Abstract
AIM
To investigate the candidate microRNA (miRNA), miR-221 as a novel biomarker for diabetic retinopathy (DR) in patients associated with type 2 diabetes (T2D).
METHODS
The subjects involved were divided into four groups: healthy control (HC), no diabetic retinopathy (NDR), non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) group. Serum miR-221 was validated by real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR). Also, serum angiotensin II (Ang II) and vascular endothelial growth factor (VEGF) were examined by enzyme-linked immunosorbent assay. In addition, receiver operating characteristic (ROC) curve was performed to explore the diagnostic accuracy of miR-221, Ang II and VEGF for DR in patients with T2D. Spearman's rank correlation coefficient was executed to estimate the correlations of serum miR-221 with metabolic parameters and serum markers in patients with T2D.
RESULTS
Primarily, serum miR-221, Ang II and VEGF were increased significantly in T2D patients compared to HC participant respectively, and progressive up-regulated in NDR, NPDR and PDR groups (P<0.001). Additionally, miR-221 in serum was remarkably positively correlated with metabolic parameters such as glycated hemoglobin (r=0.310, P=0.002) and homeostasis model assessment for insulin resistance (r=0.413, P<0.001), as well as serum markers for instance Ang II (r=0.667, P<0.001) and VEGF (r=0.499, P<0.001). Furthermore, serum miR-221 (AUC, 0.894; 95%CI, 0.833-0.955; P<0.001), Ang II (AUC, 0.888; 95%CI, 0.828-0.949; P<0.001) and VEGF (AUC, 0.785; 95%CI, 0.695-0.875; P<0.001) had evidently diagnostic efficiency in DR, and miR-221 is the most effective among them.
CONCLUSION
Serum miR-221 as a potential biomarker could be related to not only occurrence but also progression for DR in patients with T2D. However, a prospective clinical trial is warranted.
Keywords: microRNA-221, biomarker, diabetic retinopathy, type 2 diabetes
INTRODUCTION
With the increasing global morbidity of diabetes, diabetic retinopathy (DR) is still the primary reason for incurable blindness of diabetic patients, which is featured as progressive microvascular complications owing to chronically hyperglycemia from diabetes[1]. About 1/3 have symptoms of DR in diabetic patients, moreover 1/3 of these maybe have visual impaired retinal pathology, realized as proliferative diabetic retinopathy (PDR)[2]. Furthermore, the manifestation of DR implies a dangerous factor of health-threatening systemic vascular complications[3].
Epidemiological and genetic researches have furthered our comprehension of the pathophysiology on DR[4]–[6]. Moreover, recent studies have unveiled an influential and unexpected role of epigenetics, notably microRNAs (miRNAs) in main biological processes, providing a unique perspective opportunity to translate this theoretical hypothesis into the clinical practice in the form of miRNA-based pathogenesis, diagnosis, treatment and prognosis for DR[7]–[8]. miRNAs are short (about 22 nucleotides long) and highly conserved sequences of endogenous RNA that do not encode for any protein and represent a new powerful class of gene modulators[9]. They are expressed in all human cell-types and are involved in the main biological processes including cell growth, differentiation, and apoptosis[10].
Previous studies focusing on miR-221, suggested that the expression of miR-221 were significantly upregulated and may involve in the physiopathology of diabetes and macrovascular complications associated with type 2 diabetes (T2D) via specific target genes[11]–[13]. To validate the hypothesis based on previous studies, a cross-sectional study was conducted to identify whether serum miR-221 as a novel biomarker with high sensitivity and specificity for genetic diagnosis and biologic therapy in patients with DR according to the epigenetic methodology.
SUBJECTS AND METHODS
The current study was conformed to the contents of the Declaration of Helsinki, meanwhile was authorized by the Institutional Review Board/Ethics Committee of Shengjing Hospital, China Medical University. All subjects had written the informed consent before enrollment.
Participants
Participants were consecutively recruited from Shengjing Hospital, China Medical University between January 2015 and January 2016, afterwards divided to four study groups in accordance to Diabetic Retinopathy Disease Severity Scale based on fundus fluorescein angiography: healthy control (HC) group, HC participants; no diabetic retinopathy (NDR) group, T2D patients without DR; non-proliferative diabetic retinopathy (NPDR) group, T2D patients with NPDR; PDR group, T2D patients with PDR[14]. Exclusion criteria were other serious ocular and systemic diseases. Clinical history and medications were evaluated. All participants experienced ophthalmologic examination.
Samples Collection
Serum samples were collected and stored according to our previous study until further processing[15].
miRNA Quantification
The expression level of serum miRNA was quantified by real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR) according to our previous study[15]. All the experiments were run in triplicate.
Serum Factor Quantification
The expression levels of serum factors were quantified by enzyme-linked immunosorbent assay (ELISA). Serum concentration of angiotensin II (Ang II) was measured by using ELISA kit for Ang II (Wuhan USCN Business Co., Ltd., Wuhan, China) according to manufacturer protocol. Serum level of vascular endothelial growth factor (VEGF) was assessed by using Human Vascular Endothelial Cell Growth Factor ELISA Kit (CUSABIO Technology LLC, Houston, TX, USA) following manufacturer instruction. All the values obtained were in the expected range.
Statistical Analysis
SPSS 22.0 (IBM Corp, Armonk, NY, USA) was used to calculate data. Values were expressed as the mean±standard deviation (SD) for normally distributed values and median (interquartile range) for abnormal distribution values. Continuous variables were evaluated by analysis of variance, followed by Fisher's least significant difference (LSD) test for Gaussian distribution and Kruskal-Wallis test for non-Gaussian distribution. Categorical variables were assessed by Chi-squared test. Correlations were performed by either Pearson correlation coefficient or Spearman's rank correlation coefficient. The diagnostic efficiency was detected by receiver operating characteristic (ROC) curve. P<0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
No significant differences were found in age, gender, body mass index, diastolic blood pressure, systolic blood pressure, total cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, serum creatinine and blood urea nitrogen among these four groups (P>0.05). However, diabetes duration, fasting blood glucose (FBG), glycated hemoglobin (HbA1c) and homeostasis model assessment for insulin resistance (HOMA-IR) in patients with T2D were increased significantly compared to HC group (P<0.001). Additionally, of patients with T2D, diabetes duration, HbA1c and HOMA-IR in PDR and NPDR group were increased significantly compared to NDR group (P<0.05). Furthermore, diabetes duration and HbA1c in PDR group were increased significantly compared to NPDR group (P<0.05). Baseline characteristics of the recruited subjects are listed in Table 1.
Table 1. Baseline characteristics of the recruited participants in the cross-sectional study.
| Variable | HC group | NDR group | NPDR group | PDR group | P |
| Participant (n) | 33 | 37 | 34 | 30 | NA |
| Age (y) | 63.52±4.91 | 66.14±3.96 | 63.50±4.99 | 64.67±5.17 | 0.067 |
| Gender (M/F) | 23/10 | 24/13 | 20/14 | 16/14 | 0.560 |
| BMI (kg/m2) | 27.73±3.82 | 26.16±3.72 | 27.06±4.15 | 27.43±2.70 | 0.308 |
| DBP (mm Hg) | 84.58±8.02 | 88.70±9.35 | 86.56±9.38 | 89.83±7.64 | 0.077 |
| SBP (mm Hg) | 131.58±11.90 | 137.03±11.15 | 135.74±12.07 | 139.50±10.84 | 0.051 |
| Diabetes duration (y) | NA | 5.19±2.88 | 8.44±3.69b | 11.77±4.09b,c | 0.000 |
| FBG (mmol/L) | 4.75±0.85 | 9.01±1.16a | 9.14±1.76a | 8.77±1.42a | 0.000 |
| HbA1c (%) | 5.19±0.84 | 7.31±1.18a | 7.95±1.05a,b | 8.51±1.22a,b,c | 0.000 |
| HOMA-IR | 1.39±0.81 | 4.86±1.15a | 5.58±1.25a,b | 6.80±1.13a,b,c | 0.000 |
| TC (mmol/L) | 4.55±0.62 | 4.37±0.66 | 4.70±0.60 | 4.41±0.56 | 0.101 |
| TG (mmol/L) | 1.71±0.93 | 1.58±0.76 | 1.92±0.81 | 1.49±0.77 | 0.179 |
| HDL-C (mmol/L) | 1.27±0.19 | 1.34±0.16 | 1.22±0.14 | 1.29±0.18 | 0.052 |
| LDL-C (mmol/L) | 2.78±0.46 | 2.96±0.57 | 3.06±0.58 | 2.93±0.40 | 0.155 |
| Scr (µmol/L) | 56.18±13.83 | 59.14±12.32 | 53.74±11.23 | 61.40±10.38 | 0.061 |
| BUN (mmol/L) | 5.25±1.45 | 5.30±1.03 | 5.13±1.08 | 5.62±1.15 | 0.416 |
BMI: Body mass index; BUN: Blood urea nitrogen; DBP: Diastolic blood pressure; FPG: Fasting plasma glucose; HbA1c: Glycated hemoglobin; HC: Healthy control; HDL-C: High-density lipoprotein cholesterol; HOMA-IR: Homeostatic model assessment of insulin resistance; LDL-C: Low-density lipoprotein cholesterol; NA: No available; NDR: No diabetic retinopathy; NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy; SBP: Systolic blood pressure; Scr: Serum creatinine; TC: Total cholesterol; TG: Triglyceride. aP<0.05 versus HC group; bP<0.05 versus NDR group; cP<0.05 versus NPDR group.
Relative Expression of Serum miR-221
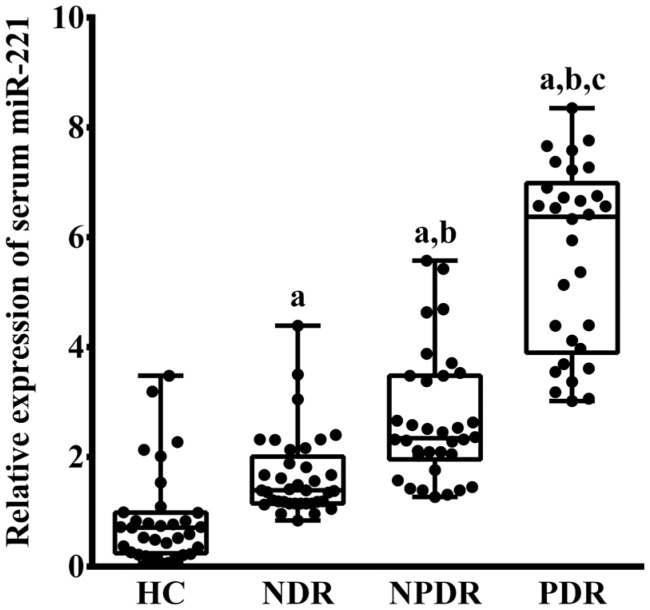
There was a significantly difference in serum miR-221 level among four groups (P<0.001; Figure 1). The serum miR-221 in patients with T2D were significantly increased compared to HC group (PHC vs NDR=0.015, PHC vs NPDR<0.001, PHC vs PDR<0.001). Moreover, in patients with T2D, the serum miR-221 in NDR, NPDR and PDR groups were gradually increased, respectively (PNDR vs NPDR=0.025, PNDR vs PDR<0.001, PNPDR vs PDR=0.001).
Figure 1. Relative expression of serum miR-221 in HC, NDR, NPDR and PDR groups.
aP<0.05 versus HC group; bP<0.05 versus NDR group; cP<0.05 versus NPDR group.
Relative Expression of Serum Makers
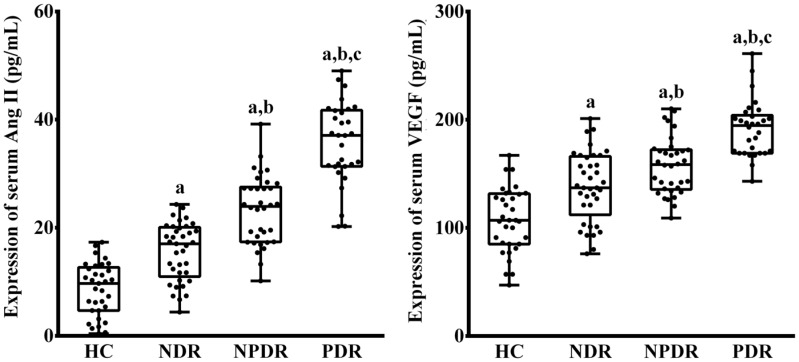
There was a significant difference in serum Ang II level among the four groups, respectively (P<0.001; Figure 2). The serum Ang II in patients with T2D were significantly increased compared to HC group, respectively (PHC vs NDR<0.001, PHC vs NPDR<0.001, PHC vs PDR<0.001). Furthermore, in patients with T2D, the serum Ang II were stepwise increased in NDR, NPDR and PDR groups (PNDR vs NPDR<0.001, PNDR vs PDR<0.001, PNPDR vs PDR<0.001).
Figure 2. Expression of serum Ang II and VEGF in HC, NDR, NPDR and PDR groups.
aP<0.05 versus HC group; bP<0.05 versus NDR group; cP<0.05 versus NPDR group.
There was a significant difference in serum VEGF level among the four groups, respectively (P<0.001; Figure 2). The serum VEGF in patients with T2D were significantly increased compared to HC group, respectively (PHC vs NDR<0.001, PHC vs NPDR<0.001, PHC vs PDR<0.001). Furthermore, in patients with T2D, the serum VEGF were stepwise increased in NDR, NPDR and PDR groups (PNDR vs NPDR=0.027, PNDR vs PDR<0.001, PNPDR vs PDR<0.001).
Correlations of Serum miR-221 with Metabolic Parameters and Serum Markers
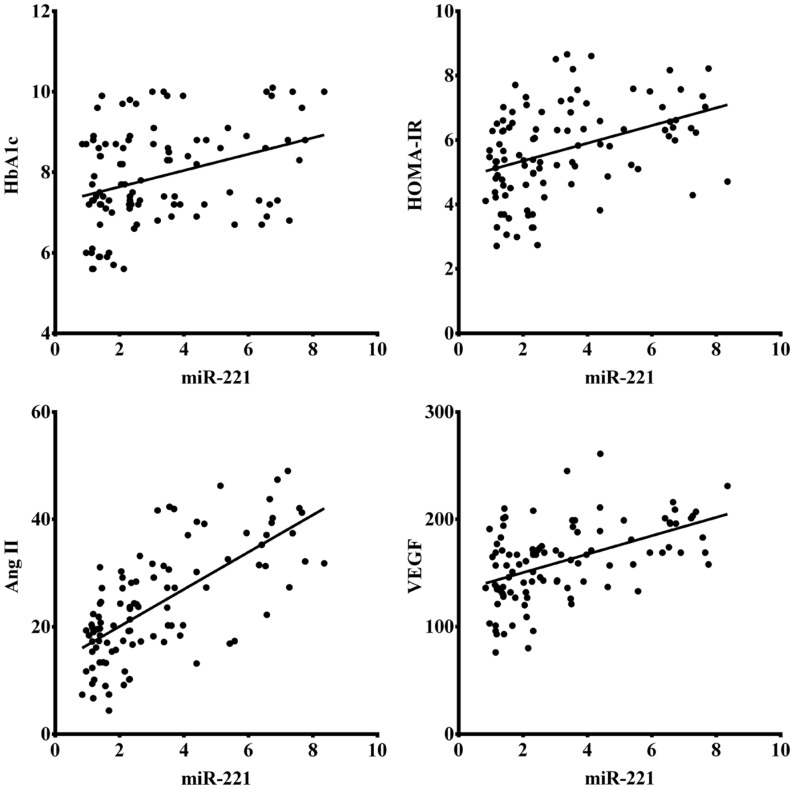
There was a significant positive correlation of serum miR-221 with metabolic parameters (Figure 3), including HbA1c (r=0.310, P=0.002) and HOMA-IR (r=0.413, P<0.001). Meanwhile, serum miR-221 were also found to be significantly positive correlated with serum markers (Figure 3), involving Ang II (r=0.667, P<0.001) and VEGF (r=0.499, P<0.001).
Figure 3. Correlation of serum miR-221 with metabolic parameters including HbA1c and HOMA-IR, as well as serum markers involving Ang II and VEGF in patients with T2D.
Diagnostic Accuracy of Serum miR-221 and Serum Makers
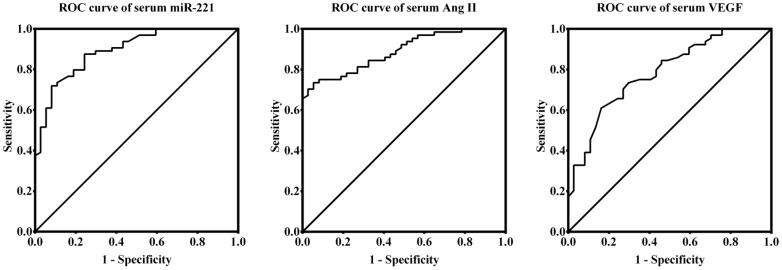
ROC curves, the AUC values of miR-221, Ang II and VEGF were 0.894 (95%CI, 0.833-0.955; P<0.001), 0.888 (95%CI, 0.828-0.949; P<0.001) and 0.785 (95%CI, 0.695-0.875; P<0.001), respectively, with significantly diagnostic accuracy for patients with DR, in which miR-221 is the most powerful (Figure 4).
Figure 4. ROC curves of miR-221, Ang II and VEGF for DR in patients with T2D.
DISCUSSION
This cross-sectional study yielded almost consentaneous results that serum miR-221 was remarkably gradually increased with the severity of DR in T2D patients compared with HC subjects, and positive correlated with serum Ang II and VEGF in DR associated with T2D, moreover with a strong diagnostic efficiency for DR, give rise to identical recommendation with regard to miR-221 as a novel biomarker for on-set and development of DR associated with T2D.
The pathogenesis of DR is a multifactorial affected by genetic and environmental factors[1]–[2]. Hyperglycemia-related pathways have been proposed to initiate a cascade of interactions between physiological and pathological alterations, ultimately leading to retinal microvascular functional disorder, which was implied in the occurrence or progression of DR[1]–[2]. Epidemiological studies, clinical trials and experimental researches have indicated that previous experience in hyperglycemia has long-lasting outcomes, and DR continues to develop even after the euglycemia is initial, therefore implying a novel biomarker is necessary to identify individuals with high risk of progressive sight-threatening DR in patients with T2D[16].
Serum miRNA is apparently steady both in vivo and in vitro circumstance with multiple external conditions such as pH, temperature and pressure[17]. Generally, DR was characterized by microvascular structural and functional abnormalities, involving occlusion and leakage of retinal vessels, resulting in macular edema in the non-proliferative phase and angiogenesis and to tufts of highly permeable vessels in the proliferative phase. As a consequence, specific miRNAs could be selectively excessively secreted into the circulatory system through destroyed blood-retinal barrier via small membrane vesicles, such as exosomes, that are released into the extracellular environment[15]. Recently, there is growing evidence that the expression patterns and levels of specific miRNAs could reveal altered physiological and pathological conditions in both the microvascular and macrovascular complications of patients with T2D, and circulating miRNA is widely recognized to be as an attractive novel biomarker[18].
miR-221, that targets and binds to substantial genes and key factors of insulin/insulin-like signaling pathway, has been previously verified to be related to metabolic syndrome, diabetes and its complications[19]. Furthermore, under hyperglycemic conditions, significantly up-regulated miR-221 could induce cell proliferation, migration and apoptosis, lead to facilitate retinal vascular endothelial dysfunction, promote retinal vascular hyperplasia and occlusion to pursue retinal ischemia as well hypoxia, and expedite retinal neovascularization[20]. Consequently, aberrantly increased miR-221 can be detected in serum of patients with T2D, then gradually increased with the severity of DR could attributed to exacerbate blood-retinal barrier breakdown to aggravate casual retinal vascular damage and dysfunction exposed to hyperglycemic environment. Therefore, it has been implied that miR-221 may involve in angiogenesis, suggesting a new theory for DR in patients with T2D.
The current quantitative results indicated that serum miR-221, Ang II and VEGF was significantly increased in T2D patients compared with HC subjects, and was regularly and markedly up-regulated in NDR, NPDR and PDR groups as well. The present qualitative results, ROC curves implied that miRNA-221, Ang II and VEGF with reasonable sensitivity and specificity have a promising diagnostic efficiency in DR respectively, among which miR-221 is the more noteworthy than others. The nowaday correlative results revealed that a significant positive correlation between miR-221 and metabolic parameters such as HbA1c and HOMA-IR, as well as serum markers for instance, Ang II and VEGF, respectively, suggesting miR-221 may involve in pathogenesis mechanism of DR in T2D patients.
Ang II, the main component in rennin-angiotensin system, which is considered as modulating retinal vascular architecture and function via role in vasoconstriction and angiogenesis has been implicated in DR[21]. VEGF, the pivotal angiogenic growth factor, which is thought to regulate retinal vascular structure as well as potence by effect on vasculogenesis and angiogenesis has been referred to DR[22]. Previous related studies could establish hypothesis that endothelial dysfunction associated with the development of DR is worsened by increased Ang II, which was induced by hyperglycemia, leading to activate JNK/c-Jun/mTOR signaling pathways, and thereby enhancing miR-221 expression through targeting cyclin-dependent kinase inhibitor 1B, furthermore, Ang II could potentiate the actions of VEGF-induced endothelial cell proliferation and migration via rennin-angiotensin system mediated mechanism, implying that Ang II may exacerbate or accentuate the expression and effect of VEGF via NADPH oxidase on retinal vascular endothelial cells[23]–[26]. These implicated that miR-221 was highly associated with Ang II and VEGF resulting in the retinal vascular endothelial dysfunction under the hyperglycemia condition.
The current study has certain limitations. First of all, the study design is comparatively restricted, and the effect of investigative biomarkers in the progression of DR is still unknown due to lack of follow up. Second, the sample size is relatively limited, and the obtained results should be interpreted restrainedly and considered preliminary. Finally, the methodology is somewhat unilateral, and the observed correlation need to further validate via molecular mechanistic experiments.
The current cross-sectional study implies that miR-221 as a potential biomarker could be related to occurrence and progression of DR in patients with T2D. Nevertheless, a prospective, high-quality, large sample, long-term clinical trial coincided with experimental research is warranted to be further investigated.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.81371045; No.81570866); Science and Technology Program of Liaoning Province, China (No. 201002196; No.2013225049); Science and Technology Program of Shenyang Municipality, China (No.F13-221-9-37; No.18-014-4-41).
Conflicts of Interest: Liu HN, None; Li X, None; Wu N, None; Tong MM, None; Chen S, None; Zhu SS, None; Qian W, None; Chen XL, None.
REFERENCES
- 1.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 2.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 3.Cheung N, Wong TY. Diabetic retinopathy and systemic vascular complications. Prog Retin Eye Res. 2008;27(2):161–176. doi: 10.1016/j.preteyeres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Cunha-Vaz J, Ribeiro L, Lobo C. Phenotypes and biomarkers of diabetic retinopathy. Prog Retin Eye Res. 2014;41:90–111. doi: 10.1016/j.preteyeres.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Simó R, Hernández C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retin Eye Res. 2015;48:160–180. doi: 10.1016/j.preteyeres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes HP, Simó R, Lois N. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016;51:156–186. doi: 10.1016/j.preteyeres.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Mastropasqua R, Toto L, Cipollone F, Santovito D, Carpineto P, Mastropasqua L. Role of microRNAs in the modulation of diabetic retinopathy. Prog Retin Eye Res. 2014;43:92–107. doi: 10.1016/j.preteyeres.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schirle NT, Sheu-Gruttadauria J, MacRae IJ. Structural basis for microRNA targeting. Science. 2014;346(6209):608–613. doi: 10.1126/science.1258040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153(3):516–519. doi: 10.1016/j.cell.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Fiorentino L, Cavalera M, Mavilio M, Conserva F, Menghini R, Gesualdo L, Federici M. Regulation of TIMP3 in diabetic nephropathy: a role for microRNAs. Acta Diabetol. 2013;50(6):965–969. doi: 10.1007/s00592-013-0492-8. [DOI] [PubMed] [Google Scholar]
- 12.Costantino S, Paneni F, Lüscher TF, Cosentino F. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur Heart J. 2016;37(6):572–576. doi: 10.1093/eurheartj/ehv599. [DOI] [PubMed] [Google Scholar]
- 13.Lightell DJ, Jr, Moss SC, Woods TC. Upregulation of miR-221 and -222 in response to increased extracellular signal-regulated kinases 1/2 activity exacerbates neointimal hyperplasia in diabetes mellitus. Atherosclerosis. 2018;269:71–78. doi: 10.1016/j.atherosclerosis.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT, Global Diabetic Retinopathy Project Group Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 15.Liu HN, Cao NJ, Li X, Qian W, Chen XL. Serum microRNA-211 as a biomarker for diabetic retinopathy via modulating Sirtuin 1. Biochem Biophys Res Commun. 2018;505(4):1236–1243. doi: 10.1016/j.bbrc.2018.10.052. [DOI] [PubMed] [Google Scholar]
- 16.Kowluru RA. Diabetic retinopathy, metabolic memory and epigenetic modifications. Vision Res. 2017;139:30–38. doi: 10.1016/j.visres.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Blondal T, Jensby Nielsen S, Baker A, Andreasen D, Mouritzen P, Wrang Teilum M, Dahlsveen IK. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods. 2013;59(1):S1–S6. doi: 10.1016/j.ymeth.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 18.La VM, Fierabracci A. Insights into the diagnostic potential of extracellular vesicles and their miRNA signature from liquid biopsy as early biomarkers of diabetic micro/macrovascular complications. Int J Mol Sci. 2017;18(9):pii: E1974. doi: 10.3390/ijms18091974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deiuliis JA. MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. Int J Obes (Lond) 2016;40(1):88–101. doi: 10.1038/ijo.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Song YH, Li F, Yang T, Lu YW, Geng YJ. MicroRNA-221 regulates high glucose-induced endothelial dysfunction. Biochem Biophys Res Commun. 2009;381(1):81–83. doi: 10.1016/j.bbrc.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fletcher EL, Phipps JA, Ward MM, Vessey KA, Wilkinson-Berka JL. The renin-angiotensin system in retinal health and disease: Its influence on neurons, glia and the vasculature. Prog Retin Eye Res. 2010;29(4):284–311. doi: 10.1016/j.preteyeres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Grigsby JG, Allen DM, Ferrigno AS, Vellanki S, Pouw CE, Hejny WA, Tsin ATC. Autocrine and paracrine secretion of vascular endothelial growth factor in the pre-hypoxic diabetic retina. Curr Diabetes Rev. 2017;13(2):161–174. doi: 10.2174/1573399812666161007165944. [DOI] [PubMed] [Google Scholar]
- 23.Fukumoto M, Takai S, Ishizaki E, Sugiyama T, Oku H, Jin D, Sakaguchi M, Sakonjo H, Ikeda T, Miyazaki M. Involvement of angiotensin II-dependent vascular endothelial growth factor gene expression via NADPH oxidase in the retina in a type 2 diabetic rat model. Curr Eye Res. 2008;33(10):885–891. doi: 10.1080/02713680802389851. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Kim JH, Yu YS, Cho CS, Kim KW. Blockade of angiotensin II attenuates VEGF-mediated blood-retinal barrier breakdown in diabetic retinopathy. J Cereb Blood Flow Metab. 2009;29(3):621–628. doi: 10.1038/jcbfm.2008.154. [DOI] [PubMed] [Google Scholar]
- 25.Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, Guo Z, Liu J, Wang Y, Yuan W, Qin Y. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215(2):286–293. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Qian LB, Jiang SZ, Tang XQ, Zhang J, Liang YQ, Yu HT, Chen J, Xu Z, Liu RM, Keller BB, Ji HL, Cai L. Exacerbation of diabetic cardiac hypertrophy in OVE26 mice by angiotensin II is associated with JNK/c-Jun/miR-221-mediated autophagy inhibition. Oncotarget. 2017;8(63):106661–106671. doi: 10.18632/oncotarget.21302. [DOI] [PMC free article] [PubMed] [Google Scholar]