Abstract
Background
Metabolic syndrome/epicardial adipose tissue (EAT) plays an important role in atrial fibrillation (AF). Although reverse atrial remodeling (RAR) often occurs after AF ablation, the effects of EAT on RAR remain unknown.
Methods
Study subjects were 104 patients in whom transthoracic echocardiography (TTE) was performed before AF ablation and 3, 6, and 12 months afterward. EAT was assessed in terms of its thickness adjacent to the right ventricular anterior wall in the TTE parasternal view. RAR was defined as >10% reduction in the left atrial volume (LAV) index by the 3‐month follow‐up examination.
Results
Postablation RAR occurred in 57/104 (55%) patients. RAR absence was associated with a relatively thick EAT (4.92 ± 1.65 vs. 3.92 ± 1.17 mm, P = 0.0005), small LAV index (24.6 ± 7.5 vs. 28.8 ± 10.6 mL/m2, P = 0.0233), and metabolic syndrome (62% vs. 28%, P = 0.0006). Metabolic syndrome and EAT were shown to be independent predictors of RAR absence. Thick EAT was significantly associated with AF recurrence after ablation (5.05 ± 2.19 mm vs. 4.17 ± 1.16 mm for no AF recurrence group, P = 0.0116), but metabolic syndrome was not (48% vs. 42%, P = 0.6189). Despite no change in body weight, EAT thickness decreased significantly by 12 months in patients without AF recurrence (4.17 ± 1.16 vs. 3.65 ± 1.16 mm, P < 0.0001).
Conclusions
EAT and metabolic syndrome appear to be strongly associated with RAR absence, but only the thick EAT was significantly associated with the postablation AF recurrence. Our findings, especially the thinning of EAT, suggest that thick EAT lead to AF vulnerability but that EAT reduction favorably affects ablation outcome.
Keywords: atrial fibrillation, atrial remodeling, echocardiography, epicardial adipose tissue, metabolic syndrome
1. INTRODUCTION
Pulmonary vein isolation (PVI) is a widely accepted therapeutic option for symptomatic atrial fibrillation (AF).1 Despite advances in ablation technologies and strategies, some patients suffer AF recurrence due to pulmonary vein (PV) reconnections or to non‐PV triggers. Another contributor to AF recurrence is atrial remodeling as manifested by atrial dilatation or fibrosis.2 Maintenance of sinus rhythm after successful PVI leads to a reduction in left atrial volume (LAV), so‐called reverse atrial remodeling (RAR). Although RAR is clearly associated with long‐term maintenance of sinus rhythm3, the mechanism responsible for RAR that occurs after ablation remains uncertain. Clarifying the mechanism underlying RAR after ablation may be beneficial in terms of patient management after AF ablation.
Metabolic syndrome, which refers to a group of risk factors for cardiovascular disease, such as obesity, hypertension, and dyslipidemia, plus high blood sugar is one of several modifiable risk factors for AF.4, 5, 6, 7, 8, 9, 10 Patients such as those with metabolic syndrome tend to have increased visceral fat.11, 12, 13 Pericardial adipose tissue is a type of visceral fat that consists of paracardial fat located outside the parietal pericardium and epicardial fat/epicardial adipose tissue (EAT) between the visceral pericardium and the myocardium. EAT in particular has emerged as an important factor in the pathogenesis of metabolic syndrome, and it has also been linked to the progression of AF.14, 15, 16 We hypothesized that EAT and the presence of metabolic syndrome may impede RAR and sinus rhythm maintenance after ablation. EAT is often assessed by mean of computed tomography (CT)‐ or magnetic resonance imaging (MRI)‐based measurement17, but such measurement, performed with the use of specific offline software is burdensome. Transthoracic echocardiography (TTE) is generally used in the management of AF, and it can be used to determine EAT thickness simply and noninvasively. Several reported studies have shown that TTE‐determined EAT thickness, defined by the echo‐free space between the right ventricle and pericardium, correlates strongly with that measured on CT images and that it is strongly associated with ablation outcome as well as the postablation recurrence of AF.18, 19 We, in the study described herein, explored the effects of TTE‐measured EAT and metabolic syndrome on RAR and ablation outcome.
2. METHODS
2.1. Study design
Included in the study were 104 consecutive patients (75 men, 29 women, aged 63 ± 10 years) who underwent ablation for AF between June 2015 and November 2016. In all 104 patients, TTE was performed 1 day before ablation and 3, 6, and 12 months after ablation. The study protocol was approved by the institutional review board of Nihon University Itabashi Hospital (Approval no: RK‐180213‐12; Approval date: February 14, 2018). All patients provided written informed consent for the electrophysiologic study, ablation procedure, and use of their anonymized data for study purposes. Adequate oral anticoagulation was given for at least 1 month prior to the ablation procedure, and all antiarrhythmic drugs were stopped for at least 5 half‐lives prior to the procedure.
2.2. Blood chemistry tests
Cardiac catheterization was performed in preparation for the ablation procedure, and blood samples were drawn from the patient's jugular vein via the sheath placed for the coronary sinus catheter. The blood samples were obtained for evaluation of serum high‐sensitivity C‐reactive protein (hs‐CRP), which was measured by particle‐enhanced immunonephelometry (BN II System, Siemens Healthcare Diagnostics Inc., Marburg, Germany), and serum N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), which was measured by sandwich chemiluminescent enzyme immunoassay (Roche Diagnostics., Mannheim, Germany).
2.3. Ablation procedure
PVI was performed by using contact force (CF)‐based ablation or cryoballoon ablation (CBA) under sedation induced with intravenous propofol and fentanyl, as previously reported.20 In brief, vascular access was obtained through the right internal jugular vein and both femoral veins. A 14‐pole catheter (7 4‐mm proximal electrodes, 2‐mm spacing; 7 4‐mm distal electrodes, 2‐mm spacing; Irvine Biomedical Inc, St. Jude Medical, Irvine, CA) was placed in the coronary sinus (CS) through the right internal jugular vein. After a single transseptal puncture, heparin was administered intravenously to maintain an activated clotting time above 300 seconds. In patients who underwent CF‐based ablation, PVI was performed, guided by a double Lasso catheter and the 3D geometric map reconstructed with the CARTO mapping system (Biosense Webster, Inc., Diamond Bar, CA). The point‐by‐point ablation method was used by a CF‐sensing irrigated tip catheter with 2‐5‐2 mm spacing (Thermocool Smart Touch; Biosense Webster) under VisiTag system guidance. RF energy was delivered at a maximum power output of 25‐30 W and a target CF of 10‐20 g with a force‐time integral of >400 gs. The upper temperature limit was set to 43°C at a saline irrigation rate of 17‐30 mL/min (CoolFlow Pump; Biosense Webster). In patients who underwent CBA, the 3D geometry of the left atrium and PVs was reconstructed with an EnSite NavX mapping system (St. Jude Medical) from data obtained with a 20‐pole circular mapping catheter (4‐mm interelectrode spacing; Inquiry AFocus II EB catheter; St. Jude Medical). An Artic Front Advance 28‐mm cyroballoon (CB‐Adv) (Medtronic, Inc., Minneapolis, MN) with an Achieve inner lumen mapping catheter was placed in the left atrium through the steerable 15Fr deflectable sheath (Flexcath, Medtronic, Inc., Minneapolis, MN). The CB‐Adv was then inflated and advanced successively to each PV ostium to establish optimal PV occlusion, determined by the absence of contrast leakage. Cryoenergy was delivered to each PV after occlusion was established. Ablation of each PV antrum was performed with a 240‐s application for left superior PV and a 180‐s application for the remaining PVs. After each CBA procedure, PVI was confirmed with the 20‐pole circular mapping catheter. If residual PV potentials were revealed, an additional cryoenergy was delivered. In all patients, the endpoint of PVI was the elimination or dissociation of all PV potentials and complete entrance block. If the PV remained connected, additional touch‐up ablation lesions were created until PVI was achieved. Linear ablation of the cavotricuspid valve isthmus was performed in patients in whom common AF was documented clinically.
2.4. Echocardiographic evaluation
TTE was performed 1 day before the ablation procedure (baseline examination) and 3, 6, and 12 months after the procedure (follow‐up examinations) with a Vivid 7 cardiovascular ultrasound system (GE Vingmed Ultrasound A.S., Horten, Norway). Standard measurements were obtained: maximum LAV, which was measured by the prolate ellipsoid method21, and left ventricular ejection fraction (LVEF), which was determined by means of M‐mode echocardiography (Teichholz's method). To standardize measures, LAV was indexed to body surface area. EAT was assessed as the space between the epicardial wall of the myocardium and the visceral layer of the pericardium at end systole in both the parasternal long‐axis and short‐axis views (Figure 1). EAT was distinguished from pericardial effusion by its inhomogeneous, whitish‐speckled appearance. The measurement was performed on the free wall of the right ventricle for two reasons: this is the anatomically recognized site of the thickest portion of epicardial fat, and use of both the long and short parasternal axes allows for the most accurate EAT measurement at that site. Intrarobserver and interobserver agreement in the measurement of EAT was tested by calculation of the intraclass correlation coefficient. The mitral annulus was visualized by means of Doppler tissue imaging from the apical 4‐chamber view, and the following indices of left ventricular diastolic and left atrial function were determined: the ratio of the mitral inflow early filling velocity (E) to atrial filling velocity (A) (E/A ratio), the ratio of E to the velocity of the septal early mitral annular ascent (e′), and the velocity of the a′ wave on the lateral left atrial tissue Doppler tracing (lateral a′). The short and long axes of the left atrium were measured in the apical 2‐ and 4‐chamber views. RAR was defined as a reduction in the LAV index of >10% from the baseline value to the 3‐month follow‐up value.22, 23
Figure 1.
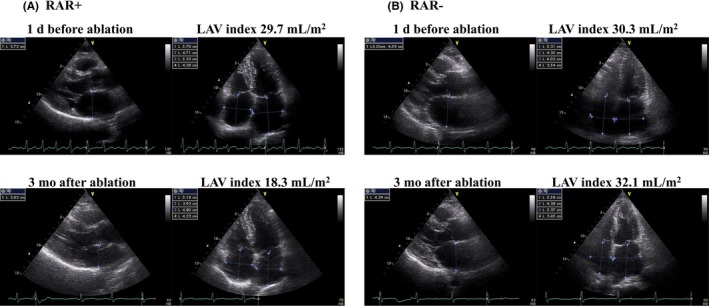
Representative transthoracic echocardiograms showing the presence (A) and the absence (B) of left atrial volume (LAV) reduction after ablation. (A) The LAV index decreased from 29.7 mL/m2 to 18.3 mL/m2 (32% reduction). (B) The LAV index did not decrease after ablation (6% increase). Reverse atrial remodeling was defined as >10% reduction in the LAV index
2.5. Validation of TTE‐based EAT measurement
To prove the reliability of TTE derived EAT thickness, the correlation between the TTE‐based EAT thickness and 3D CT‐based EAT volume was verified. The correlation between the TTE‐based EAT thickness and total EAT volume and peri‐LA EAT volume calculated from the 3D CT was examined in all patients. The EAT volumes were calculated from the noncontrast images obtained with a 3D spiral CT scanner (320‐row detector, dynamic volume CT scanner; Aquilion One, Toshiba Medical Systems, Tokyo, Japan; 0.35‐s gantry rotation time, 120 kV, and 350‐450 mA). On a workstation (Zio M900 Quadra; Amin, Tokyo, Japan), the total EAT was detected by assigning Hounsfield units from −50 to −200 to fat. The details of the measurement have been described in our previous publication.16
2.6. Data collection
Clinical data, laboratory data immediately before the ablation procedure, and echocardiographic variables were obtained 1 day before the ablation procedure, and left atrial pressure was measured immediately after the transseptal puncture during the ablation procedure. Metabolic syndrome was defined according to the following Japanese diagnostic criteria24: visceral obesity (waist circumference ≥85 cm in males and ≥90 cm in females) plus at least two of the following three components: (1) HDL‐C < 40 mg/dL, TG ≥150 mg/dL or the use of medication for dyslipidemia; (2) FPG ≥110 mg/dL or use of medication for diabetes; and (3) blood pressure ≥130/85 mmHg or use of antihypertensive medication.
2.7. Postablation follow‐up
After ablation, all patients were followed‐up regularly at 1, 3, 6 , and every 6 months thereafter at the outpatient clinic. Routine electrocardiography (ECG) was performed at each visit, and 24‐hour Holter monitoring was scheduled to follow the 6‐ and 12‐month follow‐up visits. A blanking period of 3 months was established, and recurrence of AF was defined as any ECG recording of AF or any Holter recording of AF lasting more than 30 seconds.
2.8. Statistical analysis
Continuous variables are expressed as mean ± SD, with the exception of follow‐up time, duration of AF, and biomarkers levels, which are expressed as median values and interquartile ranges. Variables were compared between patients in whom RAR developed and patients in whom RAR did not develop or between patients in whom AF recurred and those in whom it did not recur, and differences in continuous variables were analyzed by 2‐tailed t test or Mann‐Whitney U test. Categorical variables are expressed as percentages, and differences were analyzed by chi‐square test or Fisher's exact test. Within‐group differences in continuous variables measured at different time points were analyzed by paired t test. Factors with a P < 0.1 were entered into a multivariable analysis, but the TTE‐based EAT thickness was used rather than the 3D CT‐based EAT volumes to avoid any multicollinearity. Bland‐Altman analysis was performed to determine bias and limits of agreement between the intraobserver and interobserver measurements. P < 0.05 was considered statistically significant. Statistical analyses were performed with MedCalc Software Version 16.4.1 (Mariakerke, Belgium).
3. RESULTS
3.1. Patient characteristics
Clinical characteristics of the total patients and of those with and without RAR are summarized in Table 1. Seventy‐five (72%) patients were male, and patients’ mean age was 63 ± 10 years. Mean body mass index (BMI) was 23.9 ± 3.7 kg/m2, 55 (53%) patients had high blood pressure, and 21 (20%) patients had diabetes mellitus (DM). The AF was diagnosed as persistent in 46 (44%) of the 104 patients, and the mean CHADS2 score was 0.99 ± 0.91. Among the total 104 patients, the LAV index decreased significantly by 3 months after ablation (from the baseline value of 26.9 ± 9.5 mL/m2 to 23.6 ± 8.37 mL/m2, P < 0.0001). RAR occurred in 57 (55%) of the 104 patients. AF recurred in 23 (22%) of the 104 patients.
Table 1.
Clinical characteristics and laboratory, echocardiographic, and catheterization data for the total patients and per patients in whom reverse atrial remodeling did and did not occur
| Total (n = 104) | RAR (+) (n = 57) | RAR (−) (n = 47) | P value | |
|---|---|---|---|---|
| Clinical data | ||||
| Age, years | 63 ± 10 | 63 ± 10 | 64 ± 10 | 0.6449 |
| Male, n | 75 (72%) | 40 (70%) | 35 (74%) | 0.6287 |
| BMI, kg/m2 | 23.9 ± 3.7 | 23.0 ± 2.7 | 25.0 ± 4.5 | 0.0076 |
| HTN, n | 55 (53%) | 27 (47%) | 28 (60%) | 0.2168 |
| DM, n | 21 (20%) | 9 (16%) | 12 (26%) | 0.2203 |
| Metabolic syndrome, n | 45 (43%) | 16 (28%) | 29 (62%) | 0.0006 |
| Persistent AF, n | 46 (44%) | 26 (46%) | 20 (43%) | 0.7556 |
| CHADS2 score | 0.99 ± 0.91 | 0.84 ± 0.94 | 1.17 ± 0.84 | 0.0665 |
| CHA2DS2‐VASc score | 1.51 ± 1.17 | 1.35 ± 1.23 | 1.70 ± 1.08 | 0.1296 |
| Laboratory data | ||||
| NT‐proBNP, pg/mL | 218 (59–551) | 183 (59–423) | 255 (52–586) | 0.8647 |
| Cr, mg/dL | 0.83 (0.70–0.95) | 0.84 (0.70–0.95) | 0.82 (0.69–0.95) | 0.3794 |
| Hs‐CRP, mg/dL | 0.04 (0.02–0.10) | 0.04 (0.02–0.08) | 0.07 (0.03–0.18) | 0.0547 |
| Insulin, μU/mL | 4.3 (3.1–6.4) | 3.5 (2.6–5.5) | 5.3 (3.8–7.3) | 0.0431 |
| Echocardiographic data | ||||
| LVEF, % | 67.6 ± 8.3 | 66.7 ± 9.0 | 68.7 ± 7.4 | 0.2178 |
| LAD, mm | 39.2 ± 5.7 | 39.4 ± 5.3 | 38.5 ± 6.3 | 0.7587 |
| LAV, mL | 46.3 ± 16.2 | 48.4 ± 17.0 | 43.7 ± 15.0 | 0.1415 |
| LAV index, mL/m2 | 26.9 ± 9.5 | 28.8 ± 10.6 | 24.6 ± 7.5 | 0.0233 |
| Mitral E wave, m/sec | 80.6 (64.1–95.3) | 80.1 (65.1–91.5) | 82.8 (62.7–96.2) | 0.8196 |
| E/e′ ratio | 9.9 (7.9–12.5) | 10.4 (8.0–12.4) | 9.7 (7.7–12.7) | 0.9488 |
| Epicardial adipose tissue, mm | 4.37 ± 1.48 | 3.92 ± 1.17 | 4.92 ± 1.65 | 0.0005 |
| Computed tomography data | ||||
| Total epicardial adipose tissue volume, cm3 | 205 ± 67 | 173 ± 53 | 245 ± 61 | <0.0001 |
| Peri‐LA epicardial adipose tissue volume, cm3 | 61 ± 24 | 51 ± 20 | 74 ± 22 | <0.0001 |
| Ablation data | ||||
| CF‐based ablation/CBA, n | 60(58%) / 44(42%) | 38(67%)/19(33%) | 22(47%) /25(53%) | 0.0423 |
| LAP, mmHg | 8 (6–11) | 8 (6–10) | 8 (7–11) | 0.3447 |
| AF recurrence, n | 23 (22%) | 9 (16%) | 14 (30%) | 0.0885 |
Values are expressed as median (interquartile range) or mean ± SD or number (percentage) of patients.
BMI, body mass index; CBA, cryoballon ablation; CF, contact force; Cr, creatinine; DM, diabetes mellitus; Hs‐CRP, high‐sensitivity C‐reactive protein; HTN, hypertension; LAD, left atrial diameter; LAP, left atrial pressure; LAV, left atrial volume; LVEF, left ventricular ejection fraction; NT‐pro BNP, N‐terminal pro‐brain natriuretic peptide; RAR, reverse atrial remodeling.
3.2. Reproducibility and validation of TTE‐based EAT measurements
Bland‐Altman plots revealed no interobserver or intraobserver difference across the range of measurements (mean bias of 0.19 with 95% limits of agreement of −0.42 to 0.03 mm, and mean bias of 0.16 with 95% limits of agreement of −0.02 to 0.33 mm, respectively).
The EAT volume derived from noncontrast 3D CT images was 205 ± 67 cm3, and the peri‐LA EAT was 61 ± 24 cm3. The TTE‐derived EAT thickness correlated moderately with the total EAT volume (r = 0.6741, P < 0.001) and peri‐LA EAT volume (r = 0.6387, P < 0.001).
3.3. Characteristics of patients with and without RAR and major determinants of RAR
As shown in Table 1, metabolic syndrome was more prevalent among patients without RAR than among those with RAR (62% vs. 28%, respectively; P = 0.0006). The baseline LAV index was significantly lower in patients without RAR than in those with RAR (24.6 ± 7.5 mL/m2 vs. 28.8 ± 10.6 mL/m2, respectively; P = 0.0233). Representative transthoracic echocardiograms from patients with and without RAR are shown in Figure 1A and B. EAT was significantly thicker in patients without RAR than in those with RAR (4.92 ± 1.65 mm vs. 3.92 ± 1.17 mm, respectively; P = 0.0005). Similarly, the 3D CT‐derived total EAT volume and peri‐LA EAT volume were significantly larger in patients without RAR than in those with RAR (total EAT: 245 ± 61 cm3 vs. 173 ± 53 cm3, peri‐LA EAT: 74 ± 22 cm3 vs. 51 ± 20 cm3, respectively; P < 0.0001 for both). In addition, the serum insulin concentration was significantly higher in patients without RAR than in those with RAR (5.3 [3.8‐7.3] μU/mL vs. 3.5 [2.6‐5.5] μU/mL, respectively; P = 0.0431). There was a higher prevalence of patients without RAR in the CBA group than CF‐based ablation group (53% vs. 33%, respectively; P = 0.0423). No between‐group differences were observed in the age, sex, persistent AF, biomarkers, or echocardiographic variables other than the LAV index. After an adjustment for the significant variables, absence of RAR was independently associated with a relatively thick EAT (odds ratio [OR]: 1.48, 95% CI: 1.04–2.11, P = 0.0288), metabolic syndrome (OR: 5.47, 95% CI: 2.01–14.92, P = 0.0009), and relatively low baseline LAV index (OR: 0.93, 95% CI: 0.87–0.99, P = 0.0212) (Table 2).
Table 2.
Results of multivariable analysis for predictors of absence of RAR
| Odds ratio | 95% CI | P value | |||
|---|---|---|---|---|---|
| Metabolic syndrome | 5.47 | 2.01‐14.92 | 0.0009 | ||
| Thick EAT, mm | 1.48 | 1.04‐2.11 | 0.0288 | ||
| LAV index, mL/m2 | 0.93 | 0.87‐0.99 | 0.0212 | ||
| CF‐based ablation (vs. CBA) | 0.55 | 0.22‐1.38 | 0.2044 | ||
CBA, cryoballoon ablation; CF, contact force; CI, confidence interval; EAT, epicardial adipose tissue; LAV index, left atrial volume index; RAR, reverse atrial remodeling.
3.4. Influence of RAR on ablation outcome
During the median follow‐up period of 658 (373‐803) days, the AF recurred in 23 (22%) of the 104 patients. Clinical characteristics and laboratory and echocardiographic variables of patients with and without AF recurrence are summarized in Table 3. EAT was significantly thicker (5.05 ± 2.19 mm vs. 4.17 ± 1.16 mm, respectively; P = 0.0116), EAT volumes significantly larger (total EAT: 249 ± 78 cm3 vs. 192 ± 58 cm3, P = 0.0002; LA, Peri‐LA EAT: 79 ± 26 cm3 vs. 56 ± 20 cm3, P < 0.0001), CHADS2 score significantly lower (0.65 ± 0.71 vs. 1.09 ± 0.94, P = 0.0425), and absence of RAR tended to be more prevalent (61% vs. 41%, respectively; P = 0.0885) in the AF recurrence group than AF nonrecurrence group. No other factors differed between the AF recurrence group and AF nonrecurrence group, including metabolic syndrome (48% vs. 42%, respectively; P = 0.6189) and the baseline LAV index (26.0 ± 7.8 mm/m2 vs. 27.2 ± 10.0 mm/m2, respectively; P = 0.5812).
Table 3.
Clinical characteristics, laboratory, echocardiographic, and catheterization data, and clinical outcomes of the total patients and per patients with and without postablation AF recurrence
| Total (n = 104) | AF nonrecurrence (n = 81) | AF recurrence (n = 23) | P value | |
|---|---|---|---|---|
| Baseline clinical variables | ||||
| Age, years | 63 ± 10 | 63 ± 10 | 62 ± 9 | 0.6934 |
| Male, n | 75 (72%) | 60 (74%) | 15 (65%) | 0.4055 |
| BMI, kg/m2 | 23.9 ± 3.7 | 23.8 ± 3.5 | 24.1 ± 4.4 | 0.7319 |
| HTN, n | 55 (53%) | 46 (57%) | 9 (39%) | 0.1362 |
| DM, n | 21 (20%) | 17 (21%) | 4 (17%) | 0.7059 |
| Metabolic syndrome, n | 45 (43%) | 34 (42%) | 11 (48%) | 0.6189 |
| Persistent AF, n | 46 (44%) | 35 (43%) | 11 (48%) | 0.6954 |
| CHADS2 score | 0.99 ± 0.91 | 1.09 ± 0.94 | 0.65 ± 0.71 | 0.0425 |
| CHA2DS2‐VASc score | 1.51 ± 1.17 | 1.60 ± 1.22 | 1.17 ± 0.94 | 0.1208 |
| Laboratory data | ||||
| NT‐proBNP, pg/mL | 218 (59‐551) | 228 (59‐581) | 158 (60‐280) | 0.5392 |
| Cr, mg/dL | 0.83 (0.70‐0.95) | 0.83 (0.69‐0.95) | 0.83 (0.73‐0.95) | 0.7986 |
| hs‐CRP, mg/dL | 0.04 (0.02‐0.10) | 0.04 (0.02‐0.10) | 0.04 (0.02‐0.15) | 0.9507 |
| Insulin, μU/mL | 4.3 (3.1‐6.4) | 4.4 (3.3‐7.7) | 2.8 (2.8‐6.0) | 0.1786 |
| Echocardiographic data | ||||
| LVEF, % | 67.6 ± 8.3 | 67.4 ± 8.6 | 68.3 ± 7.5 | 0.6423 |
| LAD, mm | 39.2 ± 5.7 | 39.5 ± 5.9 | 38.4 ± 5.0 | 0.4007 |
| LAV, mL | 46.3 ± 16.2 | 46.3 ± 16.2 | 46.4 ± 16.5 | 0.9866 |
| LAV index, mL/m2 | 26.9 ± 9.5 | 27.2 ± 10.0 | 26.0 ± 7.8 | 0.5812 |
| Mitral E wave, m/sec | 80.6 (64.1‐95.3) | 80.8 (64.4‐96.2) | 80.5 (63.3‐91.2) | 0.9193 |
| E/e′ ratio | 9.9 (7.9‐12.5) | 10.0 (8.2‐12.4) | 9.4 (7.3‐12.3) | 0.4547 |
| Epicardial adipose tissue, mm | 4.37 ± 1.48 | 4.17 ± 1.16 | 5.05 ± 2.19 | 0.0116 |
| Absence of RAR | 47 (45%) | 33 (41%) | 14 (61%) | 0.0885 |
| Computed tomography data | ||||
| Total epicardial adipose tissue volume, cm3 | 205 ± 67 | 192 ± 58 | 249 ± 78 | 0.0002 |
| Peri‐LA epicardial adipose tissue volume, cm3 | 61 ± 24 | 56 ± 20 | 79 ± 26 | <0.0001 |
| Ablation data | ||||
| CF‐based ablation / CBA, n | 60 (58%) /44 (42%) | 45 (56%)/36 (44%) | 15 (65%)/8 (35%) | 0.4101 |
| LAP, mmHg | 8 (6‐11) | 9 (6‐11) | 8 (5‐10) | 0.3886 |
Values are expressed as median (interquartile range), mean ± SD, or number (percentage) of patients.
BMI, body mass index; CBA, cryoballon ablation; CF, contact force; Cr, creatinine; DM, diabetes mellitus; Hs‐CRP, high‐sensitivity C‐reactive protein; HTN, hypertension; LAD, left atrial diameter; LAP, left atrial pressure; LAV, left atrial volume; LVEF, left ventricular ejection fraction; NT‐pro BNP, N‐terminal pro‐brain natriuretic peptide; RAR, reverse atrial remodeling.
After the 12‐month follow‐up period, EAT thickness had decreased significantly from 4.17 ± 1.16 mm to 3.65 ± 1.16 mm in the AF nonrecurrence group (P < 0.0001), but there was not a significant change in thickness in the AF recurrence group (from 5.05 ± 2.19 mm to 4.73 ± 2.16 mm, P = 0.1892) (Figures 2 and 3). There was not a significant change in body weight during the follow‐up period in either group (AF nonrecurrence group: from 65.8 ± 12.4 kg to 65.7 ± 12.5 kg, P = 0.6223; AF recurrence group: from 67.9 ± 14.0 kg to 68.0 ± 14.0 kg, P = 0.8569). No significant correlation was observed between the reduction in the LAV index and reduction in the EAT thickness (r = −0.1778, P = 0.0710).
Figure 2.
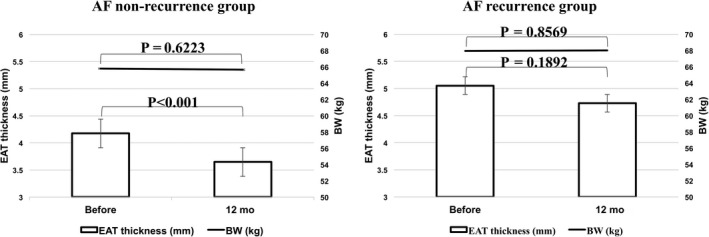
Changes in epicardial adipose tissue and body weight after ablation in the AF nonrecurrence group and AF recurrence group. AF, atrial fibrillation
Figure 3.
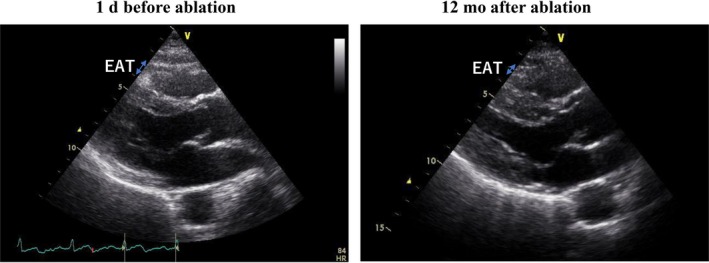
Representative transthoracic echocardiograms showing a reduction in EAT thickness 12 months after ablation. In this patient, EAT thickness decreased significantly from 5.1 mm before ablation to 2.3 mm after ablation. EAT, epicardial adipose tissue
4. DISCUSSION
This study yielded three major findings: (1) independent association between metabolic syndrome and thick EAT and absence of RAR after ablation for AF, (2) significant association between postablation AF recurrence and thick EAT, and (3) association between AF ablation success and a significant reduction in the EAT thickness 12 months after ablation.
4.1. Mechanism of RAR after AF ablation
As noted above, we found the LAV index to be decreased 3 months after ablation, and RAR was observed in 55% of the total patients. It is well known that AF ablation effectively reverses left atrial remodeling. Reported studies have shown age, hypertension, persistent AF, and a long P wave duration to be major hindrances to decreased LAV after ablation.25, 26 Our study results added to the findings of the previous studies regarding mechanistic insight into the absence of RAR after ablation. Among our study patients, metabolic syndrome and thick EAT were independently associated with the absence of RAR after AF ablation. Metabolic syndrome and EAT have been linked to the presence and progression of AF.16, 27 Increased EAT may be a manifestation of metabolic syndrome, as are obesity, hypertension, high blood sugar, and dyslipidemia, four factors that are strongly associated with left atrial remodeling.4, 5, 6, 7, 8, 9, 10, 28 Further, an increased EAT itself may lead directly to atrial remodeling by the effects of bioactive free fatty acid and a number of other bioactive molecules, such as adiponectin, resistin, and inflammatory cytokines, and by the effect of ganglionated plexi (GP).14, 15, 16, 17 These multiple mechanisms underlying EAT‐promoted left atrial remodeling modestly persist even after the ablation.22, 27 We speculated that substrates responsible for AF in patients with metabolic syndrome and EAT‐based vulnerability to AF may ultimately surpass the favorable effects of ablation, attenuating the effect of RAR.
4.2. Influence of EAT on ablation outcome
That maintenance of sinus rhythm after AF ablation led to a reduction in the EAT thickness in our patients, especially in those in whom AF did not recur, was a novel finding. Reduction in EAT has been reported to accompany weight loss29, and studies have shown that liraglutide (an analog of glucagon‐like peptide‐1), sitagliptin (dipeptidyl peptidase‐4 inhibitor), and dapagliflozin (sodium‐glucose co‐transporter‐2) reduce EAT in patients with type 2 DM.30, 31, 32 In our study patients, EAT decreased after ablation despite absence of weight loss. Therefore, our results support the notion that EAT is derived from AF, although previous reports have suggested EAT as the cause of AF.14, 15, 16, 17 There are several suggested explanations for the reduction in EAT that follows AF ablation. EAT is considered to release free fatty acids (FFAs) as energy to the myocardium in a state of a high metabolic demand.33 Animal studies have shown that the atrial oxygen consumption and coronary flow increase almost threefold after the induction of AF and lead to contractile dysfunction.34, 35 Therefore, it is possible that the greater fat depots around the LA in AF patients would be accompanied by a decreased reservoir function through augmented LA kinetic energy and the ejection force. In fact, it has been suggested that regional adipose tissues can be beneficial by preserving myocardial energy supply under certain circumstances, yet simultaneously impose certain negative effects generated by the inflammatory process.36 Conversely, the maintenance of sinus rhythm after ablation improves the atrial contractile function, and reduces the atrial oxygen consumption and coronary flow. These myocardial conditions would decrease the LA myocardium's energy supply and negative effects of the inflammatory process. In fact, we reported postablation decreases in the levels of inflammatory and fibrotic markers such as hs‐CRP, IL‐6, and matrix metalloproteinase‐2.37 In addition, endocardial ablation of EAT appears to abolish GP activity and improve ablation outcomes.27 The maintenance of sinus rhythm by ablation may decrease the myocardium's energy supply, local levels of these biomarkers, and/or GP activity, in turn attenuating the EAT activity and thus reducing the EAT thickness. Another possibility is simply a change in lifestyle among patients in whom ablation is successful. If, after ablation, patients improve their lifestyle by reducing alcohol consumption, quitting smoking, exercising, and eating regular meals, a reduction in the EAT, not represented by body weight, might occur, resulting in the maintenance of sinus rhythm. If a change in lifestyle is an important contributor to postablation maintenance of sinus rhythm, management of metabolic syndrome to reduce EAT may be crucial for preventing recurrences of AF.
4.3. Metabolic syndrome/obesity and ablation outcome
We found a strong association between metabolic syndrome and the absence of RAR after ablation, but we found no association between metabolic syndrome and postablation AF recurrence. Whether being overweight and obesity can lead to a poor outcome after AF ablation remains controversial. Reported studies showed association between body weight >200 lbs or BMI ≥25 kg/m2 and AF recurrence beyond 12 months.38, 39 In contrast, other reported studies showed BMI not to be predictive of AF recurrence after the blanking period.40, 41 One of the reasons for the contradictory results may be the difference between the studies in the proportion of overweight or obese patients. In the former two studies, the mean BMI was high and being overweight and obesity were quite prevalent, whereas in the latter two studies, the mean BMI was low, and the prevalence of being overweight and obesity was also low. The BMI in our study patients was relatively low (median: 23.9 kg/m2), which may have obscured the association between metabolic syndrome and a poor ablation outcome. Another factor at play in our study was the furtherance of the ablation technology. Ablation modalities such as CF‐based radiofrequency ablation and balloon‐based ablation have improved the ablation efficacy. These technologies may minimize the effect of metabolic syndrome in leading to an AF recurrence. Nevertheless, our results indicated that the EAT was significantly thicker, and absence of RAR tended to be more prevalent in patients with postablation AF recurrences than in those without. Therefore, EAT or the absence of RAR can be a better reflection of vulnerability predisposing to AF recurrence than metabolic syndrome/obesity. Metabolic syndrome was recently shown to be an independent predictor of a delayed postablation recurrence of AF, ie, recurrence 2 years after ablation.42 Taken together, a study that includes a very large patient group and long follow‐up period may be needed to clarify this issue.
4.4. Limitations
Our findings should be interpreted in light of our study limitations. The study was limited in size to 104 patients. Despite a relatively small number of patients, a thick EAT was strongly associated with the short‐term effect (absence of RAR) and long‐term outcome after ablation, but metabolic syndrome was only related to the short‐term effect. These findings implicate a better predictive role of EAT on AF vulnerability than that of metabolic syndrome. In addition, EAT was not assessed by 3D CT images at 12 months after the procedure because of no patient benefit when considering the radiation exposure from the CT scan. Nonetheless, the EAT thickness measured echocardiographically has been reported to be significantly correlated with the EAT volume measured by multislice CT,18, 19 and we could also find a significant correlation of the EAT thickness with the total/peri‐LA EAT volume before ablation.
5. CONCLUSIONS
In patients who underwent AF ablation, an increased EAT and presence of metabolic syndrome were independently associated with the absence of RAR. The postablation AF recurrence was significantly associated with an increased EAT, but not with metabolic syndrome. The EAT thickness decreased significantly after ablation, especially in patients for whom ablation was successful. Our data suggest that EAT may confer vulnerability to AF even after AF ablation, but that an EAT reduction has a favorable effect on the ablation outcome.
CONFLICT OF INTEREST
The authors declare no conflict of interests for this article.
ACKNOWLEDGEMENTS
The authors also thank Ms. Wendy Alexander‐Adams and Mr. John Martin for encouragement and assistance with the reporting of our findings in English.
Monno K, Okumura Y, Saito Y, et al. Effect of epicardial fat and metabolic syndrome on reverse atrial remodeling after ablation for atrial fibrillation. J Arrhythmia. 2018;34:607–616. 10.1002/joa3.12124
Funding information
This research was partially supported by Grants‐in‐Aid for Scientific Research.
References
- 1. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 2. Abhayaratna WP, Seward JB, Appleton CP, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. [DOI] [PubMed] [Google Scholar]
- 3. Kuppahally SS, Akoum N, Badger TJ, et al. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J. 2010;160:877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–495. [DOI] [PubMed] [Google Scholar]
- 5. Rosengren A, Hauptman PJ, Lappas G, Olsson L, Wilhelmsen L, Swedberg K. Big men and atrial fibrillation: effects of body size and weight gain on risk of atrial fibrillation in men. Eur Heart J. 2009;30:1113–1120. [DOI] [PubMed] [Google Scholar]
- 6. Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity–results of a meta‐analysis. Am Heart J. 2008;155:310–315. [DOI] [PubMed] [Google Scholar]
- 7. Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah AS, Habib RH. Obesity and risk of new‐onset atrial fibrillation after cardiac surgery. Circulation. 2005;112:3247–3255. [DOI] [PubMed] [Google Scholar]
- 8. Echahidi N, Mohty D, Pibarot P, et al. Obesity and metabolic syndrome are independent risk factors for atrial fibrillation after coronary artery bypass graft surgery. Circulation. 2007;116:I213–I219. [DOI] [PubMed] [Google Scholar]
- 9. Tedrow UB, Conen D, Ridker PM, et al. The long‐ and short‐term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women's health study). J Am Coll Cardiol. 2010;55:2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 11. Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity‐related health risk factors, 2001. JAMA. 2003;289:76–79. [DOI] [PubMed] [Google Scholar]
- 12. Hubert HB, Feinleib M, Mcnamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular‐disease ‐ a 26‐year follow‐up of participants in the Framingham heart‐study. Circulation. 1983;67:968–977. [DOI] [PubMed] [Google Scholar]
- 13. Morricone L, Malavazos AE, Coman C, Donati C, Hassan T, Caviezel F. Echocardiographic abnormalities in normotensive obese patients: relationship with visceral fat. Obes Res. 2002;10:489–498. [DOI] [PubMed] [Google Scholar]
- 14. Yorgun H, Canpolat U, Hazirolan T, et al. Increased epicardial fat tissue is a marker of metabolic syndrome in adult patients. Int J Cardiol. 2013;165:308–313. [DOI] [PubMed] [Google Scholar]
- 15. Thanassoulis G, Massaro JM, O'Donnell CJ, et al. Pericardial fat is associated with prevalent atrial fibrillation the framingham heart study. Circ‐Arrhythm Electrophysiol. 2010;3:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagashima K, Okumura Y, Watanabe I, et al. Association between epicardial adipose tissue volumes on 3‐dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablation. Circ J. 2011;75:2559–2565. [DOI] [PubMed] [Google Scholar]
- 17. Liang KW, Tsai IC, Lee WJ, et al. MRI measured epicardial adipose tissue thickness at the right AV Groove differentiates inflammatory status in obese men with metabolic syndrome (vol 20, pg 525, 2012). Obesity. 2012;20:1128. [DOI] [PubMed] [Google Scholar]
- 18. Chao TF, Hung CL, Tsao HM, et al. Epicardial adipose tissue thickness and ablation outcome of atrial fibrillation. PLoS ONE. 2013;8:e74926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lai YH, Yun CH, Yang FS, et al. Epicardial adipose tissue relating to anthropometrics, metabolic derangements and fatty liver disease independently contributes to serum high‐sensitivity C‐reactive protein beyond body fat composition: a study validated with computed tomography. J Am Soc Echocardiogr. 2012;25:234–241. [DOI] [PubMed] [Google Scholar]
- 20. Okumura Y, Watanabe I, Nakai T, et al. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: importance of matrix metalloproteinase‐2 as a predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2011;22:987–993. [DOI] [PubMed] [Google Scholar]
- 21. Ujino K, Barnes ME, Cha SS, et al. Two‐dimensional echocardiographic methods for assessment of left atrial volume. Am J Cardiol. 2006;98:1185–1188. [DOI] [PubMed] [Google Scholar]
- 22. Arana‐Rueda E, Pedrote A, Garcia‐Riesco L, et al. Reverse atrial remodeling following pulmonary vein isolation: the importance of the body mass index. Pacing Clin Electrophysiol. 2015;38:216–224. [DOI] [PubMed] [Google Scholar]
- 23. Kloosterman M, Rienstra M, Mulder BA, Van Gelder IC, Maass AH. Atrial reverse remodelling is associated with outcome of cardiac resynchronization therapy. Europace. 2016;18:1211–1219. [DOI] [PubMed] [Google Scholar]
- 24. Matsuzawa Y. Metabolic syndrome ‐ definition and diagnostic criteria in Japan. J Atheroscler Thromb. 2005;12:301. [DOI] [PubMed] [Google Scholar]
- 25. Yalcin MU, Gurses KM, Kocyigit D, et al. Predictors of left atrial volume index reduction following cryoballoon‐based pulmonary vein isolation. Europace. 2016;18:392–397. [DOI] [PubMed] [Google Scholar]
- 26. Okumura Y, Watanabe I, Ohkubo K, et al. Prediction of the efficacy of pulmonary vein isolation for the treatment of atrial fibrillation by the signal‐averaged P‐wave duration. Pacing Clin Electrophysiol. 2007;30:304–313. [DOI] [PubMed] [Google Scholar]
- 27. Takahashi K, Okumura Y, Watanabe I, et al. Anatomical proximity between ganglionated plexi and epicardial adipose tissue in the left atrium: implication for 3D reconstructed epicardial adipose tissue‐based ablation. J Interv Card Electrophysiol. 2016;47:203–212. [DOI] [PubMed] [Google Scholar]
- 28. Watanabe H, Tanabe N, Watanabe T, et al. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fu CP, Sheu WH, Lee IT, et al. Effects of weight loss on epicardial adipose tissue thickness and its relationship between serum soluble CD40 ligand levels in obese men. Clin Chim Acta. 2013;421:98–103. [DOI] [PubMed] [Google Scholar]
- 30. Iacobellis G, Mohseni M, Bianco SD, Banga PK. Liraglutide causes large and rapid epicardial fat reduction. Obesity (Silver Spring). 2017;25:311–316. [DOI] [PubMed] [Google Scholar]
- 31. Lima‐Martinez MM, Paoli M, Rodney M, et al. Effect of sitagliptin on epicardial fat thickness in subjects with type 2 diabetes and obesity: a pilot study. Endocrine. 2016;51:448–455. [DOI] [PubMed] [Google Scholar]
- 32. Sato T, Aizawa Y, Yuasa S, et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536. [DOI] [PubMed] [Google Scholar]
- 34. White CW, Holida MD, Marcus ML. Effects of acute atrial fibrillation on the vasodilator reserve of the canine atrium. Cardiovasc Res. 1986;20:683–689. [DOI] [PubMed] [Google Scholar]
- 35. White CW, Kerber RE, Weiss HR, Marcus ML. The effects of atrial fibrillation on atrial pressure‐volume and flow relationships. Circ Res. 1982;51:205–215. [DOI] [PubMed] [Google Scholar]
- 36. Lai YH, Yun CH, Su CH, et al. Excessive interatrial adiposity is associated with left atrial remodeling, augmented contractile performance in asymptomatic population. Echo Res Pract. 2016;3:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sasaki N, Okumura Y, Watanabe I, et al. Increased levels of inflammatory and extracellular matrix turnover biomarkers persist despite reverse atrial structural remodeling during the first year after atrial fibrillation ablation. J Interv Card Electrophysiol. 2014;39:241–249. [DOI] [PubMed] [Google Scholar]
- 38. Patel D, Mohanty P, Di Biase L, et al. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm. 2010;7:167–172. [DOI] [PubMed] [Google Scholar]
- 39. Cai L, Yin Y, Ling Z, et al. Predictors of late recurrence of atrial fibrillation after catheter ablation. Int J Cardiol. 2013;164:82–87. [DOI] [PubMed] [Google Scholar]
- 40. Richter B, Gwechenberger M, Filzmoser P, Marx M, Lercher P, Gossinger HD. Is inducibility of atrial fibrillation after radio frequency ablation really a relevant prognostic factor? Eur Heart J. 2006;27:2553–2559. [DOI] [PubMed] [Google Scholar]
- 41. Miyazaki S, Kuwahara T, Kobori A, et al. Preprocedural predictors of atrial fibrillation recurrence following pulmonary vein antrum isolation in patients with paroxysmal atrial fibrillation: long‐term follow‐up results. J Cardiovasc Electrophysiol. 2011;22:621–625. [DOI] [PubMed] [Google Scholar]
- 42. Baek YS, Yang PS, Kim TH, et al. Delayed recurrence of atrial fibrillation 2 years after catheter ablation is associated with metabolic syndrome. Int J Cardiol. 2016;223:276–281. [DOI] [PubMed] [Google Scholar]


