Abstract
AIM
To investigate the ocular hemodynamic effects of applying a hot compress to the eye.
METHODS
The right eyes of five New Zealand white rabbits, both male and female, were hot-compressed for 18min. An independently designed novel ocular contact-type temperature measuring device was used to measure the ocular surface temperature before and after the heating. Relevant retrobulbar hemodynamic parameters such as peak systolic velocity (PSV), end diastolic velocity (EDV), and resistance index (RI) of each of the central retinal artery (CRA), long posterior ciliary artery (LPCA), and ophthalmic artery (OA), as well as the mean velocity (Vm) of the central retinal vein (CRV), were measured using a color Doppler flow imaging (CDFI) technique and expressed as mean values with standard deviation (mean±SD). A statistical analysis was conducted based on a paired t-test and the Wilcoxon signed-rank test.
RESULTS
The employed real-time temperature measuring device was able to accurately measure ocular surface temperature during the hot-compress process. The temperature increased after the hot compress was applied. Analysis showed that the PSV and EDV values of the CRA and LPCA significantly increased after the application of the hot compress, as did the Vm of the CRV. There were no significant changes in the EDV of the OA nor the RI of each artery.
CONCLUSION
This experiment, which is the first of its kind, confirms that the retrobulbar blood flow velocities can increase upon heating the ocular surface. This simple method may be useful in the future.
Keywords: ocular hemodynamics, ocular surface heating, temperature detection device, color Doppler flow imaging
INTRODUCTION
Glaucoma is the second-most common eye disease that leads to blindness. Its main pathological feature is the apoptosis of retinal ganglion cells (RGCs), which causes irreversible optic nerve injury and thus poses a serious threat to human eye health. Ischemia and mechanical damage are the two most important pathological mechanisms involved in glaucoma. Specifically, ischemia is of great significance for normal intraocular pressure (IOP) glaucoma, in which IOP is within the normal range. Research has revealed that retrobulbar blood flow velocities are lower than normal in glaucoma patients. The resistance indices (RIs) of the retrobulbar blood vessels are also higher than normal[1]–[3], and this is closely related to the occurrence of visual field defects[4]–[6]. In other words, glaucoma patients have lower than normal blood supply within their eyes. For years, many glaucoma treatments have mainly focused on reducing IOP through drugs, surgery, and lasers, as well as on reducing mechanical damage to the optic nerve and assisting in its protection, while fewer treatments have been developed for blood vessel ischemia at the clinical level[7]. Physical therapies can directly improve blood flow rates of the retrobulbar blood vessels, exhibiting fewer side effects than the more invasive treatments. Therefore, it is necessary to identify a simple, safe, and effective method for improving ocular blood supply in glaucoma patients.
Temperature has always been an important indicator for health practitioners in the diagnosis and treatment of diseases. The eye is the visual organ of the body, and it has its own temperature system, which is closely related to eye diseases. It has been shown that the temperature of the ocular surface is affected by retrobulbar blood flow velocities. Higher end diastolic velocities (EDVs) of the ocular arteries imply a higher temperature of the ocular surface, and higher RI values of the ocular arteries lead to a lower temperature at the ocular surface[8]. However, it is not clear whether an increase in ocular surface temperature could lead to relative increases in retrobulbar blood flow velocities. In the current context of treatments and disease characteristics, our research team hypothesized that blood flow of the ocular surface could be increased using a hot compress on the ocular surface, and this hypothesis was tested experimentally for the first time in this study. Increasing the retrobulbar circulation through a simple and convenient physical method that is non-invasive and has no systemic side effects for patients, is explored as a new method to treat glaucoma-related ischemia. The proposed novel physical therapy method will be investigated for its therapeutic effects in glaucoma treatment in further experiments. In this experiment, the temperature of the ocular surface was measured as a function of time using a novel independently designed and fabricated contact-type temperature measuring device. With this device, it is possible to observe ocular surface temperature in real-time and assess the changes therein, which can reflect various parameters of the retrobulbar blood vessels combined with Doppler. Blood flow velocities and RI values of the different ocular blood vessels were evaluated using color Doppler flow imaging (CDFI) and spectrum Doppler detection techniques before and after the application of the hot compress.
MATERIALS AND METHODS
All the experimental procedures conformed to the Association for Research in Vision and Ophthalmology's Statement for the Use of Animals in Ophthalmic and Vision Research. The animal protocols were approved by the Animal Experimentation Ethics Committee of Harbin Medical University (Harbin, China).
Animals
Five New Zealand white rabbits aged 6-10mo and with average mass of 2.57±0.10 kg were obtained from the Laboratory Animal Research Center of Harbin Medical University (Harbin, China). The rabbits were maintained at a stable temperature of 22°C±2°C and humidity of 55%±5% under controlled illumination in a 12/12h light/dark cycle and in a non-pathogenic environment. The right eye was subjected to measurement every second day. A 3% pentobarbital sodium solution was injected at 1 mL/kg muscle. When the anesthetic effects began, the rabbit was laid on the experiment table with its head tilted to the right, and the hair around its eyelids was removed with scissors in order to avoid it affecting the ultrasound imaging. All operations were performed in a constant temperature environment. Each experiment was performed at the same place and at the same time of day. The experiments were repeated three times.
Installation and Testing of Ocular Temperature Measuring Device
The independently designed and fabricated ocular temperature measuring system consisted of five parts: temperature probe; temperature transmitter; linear power supply; single chip microcomputer; and computer screen. The microcomputer (Figure 1) recorded the instantaneous temperature, maximum and minimum temperatures, and average temperature.
Figure 1. Novel ocular contact-type temperature measuring device.
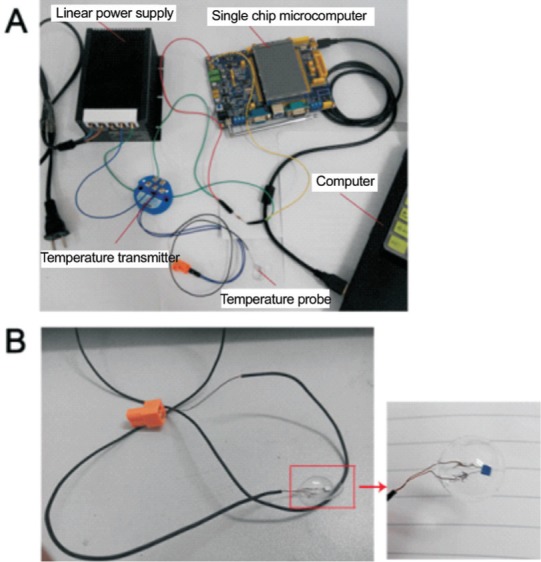
A: The device consists of five parts: the temperature probe; temperature transmitter; linear power supply; single chip microcomputer; and computer screen; B: The probe in this device is shaped similarly to a contact lens. The surface is smooth, transparent, soft, and exhibits a good fit on the eye.
The eye contact part was equipped with a thermistor fabricated in the shape of a contact lens, which used changes in resistance to infer temperature changes. The temperature transmitter and precision resistance converted the resistance changes into a voltage within the range of 0-3.3 V, which was processed by the single-chip microcomputer and displayed on the screen. After calibration, the relationship between voltage and temperature was determined.
In vitro test: When the device was connected, it was placed in a thermostatic aqueous solution and removed after stabilization for several times. The temperature curve was then analyzed, revealing the stability of the measuring device.
In vivo test: The temperature probe was placed on the surface of the cornea of a New Zealand white rabbit after the anesthesia took hold. When the temperature curve stabilized, the eye was heated and cooled, and the temperature curve of the ocular surface was observed.
Ocular Temperature Measurement Using Hot Compress Method
The probe of the temperature measuring device was able to sense the surrounding temperature, and gradually stabilized at a room temperature, as confirmed by the reading of the temperature measurement software. The eyelid was then opened and the probe was placed on the surface of the eye. The probe was slightly adjusted to allow it to fit perfectly to the eye, as shown in Figure 2. When the probe was placed on the eye surface, the temperature increased before gradually stabilizing, at which point the temperature reading represented the real-time temperature of the ocular surface. The hot compress was then placed on the eye, and the eye was heated for 18min, during which time the temperature measuring device recorded temperature changes in real time. The same dressing was used for each test, and measurements were taken at the same timepoints of the 18-min heating process.
Figure 2. Measurement of temperature in rabbit eyes.
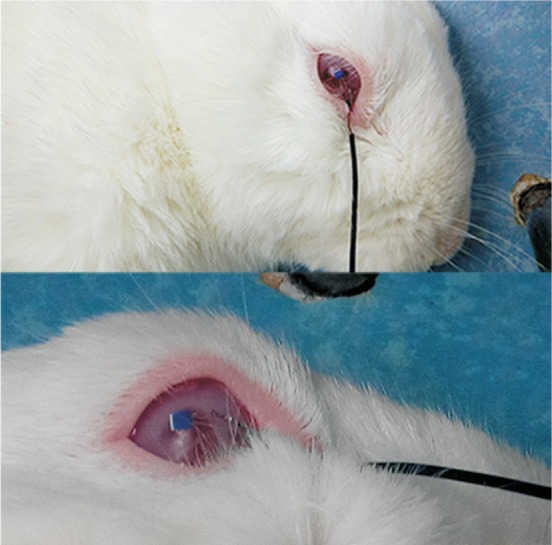
The probe can sense and measure real-time temperature changes at the ocular surface in a noninvasive manner.
Measurement of Retrobulbar Hemodynamic Parameters Before and After Application of Hot Compress
Hemodynamic parameters of the blood vessels were measured using CDFI and spectrum Doppler detection techniques, both before and after heating. A pre-warmed ultrasound gel (Aquasonic, Parker Laboratories Inc., Fairfield, NJ, USA) was used as a coupling agent on the ocular surface, after the application of two drops of topical anesthesia (tetracaine ophthalmic drops) and placement of a self-retaining wire eye speculum. The unit was attached to a stereotactic stand that allowed the application of the probe at the 6-o'clock position on the ocular surface, while avoiding any untoward pressure on the globe that could cause erroneous measurements. Then, various vessels around the optic nerve were analyzed[9]. The vessels examined included the central retinal artery (CRA), long posterior ciliary artery (LPCA), ophthalmic artery (OA), and central retinal vein (CRV). The hemodynamic parameters of interest were the peak systolic velocity (PSV) and EDV of each artery, and the mean velocity (Vm) of the CRV. The corresponding arterial RI was calculated using the system software and the formula RI=1-(PSV/EDV). In each measurement, a red image indicated blood flow in the direction of the ultrasound waves, while a blue image represented blood flow in the direction opposite to the ultrasound waves. All CDFI measurements were conducted on an UGEO WS80A ultrasound diagnostic instrument (Samsung, South Korea) by the same technician at a constant room temperature using the small organ 7-10 MHz line array probe.
Statistical Analysis
Statistical analysis was performed using the SPSS 22.0 statistical package (SPSS Inc., Chicago, IL, USA). For each eye, the Vm in the CRV and the PSV and EDV of the selected arteries were measured three times in succession. Results are given as the mean±standard deviation (SD). A paired student's t-test and Wilcoxon signed-rank test were conducted to compare the mean values, wherein values of P<0.05 were considered statistically significant.
RESULTS
Installation and Testing of Eye Temperature Measuring Device
The overall assembly of the eye temperature detection device was simple and convenient. The probe part of the device, which was similar in shape to a contact lens, was attached to the surface of the cornea, and the instantaneous temperature value was displayed on a computer screen.
In vitro test: When the probe was maintained under a constant temperature environment, the device remained stable, and the temperature of the constant environment was accurately displayed. The temperature curve is shown in Figure 3.
Figure 3. In vitro test with ocular temperature measuring device.
The temperature of the constant environment is accurately displayed when the probe is repeatedly placed in a thermostatic environment.
In vivo test: Once the installation was complete, the probe measured the temperature of the ocular surface and eventually stabilized. The corresponding temperature curve is shown in Figure 4. Using hot and cold compresses, we demonstrated that the probe was able to sense temperature variations on the ocular surface and display real-time temperature values on the computer screen, as shown in Figure 5.
Figure 4. In vivo test with ocular temperature measuring device.
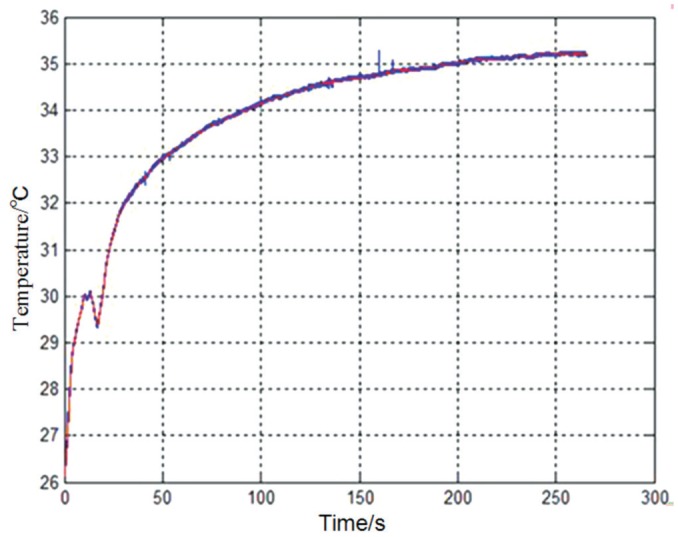
Once the probe is placed on the surface of the cornea of the New Zealand white rabbit, it can measure the temperature of the ocular surface and eventually stabilizes. The temperature value represents the temperature of the ocular surface.
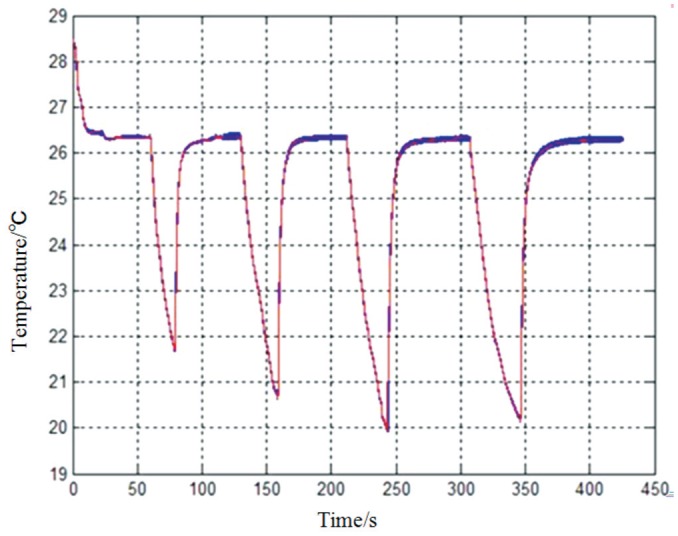
Figure 5. Real-time temperature curve for continuous hot and cold compresses.
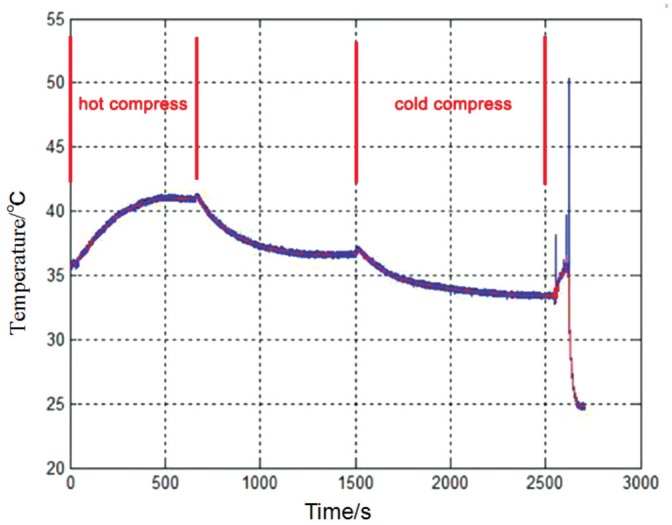
The probe can sense the temperature variations of the ocular surface. Upon application of a hot compress, the temperature gradually increases, and eventually stabilizes when the hot compress is removed. Upon application of a cold compress, the eye temperature gradually decreases, eventually stabilizing when the cold compress is removed.
Ocular Temperature Measurement During Application of the Hot Compress
The real-time eye temperature measured by the device during the application of the hot compress is shown in Figure 6. When heating was applied, the temperature rapidly increased before reaching the maximum temperature and then slowly decreasing. Based on this data, applying a hot compress to the eye significantly increased the temperature of the ocular surface and maintained it at an elevated value for 18min.
Figure 6. Real-time temperature of the ocular surface before and after application of hot compress.
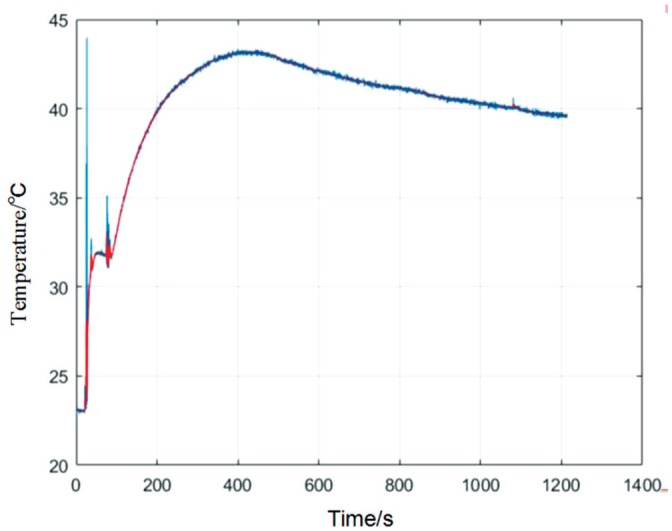
When the heating is applied, the temperature rapidly increases before reaching the maximum temperature and then slowly decreasing. The ocular surface is maintained at the elevated temperature for the duration of the application, 18min.
Measurement of Retrobulbar Hemodynamic Parameters Before and After Application of Hot Compress
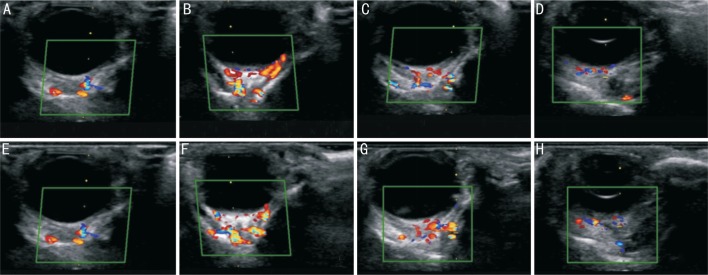
CDFI clearly showed the blood flow before and after the application of a hot compress. Figure 7 showed the contrast between the retrobulbar blood flow before and after application of the hot compress, in ultrasound images. The hemodynamic parameters for each of the vessels were listed in Table 1. In the CRA, the PSV was 22.50±1.80 mm Hg and 27.61±1.29 mm Hg before and after heating, respectively (P=0.004), and the EDV was 22.50±1.80 mm Hg and 27.61±1.29 mm Hg (P=0.044), respectively. Both of these differences were statistically significant. The corresponding RI values were 0.48±0.09 mm Hg and 0.48±0.07 mm Hg (P=0.921), respectively, a difference which was not statistically significant. In the LPCA, the PSV was 22.78±1.62 mm Hg and 25.19±1.88 mm Hg (P=0.009) before and after heating, respectively, and the EDV was 12.30±1.87 mm Hg and 14.22±1.46 mm Hg (P=0.034), respectively. Both differences were statistically significant. The RI values were 0.47±0.07 mm Hg and 0.45±0.05 mm Hg (P=0.470), respectively, a difference which was not statistically significant. In the OA, the PSV was 24.44±2.85 mm Hg and 27.44±3.79 mm Hg (P=0.026) before and after heating, respectively, which represents a statistically significant difference. The EDV was 13.29±3.14 mm Hg and 15.52±3.29 mm Hg (P=0.141), respectively, and the respective RI values were 0.46±0.10 mm Hg and 0.45±0.07 mm Hg (P=0.647). These differences were not statistically significant. In the CRV, the Vm was 5.21±0.52 mm Hg and 6.13±0.95 mm Hg (P=0.043) before and after heating, respectively, which was a statistically significant difference. The analysis showed that the PSV and EDV of the CRA and LPCA, as well as the PSV of the OA, significantly increased after the application of the hot compress. The Vm in the CRV also significantly increased after the hot compress application. There were no significant changes in the EDV of the OA nor the RI value of each artery.
Figure 7. Contrast between ultrasound images of retrobulbar blood flow before and after application of hot compress.
A-D: Ultrasonographic images of the CRA, LPCA, OA, and CRV, respectively, recorded before the hot compress application; E-H: Ultrasonographic images of the CRA, LPCA, OA, and CRV, respectively, after the hot compress application.
Table 1. Hemodynamic parameters of retrobulbar vessels before and after application of hot compress (n=5).
| Hemodynamic parameter | Before hot compress | After hot compress | P |
| CRA-PSV | 22.50±1.80 | 27.61±1.29 | 0.004 |
| CRA-EDV | 11.68±2.29 | 14.90±1.79 | 0.044 |
| CRA-RI | 0.48±0.09 | 0.48±0.07 | 0.921 |
| LPCA-PSV | 22.78±1.62 | 25.19±1.88 | 0.009 |
| LPCA-EDV | 12.30±1.87 | 14.22±1.46 | 0.034 |
| LPCA-RI | 0.47±0.07 | 0.45±0.05 | 0.470 |
| OA-PSV | 24.44±2.85 | 27.44±3.79 | 0.026 |
| OA-EDV | 13.29±3.14 | 15.52±3.29 | 0.141 |
| OA-RI | 0.46±0.10 | 0.45±0.07 | 0.647 |
| CRV-Vm | 5.21±0.52 | 6.13±0.95 | 0.043 |
CRA: Central retinal artery; LPCA: Long posterior ciliary artery; OA: Ophthalmic artery; CRV: Central retinal vein; PSV: Peak systolic velocity; EDV: End diastolic velocity; RI: Resistance index; Vm: Mean velocity.
mean±SD, cm/s
DISCUSSION
This experiment demonstrated that the application of a hot compress to the eye was able to improve the velocity of retrobulbar blood flow without affecting the RI. Applying a hot compress to the eye significantly increased the temperature of the ocular surface and maintained it at an elevated level for the duration of the application, i.e. 18min. The effect of the hot compress was evident. The results of CDFI and spectrum Doppler detection techniques showed that the temperature of the ocular surface significantly affected the retrobulbar hemodynamics. Increasing the ocular surface temperature increased the PSV and EDV of the surrounding arteries and had no effect on the RI values. The PSV and EDV of the CRA and LPCA, the PSV of the OA, and the Vm in the CRV significantly increased after application of the hot compress, whereas the EDV of the OA did not increase significantly. This may have been because the OA, which is further away from the ocular surface, had a larger blood flow, as it belongs to the large blood vessel branch of the neck. Therefore, the effect of the hot compress on this larger artery was less significant in comparison to the effect on the other vessels. In summary, the application of a hot compress may be an effective approach to reverse pathological changes in retrobulbar vessels. The results support the hypothesis that the proposed method can be used to improve retrobulbar circulation.
Our research team proposed a method for increasing retrobulbar blood flow velocities using a hot compress on the ocular surface to improve retrobulbar circulation. The hot compress method was non-traumatic; it was safe, simple, convenient, and could be performed without help from other people, which would allow patients to receive assistance at home at any time. Although this study is still in its primary stage, it provides novel insights. For many years, the treatment of glaucoma has mainly focused on reducing IOP. However, it has not been possible to alleviate the progression of optic nerve damage in many patients undergoing treatments for IOP. Ocular perfusion defects, including vasospasms, abnormal metabolic regulation, and the disturbance of myogenic regulation, have been regarded as an important factor in the pathogenesis of optic neuropathy in glaucoma, which can lead to ischemia[10]–[16]. Relevant studies have shown that hemodynamic changes in arteries such as the CRA and OA are particularly related to the appearance of glaucoma[17], with glaucoma patients often having lower PSV and EDV in posterior arteries as well as a higher RI[18]–[21].
In this experiment, a novel contact-type high-precision ocular temperature measuring device that senses temperature changes using a thermistor was independently designed and fabricated. This device has the advantage of being capable of monitoring temperature changes of the ocular surface in real time. The temperature change curve and instantaneous temperature values can be displayed directly on a computer screen. Moreover, the shape of the probe in this device is similar to that of a contact lens; the surface is smooth, transparent, soft, and fits the cornea appropriately. The device and the associated procedure are simple, fast, and noninvasive, and could be used for continuous measurements. Ultrasonography is one of the most important diagnostic methods[22]–[25]. It is very useful for ophthalmological examinations and can be employed to study intraocular foreign bodies, lens dislocation, retinal detachment, vitreous hemorrhage, traumatic retrobulbar hemorrhage, and more[26]–[31]. The CDFI and spectrum Doppler detection techniques are particularly important for the examination of retrobulbar blood flow velocities. These techniques are safe, convenient, and non-invasive, and can be used to quickly evaluate various retrobulbar hemodynamic parameters. Therefore, blood flow velocities and RIs of the retrobulbar vessels were evaluated using CDFI and spectrum Doppler detection techniques in order to provide a scientific basis for the evaluation of the therapeutic effect of the application of the hot compress.
To the best of our knowledge, similar research has not been reported. The proposed method has some challenges that should be addressed. For example, the temperature probe will be prone to oxidization over long-term use, making its regular replacement a necessity. In addition, the wire connected to the probe was too long, which was inconvenient during the experiment. Future work should also investigate whether improving retrobulbar circulation could effectively slow the progression of optic nerve damage in glaucoma patients. In the future, our group will conduct further animal experiments and develop a glaucoma model, which includes the hot compress procedure, for rabbits. In addition, we aim to develop a series of methods for molecular biology and pathology experiments in order to investigate whether the application of a hot compress can protect RGCs and reduce visual field defects, both of which are of significant importance in histology and molecular biology.
Applying a hot compress to the ocular surface increased retrobulbar blood flow velocities, and could be used to improve blood supply in this area. This simple and inexpensive treatment could become a key approach in the treatment of glaucoma, especially normal tension glaucoma, in the future.
Acknowledgments
Authors' contributions: Authors Zhou XR, Li LQ and Ren M designed the study. Li TT conducted the entired animal experiments and participated in the data collection, statistical analysis and drafted the manuscript. Shao GB participated in writing and revising the article. Jiang YL and Wang JX participated in designing the device. All authors read and pproved the final manuscript.
Foundations: Supported by the National Natural Science Funds for Young Scholar (No.81400394); Heilongjiang Province Science Foundation for Youths (No.QC08C97); Research Fund for the Doctoral Program of the Second Affiliated Hospital of Harbin Medical University (No.BS2008-23).
Conflicts of Interest: Li TT, None; Shao GB, None; Jiang YL, None; Wang JX, None; Zhou XR, None; Ren M, None; Li LQ, None.
REFERENCES
- 1.Plange N, Remky A, Arend O. Colour Doppler imaging and fluorescein filling defects of the optic disc in normal tension glaucoma. Br J Ophthalmol. 2003;87(6):731–736. doi: 10.1136/bjo.87.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abegão Pinto L, Vandewalle E, de Clerck E, Marques-Neves C, Stalmans I. Ophthalmic artery doppler waveform changes associated with increased damage in glaucoma patients. Invest Ophthalmol Vis Sci. 2012;53(4):2448–2453. doi: 10.1167/iovs.11-9388. [DOI] [PubMed] [Google Scholar]
- 3.Calvo P, Ferreras A, Polo V, Güerri N, Seral P, Fuertes-Lazaro I, Pablo LE. Predictive value of retrobulbar blood flow velocities in glaucoma suspects. Invest Ophthalmol Vis Sci. 2012;53(7):3875–3884. doi: 10.1167/iovs.11-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schumann J, Orgül S, Gugleta K, Dubler B, Flammer J. Interocular difference in progression of glaucoma correlates with interocular differences in retrobulbar circulation. Am J Ophthalmol. 2000;129(6):728–733. doi: 10.1016/s0002-9394(99)00481-x. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki Y, Drance SM. The relationship between progression of visual field defects and retrobulbar circulation in patients with glaucoma. Am J Ophthalmol. 1997;124(3):287–295. doi: 10.1016/s0002-9394(14)70820-7. [DOI] [PubMed] [Google Scholar]
- 6.Galassi F, Sodi A, Ucci F, Renieri G, Pieri B, Baccini M. Ocular hemodynamics and glaucoma prognosis: a color Doppler imaging study. Arch Ophthalmol. 2003;121(12):1711–1715. doi: 10.1001/archopht.121.12.1711. [DOI] [PubMed] [Google Scholar]
- 7.Cohen LP, Pasquale LR. Clinical characteristics and current treatment of glaucoma. Cold Spring Harb Perspect Med. 2014;4(6):a017236. doi: 10.1101/cshperspect.a017236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gugleta K, Orgül S, Flammer J. Is corneal temperature correlated with blood-flow velocity in the ophthalmic artery? Curr Eye Res. 1999;19(6):496–501. doi: 10.1076/ceyr.19.6.496.5286. [DOI] [PubMed] [Google Scholar]
- 9.Abdallah W, Fawzi A, Patel H, Dagliyan G, Matsuoka N, Grant E, Humayun M. Blood velocity measurement in the posterior segment of the rabbit eye using combined spectral Doppler and power Doppler ultrasound. Graefes Arch Clin Exp Ophthalmol. 2010;248(1):93–101. doi: 10.1007/s00417-009-1200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lip PL, Felmeden DC, Blann AD, Matheou N, Thakur S, Cunliffe IA, Lip GY. Plasma vascular endothelial growth factor, soluble VEGF receptor FLT-1, and von Willebrand factor in glaucoma. Br J Ophthalmol. 2002;86(11):1299–1302. doi: 10.1136/bjo.86.11.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leibovitch I, Kurtz S, Kesler A, Feithliher N, Shemesh G, Sela BA. C-reactive protein levels in normal tension glaucoma. J Glaucoma. 2005;14(5):384–386. doi: 10.1097/01.ijg.0000176932.06606.6e. [DOI] [PubMed] [Google Scholar]
- 12.Orgül S, Gugleta K, Flammer J. Physiology of perfusion as it relates to the optic nerve head. Surv Ophthalmol. 1999;43(Suppl 1):S17–S26. doi: 10.1016/s0039-6257(99)00009-0. [DOI] [PubMed] [Google Scholar]
- 13.Resch H, Schmidl D, Hommer A, Rensch F, Jonas JB, Fuchsjäger-Mayrl G, Garhöfer G, Vass C, Schmetterer L. Correlation of optic disc morphology and ocular perfusion parameters in patients with primary open angle glaucoma. Acta Ophthalmologica. 2011;89(7):e544–e549. doi: 10.1111/j.1755-3768.2011.02175.x. [DOI] [PubMed] [Google Scholar]
- 14.Moore D, Harris A, Wudunn D, Kheradiya N, Siesky B. Dysfunctional regulation of ocular blood flow: a risk factor for glaucoma? Clin Ophthalmol. 2008;2(4):849–861. doi: 10.2147/opth.s2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolela MT. Clinical clues of vascular dysregulation and its association with glaucoma. Can J Ophthalmol. 2008;43(3):337–341. doi: 10.3129/i08-063. [DOI] [PubMed] [Google Scholar]
- 16.Resch H, Garhofer G, Fuchsjäger-Mayrl G, Hommer A, Schmetterer L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009;87(1):4–12. doi: 10.1111/j.1755-3768.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- 17.Kanakamedala P, Harris A, Siesky B, Tyring A, Muchnik M, Eckert G, Abrams Tobe L. Optic nerve head morphology in glaucoma patients of African descent is strongly correlated to retinal blood flow. Br J Ophthalmol. 2014;98(11):1551–1554. doi: 10.1136/bjophthalmol-2013-304393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser HJ, Schoetzau A, Stümpfig D, Flammer J. Blood-flow velocities of the extraocular vessels in patients with high-tension and normal-tension primary open-angle glaucoma. Am J Ophthalmol. 1997;123(3):320–327. doi: 10.1016/s0002-9394(14)70127-8. [DOI] [PubMed] [Google Scholar]
- 19.Zeitz O, Galambos P, Wagenfeld L, Wiermann A, Wlodarsch P, Praga R, Matthiessen ET, Richard G, Klemm M. Glaucoma progression is associated with decreased blood flow velocities in the short posterior ciliary artery. Br J Ophthalmol. 2006;90(10):1245–1248. doi: 10.1136/bjo.2006.093633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber K, Plange N, Remky A, Arend O. Comparison of colour Doppler imaging and retinal scanning laser fluorescein angiography in healthy volunteers and normal pressure glaucoma patients. Acta Ophthalmol Scand. 2004;82(4):426–431. doi: 10.1111/j.1395-3907.2004.00269.x. [DOI] [PubMed] [Google Scholar]
- 21.Tobe LA, Harris A, Hussain RM, Eckert G, Huck A, Park J, Egan P, Kim NJ, Siesky B. The role of retrobulbar and retinal circulation on optic nerve head and retinal nerve fibre layer structure in patients with open-angle glaucoma over an 18-month period. Br J Ophthalmol. 2015;99(5):609–612. doi: 10.1136/bjophthalmol-2014-305780. [DOI] [PubMed] [Google Scholar]
- 22.Slimani O, Ben Temim R, Ajroudi M, Makhlouf T, Mathlouthi N, Attia L. Contribution of ultrasound in the study of ovarian fibrothecomas: a series of 47 cases. Tunis Med. 2017;95(1):29–36. [PubMed] [Google Scholar]
- 23.Xue K, Liu AL, Hui R, Zhang J, Qian J. Retrobulbar ocular blood flow changes measured by colour Doppler imaging after intra-arterial chemotherapy in retinoblastoma. Br J Ophthalmol. 2017;101(10):1419–1422. doi: 10.1136/bjophthalmol-2016-310056. [DOI] [PubMed] [Google Scholar]
- 24.Hwang IJ, Kim DW, Lee YJ, Choo HJ, Jung SJ, Baek HJ. Ultrasonographic interval changes in solid thyroid nodules after ultrasonography-guided fine-needle aspiration. Korean J Radiol. 2018;19(1):158–166. doi: 10.3348/kjr.2018.19.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu NQ, Evora M, Lam UP, Ip MF, Li JJ. Acute myocardial infarction caused by myocardial bridging alone confirmed by using intravascular ultrasonography. Chronic Dis Transl Med. 2017;3(4):260–262. doi: 10.1016/j.cdtm.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaivas M, Theodoro D, Sierzenski PR. A study of bedside ocular ultrasonography in the emergency department. Acad Emerg Med. 2002;9(8):791–799. doi: 10.1111/j.1553-2712.2002.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 27.Roque PJ, Hatch N, Barr L, Wu TS. Bedside ocular ultrasound. Crit Care Clin. 2014;30(2):227–241. doi: 10.1016/j.ccc.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Kilker BA, Holst JM, Hoffmann B. Bedside ocular ultrasound in the emergency department. Eur J Emerg Med. 2014;21(4):246–253. doi: 10.1097/MEJ.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 29.Kniess CK, Fong TC, Reilly AJ, Laoteppitaks C. Early detection of traumatic retrobulbar hemorrhage using bedside ocular ultrasound. J Emerg Med. 2015;49(1):58–60. doi: 10.1016/j.jemermed.2014.12.074. [DOI] [PubMed] [Google Scholar]
- 30.Stalmans I, Siesky B, Zeyen T, Fieuws S, Harris A. Reproducibility of color Doppler imaging. Graefes Arch Clin Exp Ophthalmol. 2009;247(11):1531–1538. doi: 10.1007/s00417-009-1178-3. [DOI] [PubMed] [Google Scholar]
- 31.Ehrlich R, Harris A, Siesky BA, Moss AM, Ramanathan M, Pickett MA, Wudunn D, Hawkes M, Shoshani YZ. Repeatability of retrobulbar blood flow velocity measured using color doppler imaging in the indianapolis glaucoma progression study. J Glaucoma. 2011;20(9):540–547. doi: 10.1097/IJG.0b013e3181f46606. [DOI] [PubMed] [Google Scholar]