and apoptosis[62]. Around 70%-80% of patients with CRC show the loss of APC[63]. A meta-analysis by Liang et al[64] evaluated the associations between three APC polymorphisms (D1822V, E1317Q, and I1307K) and the risk of CRC. The results showed a low association between E1317Q and the risk of CRC, especially for adenomas. Ashkenazi Jews I1307K-variant carriers showed a significantly increased risk of CRC compared to I1307K wild-type carriers. In this meta-analysis, there was no evidence of heterogeneity between studies; however, all the included studies were case-control studies with high likelihood of recall bias and selection bias[64]. Another recent meta-analysis highlighted that hypermethylated APC promoter was more frequent in adenoma than in normal control samples. Moreover, APC hypermethylation levels were higher in CRC patients at stage I compared to normal controls. The authors concluded that APC hypermethylation could represent an important biomarker for early CRC diagnosis and a possible treatment target for personalized therapy. Interestingly, the results did not show a significant association between APC promoter methylation and overall survival in CRC patients. The heterogeneity in the meta-analysis was 43%, and there was no publication bias. However, only publications in English and Chinese were included in the study, thus suggesting the possible existence of selection bias[65]. Another study showed that APC mutation/high miR-21 in patients with advanced CRC had poorer overall survival. The mutation of APC and expression of miR-21 might be useful clinical predictors for CRC[66-68].
microRNA
microRNAs (miRNAs) are small non-coding RNA sequences that can control the expression of genes at the post-transcriptional level[69]. miRNAs play crucial roles in cancer biology and are involved in a number of cellular processes such as proliferation, apoptosis, differentiation, invasion and metastasis[70]. There is growing evidence that carcinogenesis and tumor progression could be associated with abnormalities of miRNAs[71]; thus, miRNAs could represent valuable biomarkers for early detection of cancer, prognostic stratification and therapy-response prediction[72,73]. miRNAs can be isolated from a variety of biological samples, including blood, saliva and stools[74]. A recent study identified a set of 19 differentially expressed miRNAs. Among these, the up-regulation of hsa-miR-183-5p and hsa-miR-21-5p, and the down-regulation of hsa-miR-195-5p and hsa-miR-497-5p were associated with CRC through the interplay with the MMR pathway and transforming growth factor β, WNT, RAS, MAPK, and PI3K signaling pathways[68]. miR-21 is one of the most studied miRNAs[75]; a recent meta-analysis by Peng et al[67] investigated the role of miR-21 in CRC and reported a sensitivity of 0.64, a specificity of 0.85 and an area under the curve of 0.85, as regards diagnostic test accuracy. Samples taken from blood circulation showed corresponding values of 0.72, 0.84, and 0.86 respectively. As regards diagnostic meta-analysis of miR-21-related combination biomarkers, the above values were 0.79, 0.79 and 0.86, respectively. The highest predictive power was observed for miRNA combination markers in circulation (0.85, 0.86, and 0.92 respectively). These results suggested that circulating (especially in serum) miR-21 could represent a promising diagnostic biomarker, while tissue miR-21 could be a useful prognostic marker for CRC. Meta-regression analysis found that ethnicity, sample size, and sample source did not have a significant effect on the pooled results (P > 0.10). Also, there was no heterogeneity from the threshold effect.
OTHER PROMISING BIOMARKERS FOR CRC
Phosphatidylinositide-3-kinases
Phosphatidylinositide-3-kinases (PI3K) are lipid kinases that are involved in the regulation of cellular behavior, including proliferation, adhesion and survival[76]. PI3K signaling is a major pathway for RAS-mediated proliferation, transformation and tumor progression[77]. Abnormalities in PI3K signaling are frequently observed in human cancer[78] and mutations in the PIK3CA gene, the gene coding for the catalytic subunit p110alpha of PI3K, have been described in many cancers, including CRC[79]. These mutations in CRC are associated with right side location, mucinous histological type, KRAS mutation, loss of MGMT expression and a high degree of methylation (CIMP)[78]. PIK3CA mutation is also associated with a significant reduction in survival in CRC patients with BRAF-WT[80]. Mutations at PIK3CA exon 9 and exon 20 trigger different biologic effects and are responsible for the promotion of cancerogenesis. A genetic and biochemical analysis conducted by Zhao et al[81] demonstrated that coexistent mutations in both exons 9 and 20, but not in exon 9 or 20 alone, result in an increase of tumorigenic effects with worse cancer-specific survival. Jehan et al[82] analyzed data from 220 patients who received adjuvant chemotherapy and/or radiotherapy and showed that PI3KCA amplification could represent an independent prognostic marker for better survival and a promising marker to detect CRC patients that may benefit the most from adjuvant therapy. Recent studies proposed mutated PIK3CA as a biomarker to detect CRCs sensitive to aspirin. Liao et al[83] carried out a study in 964 patients with CRC and observed that patients with mutated-PIK3CA who started aspirin therapy after diagnosis, showed higher colorectal cancer-specific survival (multivariate hazard ratio for cancer-related death, 0.18; 95%CI, 0.06 to 0.61; P < 0.001 by the log-rank test) and overall survival (multivariate hazard ratio for death from any cause, 0.54; 95%CI, 0.31 to 0.94; P = 0.01 by the log-rank test), as compared to patients with PIK3CA-WT[83]. Domingo et al[84] studied 896 participants in the Vioxx in Colorectal Cancer Therapy: Definition of Optimal Regime trial, and confirmed the role of mutated PIK3CA as a predictive molecular biomarker in CRC patients for adjuvant aspirin therapy. A population-based cohort study of 740 stage II and III CRC patients, showed a 31% improvement in cancer-specific survival in aspirin users compared to non-users (adjusted HR = 0.69, 95%CI: 0.47–0.98). These outcomes were more evident in patients with high PTGS2 (prostaglandin-endoperoxide synthase 2, also known as cyclooxygenase-2 or COX-2) expression compared to those with low PTGS2 expression. Further trials are needed to better detect CRC patients who may receive a survival benefit from aspirin therapy[85].
PTEN
PTEN is a tumor suppressor gene that regulates the cell-survival signaling pathway initiated by PI3K. PTEN mutations are associated with advanced and metastatic tumors[86] and PTEN promoter hypermethylation is frequently observed in MSI-high sporadic CRCs[87]. Patients with PTEN expression showed significantly longer overall survival compared to patients with PTEN loss tumor[88]; other studies reported an association with poor prognosis in stage II patients only[86] or in CRC patients with liver metastasis[89]. PTEN could represent a useful predictive marker for KRAS-WT patients treated with anti-EGFR therapy[90].
TP53
The TP53 gene encodes a tumor suppressor protein that is involved in the regulation of cell cycle, apoptosis, senescence, and DNA repair. TP53 mutations may result in altered function of TP53 protein, which plays a pivotal role in tumorigenesis. TP53 mutations are observed in about 60% of colorectal tumors and can be found in both adenomas and in malignant cells[91]. There is evidence that the expression of p53 mRNA could represent a useful predictor of survival in patients with stage III CRC or rectal cancer[92].
NDST4
NDST4 is a tumor suppressor gene located at chromosome 4q26. Most CRCs showed a significant decrease in NDST4 expression compared to normal colonic mucosa and some studies showed that the loss of NDST4 was associated with higher pathological stages and poor survival[93]. NDST4 belongs to the N-deacetylase/N-sulfotransferase (heparan glucosaminyl) (NDST) family, and regulates heparan sulfate (HS) biosynthesis on a core protein to form heparan sulphate proteoglycans (HSPGs)[94]. The loss of NDST4 function could lead to an increase in the invasive ability of cancer cells through changes of the interaction between cell adhesion receptors and their ligands. The genetic loss of NDST4 could represent a biomarker of adverse prognosis for patients with CRC[95].
Chromosome 18q loss of heterozygosity
Loss of heterozygosity of chromosome 18q (18qLOH) is a genetic alteration frequently observed in CRC[94] and many key genes (i.e., DCC, SMAD2 and SMAD4), involved in CRC tumorigenesis, are located on chromosome 18q[95,96]. A study by Sarli et al[97] carried out in 118 patients, reported a decreased overall survival for patients with CRC stage III and 18qLOH compared to non-18qLOH patients. The authors concluded that 18qLOH could represent an informative genetic marker, and has the potential to be used to predict recurrences and survival in resected stage III CRCs[97]. A meta-analysis of 27 studies on the prognostic significance of 18q LOH showed that chromosome 18q allelic imbalance and DCC loss of expression could be considered as negative predictive factors for survival. In this metanalysis there was evidence of significant heterogeneity and publication bias[98]. However, these findings suggested that 18q LOH/DCC status could help to detect CRC patients who may benefit from adjuvant chemotherapy after potential curative surgery[99]. Boulay et al[100] analyzed 202 colorectal tumour biopsies from a previous randomised study of adjuvant chemotherapy, and observed that patients with the loss of 18q (and SMAD4 deletion) could obtain less benefit from adjuvant 5-FU treatment.
IGFR-1R
The type 1 insulin-like growth factor receptor (IGF-1R) is a transmembrane glycoprotein composed of two extracellular subunits and two cytoplasmic subunits with tyrosine kinase activity. Overexpression of IGFR-1R has been observed in various tumors (i.e., primary renal cancer cells, and preinvasive breast lesions), and its activation is involved in cell proliferation, differentiation, angiogenesis, and apoptosis[101,102]. IGF-1R undergoes nuclear translocation and interacts with chromatin, under the regulatory effect of IGF[103]. IGF-1R has become a target of new treatments, especially monoclonal antibodies or tyrosine kinase inhibitors. In vitro studies demonstrated that chemotherapy resistance in CRC cell lines was associated with overexpression of IGF-1R within the nuclear compartment. Recently, Codony-Servat et al[104] carried out a study in four cohorts of patients with metastatic CRC (total n = 470), and showed that IGF-1R nuclear location might lead to chemotherapy and targeted agent resistance. Metastatic CRCs presented higher levels of IGF-1R compared to untreated primary cancers and showed poor overall survival. It is noteworthy that ganitumab, an IGF-1R blocking monoclonal antibody, and dasatinib, an SRC inhibitor, augmented the nuclear localization of IGF-1R. Based on these results, IGF-R1 could represent a new potential biomarker for poor prognosis in patients with metastatic CRC[104].
CONSENSUS MOLECULAR SUBTYPES CLASSIFICATION OF CRC
The consensus molecular subtypes (CMS) classification is a recent CRC classifications based on comprehensive gene expression profiling[105,106]. CRC can be separated into 4 groups called CMS1, CMS2, CMS3 and CMS4, and each group shows a unique biology and gene expression pattern: CMS1 (MSI immune, 14%), with higher mutation levels, presence of MSI and marked immune activation; CMS2 (canonical, 37%), found in epithelial CRCs, with higher CIN, and strong WNT and MYC signaling activation; CMS3 (metabolic, 13%), observed in epithelial CRCs with evident metabolic disorders; and CMS4 (mesenchymal, 23%), with noticeable TGF-beta activation, angiogenesis and stromal invasion. The remaining 13% may show mixed characteristics due to transition phenotype or intratumoral heterogeneity[106]. A recent retrospective study by Okita et al[107] carried out in 193 patients with metastatic CRCs, showed that the biological features of CMS may affect the efficacy of chemotherapy. In fact, the results of the study demonstrated that in CMS4 subtype, chemotherapic regimens containing irinotecan showed more benefit than those containing oxaliplatin for progression-free survival [hazard ratio (HR) = 0.31, 95%CI: 0.13-0.64] and overall survival (HR = 0.45, 95%CI: 0.19-0.99). As regards anti-EGFR therapy, CMS1 showed worse progression-free survival (HR = 2.50, 95%CI: 1.31-4.39) and overall survival (HR = 4.23, 95%CI: 1.83-9.04), while CMS2 had better progression-free survival (HR = 0.67, 95%CI: 0.44-1.01) and overall survival (HR = 0.49, 95%CI: 0.27-0.87) compared to the other subtypes[107]. There is evidence that CMS classification could represent the starting point for future clinical stratification and subtype–based targeted interventions for CRC. A study by Isella et al[108] identified 5 CRC intrinsic subtypes (CRIS) characterized by unique molecular, functional and phenotypic features: (1) CRIS-A: mucinous subtype, glycolytic metabolism, with marked MSI, mutated BRAF or KRAS; (2) CRIS-B: active TGF-β signaling, epithelial–mesenchymal transition, bad prognosis; (3) CRIS-C: high EGFR signaling, and sensitivity to EGFR inhibitors (i.e., cetuximab); (4) CRIS-D: high WNT signaling, IGF2 gene amplification/overexpression (which has been involved in reduction of sensitivity to EGFR blockade in patients with KRAS-WT CRCs)[109]; and (5) CRIS-E: Paneth-like phenotype and TP53-mutated genotype. CRIS subtypes categorized independent groups of primitive and metastatic CRCs effectively, representing a great opportunity to enhance patients’ management with regard to precision medicine.
CONCLUSION
Research is moving towards a better comprehension of the mechanisms underlying the pathophysiology and the management of colorectal cancer. Recently, new treatment regimens have been developed, mainly for advanced CRC stages. Some of the most useful innovations in the management of CRC include the possibility to detect the absence of KRAS, BRAF, NRAS and PIK3CA gene mutations with the subsequent choice to administer targeted adjuvant therapy with anti-EGFR antibodies. Moreover, CRC patients can benefit from tests for MSI and for the detection of 18qLOH that can be helpful in guiding therapeutic decisions as regards the administration of 5-FU. Future therapies for CRC could include targeted therapy against membrane receptors, for example other EGFR ligands, platelet-derived growth factor receptors, and insulin-like growth factor 1 receptor. It seems reasonable to think that in the future, molecular screening will help to recognize patients suitable for specific targeted treatments and to fully characterize cancers. The objective of future research will be to detect biomarkers that could provide a cost-effective and non-invasive diagnosis of CRC; other goals are the identification of the best prognostic panel of biomarkers and the characterization of predictive biomarkers to help in the selection of the most appropriate therapy.
ACKNOWLEDGMENTS
We wish to thank the Scientific Bureau of the University of Catania for language support.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: September 1, 2018
First decision: October 11, 2018
Article in press: November 7, 2018
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Coleman HG, Linnebacher M, Wang YF S- Editor: Wang JL L- Editor: A E- Editor: Song H
Contributor Information
Marco Vacante, Department of General Surgery and Medical-Surgical Specialties, University of Catania, Catania 95123, Italy.
Antonio Maria Borzì, Department of General Surgery and Medical-Surgical Specialties, University of Catania, Catania 95123, Italy.
Francesco Basile, Department of General Surgery and Medical-Surgical Specialties, University of Catania, Catania 95123, Italy.
Antonio Biondi, Department of General Surgery and Medical-Surgical Specialties, University of Catania, Catania 95123, Italy. abiondi@unict.it.
References
- 1.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2.Biondi A, Vacante M, Ambrosino I, Cristaldi E, Pietrapertosa G, Basile F. Role of surgery for colorectal cancer in the elderly. World J Gastrointest Surg. 2016;8:606–613. doi: 10.4240/wjgs.v8.i9.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biondi A, Grosso G, Mistretta A, Marventano S, Toscano C, Drago F, Gangi S, Basile F. Laparoscopic vs. open approach for colorectal cancer: evolution over time of minimal invasive surgery. BMC Surg. 2013;13 Suppl 2:S12. doi: 10.1186/1471-2482-13-S2-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varghese AM, Saltz LB. BRAF mutation as a biomarker in colorectal cancer. Adv Genomics Genet. 2015;5:347–353. [Google Scholar]
- 5.Marventano S, Forjaz M, Grosso G, Mistretta A, Giorgianni G, Platania A, Gangi S, Basile F, Biondi A. Health related quality of life in colorectal cancer patients: state of the art. BMC Surg. 2013;13 Suppl 2:S15. doi: 10.1186/1471-2482-13-S2-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Febbo PG, Ladanyi M, Aldape KD, De Marzo AM, Hammond ME, Hayes DF, Iafrate AJ, Kelley RK, Marcucci G, Ogino S, et al. NCCN Task Force report: Evaluating the clinical utility of tumor markers in oncology. J Natl Compr Canc Netw. 2011;9 Suppl 5:S1–32; quiz S33. doi: 10.6004/jnccn.2011.0137. [DOI] [PubMed] [Google Scholar]
- 7.Deschoolmeester V, Baay M, Specenier P, Lardon F, Vermorken JB. A review of the most promising biomarkers in colorectal cancer: one step closer to targeted therapy. Oncologist. 2010;15:699–731. doi: 10.1634/theoncologist.2010-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK, Kopetz SE, Lieu C, Lindor NM, Minsky BD, et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35:1453–1486. doi: 10.1200/JCO.2016.71.9807. [DOI] [PubMed] [Google Scholar]
- 9.Tariq K, Ghias K. Colorectal cancer carcinogenesis: a review of mechanisms. Cancer Biol Med. 2016;13:120–135. doi: 10.28092/j.issn.2095-3941.2015.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 11.You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22:9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Migliore L, Migheli F, Spisni R, Coppedè F. Genetics, cytogenetics, and epigenetics of colorectal cancer. J Biomed Biotechnol. 2011;2011:792362. doi: 10.1155/2011/792362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen L, Toyota M, Kondo Y, Lin E, Zhang L, Guo Y, Hernandez NS, Chen X, Ahmed S, Konishi K, et al. Integrated genetic and epigenetic analysis identifies three different subclasses of colon cancer. Proc Natl Acad Sci U S A. 2007;104:18654–18659. doi: 10.1073/pnas.0704652104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppedè F, Lopomo A, Spisni R, Migliore L. Genetic and epigenetic biomarkers for diagnosis, prognosis and treatment of colorectal cancer. World J Gastroenterol. 2014;20:943–956. doi: 10.3748/wjg.v20.i4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizzo S, Bronte G, Fanale D, Corsini L, Silvestris N, Santini D, Gulotta G, Bazan V, Gebbia N, Fulfaro F, et al. Prognostic vs predictive molecular biomarkers in colorectal cancer: is KRAS and BRAF wild type status required for anti-EGFR therapy? Cancer Treat Rev. 2010;36 Suppl 3:S56–S61. doi: 10.1016/S0305-7372(10)70021-9. [DOI] [PubMed] [Google Scholar]
- 16.Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, Ivanova E, Watson IR, Nickerson E, Ghosh P, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012;485:502–506. doi: 10.1038/nature11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson SL, Su PF, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fransén K, Klintenäs M, Osterström A, Dimberg J, Monstein HJ, Söderkvist P. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis. 2004;25:527–533. doi: 10.1093/carcin/bgh049. [DOI] [PubMed] [Google Scholar]
- 19.Tie J, Gibbs P, Lipton L, Christie M, Jorissen RN, Burgess AW, Croxford M, Jones I, Langland R, Kosmider S, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer. 2011;128:2075–2084. doi: 10.1002/ijc.25555. [DOI] [PubMed] [Google Scholar]
- 20.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 21.Gonsalves WI, Mahoney MR, Sargent DJ, Nelson GD, Alberts SR, Sinicrope FA, Goldberg RM, Limburg PJ, Thibodeau SN, Grothey A, et al. Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J Natl Cancer Inst. 2014;106:dju106. doi: 10.1093/jnci/dju106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalady MF, Dejulius KL, Sanchez JA, Jarrar A, Liu X, Manilich E, Skacel M, Church JM. BRAF mutations in colorectal cancer are associated with distinct clinical characteristics and worse prognosis. Dis Colon Rectum. 2012;55:128–133. doi: 10.1097/DCR.0b013e31823c08b3. [DOI] [PubMed] [Google Scholar]
- 23.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 24.Yokota T, Ura T, Shibata N, Takahari D, Shitara K, Nomura M, Kondo C, Mizota A, Utsunomiya S, Muro K, et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer. 2011;104:856–862. doi: 10.1038/bjc.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, Taylor G, Barrett JH, Quirke P. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27:5931–5937. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 26.Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, Celik I, Köhne CH. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48:1466–1475. doi: 10.1016/j.ejca.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 27.Mao C, Liao RY, Qiu LX, Wang XW, Ding H, Chen Q. BRAF V600E mutation and resistance to anti-EGFR monoclonal antibodies in patients with metastatic colorectal cancer: a meta-analysis. Mol Biol Rep. 2011;38:2219–2223. doi: 10.1007/s11033-010-0351-4. [DOI] [PubMed] [Google Scholar]
- 28.Pietrantonio F, Petrelli F, Coinu A, Di Bartolomeo M, Borgonovo K, Maggi C, Cabiddu M, Iacovelli R, Bossi I, Lonati V, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51:587–594. doi: 10.1016/j.ejca.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 29.Jancík S, Drábek J, Radzioch D, Hajdúch M. Clinical relevance of KRAS in human cancers. J Biomed Biotechnol. 2010;2010:150960. doi: 10.1155/2010/150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bazan V, Agnese V, Corsale S, Calò V, Valerio MR, Latteri MA, Vieni S, Grassi N, Cicero G, Dardanoni G, Tomasino RM, Colucci G, Gebbia N, Russo A; Gruppo Oncologico dell’Italia Meridionale (GOIM) Specific TP53 and/or Ki-ras mutations as independent predictors of clinical outcome in sporadic colorectal adenocarcinomas: results of a 5-year Gruppo Oncologico dell’Italia Meridionale (GOIM) prospective study. Ann Oncol. 2005;16 Suppl 4:iv50–iv55. doi: 10.1093/annonc/mdi908. [DOI] [PubMed] [Google Scholar]
- 31.Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 32.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. J Mol Diagn. 2008;10:301–307. doi: 10.2353/jmoldx.2008.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallik D, Borrini F, Boige V, Viguier J, Jacob S, Miquel C, Sabourin JC, Ducreux M, Praz F. Microsatellite instability is a predictive factor of the tumor response to irinotecan in patients with advanced colorectal cancer. Cancer Res. 2003;63:5738–5744. [PubMed] [Google Scholar]
- 38.Des Guetz G, Uzzan B, Nicolas P, Schischmanoff O, Perret GY, Morere JF. Microsatellite instability does not predict the efficacy of chemotherapy in metastatic colorectal cancer. A systematic review and meta-analysis. Anticancer Res. 2009;29:1615–1620. [PubMed] [Google Scholar]
- 39.Losso GM, Moraes Rda S, Gentili AC, Messias-Reason IT. Microsatellite instability--MSI markers (BAT26, BAT25, D2S123, D5S346, D17S250) in rectal cancer. Arq Bras Cir Dig. 2012;25:240–244. doi: 10.1590/s0102-67202012000400006. [DOI] [PubMed] [Google Scholar]
- 40.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Shin SH, Kwon HJ, Cho NY, Kang GH. Prognostic implications of CpG island hypermethylator phenotype in colorectal cancers. Virchows Arch. 2009;455:485–494. doi: 10.1007/s00428-009-0857-0. [DOI] [PubMed] [Google Scholar]
- 42.Lee S, Cho NY, Choi M, Yoo EJ, Kim JH, Kang GH. Clinicopathological features of CpG island methylator phenotype-positive colorectal cancer and its adverse prognosis in relation to KRAS/BRAF mutation. Pathol Int. 2008;58:104–113. doi: 10.1111/j.1440-1827.2007.02197.x. [DOI] [PubMed] [Google Scholar]
- 43.Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21:5427–5440. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- 44.Rashid A, Shen L, Morris JS, Issa JP, Hamilton SR. CpG island methylation in colorectal adenomas. Am J Pathol. 2001;159:1129–1135. doi: 10.1016/S0002-9440(10)61789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 46.Nosho K, Kawasaki T, Ohnishi M, Suemoto Y, Kirkner GJ, Zepf D, Yan L, Longtine JA, Fuchs CS, Ogino S. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia. 2008;10:534–541. doi: 10.1593/neo.08336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MS, McGuffey EJ, Morris JS, Manyam G, Baladandayuthapani V, Wei W, Morris VK, Overman MJ, Maru DM, Jiang ZQ, et al. Association of CpG island methylator phenotype and EREG/AREG methylation and expression in colorectal cancer. Br J Cancer. 2016;114:1352–1361. doi: 10.1038/bjc.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itzkowitz SH, Jandorf L, Brand R, Rabeneck L, Schroy PC 3rd, Sontag S, Johnson D, Skoletsky J, Durkee K, Markowitz S, Shuber A. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007;5:111–117. doi: 10.1016/j.cgh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Itzkowitz S, Brand R, Jandorf L, Durkee K, Millholland J, Rabeneck L, Schroy PC 3rd, Sontag S, Johnson D, Markowitz S, Paszat L, Berger BM. A simplified, noninvasive stool DNA test for colorectal cancer detection. Am J Gastroenterol. 2008;103:2862–2870. doi: 10.1111/j.1572-0241.2008.02088.x. [DOI] [PubMed] [Google Scholar]
- 50.Nian J, Sun X, Ming S, Yan C, Ma Y, Feng Y, Yang L, Yu M, Zhang G, Wang X. Diagnostic Accuracy of Methylated SEPT9 for Blood-based Colorectal Cancer Detection: A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol. 2017;8:e216. doi: 10.1038/ctg.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez-Carbonell L, Balaguer F, Toiyama Y, Egoavil C, Rojas E, Guarinos C, Andreu M, Llor X, Castells A, Jover R, et al. IGFBP3 methylation is a novel diagnostic and predictive biomarker in colorectal cancer. PLoS One. 2014;9:e104285. doi: 10.1371/journal.pone.0104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu T, Pappou EP, Guzzetta AA, Calmon Mde F, Sun L, Herrera A, Li F, Wolfgang CL, Baylin SB, Iacobuzio-Donahue CA, et al. IGFBP-3 Gene Methylation in Primary Tumor Predicts Recurrence of Stage II Colorectal Cancers. Ann Surg. 2016;263:337–344. doi: 10.1097/SLA.0000000000001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang D, Liu J, Wang DR, Yu HF, Li YK, Zhang JQ. Diagnostic and prognostic value of the methylation status of secreted frizzled-related protein 2 in colorectal cancer. Clin Invest Med. 2011;34:E88–E95. doi: 10.25011/cim.v34i1.15105. [DOI] [PubMed] [Google Scholar]
- 54.Lange CP, Campan M, Hinoue T, Schmitz RF, van der Meulen-de Jong AE, Slingerland H, Kok PJ, van Dijk CM, Weisenberger DJ, Shen H, et al. Genome-scale discovery of DNA-methylation biomarkers for blood-based detection of colorectal cancer. PLoS One. 2012;7:e50266. doi: 10.1371/journal.pone.0050266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh T, Kim N, Moon Y, Kim MS, Hoehn BD, Park CH, Kim TS, Kim NK, Chung HC, An S. Genome-wide identification and validation of a novel methylation biomarker, SDC2, for blood-based detection of colorectal cancer. J Mol Diagn. 2013;15:498–507. doi: 10.1016/j.jmoldx.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Crea F, Nobili S, Paolicchi E, Perrone G, Napoli C, Landini I, Danesi R, Mini E. Epigenetics and chemoresistance in colorectal cancer: an opportunity for treatment tailoring and novel therapeutic strategies. Drug Resist Updat. 2011;14:280–296. doi: 10.1016/j.drup.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Watson RG, Muhale F, Thorne LB, Yu J, O’Neil BH, Hoskins JM, Meyers MO, Deal AM, Ibrahim JG, Hudson ML, et al. Amplification of thymidylate synthetase in metastatic colorectal cancer patients pretreated with 5-fluorouracil-based chemotherapy. Eur J Cancer. 2010;46:3358–3364. doi: 10.1016/j.ejca.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ebert MP, Tänzer M, Balluff B, Burgermeister E, Kretzschmar AK, Hughes DJ, Tetzner R, Lofton-Day C, Rosenberg R, Reinacher-Schick AC, et al. TFAP2E-DKK4 and chemoresistance in colorectal cancer. N Engl J Med. 2012;366:44–53. doi: 10.1056/NEJMoa1009473. [DOI] [PubMed] [Google Scholar]
- 59.Cheetham S, Tang MJ, Mesak F, Kennecke H, Owen D, Tai IT. SPARC promoter hypermethylation in colorectal cancers can be reversed by 5-Aza-2’deoxycytidine to increase SPARC expression and improve therapy response. Br J Cancer. 2008;98:1810–1819. doi: 10.1038/sj.bjc.6604377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amatu A, Sartore-Bianchi A, Moutinho C, Belotti A, Bencardino K, Chirico G, Cassingena A, Rusconi F, Esposito A, Nichelatti M, et al. Promoter CpG island hypermethylation of the DNA repair enzyme MGMT predicts clinical response to dacarbazine in a phase II study for metastatic colorectal cancer. Clin Cancer Res. 2013;19:2265–2272. doi: 10.1158/1078-0432.CCR-12-3518. [DOI] [PubMed] [Google Scholar]
- 61.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 62.Panarelli NC, Vaughn CP, Samowitz WS, Yantiss RK. Sporadic microsatellite instability-high colon cancers rarely display immunohistochemical evidence of Wnt signaling activation. Am J Surg Pathol. 2015;39:313–317. doi: 10.1097/PAS.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 63.Jen J, Powell SM, Papadopoulos N, Smith KJ, Hamilton SR, Vogelstein B, Kinzler KW. Molecular determinants of dysplasia in colorectal lesions. Cancer Res. 1994;54:5523–5526. [PubMed] [Google Scholar]
- 64.Liang J, Lin C, Hu F, Wang F, Zhu L, Yao X, Wang Y, Zhao Y. APC polymorphisms and the risk of colorectal neoplasia: a HuGE review and meta-analysis. Am J Epidemiol. 2013;177:1169–1179. doi: 10.1093/aje/kws382. [DOI] [PubMed] [Google Scholar]
- 65.Liang TJ, Wang HX, Zheng YY, Cao YQ, Wu X, Zhou X, Dong SX. APC hypermethylation for early diagnosis of colorectal cancer: a meta-analysis and literature review. Oncotarget. 2017;8:46468–46479. doi: 10.18632/oncotarget.17576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen TH, Chang SW, Huang CC, Wang KL, Yeh KT, Liu CN, Lee H, Lin CC, Cheng YW. The prognostic significance of APC gene mutation and miR-21 expression in advanced-stage colorectal cancer. Colorectal Dis. 2013;15:1367–1374. doi: 10.1111/codi.12318. [DOI] [PubMed] [Google Scholar]
- 67.Peng Q, Zhang X, Min M, Zou L, Shen P, Zhu Y. The clinical role of microRNA-21 as a promising biomarker in the diagnosis and prognosis of colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:44893–44909. doi: 10.18632/oncotarget.16488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falzone L, Scola L, Zanghì A, Biondi A, Di Cataldo A, Libra M, Candido S. Integrated analysis of colorectal cancer microRNA datasets: identification of microRNAs associated with tumor development. Aging (Albany NY) 2018;10:1000–1014. doi: 10.18632/aging.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 70.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferracin M, Veronese A, Negrini M. Micromarkers: miRNAs in cancer diagnosis and prognosis. Expert Rev Mol Diagn. 2010;10:297–308. doi: 10.1586/erm.10.11. [DOI] [PubMed] [Google Scholar]
- 73.Nana-Sinkam P, Croce CM. MicroRNAs in diagnosis and prognosis in cancer: what does the future hold? Pharmacogenomics. 2010;11:667–669. doi: 10.2217/pgs.10.57. [DOI] [PubMed] [Google Scholar]
- 74.Carter JV, Galbraith NJ, Yang D, Burton JF, Walker SP, Galandiuk S. Blood-based microRNAs as biomarkers for the diagnosis of colorectal cancer: a systematic review and meta-analysis. Br J Cancer. 2017;116:762–774. doi: 10.1038/bjc.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang Y, Yang YB, Zhang XH, Yu XL, Wang ZB, Cheng XC. MicroRNA-21 gene and cancer. Med Oncol. 2013;30:376. doi: 10.1007/s12032-012-0376-8. [DOI] [PubMed] [Google Scholar]
- 76.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 77.Michael JV, Wurtzel JG, Goldfinger LE. Regulation of H-Ras-driven MAPK signaling, transformation and tumorigenesis, but not PI3K signaling and tumor progression, by plasma membrane microdomains. Oncogenesis. 2016;5:e228. doi: 10.1038/oncsis.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gray RT, Cantwell MM, Coleman HG, Loughrey MB, Bankhead P, McQuaid S, O’Neill RF, Arthur K, Bingham V, McGready C, et al. Evaluation of PTGS2 Expression, PIK3CA Mutation, Aspirin Use and Colon Cancer Survival in a Population-Based Cohort Study. Clin Transl Gastroenterol. 2017;8:e91. doi: 10.1038/ctg.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94:455–459. doi: 10.1038/sj.bjc.6602970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosty C, Young JP, Walsh MD, Clendenning M, Sanderson K, Walters RJ, Parry S, Jenkins MA, Win AK, Southey MC, et al. PIK3CA activating mutation in colorectal carcinoma: associations with molecular features and survival. PLoS One. 2013;8:e65479. doi: 10.1371/journal.pone.0065479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao L, Vogt PK. Helical domain and kinase domain mutations in p110alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc Natl Acad Sci U S A. 2008;105:2652–2657. doi: 10.1073/pnas.0712169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jehan Z, Bavi P, Sultana M, Abubaker J, Bu R, Hussain A, Alsbeih G, Al-Sanea N, Abduljabbar A, Ashari LH, et al. Frequent PIK3CA gene amplification and its clinical significance in colorectal cancer. J Pathol. 2009;219:337–346. doi: 10.1002/path.2601. [DOI] [PubMed] [Google Scholar]
- 83.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Domingo E, Church DN, Sieber O, Ramamoorthy R, Yanagisawa Y, Johnstone E, Davidson B, Kerr DJ, Tomlinson IP, Midgley R. Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J Clin Oncol. 2013;31:4297–4305. doi: 10.1200/JCO.2013.50.0322. [DOI] [PubMed] [Google Scholar]
- 85.Jang KS, Song YS, Jang SH, Min KW, Na W, Jang SM, Jun YJ, Lee KH, Choi D, Paik SS. Clinicopathological significance of nuclear PTEN expression in colorectal adenocarcinoma. Histopathology. 2010;56:229–239. doi: 10.1111/j.1365-2559.2009.03468.x. [DOI] [PubMed] [Google Scholar]
- 86.Goel A, Arnold CN, Niedzwiecki D, Carethers JM, Dowell JM, Wasserman L, Compton C, Mayer RJ, Bertagnolli MM, Boland CR. Frequent inactivation of PTEN by promoter hypermethylation in microsatellite instability-high sporadic colorectal cancers. Cancer Res. 2004;64:3014–3021. doi: 10.1158/0008-5472.can-2401-2. [DOI] [PubMed] [Google Scholar]
- 87.Tural D, Batur S, Erdamar S, Akar E, Kepil N, Mandel NM, Serdengeçti S. Analysis of PTEN, BRAF and PI3K status for determination of benefit from cetuximab therapy in metastatic colorectal cancer patients refractory to chemotherapy with wild-type KRAS. Tumour Biol. 2014;35:1041–1049. doi: 10.1007/s13277-013-1138-8. [DOI] [PubMed] [Google Scholar]
- 88.Sawai H, Yasuda A, Ochi N, Ma J, Matsuo Y, Wakasugi T, Takahashi H, Funahashi H, Sato M, Takeyama H. Loss of PTEN expression is associated with colorectal cancer liver metastasis and poor patient survival. BMC Gastroenterol. 2008;8:56. doi: 10.1186/1471-230X-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet JB, Lecomte T, Rougier P, Lievre A, Landi B, Boige V, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924–5930. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]
- 90.Scalise JR, Poças RC, Caneloi TP, Lopes CO, Kanno DT, Marques MG, Valdivia JC, Maximo FR, Pereira JA, Ribeiro ML, et al. DNA Damage Is a Potential Marker for TP53 Mutation in Colorectal Carcinogenesis. J Gastrointest Cancer. 2016;47:409–416. doi: 10.1007/s12029-016-9846-0. [DOI] [PubMed] [Google Scholar]
- 91.Huh JW, Kim HR, Kim YJ. Prognostic role of p53 messenger ribonucleic acid expression in patients after curative resection for stage I to III colorectal cancer: association with colon cancer stem cell markers. J Am Coll Surg. 2013;216:1063–1069. doi: 10.1016/j.jamcollsurg.2013.01.058. [DOI] [PubMed] [Google Scholar]
- 92.Tzeng ST, Tsai MH, Chen CL, Lee JX, Jao TM, Yu SL, Yen SJ, Yang YC. NDST4 is a novel candidate tumor suppressor gene at chromosome 4q26 and its genetic loss predicts adverse prognosis in colorectal cancer. PLoS One. 2013;8:e67040. doi: 10.1371/journal.pone.0067040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kjellén L. Glucosaminyl N-deacetylase/N-sulphotransferases in heparan sulphate biosynthesis and biology. Biochem Soc Trans. 2003;31:340–342. doi: 10.1042/bst0310340. [DOI] [PubMed] [Google Scholar]
- 94.Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR, Preisinger AC, Thomas G, Kinzler KW. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- 95.Riggins GJ, Thiagalingam S, Rozenblum E, Weinstein CL, Kern SE, Hamilton SR, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mad-related genes in the human. Nat Genet. 1996;13:347–349. doi: 10.1038/ng0796-347. [DOI] [PubMed] [Google Scholar]
- 96.Howe JR, Roth S, Ringold JC, Summers RW, Järvinen HJ, Sistonen P, Tomlinson IP, Houlston RS, Bevan S, Mitros FA, et al. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998;280:1086–1088. doi: 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- 97.Sarli L, Bottarelli L, Bader G, Iusco D, Pizzi S, Costi R, D’Adda T, Bertolani M, Roncoroni L, Bordi C. Association between recurrence of sporadic colorectal cancer, high level of microsatellite instability, and loss of heterozygosity at chromosome 18q. Dis Colon Rectum. 2004;47:1467–1482. doi: 10.1007/s10350-004-0628-6. [DOI] [PubMed] [Google Scholar]
- 98.Popat S, Houlston RS. A systematic review and meta-analysis of the relationship between chromosome 18q genotype, DCC status and colorectal cancer prognosis. Eur J Cancer. 2005;41:2060–2070. doi: 10.1016/j.ejca.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 99.Popat S, Zhao D, Chen Z, Pan H, Shao Y, Chandler I, Houlston RS. Relationship between chromosome 18q status and colorectal cancer prognosis: a prospective, blinded analysis of 280 patients. Anticancer Res. 2007;27:627–633. [PubMed] [Google Scholar]
- 100.Boulay JL, Mild G, Lowy A, Reuter J, Lagrange M, Terracciano L, Laffer U, Herrmann R, Rochlitz C. SMAD4 is a predictive marker for 5-fluorouracil-based chemotherapy in patients with colorectal cancer. Br J Cancer. 2002;87:630–634. doi: 10.1038/sj.bjc.6600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baserga R. The IGF-I receptor in cancer research. Exp Cell Res. 1999;253:1–6. doi: 10.1006/excr.1999.4667. [DOI] [PubMed] [Google Scholar]
- 102.Girnita L, Girnita A, Brodin B, Xie Y, Nilsson G, Dricu A, Lundeberg J, Wejde J, Bartolazzi A, Wiman KG, et al. Increased expression of insulin-like growth factor I receptor in malignant cells expressing aberrant p53: functional impact. Cancer Res. 2000;60:5278–5283. [PubMed] [Google Scholar]
- 103.Aleksic T, Chitnis MM, Perestenko OV, Gao S, Thomas PH, Turner GD, Protheroe AS, Howarth M, Macaulay VM. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010;70:6412–6419. doi: 10.1158/0008-5472.CAN-10-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Codony-Servat J, Cuatrecasas M, Asensio E, Montironi C, Martínez-Cardús A, Marín-Aguilera M, Horndler C, Martínez-Balibrea E, Rubini M, Jares P, et al. Nuclear IGF-1R predicts chemotherapy and targeted therapy resistance in metastatic colorectal cancer. Br J Cancer. 2017;117:1777–1786. doi: 10.1038/bjc.2017.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Linnekamp JF, Hooff SRV, Prasetyanti PR, Kandimalla R, Buikhuisen JY, Fessler E, Ramesh P, Lee KAST, Bochove GGW, de Jong JH, et al. Consensus molecular subtypes of colorectal cancer are recapitulated in in vitro and in vivo models. Cell Death Differ. 2018;25:616–633. doi: 10.1038/s41418-017-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Okita A, Takahashi S, Ouchi K, Inoue M, Watanabe M, Endo M, Honda H, Yamada Y, Ishioka C. Consensus molecular subtypes classification of colorectal cancer as a predictive factor for chemotherapeutic efficacy against metastatic colorectal cancer. Oncotarget. 2018;9:18698–18711. doi: 10.18632/oncotarget.24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Isella C, Brundu F, Bellomo SE, Galimi F, Zanella E, Porporato R, Petti C, Fiori A, Orzan F, Senetta R, et al. Selective analysis of cancer-cell intrinsic transcriptional traits defines novel clinically relevant subtypes of colorectal cancer. Nat Commun. 2017;8:15107. doi: 10.1038/ncomms15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zanella ER, Galimi F, Sassi F, Migliardi G, Cottino F, Leto SM, Lupo B, Erriquez J, Isella C, Comoglio PM, et al. IGF2 is an actionable target that identifies a distinct subpopulation of colorectal cancer patients with marginal response to anti-EGFR therapies. Sci Transl Med. 2015;7:272ra12. doi: 10.1126/scitranslmed.3010445. [DOI] [PubMed] [Google Scholar]
- 110.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 111.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC Jr; ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]


