Abstract
Background
Brugada syndrome is an ion channelopathy that predisposes affected subjects to ventricular tachycardia/fibrillation (VT/VF), potentially leading to sudden cardiac death (SCD). Tpeak‐Tend intervals, (Tpeak‐Tend)/QT ratio and Tpeak‐Tend dispersion have been proposed for risk stratification, but their predictive values in Brugada syndrome have been challenged recently.
Methods
A systematic review and meta‐analysis was conducted to examine their values in predicting arrhythmic and mortality outcomes in Brugada Syndrome. PubMed and Embase databases were searched until 1 May 2018, identifying 29 and 57 studies.
Results
Nine studies involving 1740 subjects (mean age 45 years old, 80% male, mean follow‐up duration was 68 ± 27 months) were included. The mean Tpeak‐Tend interval was 98.9 ms (95% CI: 90.5‐107.2 ms) for patients with adverse events (ventricular arrhythmias or SCD) compared to 87.7 ms (95% CI: 80.5‐94.9 ms) for those without such events, with a mean difference of 11.9 ms (95% CI: 3.6‐20.2 ms, P = 0.005; I 2 = 86%). Higher (Tpeak‐Tend)/QT ratios (mean difference = 0.019, 95% CI: 0.003‐0.036, P = 0.024; I 2 = 74%) and Tpeak‐Tend dispersion (mean difference = 7.8 ms, 95% CI: 2.1‐13.4 ms, P = 0.007; I 2 = 80%) were observed for the event‐positive group.
Conclusion
Tpeak‐Tend interval, (Tpeak‐Tend)/QT ratio and Tpeak‐Tend dispersion were higher in high‐risk than low‐risk Brugada subjects, and thus offer incremental value for risk stratification.
Keywords: Brugada syndrome, risk stratification, sudden cardiac death, Tpeak‐Tend, ventricular arrhythmia
1. INTRODUCTION
Brugada syndrome is a used to describe the combination of specific ECG changes, the Brugada pattern, in addition to life threatening arrhythmias and sudden cardiac death (SCD).1 Traditionally, it has been considered a congenital ion channelopathy linked to abnormalities in the cardiac sodium channel.2, 3 Recently, pathogenic mutations in other ion channels have been described. Mechanisms of arrhythmogenesis can be broadly divided into triggered activity and re‐entry. Of these, re‐entry is thought to be the predominant mechanism underlying increased arrhythmogenicity in Brugada syndrome requiring an increased spatial dispersion of repolarization. Such re‐entrant activity may involve direct electrotonic activation during phase 2 of the cardiac action potential, as shown in pre‐clinical studies using arterially perfused, canine wedge preparations,4 or circus‐type/spiral wave activity around an anatomical or functional obstacle. Regardless of the precise underlying mechanism for re‐entry, this transmural dispersion of repolarization can be quantified electrocardiographically by the interval from the peak to the end of the T‐wave (Tpeak‐Tend interval), (Tpeak‐Tend)/QT ratio and Tpeak‐Tend dispersion.5, 6
However, not all studies have shown an association between higher Tpeak‐Tend intervals, (Tpeak‐Tend)/QT ratio or Tpeak‐Tend dispersion with an arrhythmogenic phenotype in Brugada Syndrome. Recently, Mugnai and colleagues conducted one of the largest retrospective studies to date, including a total of 448 patients with spontaneous or drug induced type 1 Brugada pattern.7 They found no statistically significant difference in all three indices between asymptomatic subjects and patients with syncope and malignant arrhythmias. Morita and colleagues also found in 471 patients no difference in Tpeak‐Tend intervals between patients with syncope or VT/VF and those who were asymptomatic.8 These findings contrast with a meta‐analysis published previously by some members of our group, which extracted and pooled odds or hazard ratios for the relationship between Tpeak‐Tend and arrhythmic and/or mortality outcomes in various clinical conditions, including Brugada Syndrome.9 This demonstrated prolonged Tpeak‐Tend interval was associated with an increased risk of ventricular arrhythmias and SCD in Brugada Syndrome.
However, our previous study did not determine the absolute mean values for Tpeak‐Tend, nor was it possible to include the largest dataset from Mugnai and colleagues. Moreover, it did not investigate the utility of other indices such as (Tpeak‐Tend)/QT ratio or Tpeak‐Tend dispersion. Therefore, we conducted a systematic review with meta‐analysis into the relationships between Tpeak‐Tend interval, (Tpeak‐Tend)/QT ratio and Tpeak‐Tend dispersion and arrhythmic and/or mortality endpoints in Brugada Syndrome.
2. METHODS
2.1. Search strategy, inclusion and exclusion criteria
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISM) statement. PubMed and Embase were searched for studies that investigated the association between Tpeak‐Tend or Tpeak‐Tend /QT with arrhythmic or mortality endpoints in Brugada syndrome. The following search terms were used for both databases: [“Tpeak‐Tend” or “Tpeak‐end” or “Tp‐e” AND Brugada]. The databases were searched until 1 May 2018 without language restrictions. The following inclusion criteria were used: (a) the study was a case‐control, prospective or retrospective cohort study in human subjects with a Brugada phenotype, (b) Tpeak‐Tend intervals or (Tpeak‐Tend) /QT ratios were provided; (c) predefined adverse events (appropriate implantable cardioverter‐defibrillator therapy [ICD], syncope, ventricular tachycardia/fibrillation [VT/VF], SCD, cardiovascular death [CVD], major adverse cardiac events [MACE]) or all‐cause mortality were reported. In cases of incomplete data from the published studies, the original authors were contacted, but no replies were received.
The Newcastle‐Ottawa Quality Assessment Scale (NOS) was used for quality assessment of the included studies.10 The NOS system evaluated the categories of study participant selection, results comparability, and quality of the outcomes. Specifically, the following characteristics were assessed: (a) representativeness of the exposed cohort; (b) selection of the non‐exposed cohort; (c) ascertainment of exposure; (d) demonstration that outcome of interest was not present at the start of study; (e) comparability of cohorts based on study design or analysis; (f) assessment of outcomes; (g) follow‐up periods that were sufficiently long for outcomes to occur; and (h) adequacy of follow‐up of cohorts. This scale varied from zero to nine stars, which indicated that studies were graded as poor quality if the score was <5, fair if the score was 5‐7, and good if the score was >8. Studies with a score equal to or higher than six were included. The details of the NOS quality assessment are shown in Tables S1 and S2.
2.2. Data extraction and statistical analysis
Data from the different studies were entered in pre‐specified spreadsheets in Microsoft Excel. All potentially relevant studies were retrieved as complete manuscripts, which were assessed fully to determine their compliance with the inclusion criteria. We extracted the following data from the included studies: (a) publication details: last name of first author, publication year and locations; (b) study design; (c) endpoint(s); (d) quality score; and (e) characteristics of the population including sample size, gender, age and number of subjects. Two reviewers (GT and MG) reviewed each included study independently. Disagreements were resolved by adjudication with input from a third reviewer (TL).
Adverse events were defined as ventricular arrhythmias (VT/VF), SCD, cardiovascular death, MACE or all‐cause mortality. If more than one mortality endpoint was described, then SCD was preferentially used for analysis, followed by cardiovascular and all‐cause mortality in this order. Mean differences between event‐positive and event‐negative groups, with 95% confidence intervals (CIs) for Tpeak‐Tend interval, (Tpeak‐Tend)/QT ratio and Tpeak‐Tend dispersion were extracted and subsequently combined to generate a pooled estimate.
Heterogeneity between studies was quantified using The Cochran's Q value and the I 2 statistic from the standard chi‐square test, which describes the percentage of the variability in effect estimates resulting from heterogeneity. I 2 > 50% was considered to reflect significant statistical heterogeneity. A fixed effects model was used if I 2 < 50%. The random‐effect model using the inverse variance heterogeneity method was used when I 2 > 50%. To locate the origin of the heterogeneity, sensitivity analysis by excluding one study at a time, and subgroup analyses based on different disease conditions and different endpoints were performed. Funnel plots, Begg and Mazumdar rank correlation test and Egger's test were used to detect publication bias.
3. RESULTS
Figure 1 shows a flow diagram detailing the above search terms with inclusion and exclusion criteria. A total of 29 and 57 entries were retrieved from PubMed and Embase, respectively. Nine studies met the inclusion criteria and were included in our final meta‐analysis.6, 7, 11, 12, 13, 14, 15, 16, 17 In this meta‐analysis, a total of 1740 subjects with Brugada Syndrome were included (mean age 45 years old, 80% male). The mean follow‐up duration was 68 ± 27 months. Of the entire cohort, 40% had a spontaneous Type 1 pattern and 19% were positive for SCN5a mutation. The baseline characteristics of these studies and of the study populations are shown in Table 1.
Figure 1.
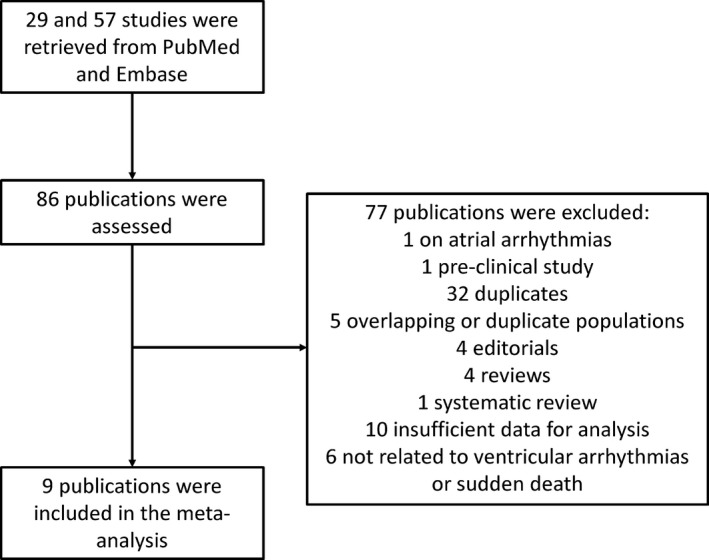
Flow diagram of the study selection process
Table 1.
Characteristics of the nine studies included in this meta‐analysis
| First author/year | Sample size (n) | Tpeak‐Tend measurement: method and leads | Age (SD) | No. of males (%) | No. of Sp. type 1 patients (%) | No. of SCN5a positive patients (%) | Endpoints | Comparisons | No. of patients with adverse events /without adverse events/%/% per year | Follow‐up duration (months) | Quality score | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morita 2017 | 471 | Tangent method; V1, V2, V3, V5 | 47 (19) | 447 (95) | 118 (25) | 27 (15) | Syncope or VT/VF | Syncope/VT/VF vs asymptomatic | 145/326/31/4.09 | 91 | 7 | 16 |
| Mugnai 2017 | 448 | End of the T‐wave; V1 to V6 | 45 (16) | 273 (61) | 96 (21) | 55 (22) | Spontaneous VF or SCD | AT/SD vs asymptomatic | 43/290/13/1.67 | 93 | 6 | 7 |
| Kawazoe 2016 | 143 | Tangent method; V1 to V6 | 46 (12) | 140 (98) | 84 (59) | – | VF | VF vs no VF | 35/108/24/1.9 | 105 | 7 | 17 |
| Zumhagen 2016 | 78 | Tangent method; V1 | 45 (14) | 57 (73) | 22 (28) | 17 (22) | Spontaneous VT/VF | VT/VF/aborted SCD vs asymptomatic/syncope | 22/54/29/‐ | – | 6 | 14 |
| Maury 2015 | 325 | Tangent method; V1 to V4 | 47 (13) | 260 (80) | 143 (44) | 43 (13) | Spontaneous VT/VF | AT/SD vs asymptomatic | 26/226/10/2.50 | 48 | 7 | 11 |
| Letsas 2010 | 23 | End of the T‐wave; V2, V6 | 43 (15) | 19 (83) | 10 (43) | – | Inducible VT/VF | Inducible VT vs no inducible VT | 17/6/74/16.15 | 55 | 6 | 12 |
| Junttila 2008 | 200 | End of the T‐wave; V2, II | 40 (16) | 143 (72) | 200 (100) | 25 (50) | Syncope, VT/VF, SCD | Syncope/VT/VF/aborted SCD vs asymptomatic | 66/134/33/‐ | – | 7 | 15 |
| Wang 2007 | 23 | End of the T‐wave; Max from V1 to V6 | 45 (8) | 23 (100) | – | – | Spontaneous VT/VF | Syncope/VT/VF/inducible VT vs asymptomatic | 11/9/55/5.12 | 43 | 8 | 13 |
| Castro Hevia 2006 | 29 | Tangent method, Max from V1 to V6 | 41 (12) | 25 (86) | 15 (52) | – | Spontaneous VT/VF | Presyncope/syncope/aborted SCD vs asymptomatic | 12/17/41/3.81 | 43 | 8 | 6 |
SCD: sudden cardiac death; VT: ventricular tachycardia; VF: ventricular fibrillation; Sp.: spontaneous.
3.1. Tpeak‐Tend
For determining Tend, the tangent method and the return of the voltage to baseline method were used. Tpeak‐Tend intervals from different leads and the maximum of these measurements have been presented by most studies. Regarding maximum Tpeak‐Tend intervals, the mean value for the event‐positive group was 98.9 ms (95% CI: 90.5‐107.2 ms) (Figure 2A) and event‐negative group was 87.7 ms (95% CI: 80.5‐94.9 ms) (Figure 2B). Five studies reported longer values in the event‐positive compared to event‐negative groups, whereas four studies reported no significant difference (Figure 2C). Tpeak‐Tend intervals were 11.9 ms longer (95% CI: 3.6‐20.2 ms, P = 0.005) in event‐positive patients than in event‐negative patients. The Cochran's Q value was greater than the degrees of freedom (56 vs 8), indicating that the true effect size was different between studies. I 2 took a value of 86%, suggesting the presence of substantial heterogeneity. A funnel plot plotting standard errors against differences in means is shown in Figure S1. Begg and Mazumdar rank correlation analysis demonstrated that Kendall's Tau took a value of 0.3 with P = 0.30, which suggests no significant publication bias. Egger's test demonstrated no significant asymmetry (intercept 2.4, t‐value 1.2; P = 0.25). To identify the source of the heterogeneity, sensitivity analysis was performed by removing one study at a time, but this did not significantly influence the mean difference (Figure S2), suggesting that no single study was responsible for the heterogeneity observed in this meta‐analysis. Subgroup analysis based on the method of Tend determination was performed. For the tangent method, the Tpeak‐Tend mean difference was 15.5 ms (95% CI: 3.9‐27.2 ms; P = 0.009) and I 2 remained high at 90%. For full recovery of voltage to baseline, the mean difference was 6.0 ms (95% CI: 0.7‐11.4 ms; P = 0.006) and I 2 remained high at 76%. Therefore, different methods of Tend determination did not introduce significant heterogeneity to the pooled effect estimate.
Figure 2.
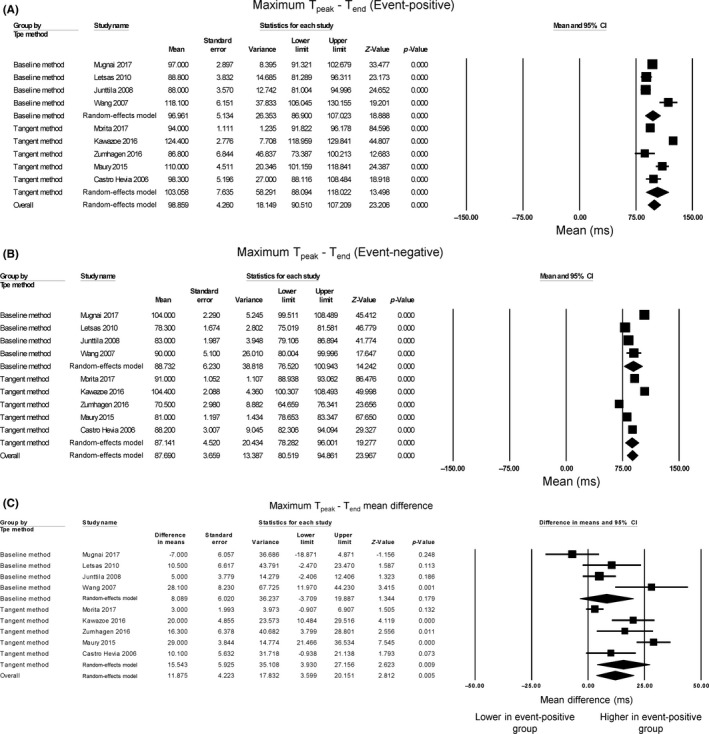
Forest plot demonstrating Tpeak‐Tend intervals obtained from event‐positive (A) and event‐negative (B) groups and the mean difference between both groups (C) in Brugada Syndrome
3.2. (Tpeak‐Tend)/QT ratio
Regarding maximum (Tpeak‐Tend)/QT ratio, the mean value for the event‐positive group was 0.221 (95% CI: 0.208‐0.234) (Figure 3A) and event‐negative group was 0.210 (95% CI: 0.205‐0.214) (Figure 3B). Two studies reported higher values in Brugada subjects with positive events compared to those without such events, whereas four studies demonstrated no significance between the groups (Figure 3C). Pooling of the mean values demonstrated significantly higher (Tpeak‐Tend)/QT ratios in the event‐positive group than in the event‐negative group (mean difference = 0.019, 95% CI: 0.003‐0.036, P = 0.024). The Cochran's Q value was greater than the degrees of freedom (19 vs 5), indicating that the true effect size was different between studies. I 2 took a value of 74%, suggesting significant heterogeneity. A funnel plot plotting standard errors against differences in means is shown in Figure S3. Begg and Mazumdar rank correlation analysis demonstrated that Kendall's Tau took a value of 0.07 with P = 1, which suggested no significant publication bias. Egger's test demonstrated no significant asymmetry (intercept 3.5, t‐value 1.1; P = 0.31). To identify the source of the heterogeneity, sensitivity analysis was performed by removing one study at a time, but this did not significantly influence the mean difference (Figure S4), suggesting that no single study was responsible for the heterogeneity observed in this meta‐analysis. Subgroup analysis based on the method of Tend determination was performed. For the tangent method, the mean difference of (Tpeak‐Tend)/QT ratio was 0.03 (95% CI: 0.01‐0.05; P < 0.05) and I 2 was lowered to 55%. For full recovery of voltage to baseline, the mean difference was only 0.004 (95% CI: −0.03 to 0.03 ms; P = 0.81) and I 2 remained high at 74%. Therefore, different method of Tend determination appeared to contribute partially to the heterogeneity of the pooled effect estimate. Moreover, statistical significance was achieved when the tangent method was used, but was lost when the return to baseline method was used, which may suggest the former approach may be more sensitive.
Figure 3.
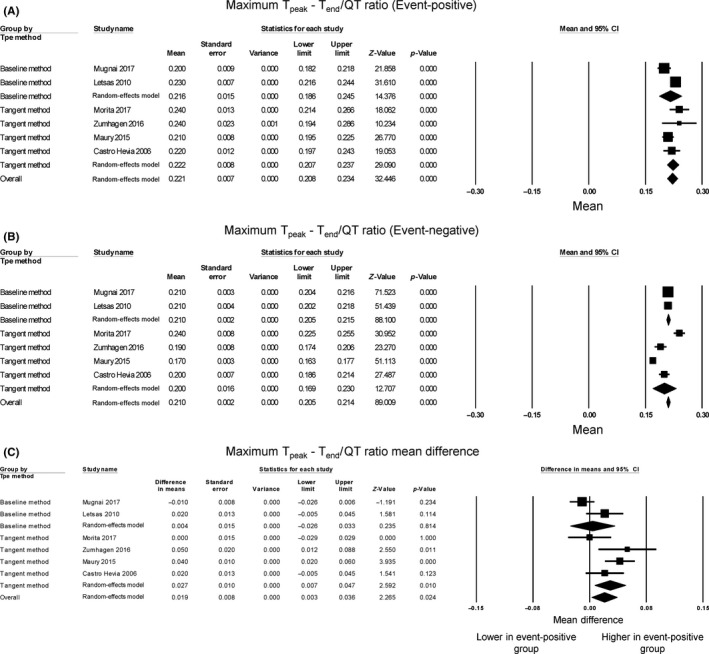
Forest plot demonstrating Tpeak‐Tend/QT ratios obtained from event‐positive (A) and event‐negative (B) groups and the mean difference between both groups (C) in Brugada Syndrome
3.3. Tpeak‐Tend dispersion
Regarding maximum Tpeak‐Tend dispersion, the mean value for the event‐positive group was 40.8 ms (95% CI: 26.9‐54.8 ms) (Figure 4A) and event‐negative group was 29.7 ms (95% CI: 24.5‐34.8 ms) (Figure 4B). Regarding Tpeak‐Tend dispersion, two studies reported longer values in event‐positive group compared to event‐negative groups, whereas three studies found no significant difference (Figure 4C). Overall, pooling of the data showed that Tpeak‐Tend dispersion was significantly higher in the event‐positive than in the event‐negative groups (mean difference = 7.8 ms, 95% CI: 2.1 to 13.4 ms, P = 0.007). The Cochran's Q value was greater than the degrees of freedom (20 vs 4), indicating that the true effect size was different between studies. I 2 took a value of 80%, suggesting significant heterogeneity. A funnel plot plotting standard errors against differences in means is shown in Figure S5. Begg and Mazumdar rank correlation analysis demonstrated that Kendall's Tau took a value of −2 with P = 0.62, which suggests no significant publication bias. Egger's test demonstrated no significant asymmetry (intercept −5.4, t‐value 0.8; P = 0.48). To identify the source of the heterogeneity, sensitivity analysis was performed by removing one study at a time, but this did not significantly influence the mean difference between event‐positive and event‐negative groups (Figure S6), suggesting that no single study was responsible for the heterogeneity observed in this meta‐analysis. Subgroup analysis based on the method of Tend determination was performed. For the tangent method, the mean difference of Tpeak‐Tend dispersion was 16.2 ms (95% CI: 7.9‐24.5 ms; P < 0.0001) and I 2 was 65%. For full recovery of voltage to baseline, the mean difference was 0.4 ms (95% CI: −7.3 to 8.2 ms; P = 0.91) and I 2 was reduced to 19%. Therefore, different method of Tend determination contributed heterogeneity to the pooled effect estimate. Moreover, statistical significance was achieved when the tangent method was used, but was lost when the return to baseline method was used, which may suggest the former approach may be more sensitive.
Figure 4.
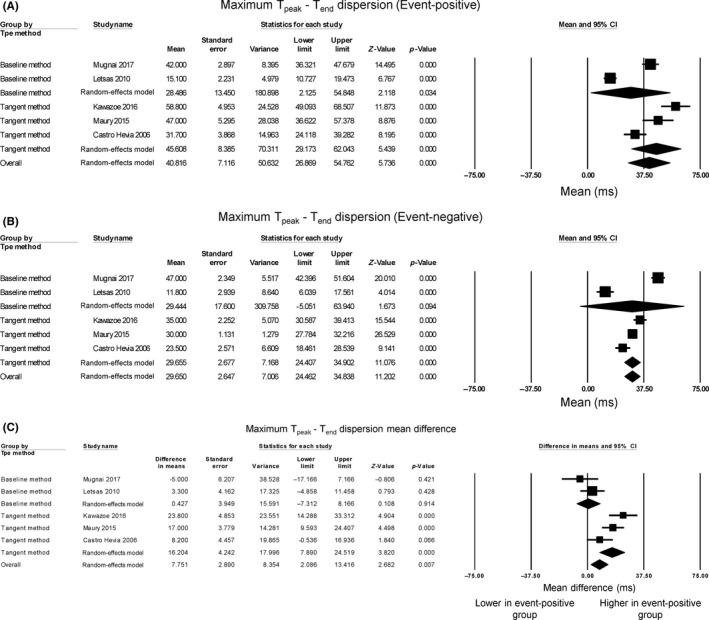
Forest plot demonstrating Tpeak‐Tend dispersion obtained from event‐positive (A) and event‐negative (B) groups and the mean difference between both groups (C) in Brugada Syndrome
3.4. Comparisons between patients with and without SCN5A mutations
SCN5A is the commonest ion channel gene that is mutated in Brugada syndrome.2, 3 Separate meta‐analyses were conducted to compare the different Tpeak‐Tend parameters between patients with and without SCN5A mutations. Two of the included studies provided sufficient information for such analyses.7, 14 No significant difference in Tpeak‐Tend (mean difference = 8.2 ms, 95% CI: −6.7 to 23.2 ms, P = 0.28; I 2 = 59%; Figure S7), Tpeak‐Tend/QT ratio (mean difference = −0.006 ms, 95% CI: −0.023 to 0.011 ms, P = 0.47; I 2 = 24%; Figure S8) or Tpeak‐Tend dispersion (mean difference = 5.2 ms, 95% CI: −2.9 to 13.2 ms, P = 0.21; I 2 = 31%; Figure S9) was observed between patients with and without SCN5A mutations.
4. DISCUSSION
The main findings of our meta‐analysis, which included 1597 Brugada subjects, are (a) Tpeak‐Tend intervals, (b) (Tpeak‐Tend)/QT ratio and (c) Tpeak‐Tend dispersion are higher in Brugada subjects with adverse cardiac events (ventricular tachy‐arrhythmias and SCD) when compared to Brugada subjects free from such events.
The presence of pre‐existing electrophysiological heterogeneities is important for mediating the normal, unidirectional spread of action potentials in the heart.18, 19 These are attributed to differences in repolarization times of the different cell types, which are responsible for generation of the T‐wave on the electrocardiogram (ECG).20, 21 However, exacerbation of such differences has been associated with ventricular tachy‐arrhythmias in different conditions, thereby generating a pro‐arrhythmic phenotype. These include congenital ion channelopathies such as long QT syndrome and Brugada syndrome22, 23, 24 and acquired cardiac diseases such as myocardial infarction.25, 26 These heterogeneities can occur locally or across the myocardial wall,27 potentially causing arrhythmias by inducing unidirectional conduction block and therefore circus‐type or spiral wave re‐entry.28, 29 Moreover, a greater epicardial‐endocardial repolarization time difference may increase the propensity of phase 2 re‐entry, which is hypothesized to generate extrasystolic activity in Brugada syndrome.30 This occurs when sites with an action potential dome to sites which a dome morphology, leading to direct depolarization of the downstream sites.31 Once an extrasystole is generated, together with a favorable re‐entrant substrate, ventricular tachycardia and fibrillation can result.32
A number of electrocardiographic indices have been proposed for stratification of arrhythmic or mortality risk.33, 34 Of these, Yan and Antzelevitch were the first to propose the use of the difference between the peak and the end of the T‐wave (the Tpeak‐Tend interval) as a measure of transmural dispersion of repolarization.20, 35, 36, 37 Subsequent clinical studies have demonstrated that, confirmed recently in a systematic review and meta‐analysis from our group,9 that Tpeak‐Tend prolongation significantly elevated the risk of ventricular tachy‐arrhythmias and/or SCD in heart failure, ischemic heart disease, Brugada syndrome, hypertension, and the general population. Recently, Mugnai and colleagues in a total of 448 subjects found no significant differences Tpeak‐Tend intervals, (Tpeak‐Tend)/QT ratio or Tpeak‐Tend dispersion between patients with VT/VF requiring anti‐tachycardia pacing or with sudden death, and those who were asymptomatic.7 Similarly, in a separate population of 471 subjects, Morita and colleagues found no significance difference in Tpeak‐Tend intervals between patients with syncope or VT/VF and asymptomatic patients.16 Publication of these two studies prompted us to conduct this meta‐analysis, which confirms the value of Tpeak‐Tend interval, (Tpeak‐Tend)/QT ratio and Tpeak‐Tend dispersion, in distinguishing high‐risk patients from low‐risk patients.
In the Mugnai study, the largest study to date, the percentage of patients with adverse events were the lowest at 13%.7 Male gender, a spontaneous Type 1 Brugada pattern and SCN5a mutation positive status were significantly associated with ventricular arrhythmias.38 Therefore, the lower percentage of patients with adverse events can be explained by the lower percentage of Type 1 Brugada patients (21% vs 28%‐100% in the remaining studies) and lower percentage male patients (61% vs 72%‐100%) despite similar percentage with SCN5a positive status (22% vs 13%‐50%). While these differences in patient characteristics affect the likelihood of adverse events occurring, they should not explain the lack of difference in Tpeak‐Tend intervals between event‐positive and event‐negative groups in the Morita study16 or the Mugnai study. Interestingly, Mugnai and colleagues found a non‐statistically significant lower Tpeak‐Tend intervals in event‐positive groups. Of the remaining six studies, five studies had reported significantly higher Tpeak‐Tend intervals and one study reported no difference.15 A recent epidemiological study reported a U‐shaped relationship between Tpeak‐Tend intervals and increased mortality.39 Autonomic modulation, which is part of Coumel's triad for arrhythmogenesis,40 is known to modulate the re‐entrant substrate. Increased activity of the parasympathetic nervous system may reduce Tpeak‐Tend intervals, which may also be pro‐arrhythmic.41 By contrast, exercise, during which sympathetic activity is increased, can exacerbate pre‐existing heterogeneities, such as producing conduction slowing42 and increasing the dispersion of repolarization.43
In our previous meta‐analysis pooling together studies that reported odds ratios or hazard ratios, the average cut‐off for Tpeak‐Tend was 95.8 ms across different clinical conditions.9 The present meta‐analysis pooling mean values for event‐positive and ‐negative groups clearly indicates that the 100 ms cut‐off is too high for Brugada syndrome. Our data would support a lower cut‐off value between 88 and 99 ms to be used. This cut‐off will also be method‐dependent for determining Tend in the case of the Tpeak‐Tend intervals. Previously, it was shown that in a cohort of high‐risk Brugada subjects, only 10 of 16 studies reported a Tpeak‐Tend longer than 100 ms, supporting our notion that this cut‐off value may be too high.44 Moreover, different studies measured Tpeak‐Tend from different leads. Some had measured it from all 12 leads and taken the mean values while others have done so for V1 to V3 only. While there is no consensus as to which leads are most appropriate for measurement, obtaining it from all 12 leads is likely to be less useful clinically due to the time‐consuming nature. To simplify Tpeak‐Tend determination, we would thus propose measuring it from the right precordial leads given BrS is primarily a right ventricular disorder.
While it may appear that the difference in Tpeak‐Tend between high‐risk and low‐risk Brugada patients was only small, at around 12 ms, it should be emphasized that increased transmural dispersion of repolarization is only one mechanism by which re‐entrant arrhythmogenesis is generated. Other mechanisms, such as reduced conduction velocity, increased dispersion of conduction45 or dynamic substrates such as steep action potential restitution,46 in which normal Tpeak‐Tend interval, Tpeak‐Tend/QT ratio or Tpeak‐Tend dispersion may be observed, also contribute to arrhythmogenesis in Brugada syndrome. Therefore, better risk stratification scores will need to incorporate a combination of repolarization and conduction indices. Moreover, some of these dynamic changes may not be detectable on the ECG and may require additional tests such as non‐invasive ECG imaging (ECGi),43 or only becomes detectable only under stressful conditions such as exercise.43
4.1. Limitations
The following limitations of this meta‐analysis should be noted. First, there is marked heterogeneity between the included studies. The method of Tpeak‐Tend determination across the studies was split even between the tangent method and full recovery of the voltage to baseline. Subgroup analysis based on the method used did not reduce the heterogeneity observed. Therefore, measurement method was unlikely to have significantly contributed to the heterogeneity observed. Moreover, the Letsas 2010 study12 used a different endpoint of inducible VT compared to the remaining studies, but its exclusion did not significant affect the mean Tpeak‐Tend values for event‐positive group, event‐negative group, and mean difference between these groups. Second, retrospective studies may have more bias than prospective studies. Finally, it should be acknowledged that there is overlap between event‐postiive and event‐negative groups irrespective of the method of measuring Tend. This would suggest as a single measurement, Tpeak‐Tend is unlikely to be useful in its own right. Indeed, accurate risk stratification will require a composite scoring system assessing not only dispersion of repolarization, but that of conduction, clinical symptoms, family history, the type of Brugada pattern, genetic background, electrical and drug provocation testing as well as electrophysiological mapping.38, 41, 45, 47, 48, 49
5. CONCLUSIONS
Tpeak‐Tend interval, Tpeak‐Tend/QT ratio and Tpeak‐Tend dispersion were higher in high‐risk than low‐risk Brugada subjects, and thus offer incremental value for risk stratification.
CONFLICTS OF INTERESTS
Authors declare no Conflict of Interests for this article.
Supporting information
ACKNOWLEDGEMENTS
GT thanks the Croucher Foundation of Hong Kong for the support of a clinical assistant professorship.
Tse G, Gong M, Li CKH, et al. Tpeak‐Tend, Tpeak‐Tend/QT ratio and Tpeak‐Tend dispersion for risk stratification in Brugada Syndrome: A systematic review and meta‐analysis. J Arrhythmia. 2018;34:587–597. 10.1002/joa3.12118
REFERENCES
- 1. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20(6):1391–1396. [DOI] [PubMed] [Google Scholar]
- 2. Chen Q, Kirsch GE, Zhang D, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392(6673):293–296. [DOI] [PubMed] [Google Scholar]
- 3. Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST‐segment elevation. Circulation. 1999;100(15):1660–1666. [DOI] [PubMed] [Google Scholar]
- 4. Burashnikov A, Antzelevitch C. Differences in the electrophysiologic response of four canine ventricular cell types to alpha 1‐adrenergic agonists. Cardiovasc Res. 1999;43(4):901–908. [DOI] [PubMed] [Google Scholar]
- 5. Xia Y, Liang Y, Kongstad O, Holm M, Olsson B, Yuan S. Tpeak‐Tend interval as an index of global dispersion of ventricular repolarization: evaluations using monophasic action potential mapping of the epi‐ and endocardium in swine. J Interv Card Electrophysiol. 2005;14(2):79–87. [DOI] [PubMed] [Google Scholar]
- 6. Castro Hevia J, Antzelevitch C, Tornes Barzaga F, et al. Tpeak‐Tend and Tpeak‐Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47(9):1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mugnai G, Hunuk B, Hernandez‐Ojeda J, et al. Role of electrocardiographic Tpeak‐Tend for the prediction of ventricular arrhythmic events in the Brugada syndrome. Am J Cardiol. 2017;120(8):1332–1337. [DOI] [PubMed] [Google Scholar]
- 8. Morita H, Watanabe A, Kawada S, et al. Identification of electrocardiographic risk markers for the initial and recurrent episodes of ventricular fibrillation in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2018;29(1):107–114. [DOI] [PubMed] [Google Scholar]
- 9. Tse G, Gong M, Wong WT, et al. The Tpeak – Tend interval as an electrocardiographic risk marker of arrhythmic and mortality outcomes: a systematic review and meta‐analysis. Heart Rhythm. 2017;14(8):1131–1137. [DOI] [PubMed] [Google Scholar]
- 10. Marshall SC, Molnar F, Man‐Son‐Hing M, et al. Predictors of driving ability following stroke: a systematic review. Top Stroke Rehabil. 2007;14(1):98–114. [DOI] [PubMed] [Google Scholar]
- 11. Maury P, Sacher F, Gourraud JB, et al. Increased Tpeak‐Tend interval is highly and independently related to arrhythmic events in Brugada syndrome. Heart Rhythm. 2015;12(12):2469–2476. [DOI] [PubMed] [Google Scholar]
- 12. Letsas KP, Weber R, Astheimer K, Kalusche D, Arentz T. Tpeak‐Tend interval and Tpeak‐Tend/QT ratio as markers of ventricular tachycardia inducibility in subjects with Brugada ECG phenotype. Europace. 2010;12(2):271–274. [DOI] [PubMed] [Google Scholar]
- 13. Wang JF, Shan QJ, Yang B, et al. Tpeak‐Tend interval and risk of cardiac events in patients with Brugada syndrome. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35(7):629–632. [PubMed] [Google Scholar]
- 14. Zumhagen S, Zeidler EM, Stallmeyer B, Ernsting M, Eckardt L, Schulze‐Bahr E. Tpeak‐Tend interval and Tpeak‐Tend/QT ratio in patients with Brugada syndrome. Europace. 2016;18(12):1866–1872. [DOI] [PubMed] [Google Scholar]
- 15. Junttila MJ, Brugada P, Hong K, et al. Differences in 12‐lead electrocardiogram between symptomatic and asymptomatic Brugada syndrome patients. J Cardiovasc Electrophysiol. 2008;19(4):380–383. [DOI] [PubMed] [Google Scholar]
- 16. Morita H, Watanabe A, Kawada S, et al. Identification of electrocardiographic risk markers for the initial and recurrent episodes of ventricular fibrillation in patients with Brugada syndrome. J Cardiovasc Electrophysiol 2017;29:107–114. [DOI] [PubMed] [Google Scholar]
- 17. Kawazoe H, Nakano Y, Ochi H, et al. Risk stratification of ventricular fibrillation in Brugada syndrome using noninvasive scoring methods. Heart Rhythm. 2016;13(10):1947–1954. [DOI] [PubMed] [Google Scholar]
- 18. Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85(4):1205–1253. [DOI] [PubMed] [Google Scholar]
- 19. Nerbonne JM, Guo W. Heterogeneous expression of voltage‐gated potassium channels in the heart: roles in normal excitation and arrhythmias. J Cardiovasc Electrophysiol. 2002;13(4):406–409. [DOI] [PubMed] [Google Scholar]
- 20. Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm. 2007;4(7):964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Antzelevitch C, Shimizu W, Yan GX, Sicouri S. Cellular basis for QT dispersion. J Electrocardiol. 1998;30(Suppl):168–175. [DOI] [PubMed] [Google Scholar]
- 22. Antzelevitch C, Yan GX, Ackerman MJ, et al. J‐Wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge: endorsed by the Asia Pacific Heart Rhythm Society (APHRS), the European Heart Rhythm Association (EHRA), the Heart Rhythm Society (HRS), and the Latin American Society of Cardiac Pacing and Electrophysiology (Sociedad Latinoamericana de Estimulacifin Cardiaca y Electro fi siologia [SOLAECE]). Europace. 2017;19(4):665–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu T, Zheng J, Yan GX. J wave syndromes: history and current controversies. Korean Circ J. 2016;46(5):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tse G, Chan YW, Keung W, Yan BP. Electrophysiological mechanisms of long and short QT syndromes. Int J Cardiol Heart Vasc. 2017;14:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erikssen G, Liestol K, Gullestad L, Haugaa KH, Bendz B, Amlie JP. The terminal part of the QT interval (T peak to T end): a predictor of mortality after acute myocardial infarction. Ann Noninvasive Electrocardiol. 2012;17(2):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jani Y, Kamberi A, Xhunga S, et al. The influence of type 2 diabetes and gender on ventricular repolarization dispersion in patients with sub‐clinic left ventricular diastolic dysfunction. Am J Cardiovas Dis. 2015;5(4):155–166. [PMC free article] [PubMed] [Google Scholar]
- 27. Dogan M, Yiginer O, Degirmencioglu G, Un H. Transmural dispersion of repolarization: a complementary index for cardiac inhomogeneity. J Geriatr Cardiol. 2016;13(1):99–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shaw RM, Rudy Y. The vulnerable window for unidirectional block in cardiac tissue: characterization and dependence on membrane excitability and intercellular coupling. J Cardiovasc Electrophysiol. 1995;6(2):115–131. [DOI] [PubMed] [Google Scholar]
- 29. Tse G, Wong ST, Tse V, Lee YT, Lin HY, Yeo JM. Cardiac dynamics: alternans and arrhythmogenesis. J Arrhythm. 2016;32(5):411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tse G, Liu T, Li KH, et al. Electrophysiological mechanisms of Brugada syndrome: insights from pre‐clinical and clinical studies. Front Physiol. 2016;7:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shimizu W, Aiba T, Kamakura S. Mechanisms of disease: current understanding and future challenges in Brugada syndrome. Nat Clin Pract Cardiovasc Med. 2005;2(8):408–414. [DOI] [PubMed] [Google Scholar]
- 32. Rodriguez‐Manero M, Sacher F, de Asmundis C, et al. Monomorphic ventricular tachycardia in patients with Brugada syndrome: a multicenter retrospective study. Heart Rhythm. 2016;13(3):669–682. [DOI] [PubMed] [Google Scholar]
- 33. Robyns T, Lu HR, Gallacher DJ, et al. Evaluation of index of cardio‐electrophysiological balance (iCEB) as a new biomarker for the identification of patients at increased arrhythmic risk. Ann Noninvasive Electrocardiol. 2016;21(3):294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tse G. Both transmural dispersion of repolarization and of refractoriness are poor predictors of arrhythmogenicity: a role for iCEB (QT/QRS)? J Geriatr Cardiol. 2016;13(9):813–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shimizu W, McMahon B, Antzelevitch C. Sodium pentobarbital reduces transmural dispersion of repolarization and prevents torsades de Pointes in models of acquired and congenital long QT syndrome. J Cardiovasc Electrophysiol. 1999;10(2):154–164. [DOI] [PubMed] [Google Scholar]
- 36. Emori T, Antzelevitch C. Cellular basis for complex T waves and arrhythmic activity following combined I(Kr) and I(Ks) block. J Cardiovasc Electrophysiol. 2001;12(12):1369–1378. [DOI] [PubMed] [Google Scholar]
- 37. Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long‐QT syndrome. Circulation. 1998;98(18):1928–1936. [DOI] [PubMed] [Google Scholar]
- 38. Letsas KP, Asvestas D, Baranchuk A, et al. Prognosis, risk stratification, and management of asymptomatic individuals with Brugada syndrome: a systematic review. Pacing Clin Electrophysiol. 2017;40(12):1332–1345. [DOI] [PubMed] [Google Scholar]
- 39. Bachmann TN, Skov MW, Rasmussen PV, et al. Electrocardiographic Tpeak‐Tend interval and risk of cardiovascular morbidity and mortality: results from the Copenhagen ECG study. Heart Rhythm. 2016;13(4):915–924. [DOI] [PubMed] [Google Scholar]
- 40. Coumel P. Cardiac arrhythmias and the autonomic nervous system. J Cardiovasc Electrophysiol. 1993;4(3):338–355. [DOI] [PubMed] [Google Scholar]
- 41. Lambiase PD. Tpeak – Tend interval and Tpeak – Tend /QT ratio as markers of ventricular tachycardia inducibility in subjects with Brugada ECG phenotype. EP Europace. 2010;12(2):158–159. [DOI] [PubMed] [Google Scholar]
- 42. Amin AS, de Groot EA, Ruijter JM, Wilde AA, Tan HL. Exercise‐induced ECG changes in Brugada syndrome. Circ Arrhythm Electrophysiol. 2009;2(5):531–539. [DOI] [PubMed] [Google Scholar]
- 43. Leong KM, Ng FS, Roney C, et al. Repolarization abnormalities unmasked with exercise in sudden cardiac death survivors with structurally normal hearts. J Cardiovasc Electrophysiol. 2017;29:115–126. [DOI] [PubMed] [Google Scholar]
- 44. Rivard L, Roux A, Nault I, et al. Predictors of ventricular arrhythmias and sudden death in a Quebec cohort with Brugada syndrome. Can J Cardiol. 2016;32(11):1355.e1–1355.e7. [DOI] [PubMed] [Google Scholar]
- 45. Meng L, Letsas KP, Baranchuk A, et al. Meta‐analysis of fragmented QRS as an electrocardiographic predictor for arrhythmic events in patients with Brugada syndrome. Front Physiol. 2017;8:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lambiase PD, Ahmed AK, Ciaccio EJ, et al. High‐density substrate mapping in Brugada syndrome: combined role of conduction and repolarization heterogeneities in arrhythmogenesis. Circulation. 2009;120(2):106–117, 1‐4. [DOI] [PubMed] [Google Scholar]
- 47. Asvestas D, Tse G, Baranchuk A, et al. High risk electrocardiographic markers in Brugada syndrome. IJC Heart Vasc. 2018;18:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bayoumy A, Gong MQ, Christien Li KH, et al. Spontaneous type 1 pattern, ventricular arrhythmias and sudden cardiac death in Brugada Syndrome: an updated systematic review and meta‐analysis. J Geriatr Cardiol. 2017;14(10):639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nunn L, Bhar‐Amato J, Lambiase P. Brugada syndrome: controversies in risk stratification and management. Indian Pacing Electrophysiol J. 2010;10(9):400–409. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


