Abstract
AIM
To report real-life data on the use of an intravitreal fluocinolone acetonide implant in the treatment of refractory diabetic macular edema (DME) in pars plana vitrectomized (PPV) and non-PPV eyes.
METHODS
This was a comparative retrospective observational study of 23 eyes with chronic DME. Best-corrected visual acuity (BCVA) and central macular thickness (CMT) were recorded at baseline, 1, 4 and 12mo. Descriptive statistics and non-parametric tests were performed to analyze and compare PPV and non-PPV eyes.
RESULTS
Seven PPV and 16 non-PPV eyes were included in the study. Median BCVA in the non-PPV group varied from 0.65 logMAR [Interquartile range (IQR): 0.40] at baseline to 0.42 logMAR (IQR: 0.40) at 12mo. Median CMT varied from 430 µm (IQR: 131.3) at baseline to 317 µm (IQR: 107.5) at 12mo. Median BCVA in the PPV group varied from 0.60 logMAR (IQR: 0.62) at baseline to 0.74 logMAR (IQR: 0.34) at 12mo. Median CMT varied from 483 µm (IQR: 146) at baseline to 397 µm (IQR: 132) at 12mo. Of 0/7 eyes and 1/16 eyes in the PPV and non-PPV eyes respectively had a baseline visual acuity of 6/12 or better (0.3 logMAR). At last follow up, 1/7 and 5/16 eyes in the PPV and non-PPV group respectively achieved a visual acuity of 6/12 or better.
CONCLUSION
Visual outcomes are modest following the use of the fluocinolone acetonide implant for chronic DME. The steroid implant is a useful treatment option in the management of refractory DME in vitrectomized and non-vitrectmized eyes.
Keywords: diabetic macular edema, fluocinolone acetonide, intravitreal implant, steroid, vitrectomy
INTRODUCTION
Diabetic macular edema (DME) is a leading cause of visual impairment today[1]–[2]. Between 2012 and 2013 diabetic retinopathy and maculopathy accounted for 5.4% of severe sight impairment (SSI) and 6.3% partial sight impairment (PSI) registrations in England and Wales[3]. Several options are currently available for treatment, most of which are based on intravitreal injections of therapeutic agents. Anti-vascular endothelial growth factor (VEGF) drugs are generally first line agents for the treatment of center-involving DME in phakic patients according to the National Institute for Health and Care Excellence (NICE) guidelines in the UK[4]. The use of intravitreal steroids is the standard second line treatment in pseudophakic patients due to their known negative effect on lens transparency. Another well-documented side effect of steroid drugs is the increase in intraocular pressure (IOP), which is generally treated medically.
Treatment of DME in vitrectomized patients is not clearly defined. Evidence suggests that eyes that underwent previous pars plana vitrectomy (PPV) show a good response to intravitreal ranibizumab for DME, although more injections may be required and OCT change may be lagging compared to patients with no previous history of PPV (non-PPV)[5].
Three different steroid formulations are currently available in the UK market. Triamcinolone acetonide (TA) is a synthetic steroid available as an injectable suspension, unlicensed for intravitreal administration. Its use in ophthalmology was first described in the 1980s and today it is an inexpensive option for the treatment of macular edema caused by several vascular diseases as well as numerous non-infectious inflammatory ocular conditions[6]. Ozurdex® (Allergan, Inc., Irvine, CA, USA) is an injectable implant containing 700 µg of dexamethasone. It is licensed in the UK for the treatment of pseudophakic patients affected by DME or macular edema secondary to retinal vein occlusion with good results both in large clinical trials as well as in real-world series[7]–[8]. Iluvien® (Alimera Sciences Limited, Aldershot, UK) is an intravitreal implant releasing a sustained dose of 0.2 µg/d of fluocinolone acetonide (FAc). It has been introduced in the UK in 2013 and it is currently approved by NICE for the treatment of chronic DME insufficiently responsive to available therapies. The major advantage of the FAc intravitreal implant is that it allows for the sustained release of the active agent for up to 36mo compared to TA, which requires frequent injection. Furthermore, numerous publications have demonstrated the efficacy of Iluvien® in the treatment of patients affected by DME in a real-world setting[9]–[11].
The purpose of the present study is to report on real life outcomes of the treatment of chronic DME including eyes with treated proliferative diabetic retinopathy (PDR) and previous vitrectomy. This report takes into consideration the impact of previous vitreous surgery, which accounts for a non-negligible number of patients with advanced diabetic eye disease, on drug response.
SUBJECTS AND METHODS
The present study is a retrospective observational case-series. The study location was the Eye Treatment Centre at Whipps Cross University Hospital in London. Approval for data collection and analysis was obtained from the Clinical Effectiveness Unit at Whipps Cross University Hospital and adhered to the tenets set forth in the Declaration of Helsinki. Patient demographics and clinical data were obtained from Medisoft® (Medisoft Ltd., Leeds, UK), an electronic patient records database used in our daily clinical practice.
All eyes that received the intravitreal FAc implant had refractory DME, characterized by cystoid macular edema (CME) defined as the accumulation of fluid in well-defined round hyporeflective spaces within the outer plexiform layer on spectral-domain optical coherence tomography (SD-OCT) associated with loss of the normal foveal depression and insufficiently responsive to other therapies.
All eyes were pseudophakic. Exclusion criteria included coexisting pathology that could cause macular edema such as recent cataract extraction, retinal vascular disease other than diabetic retinopathy, retinal dystrophies and degeneration, ocular inflammatory disease and known allergies to one or more of the excipients contained in the implant. Previous vitrectomy surgery was not an exclusion for the study.
LogMAR best-corrected visual acuity (BCVA) and full clinical examination were recorded at baseline, 1, 4 and 12mo following intravitreal implant placement. SD-OCT imaging was performed using two instruments: the Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) and the Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA, USA) systems. Central macular thickness (CMT), defined as retinal thickness in the inner circle of the Spectralis macular thickness map and central subfield thickness on the cirrus macular thickness analysis report, was recorded at each time point. The well-known discrepancy in macular thickness measurement between machines was overcome by imaging one patient with the same device at each follow up visit[12].
Visual acuity and OCT data were analyzed retrospectively at corresponding time points at baseline, 1, 4 and 12mo. Individual SD-OCT images were analyzed in a masked fashion in order to evaluate subjective changes in macular profiles. Statistical analyses were performed with Stata 14.1 (StataCorp LP) software. Descriptive statistics and non-parametric tests using Wilcoxon sign-rank and Kruskal-Wallis tests were performed to analyze the data.
RESULTS
Patient demographics are summarized in Table 1.
Table 1. Patient demographics.
| Parameters | Total | PPV | Non-PPV |
| Gender | |||
| M | 14 | 6 | 8 |
| F | 9 | 1 | 8 |
| Ethnicity | |||
| White/any other white background | 4 | 0 | 4 |
| Asian/any other Asian background | 10 | 4 | 6 |
| Black/any other black background | 2 | 1 | 1 |
| Other/unknown | 7 | 2 | 5 |
| Diabetes control | |||
| Insulin | 3 | 1 | 2 |
| Tablets | 6 | 2 | 4 |
| Insulin+tablets | 11 | 4 | 7 |
| Unknown | 3 | 0 | 3 |
| PPV indication | |||
| ERM peel | 2 | 2 | N/A |
| VH | 4 | 4 | N/A |
| TRD | 1 | 1 | N/A |
| Previous PRP | 8 | 6 | 2 |
| Pre-Iluvien® maculopathy treatments | |||
| Intravitreal anti-VEGF | 11 | 2 | 9 |
| Intravitreal TA | 6 | 0 | 6 |
| Macular laser | 12 | 1 | 11 |
| Pre-Iluvien® OCT features | |||
| CME | 11 | 2 | 9 |
| Hyper-reflective epiretinal band | 12 | 4 | 8 |
| Intact ellipsoid | 9 | 2 | 7 |
| IOP lowering treatment | |||
| Pre-Iluvien® | 6 | 5 | 1 |
| Started after Iluvien® | 3 | 0 | 3 |
PPV: Pars plana vitrectomy; ERM: Epiretinal membrane; VH: Vitreous hemorrhage; TRD: Tractional retinal detachment; N/A: Not applicable; PRP: Panretinal laser photocoagulation; VEGF: Vascular endothelial growth factor; TA: Triamcinolone acetonide; OCT: Optical coherence tomography; CME: Cystoid macular edema; ELM: External limiting membrane; IOP: Intraocular pressure.
Twenty-three eyes were enrolled in the study. Mean patient age was 68.1y (range 46-83). Seven vitrectomized eyes and 16 non-vitrectomized eyes received an Iluvien® implant for refractory DME. The main indication for PPV was vitreous hemorrhage, followed by epiretinal membrane and tractional retinal detachment. The mean (range) interval between vitrectomy and implant placement was 25.8 (3-74)mo. Approximately one third of the cohort had previous panretinal photocoagulation (PRP) with preponderance in the PPV group (6 out of 7 eyes) compared to the non-PPV group (2/16 eyes). This could be explained by the significant difference in baseline diabetic retinopathy grading between the studied groups, as PPV eyes were more likely to have active PDR compared to non-PPV eyes. A small number of patients involved in the study were prescribed IOP lowering drops following implant placement, none required surgical treatment to reduce the IOP after Iluvien® was administered.
Subgroup Analysis
Overall, 5 of 7 patients in the PPV group experienced an improvement in BCVA and 2 patients showed a decrease in BCVA 12mo after implant placement. This compares to 11 of 16 patients in the non-PPV group who experienced an improvement in BCVA, 4 patients who showed a reduction in BCVA and 1 patient who showed no change from baseline to follow-up at 12mo.
Median BCVA in the non-PPV group varied from 0.65 logMAR [Interquartile range (IQR): 0.40] at baseline to 0.42 logMAR (IQR: 0.40) at the 12mo final assessment (Z=2.121, P=0.034). Median CMT varied from 430 µm (IQR: 131.3) at baseline to 317 µm (IQR: 107.5) at the 12mo final assessment (Z=2.405, P=0.016). Totally 12 of 16 non-PPV patients noted a reduction in CMT and 4 of 16 patients noted an increase in CMT at final follow-up. Median BCVA in the PPV group varied from 0.60 logMAR (IQR: 0.62) at baseline to 0.74 logMAR (IQR: 0.34) at the 12mo final assessment (Z=1.352, P>0.05). Median CMT varied from 483 µm (IQR: 146) at baseline to 397 µm (IQR: 132) at the 12mo final assessment (Z=1.690, P>0.05). Six of 7 PPV patients had a reduction in CMT on OCT imaging at 12-month follow-up. Only 1 vitrectomized eye experienced a worsening of CMT (from 305 µm at baseline to 397 µm at 6-12mo).
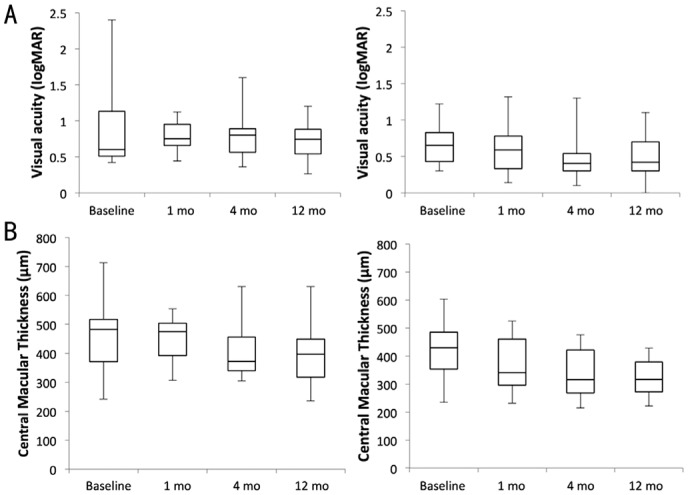
Mean BCVA gain in the PPV group at 12mo was 0.24 logMAR compared with 0.17 logMAR in the non-PPV group. Mean improvement in CMT in the PPV group from baseline to 12mo was 60 µm compared with 72 µm in the non-PPV group. Kruskal-Wallis tests did not demonstrate difference in mean ranks for BCVA or CMT over the study periods for both the PPV and non-PPV group. Overall, 0/7 eyes and 1/16 (6.3%) eyes in the PPV and non-PPV groups respectively had a baseline visual of 6/12 or better (0.3 logMAR). At last follow up, 1/7 (14.3%) and 5/16 (31.3%) eyes in the PPV and non-PPV eyes respectively achieved a visual acuity of 6/12 or better. Figure 1 and Table 2 summarize BCVA and CMT change in both subgroups from baseline to 12mo follow-up visit.
Figure 1. Functional and anatomical results after intraviteal FAc treatment in PPV and non-PPV eyes.
A: BCVA in logMAR; B: CMT in µm in vitrectomized and non-vitrectomized eyes over the studied period. The boxes represent the IQR, the horizontal lines within the boxes indicate the median and the whiskers represent minimum and maximum values.
Table 2. Mean/median BCVA and mean/median CMT according to time period following Iluvien® implant insertion in vitrectomized and non-vitrectomized eyes.
| Parameters | BCVA (logMAR) |
CMT (µm) |
||||||
| n | Mean | Median | Range | n | Mean | Median | Range | |
| PPV | ||||||||
| Pre-injection | 7 | 0.96 | 0.60 | 0.50-2.40 | 7 | 459 | 483 | 242-713 |
| 1mo | 6 | 0.78 | 0.75 | 0.44-1.12 | 7 | 447 | 475 | 307-554 |
| 4mo | 7 | 0.81 | 0.80 | 0.36-1.60 | 7 | 414 | 372 | 305-631 |
| 12mo | 7 | 0.72 | 0.74 | 0.26-1.20 | 7 | 399 | 397 | 236-631 |
| Non-PPV | ||||||||
| Pre-injection | 16 | 0.67 | 0.65 | 0.30-1.22 | 16 | 416 | 430 | 235-584 |
| 1mo | 14 | 0.60 | 0.59 | 0.14-1.32 | 15 | 380 | 341 | 232-652 |
| 4mo | 13 | 0.44 | 0.40 | 0.10-1.30 | 13 | 343 | 316 | 215-483 |
| 12mo | 16 | 0.51 | 0.42 | 0.00-1.10 | 16 | 344 | 317 | 222-732 |
PPV: Pars plana vitrectomy; BCVA: Best-corrected visual acuity; CMT: Central macular thickness.
In our PPV cohort, all patients, except one, received the FAc implant not earlier than 6mo after surgery and in two cases, more than 3y after vitrectomy. Individual SD-OCT images showed persistent hyporeflective cystic spaces in 7/7 eyes in the PPV group. There was a persistent epiretinal hyper-reflective line at the macula suggestive of residual cortical vitreous or a pre-retinal membrane in 4/7 eyes in the PPV group and 9/16 eyes in the non-PPV group. We hereby present three representative cases to highlight our findings.
Case Presentations
Case A (non-PPV group)
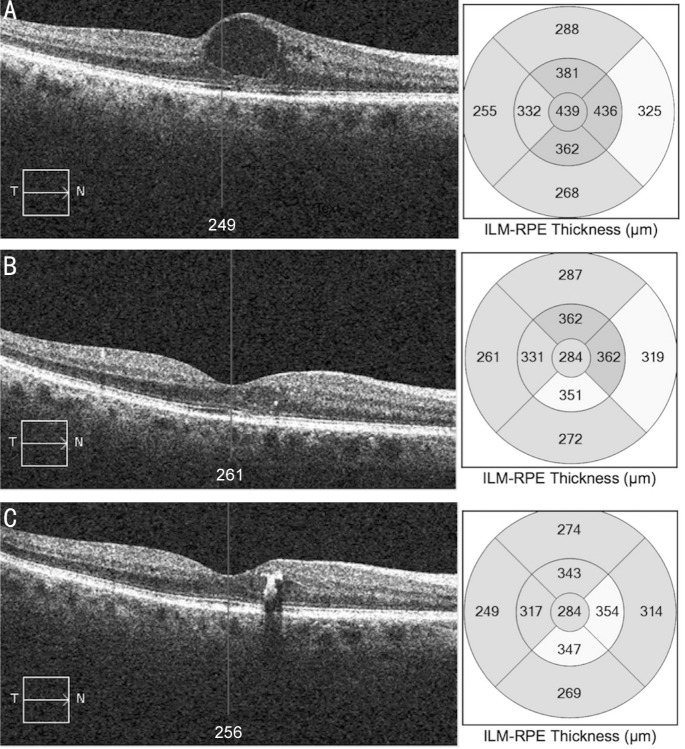
A 62-year-old Asian, male, with type 2 diabetes mellitus treated with insulin and tablets was referred to our medical retina service for the management of DME in his right eye. A course of monthly intravitreal injections of ranibizumab over a period of five months was given. BCVA after anti-VEGF treatment was 0.3 logMAR and OCT images showed persistent DME. The FAc implant was inserted three months later and BCVA improved to 0.14 logMAR 1mo following the FAc implant injection and remained stable 12mo after treatment. CMT improved from 439 to 284 µm and remained unchanged over a period of 12mo (Figure 2).
Figure 2. OCT scans of case A (non-PPV group).
A: Serial macular OCT scans and CMT maps at baseline; B: 1mo from the FAc implant; C: 12mo from the FAc implant.
Case B (PPV group)
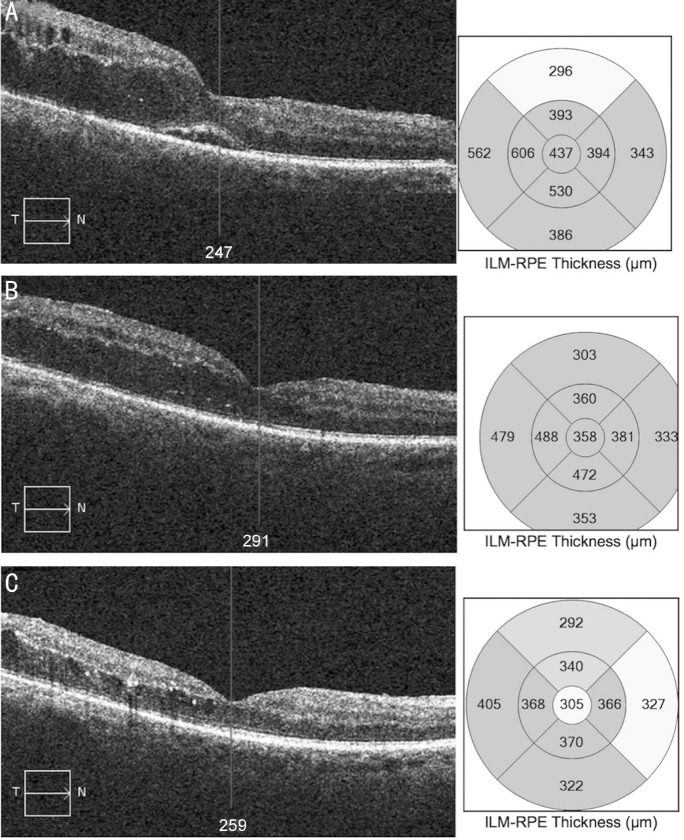
A 74-year-old Asian, male, affected by type 2 diabetes mellitus on oral treatment, was referred to our vitreoretinal service for the surgical management of a recent episode of vitreous hemorrhage to his right eye secondary to PDR. He was previously treated with PRP but gave no past history of DME. He was noted to have significant macular fluid after successful vitrectomy and fill-in PRP. BCVA was 0.52 logMAR five months after surgery. BCVA was unchanged at 0.5 logMAR two months postoperatively. The FAc implant was inserted one month later and BCVA was 0.36 logMAR at 1mo and 0.46 logMAR at 12mo after implant placement. Baseline CMT was 437 µm, which improved to 358 and 305 µm, 4wk and 12mo respectively after FAc implant insertion (Figure 3).
Figure 3. OCT scans of case B (PPV group).
A: Serial macular OCT scans and CMT maps at baseline; B: 1mo from the FAc implant; C: 12mo from the FAc implant.
Case C (PPV group)
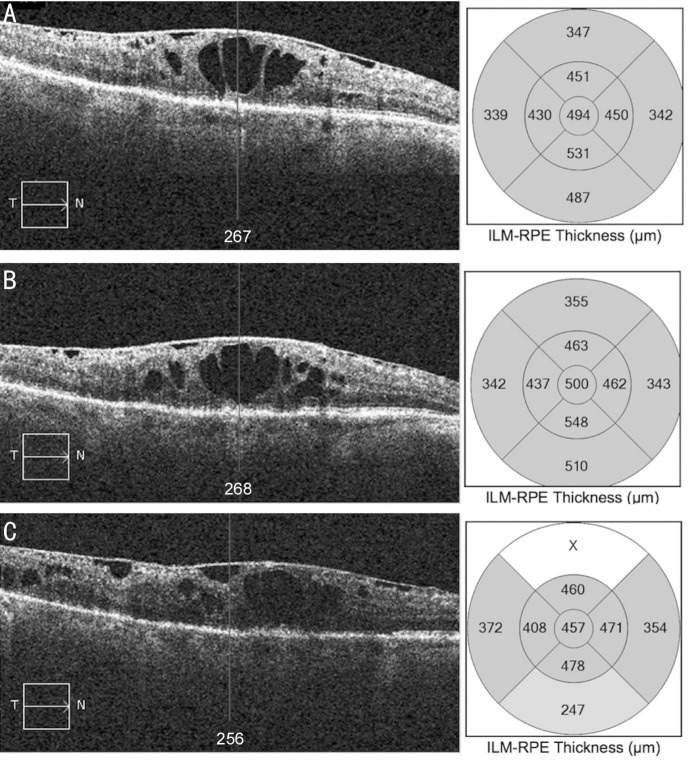
A 72-year-old, Indian, male, with type 2 diabetes mellitus on combined insulin and oral treatment, underwent PPV for the treatment of a diabetic vitreous hemorrhage in his right eye under the care of our vitreoretinal service. After successful surgery, he received repeat intravitreal injections for the treatment of DME with a limited response. Six years later he was offered an Iluvien® implant. His baseline BCVA was 1.24 logMAR, which improved to 1.12 logMAR and 0.86 logMAR at 4wk and 12mo from the injection respectively. However, BCVA improvement was not mirrored by similar improvements on OCT. CMT remained essentially unchanged 4wk after implant placement and a non-significant reduction of 50 µm was noted 12mo after Iluvien®. A hyper-reflective epiretinal band is clearly visible on OCT imaging (Figure 4).
Figure 4. OCT scans of case C (PPV group).
A: Serial macular OCT scans and CMT maps at baseline; B: 1mo from the FAc implant; C: 12mo from the FAc implant.
DISCUSSION
We present a single center, real world report on a cohort of vitrectomized and non-vitrectomized eyes treated with an FAc implant for refractory DME. Our findings show little change in visual acuity and macular thickness in PPV eyes, however better visual outcomes and a corresponding reduction in macular thickness were noted in non-PPV eyes. We believe this might be related to the presence of pre-retinal hyper-reflective tissue that may act as a barrier to the diffusion of the intravitreal steroid to the retina leading to the persistent pooling of fluid at the macula, which was more frequently noted in vitrectomized eyes. Thus further surgery and peeling of the pre-retinal membrane may be indicated in such eyes. Whilst our data is consistent with findings from the original FAME trials, as well as other reports, which demonstrated efficacy of FAc implants in the treatment of DME both in PPV and non-PPV eyes, we have not observed a clear visual benefit in PPV eyes which we attribute to the advanced stage of PDR as noted at the time of PPV surgery[9]–[11],[13]–[17].
Real life reports and study outcomes in general are markedly dependent on the study population, which in turn reduces the value of any comparison between studies. Thus, the indication for PPV surgery, presence or absence of PDR, any pre-existing DME, surgical technique and interval between vitrectomy and Iluvien® placement may all affect the response to treatment with the Iluvien® implant. The main difference between PPV and non-PPV eyes in the present investigation relates to the fact that PPV eyes were much more likely to have PDR, which in itself would limit visual outcomes in this subgroup.
Steroid implants are generally considered as a second line treatment option for eyes with DME non-responsive to conventional treatments including macular laser and anti-VEGF agents, which is at least in part justified by economic considerations. Furthermore, a recent report confirmed that Iluvien® is a cost and time-effective procedure, while showing non-inferiority compared to other DME treatments[18]. The risk of a potential long-term steroid response is another factor that might justify the use of Iluvien® as a last resort for refractory DME[19].
Most encouraging, is that we have only observed a negligible IOP elevation following the intravitreal FAc implant. None of our patients suffered vision-threatening complications, which is in line with previous reports. However, one of our PPV patients with a history of complicated cataract surgery, experienced late migration of the FAc implant in the anterior chamber that required implant removal[20]. Thus, the intravitreal fluocinolone implant should be avoided if not contraindicated in eyes with a posterior capsulotomy.
Visual outcomes are limited in PPV eyes, which can at least in part be explained by the baseline difference in the rate of PDR as 6/7 PPV eyes and only 2/16 non-PPV eyes had previous PRP for PDR. PRP laser could exacerbate pre-existing DME and promote the formation of epi-macular tissue that may contribute to the reduced efficacy of intravitreal treatments.
In summary, the management of chronic DME can be fraught with complications including treatment delays due to patient factors such as the reluctance to have any treatment, missed clinic follow up visits, due to numerous other hospital appointments therefore resulting in a degree of selection bias whereby patients with worse diabetes and longstanding DME may receive delayed or inappropriate treatment. The timing of the administration of the FAc implant for DME is not clearly defined and evidence is mounting for the earlier intervention with a steroid implant if there is a limited response to other therapies.
The small sample size in this investigation and its retrospective nature are important caveats of the study. This would limit the value of any statistical analysis and the data is therefore better viewed using descriptive analyses. Prospective randomized controlled trials are required to validate preliminary real world findings and clarify the role and optimal timing of the administration of Iluvien® in the treatment of chronic DME.
In conclusion, the Iluvien® implant is a potentially useful treatment option in the management of refractory DME for both PPV and non-PPV eyes. Visual and morphological outcomes are limited in PPV eyes, although anatomic and functional improvements were observed in non-PPV eyes.
Acknowledgments
Conflicts of Interest: La Mantia A, None; Hawrami A, None; Laviers H, None; Patra S, None; Zambarakji H has acted as advisory Board Member for Alimera Sciences.
REFERENCES
- 1.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prokofyeva E, Zrenner E. Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic Res. 2012;47(4):171–188. doi: 10.1159/000329603. [DOI] [PubMed] [Google Scholar]
- 3.Quartilho A, Simkiss P, Zekite A, Xing W, Wormald R, Bunce C. Leading causes of certifiable visual loss in England and Wales during the year ending 31 March 2013. Eye (Lond) 2016;30(4):602–607. doi: 10.1038/eye.2015.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev. 2014;(10):CD007419. doi: 10.1002/14651858.CD007419.pub4. [DOI] [PubMed] [Google Scholar]
- 5.Bressler SB, Melia M, Glassman AR, Almukhtar T, Jampol LM, Shami M, Berger BB, Bressler NM, Diabetic Retinopathy Clinical Research Network Ranibizumab plus prompt or deferred laser for diabetic macular edema in eyes with vitrectomy before anti-vascular endothelial growth factor therapy. Retina. 2015;35(12):2516–2528. doi: 10.1097/IAE.0000000000000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lattanzio R, Cicinelli MV, Bandello F. Intravitreal steroids in diabetic macular edema. Dev Ophthalmol. 2017;60:78–90. doi: 10.1159/000459691. [DOI] [PubMed] [Google Scholar]
- 7.Malcles A, Dot C, Voirin N, Agard E, Vie AL, Bellocq D, Denis P, Kodjikian L. Real-life study in diabetic macular edema treated with dexamethasone implant: the Reldex study. Retina. 2017;37(4):753–760. doi: 10.1097/IAE.0000000000001234. [DOI] [PubMed] [Google Scholar]
- 8.Eter N, Mohr A, Wachtlin J, Feltgen N, Shirlaw A, Leaback R, German Ozurdex in RVO Real World Study Group Dexamethasone intravitreal implant in retinal vein occlusion: real-life data from a prospective, multicenter clinical trial. Graefes Arch Clin Exp Ophthalmol. 2017;255(1):77–87. doi: 10.1007/s00417-016-3431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massin P, Erginay A, Dupas B, Couturier A, Tadayoni R. Efficacy and safety of sustained-delivery fluocinolone acetonide intravitreal implant in patients with chronic diabetic macular edema insufficiently responsive to available therapies: a real-life study. Clin Ophthalmol. 2016;10:1257–1264. doi: 10.2147/OPTH.S105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Ghrably I, Steel DHW, Habib M, Vaideanu-Collins D, Manvikar S, Hillier RJ. Diabetic macular edema outcomes in eyes treated with fluocinolone acetonide 0.2 µg/d intravitreal implant: real-world UK experience. Eur J Ophthalmol. 2017;27(3):357–362. doi: 10.5301/ejo.5000929. [DOI] [PubMed] [Google Scholar]
- 11.Bailey C, Chakravarthy U, Lotery A, Menon G, Talks J, Medisoft Audit Group Real-world experience with 0.2 µg/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom. Eye (Lond) 2017;31(12):1707–1715. doi: 10.1038/eye.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, Iliev ME, Frey M, Rothenbuehler SP, Enzmann V, Wolf S. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009;50(7):3432–3437. doi: 10.1167/iovs.08-2970. [DOI] [PubMed] [Google Scholar]
- 13.Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, Tolentino M, Gupta A, Duarte L, Madreperla S, Gonder J, Kapik B, Billman K, Kane FE, FAME Study Group Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626–635. doi: 10.1016/j.ophtha.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Holden SE, Currie CJ, Owens DR. Evaluation of the clinical effectiveness in routine practice of fluocinolone acetonide 190 µg intravitreal implant in people with diabetic macular edema. Curr Med Res Opin. 2017;33(sup2):5–17. doi: 10.1080/03007995.2017.1366645. [DOI] [PubMed] [Google Scholar]
- 15.Schmit-Eilenberger VK. A novel intravitreal Fluocinolone Acetonide implant (Iluvien®) in the treatment of patients with chronic diabetic macular edema that is insufficiently responsive to other medical treatment options: a case series. Clin Ophthalmol. 2015;9:801–811. doi: 10.2147/OPTH.S79785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meireles A, Goldsmith C, El-Ghrably I, Erginay A, Habib M, Pessoa B, Coelho J, Patel T, Tadayoni R, Massin P, Atorf J, Augustin AJ. Efficacy of 0.2 µg/day fluocinolone acetonide implant (ILUVIEN) in eyes with diabetic macular edema and prior vitrectomy. Eye (Lond) 2017;31(5):684–690. doi: 10.1038/eye.2016.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pessoa B, Coelho J, Correia N, Ferreira N, Beirao M, Meireles A. Fluocinolone acetonide intravitreal implant 190 µg (ILUVIEN®) in vitrectomized versus nonvitrectomized eyes for the treatment of chronic diabetic macular edema. Ophthalmic Res. 2018;59(2):68–75. doi: 10.1159/000484091. [DOI] [PubMed] [Google Scholar]
- 18.Ch'ng SW, Brent AJ, Empeslidis T, Konidaris V, Banerjee S. Real-world cost savings demonstrated by switching patients with refractory diabetic macular edema to intravitreal fluocinolone acetonide (Iluvien): a retrospective cost analysis study. Ophthalmol Ther. 2018;7(1):75–82. doi: 10.1007/s40123-017-0114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parrish RK, 2nd, Campochiaro PA, Pearson PA, Green K, Traverso CE, FAME Study Group Characterization of intraocular pressure increases and management strategies following treatment with fluocinolone acetonide intravitreal implants in the FAME trials. Ophthalmic Surg Lasers Imaging Retina. 2016;47(5):426–435. doi: 10.3928/23258160-20160419-05. [DOI] [PubMed] [Google Scholar]
- 20.Papastavrou VT, Zambarakji H, Dooley I, Eleftheriadis H, Jackson TL. Observation: fluocinolone acetonide (Iluvien) implant migration into the anterior chamber. Retin Cases Brief Rep. 2017;11(1):44–46. doi: 10.1097/ICB.0000000000000284. [DOI] [PubMed] [Google Scholar]