Abstract
Background
Despite the long experience of cardiac implantable electronic device (CIED) implantation in Thailand, epidemiology of CIED infection in Thailand has never been studied.
Methods
A retrospective cohort study was conducted at the cardiac referral center in Thailand to investigate incidence of CIED infection and causative organisms between October 2002 and December 2017. A matched case‐control study was performed to determine the factors associated with CIED infection.
Results
Incidence of CIED infection was 0.9% with a stable trend during the studied period. There were 54 episodes of CIED infection. The median (interquartile range) age of the patients was 67.5 (53.0‐75.0) years. A total of 29 (53.7%), 18 (33.3%), and 7 (13.0%) were permanent pacemaker, automatic implantable cardioverter‐defibrillator, and cardio‐resynchronization therapy‐related infection, respectively. Gram‐positive cocci were the most common organism (24 episodes, 44.4%). Gram‐negative bacilli were isolated in six episodes (11.1%). About 9.3% were polymicrobial and 35.2% were culture negative. Multivariate analysis showed that previous CIED infection and generator revision procedure were associated with CIED infection (odds ratio [OR] 48.56, 95% confidence interval [CI] 3.72‐633.62; P = 0.003 and OR 19.99, 95% CI 1.28‐333.24; P = 0.033 respectively). Forty (74.1%) cases were cured. Leaving device in situ was the only factor significantly associated with poor outcome (OR 11.40, 95% CI 1.52‐85.73; P = 0.018).
Conclusions
In Thailand, while CIED implantation is rising, incidence of CIED infection is stable. Microbiology of CIED infection in Thailand is similar to western countries, albeit a higher proportion of negative culture. Previous CIED infection and generator revision procedure are associated with CIED infection.
Keywords: cardiac implantable electronic device infection, microbiology, risk factors, Thailand, treatment outcome
1. INTRODUCTION
Cardiac implantable electronic devices (CIEDs), that is, pacemakers (PPMs), implantable cardioverter‐defibrillators (ICDs) and cardiac resynchronization therapy with and without defibrillator (CRT‐D/CRT‐P), are indicated for a growing number of patients with tachyarrhythmia, bradyarrhythmia or severely reduced ejection fraction, can become infected.1 CIED infection is associated with a marked increase in mortality and in‐hospital financial charges.2 The true incidence of CIED infection is difficult to determine due to lack of a comprehensive registry or mandatory reporting. In a review of 22 studies that included at least 1,000 patients from North America, European countries, and Australia, the rate of infections ranged from 0.5% to 2.2%.3 Annual rate of CIED infection in the United States markedly increased since 2004.2 This coincided with increased implantation in older patients with comorbidities. An increased rate of CIED infection in the United Kingdom was also reported.4
Previous studies showed risk factors associated with CIED infection included early CIED re‐intervention,5, 6 postoperative hematoma,7, 8, 9 CIED replacement,9, 10, 11 more complex CIED,6, 12, 13 use of temporary pacemaker prior to implantation,5, 9 a large number of prior procedures,5 prior CIED infection,14, 15 lack of proper preoperative antibiotics,14 longer procedure time,6, 14 fever or systemic infection,5, 14 chronic kidney disease,6, 14, 16, 17 hemodialysis,6 chronic skin disease,14 corticosteroid treatment,9 chronic obstructive pulmonary disease,9, 14, 15 diabetes,9, 14 malignancy,14 male gender,10, 16 and younger age.10, 12
In western countries, gram‐positive cocci are major types of CIED infection (from 67.5% of patients to 92.5% of isolates across ten studies).3 The most commonly isolated microorganisms included coagulase‐negative Staphylococci, and Staphylococcus aureus. Gram‐negative bacilli were isolated in 1%‐17% of episodes,3 and polymicrobial infection was reported with a range from 2% to 24.5%.3 Fungal and Mycobacterium spp. infection were very rare.3, 18
In Asian countries, gram‐negative bacilli have surpassed gram‐positive cocci in many types of infections including infections in patients with foreign materials. Gram‐negative bacilli accounted for 36%‐60% of causative pathogens of central venous catheter‐related blood stream infections in a tertiary hospital in India,19, 20 whereas gram‐negative bacilli accounted for only 19%‐21% of these infections in the western countries.21, 22 In prosthetic joint infection, gram‐negative organisms accounted for 20% in Taiwan23 and 6%‐23% in India,24 whereas only 6%‐11% in North America.25 However, data on microbiology of CIED infection in Asian countries are limited. Studies from Japan and China showed that Staphylococcus remained the predominant pathogen associated with CIED infection.26, 27, 28
In Thailand, CIED implantation has been increasingly performed. Nevertheless, incidence of CIED infection has never been studied. Microbiological data of CIED infection in Thailand as well as Southeast Asia are scarce and might be different from western countries. Study of epidemiology and microbiology of CIED infection in Thailand would provide beneficial information that could result in the proper antibiotic use for prophylaxis and empirical treatment of CIED infection in Southeast Asia countries.
2. METHODS
2.1. Patient population and case definitions
Patients with evidence of CIED infection that presented at or were referred to Ramathibodi hospital, a 1300‐bed university hospital and a cardiac referral center in Bangkok, Thailand from October 2002 to January 2018 were included. A case‐control study was performed to identify risk factors associated with CIED infection. The control was the patient who received CIED implantation but had no evidence or history of CIED infection. Two controls were identified for each case and were matched for age and sex. CIED infection was defined as the presence of generator pocket infections, CIED‐related lead infection (CIED‐LI) or infectious endocarditis (CIED‐IE), as previously described.3 A case of generator pocket infection was defined as erythema affecting the box implantation incision site, or incision site purulent exudate, or wound dehiscence, or erosion through skin with exposure of the generator or leads or fluctuation (abscess) or fistula formation and no systemic symptoms and negative blood culture. CIED‐LI was defined as symptoms or signs of systemic infection, echocardiography consistent with vegetation(s) attached to leads and the presence of major Duke microbiological criteria.29 On the other hand, if echocardiography showed valve involvement, CIED‐IE was defined. Most patients with device‐related infections were identified at the time of follow‐up at the outpatient department (OPD) and using a hospital‐based electronic database where all staff physicians, trainees, and nurses involved with pacemaker and ICD implantation reported any cases of suspected or confirmed device‐related infection. Early CIED infection was defined as an infection less than 6 months after last intervention.3 Study protocol was reviewed and approved by the Ethical Clearance Committee on Human Right Related to Research Involving Human Subjects of the Faculty of Medicine Ramathibodi Hospital, Mahidol University.
2.2. Specimen collection and culture
Pocket tissue and tips of medical device were collected during device extraction. Each specimen was obtained using an aseptic technique. Pocket tissue samples and tips of medical device were placed in sterile bottles and transported to the clinical microbiology laboratory. Samples from pocket tissues were inoculated onto solid media (blood agar, MacConkey agar) and thioglycolate broth. Samples from medical devices were inoculated onto blood agar. In some patients with CIED infection whose device was retained, the sample was collected from wound swab culture using STUART transport medium tube and then inoculated onto solid media (blood agar, MacConkey agar). Bacterial identification and antimicrobial susceptibility were performed using the biochemical testing30 and matrix assisted laser desorption ionization‐time of flight mass spectrometry (MALDI‐TOF MS). Minimum inhibitory concentrations (MICs) were determined according to Clinical and Laboratory Standards Institute (CLSI) breakpoints.31
2.3. Statistical analysis
Descriptive statistics were presented as a number (percent) for categorical variables and median (interquartile range; IQR) for continuous variables. Chi‐squared or Fisher's exact test were used for categorical variables. Mann‐Whitney U‐tests were used to compare continuous variables. Kruskal‐Wallis test was used to compare time to infection among different cardiac devices. A 1:2 matched case‐controlled study and logistic regression were used to determine the risk factors associated with CIED infection. Variables that presented a P < 0.2 from univariate logistic regression were considered in a multivariate logistic regression model. Odds ratio (OR) and its 95% confidence interval (CI) were estimated. A P < 0.05 was considered statistically significant. All statistical analyses were performed using the Stata statistical software version 12.0.
3. RESULTS
3.1. Incidence
The rate of CIED implantation at Ramathibodi Hospital was gradually increased from 84 to 328 implantations per year. Between 2003 and 2017, 2144 primary implantations of PPM, 651 automatic implantable cardioverter‐defibrillators (AICD), 1 subcutaneous implantable cardioverter‐defibrillator (S‐ICD), and 339 CRT‐P/D were recorded. The majority of this increase was due to a large increase in annual PPM (DDD) implantation (from 32 to 147 implantations per year; 459.4%) and the annual AICD implantation increased by 407.1% (from 14 to 57 implantations per year) during this period (Figure 1). During the study period, 28 cases of CIED infection following implantation in Ramathibodi Hospital were identified (0.9% incidence). The annual rate of infection was stable (< 2.4%) (Figure 2). Consideration as to the type of devices, incidence of PPM, AICD, and CRT infection were 0.6%, 1.3%, and 1.7%, respectively.
Figure 1.
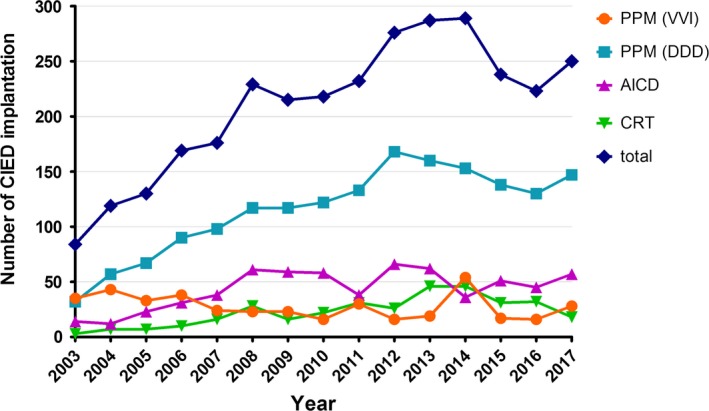
Annual number of CIED implantation in Ramathibodi Hospital
Figure 2.
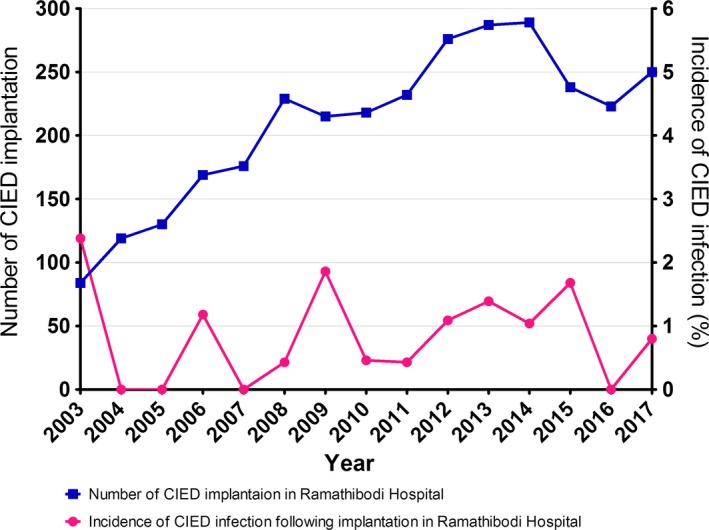
Incidence of CIED infection comparing to annual number of CIED implantation
3.2. Case characteristics
A total of 54 episodes of CIED infection were included in this study (28 and 26 episodes of infection following CIED implantation in Ramathibodi Hospital and outside hospitals, respectively). Of all CIED infection, 44 (81.5%) patients had generator pocket infections, 4 (7.4%) patients had CIED‐LI, and 6 (11.1%) patients had CIED‐IE. 108 matched patients were included in this evaluation. Demographic data of patients with and without CIED infections are shown in Table 1. Overall, the median (IQR) age of the CIED infected patients was 67.5 (53.0‐75.0) years and 75.9% were male. There was no statistically significant difference between the two groups in age, sex, body mass index (BMI), underlying diseases, anticoagulant use and indications for the implantation. The CIED infection group consisted of 53.7% pacemaker patients, 33.3% AICD patients, and 13.0% CRT‐P/D patients. The control group consisted of 33.4% pacemaker patients, 52.8% AICD patients, and 13.0% CRT‐P/D patients (Table 2). The type of CIED in the two groups was significantly different; the number of PPM (VVI) implantation was higher in the CIED infection group, whereas the number of AICD implantations was higher in control group. The median (IQR) time to infection of each device was 1.99 (0.27‐5.75), 0.82 (0.33‐4.55), 0.30 (0.21‐0.85) years in PPM, AICD, and CRT, respectively. We found no statistical significance among time to infection of the devices (P = 0.118).
Table 1.
Patients’ characteristics
| Infection (n = 54) | No infection (n = 108) | P‐value | |
|---|---|---|---|
| Demographics | |||
| Median age, years (IQR) | 67.5 (53.0‐75.0) | 61.0 (46.0‐71.5) | 0.094 |
| Male | 41 (75.9%) | 80 (74.1%) | 0.798 |
| Body mass index, kg/m2 (IQR) | 23.1 (20.9‐24.5) | 23.5 (20.7‐26.5) | 0.148 |
| LVEF, % (IQR) | 55 (26‐69) | 41 (30‐60) | 0.365 |
| Pacemaker dependent | 22 (62.9%) | 36 (39.1%) | 0.016 |
| Indication for implantation | |||
| CAD with poor LVEF | 8 (14.8%) | 19 (17.6%) | 0.655 |
| Dilated cardiomyopathy | 8 (14.8%) | 15 (13.9%) | 0.874 |
| Long QT syndrome | 1 (1.85%) | 1 (0.93%) | >0.999 |
| Hypertrophic cardiomyopathy | 0 (0.0%) | 5 (4.6%) | 0.170 |
| Brugada syndrome | 7 (13.0%) | 21 (19.4%) | 0.304 |
| ARVD | 0 (0.0%) | 2 (1.9%) | 0.553 |
| Sick sinus syndrome | 15 (27.8%) | 21 (19.4%) | 0.229 |
| Atrioventricular block | 17 (31.5%) | 21 (19.4%) | 0.088 |
| Syncope | 0 (0.0%) | 1 (0.9%) | >0.999 |
| Others | 2 (3.7%) | 6 (5.6%) | 0.720 |
| History of CIED infection | 17 (40.5%) | 2 (1.9%) | <0.001 |
| Underlying diseases | |||
| Diabetes mellitus | 16 (29.6%) | 26 (24.1%) | 0.447 |
| Hypertension | 29 (53.7%) | 48 (44.4%) | 0.266 |
| Dyslipidemia | 22 (40.7%) | 31 (28.7%) | 0.124 |
| Old CVA | 6 (11.1%) | 9 (8.3%) | 0.565 |
| COPD | 4 (7.4%) | 6 (5.6%) | 0.732 |
| Cirrhosis | 1 (1.9%) | 2 (1.9%) | >0.999 |
| Malignancy | 1 (1.9%) | 1 (0.9%) | >0.999 |
| Chronic kidney disease | 4 (7.4%) | 10 (9.3%) | 0.776 |
| ESRD with HD | 1 (2.3%) | 6 (5.6%) | 0.426 |
| ESRD with PD | 0 (0.0%) | 1 (0.9%) | >0.999 |
| Autoimmune diseases | 4 (7.4%) | 2 (1.9%) | 0.096 |
| CAD | 16 (29.6%) | 34 (31.5%) | 0.810 |
| Prosthetic valve | 1 (1.9%) | 2 (1.9%) | >0.999 |
| Congestive heart failure | 5 (9.3%) | 13 (12.0%) | 0.596 |
| HIV infection | 0 (0.0%) | 1 (0.9%) | >0.999 |
| Medical conditions | |||
| Central line | 0 (0.0%) | 1 (0.9%) | >0.999 |
| Steroid use | 2 (3.7%) | 0 (0.0%) | 0.110 |
| Anticoagulants | |||
| NOAC | 2 (1.9%) | 2 (1.9%) | 0.112 |
| VKA | 6 (11.1%) | 12 (11.1%) | |
| Enoxaparin | 3 (5.6%) | 0 (0.0%) | |
ARVD, arrhythmogenic ventricular dysplasia; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; ESRD, end‐stage renal disease; HD, hemodialysis; HIV, human immunodeficiency virus; IQR, interquartile range; LVEF, left ventricular ejection fraction; NOAC, novel oral anticoagulant; PD, peritoneal dialysis; VKA, vitamin K antagonist.
Table 2.
Device features and implantation characteristics
| Infection (n = 54) | No infection (n = 108) | P‐value | |
|---|---|---|---|
| Cardiac device | |||
| PPM (DDD) | 15 (27.8%) | 33 (30.6%) | <0.001 |
| PPM (VVI) | 14 (25.9%) | 3 (2.8%) | |
| AICD | 18 (33.3%) | 57 (52.8%) | |
| S‐ICD | 0 (0.0%) | 1 (0.9%) | |
| CRT‐P | 5 (9.3%) | 2 (1.9%) | |
| CRT‐D | 2 (3.7%) | 12 (11.1%) | |
| Number of lead | |||
| 1 | 30 (56.6%) | 57 (52.8%) | 0.871 |
| 2 | 16 (30.2%) | 37 (34.3%) | |
| 3 | 7 (13.2%) | 14 (13.0%) | |
| Type of the last procedure | |||
| Primary implantation | 28 (56.0%) | 60 (56.1%) | 0.030 |
| Generator change | 8 (16.0%) | 27 (25.2%) | |
| Lead revision | 1 (2.0%) | 2 (1.9%) | |
| Generator revision | 5 (10.0%) | 1 (0.9%) | |
| Device upgrade | 0 (0.0%) | 6 (5.6%) | |
| Re‐implantation | 8 (16.0%) | 11 (10.3%) | |
| Duration of operation, hours | 119.5 (87.0‐160.0) | 90.0 (70.0‐128.0) | 0.017 |
| Perioperative antibiotics | |||
| Cefazolin + cephalexin | 23 (82.1%) | 99 (92.5%) | 0.152 |
| Vancomycin + clindamycin | 4 (14.3%) | 7 (6.5%) | |
| Others | 1 (3.6%) | 1 (0.9%) | |
| Duration of antibiotic, days | 7.0 (7.0‐8.5) | 7.0 (7.0‐7.0) | 0.003 |
| Hematoma | 3 (10.0%) | 4 (3.7%) | 0.177 |
| Temporary pacemaker | 3 (10.0%) | 4 (3.7%) | 0.177 |
AICD, automatic implantable cardioverter‐defibrillators; CRT‐D, cardiac resynchronization therapy defibrillator; CRT‐P, cardiac resynchronization therapy pacemaker; PPM, permanent pacemaker; S‐ICD, subcutaneous implantable cardioverter‐defibrillators.
3.3. Microbiological characteristics of CIED infections
In all 54 cases of diagnosed CIED infection, 30 (55.6%) cases were monomicrobial infections, five (9.3%) cases were polymicrobial infections, and 19 (35.2%) cases were culture negative (Figure 3). Coagulase‐negative Staphylococci (CoNS) were the most frequently isolated pathogens, accounting for 22.2% of all cases, followed by Staphylococcus aureus (18.5%) and gram‐negative organisms (11.1%). Twenty of 24 cases (83.3%) of gram‐positive CIED infection were methicillin‐susceptible. Two other gram‐positive organisms were Streptococcus bovis and Bravebacillus spp.
Figure 3.
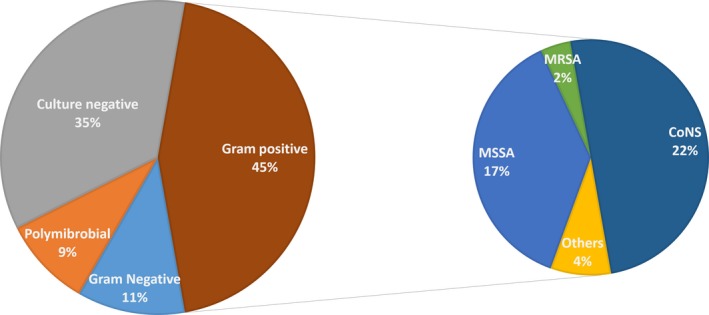
Microbiology of CIED infection (n = 54). CoNS, coagulase‐negative Staphylococcus; MRSA, methicillin‐resistant Staphylococcus aureus; MSSA, methicillin‐susceptible Staphylococcus aureus
Of the gram‐negative organisms, three of six cases (50.0%) grew Pseudomonas aeruginosa. Other gram‐negative organisms included Pseudomonas stutzeri, Serratia marcescens, and Acinetobacter baumanii. The polymicrobials identified in these five cases were (a) Enterobacter cloacae and Citrobacter freundii complex, (b) Escherichia coli and Mycobacterium fortuitum, (c) CoNS, Klebsiella pneumoniae and Achromobacter xylososidans, (d) Klebsiella oxytoca, E. coli, A. xylososidans, P. stutzeri, Stenotrophomonas maltophilia, Pannonibacter phragmitetus and M. fortuitum, (e) P. aeruginosa, S. aureus and K. pneumoniae.
There were no statistically significant differences in microbiology of early‐ vs late‐onset infection in this cohort (Table 3). Eleven (20.4%) patients had bacteremia. The proportion of bloodstream infection of each pathogen was not significantly different statistically.
Table 3.
Microbiology of early versus late CIED infection
| Early infection | Late infection | P‐value | |
|---|---|---|---|
| Bacterial infection | |||
| Staphylococcus aureus | 6 (24.0%) | 4 (13.8%) | 0.3252 |
| CoNS | 3 (12.0%) | 9 (31.0%) | |
| Other gram‐positive | 2 (8.0%) | 0 (0.0%) | |
| Gram‐negative | 3 (12.0%) | 3 (10.3%) | |
| Polymicrobial | 3 (12.0%) | 2 (6.9%) | |
| Negative culture | 8 (32.0%) | 11 (37.9%) | |
CIED, cardiac implantable electronic devices; CoNS, coagulase‐negative Staphylococcus.
3.4. Risk factors
Possible factors associated with CIED infection including age, BMI, comorbidities, prior procedure use of temporary pacemaker, previous history of CIED infection, hematoma, perioperative antibiotic use, duration of operation, cardiac device, and type of the last procedure were analyzed (Table 4). By univariate logistic regression, factors with a P < 0.2 included age, BMI, underlying autoimmune diseases, history of CIED infection, use of vancomycin and clindamycin as perioperative antibiotics, duration of perioperative antibiotics, duration of operation, hematoma, prior procedure use of a temporary pacemaker, PPM (VVI), and generator revision procedure. By multivariate logistic regression, a history of CIED infection and generator revision procedure were independent factors associated with CIED infection (OR 48.56, 95% CI 3.72‐633.62; P = 0.003 and OR 19.99, 95% CI 1.28‐313.24; P = 0.033, respectively).
Table 4.
Univariate analysis for risk factors of CIED infection
| OR | P‐value | |
|---|---|---|
| Age | 1.01 (1.00‐1.03) | 0.155 |
| Body mass index | 0.94 (0.87‐1.02) | 0.145 |
| Autoimmune diseases | 4.24 (0.75‐23.93) | 0.102 |
| History of CIED infection | 35.7 (7.74‐164.66) | <0.001 |
| Vancomycin + clindamycin | 2.38 (0.64‐8.80) | 0.193 |
| Duration of perioperative antibiotics (days) | 1.26 (1.02‐1.54) | 0.030 |
| Duration of operation (hours) | 1.62 (1.10‐2.40) | 0.015 |
| Hematoma | 2.86 (0.60‐13.56) | 0.185 |
| Temporary pacemaker | 2.86 (0.60‐13.56) | 0.185 |
| PPM (VVI) | 12.25 (3.34‐44.91) | <0.001 |
| Type of the last procedure | ||
| Primary implantation | 1.00 | |
| Generator change | 0.63 (0.25‐1.57) | 0.327 |
| Lead revision | 1.07 (0.93‐12.34) | 0.956 |
| Generator revision | 10.71 (1.19‐96.06) | 0.034 |
| Re‐implantation | 1.56 (0.56‐4.30) | 0.392 |
CIED, cardiac implantable electronic devices; PPM, permanent pacemaker.
3.5. Management and outcomes
Prior to infected device removal, all patients were assessed for a need of ongoing CIED therapy. Twenty‐three of 54 cases required re‐implantation of a new device, of which 14 replacements were PPMs, 7 were AICDs, and 2 were CRTs. In the CIED infected cases, 44 (81.5%) of the devices were completely removed (both generator and leads), 2 (3.7%) removed only the generator but left the leads, and 8 (14.8%) left the device in situ. All replacements were placed at a median (IQR) of 28 (14‐43) days following removal of the infected device. Outcomes were as follows: 40 cases were cured, seven cases were relapse of CIED infection, and one died because of hospital acquired pneumonia. There were six cases that the outcome could not be traced due to referral back to outside hospitals. Leaving device in situ was the only factor significantly associated with poor outcome (relapse of the infection or death) (OR 11.40, 95% CI 1.52‐85.73; P = 0.018).
4. DISCUSSION
At Ramathibodi Hospital, the majority of devices that increased were PPM. The marked increased rate of implantation might be the result of the start of Thailand's Universal Coverage Scheme in 2002, which covered approximately 75% of the entire Thai population, making more patients able to access the appropriate treatment.32 Our study demonstrated that the annual incidence of CIED infection at Ramathibodi Hospital was stable at approximately 0.9% despite a continuous rise in CIED implantation. In our series, the incidence of the infection was comparable to previous reports from Japan (1.1%),33 and China (1.9%).34 During study period, the method of CIED implantation and perioperative antibiotics were not changed. However, after a case of mycobacterial infection, skin preparation technique was changed since 2015. This included preprocedure povidone‐iodine scrub for one minute instead of unmeasured time, the use of sterile drape and a new bottle of sterile water for each patient. These might result in a slight decrease in CIED infection rate thereafter.
Most of the positive culture in this study were gram‐positive organisms similar to western countries, but in a lower proportion (44.4% vs 68%‐93%).3 CoNS, which are one of the normal skin flora, were the most frequent cause of the CIED infections, indicating that most CIED infections were inoculated at the time of implantations.28, 35, 36 Although monomicrobial gram‐negative CIED infection composed only 11.1% in this study, all polymicrobial CIED infection patients, composed of gram‐negative bacilli. Two episodes (3.7%) of Mycobacterium fortuitum, which is a rapid growing mycobacterium (RGM), were found in this study. One grew from wound tissue and another grew from blood culture. Mycobacterium related CIED infection was rarely found. There were case reports both from Asian and western countries.18, 37, 38, 39 The most common isolated Mycobacterial organisms were in the M. fortuitum group, which account for approximately 50% of mycobacterial infections.18 The rate of negative culture was quite high in our study. This might be due to some patients receiving antibiotic therapy before they presented to our hospital,27, 40 and thus potentially confounded the microbiological data in this study.
At our center, cefazolin followed by cephalexin for a 7‐day course was the most common antibiotics prescribed as perioperative antibiotics. Because of the high proportion of methicillin‐susceptible pathogen and gram‐negative organisms, cefazolin and cephalexin were considered an appropriate prophylaxis for CIED implantation in Thailand. For empirical treatment of CIED infection, chosen antibiotics should have a broad spectrum to cover staphylococci and gram‐negative organisms including Pseudomonas species.
Diverse predisposing risk factors for CIED infection were identified, but significant risk factors in one study did not reach significance in others and vice versa. The more complex devices had more opportunity to be infected.6, 12, 13 Not only was the complexity of the device itself a risk factor, but also a longer operation time was as well. If the device had multifunction and numerous leads, it was usually associated with a longer operation time. We found increased OR of infection associated with a longer duration of operation. However, this did not reach statistical significance in multivariate analysis, likely due to the small sample size. In univariate analysis, a longer duration of perioperative antibiotic is associated with CIED infection. However, this did not reach statistical significance in the multivariate model. We found a statistically significant association between a longer duration of antibiotic use and the history of CIED infection group (P < 0.001). It is possible that the patients who had a history of CIED infection received a longer duration of perioperative antibiotic in the subsequent procedure. The only significant risk factors associated with CIED infection from this study were history of CIED infection and generator revision procedure, which supports the earlier studies.9, 15, 41
In patients with pocket infection without systemic infection and complete device removal was not feasible due to patient's condition/preference or limited availability of care, prolonged course of oral antibiotic was provided to the patients as an alternative. Our result revealed poor outcome in the group, which the system was not completely removed. In our study, the rate of relapse of CIED infection was 6.5% in the completely removed group that underwent re‐implantation after finishing a 14‐21‐day course of antibiotics, healed explanted site and a negative repeat hemoculture. Telemetry monitoring or exteriorized pacemaker could provide a temporary solution that would enable delay of the re‐implantation procedure and minimize the possibility of relapse infection in these high‐risk patients.41 Subcutaneous implantable cardioverter‐defibrillator may be the appropriate choice in selected patients, because the rate of recurrent infection is low, even in the previous CIED infected patient.42
To our knowledge, this is the first study to describe CIED infection in Thailand. The strength of our study was that a majority of infected patients in other parts of Thailand were referred to our hospital because only a few hospitals in Thailand could provide laser lead extraction for the CIED infected patients. Our study had some limitations. We were unable to reach medical records of some patients, and information of some procedure‐related risk factors in the patients referred from outside hospitals.
5. CONCLUSIONS
In conclusion, incidence of CIED infection in Thailand shows a stable trend while the rate of CIED implantation continues to rise. Microbiology of CIED infection in Thailand is quite similar to western countries. Perioperative antibiotic spectrum should cover both gram‐positive and gram‐negative organisms. Broad‐spectrum antibiotics with staphylococci and gram‐negative organisms including Pseudomonas species coverage should be used as empirical antibiotics for CIED infection in Thailand. Risk factors associated with CIED infection are previous history of CIED infection and generator revision procedure. Therefore, patients with these risk factors must be closely monitored. A multicenter study of CIED infection in Thailand should be pursued.
CONFLICT OF INTEREST
Authors declare no conflict of interests for this article.
ACKNOWLEDGEMENTS
We are grateful to Sineenart Likitratcharoen, chief nurse of cardiology unit, and all Medical Record Department staff members at Ramathibodi Hospital for their assistance in medical record gathering for our review.
Korkerdsup T, Ngarmukos T, Sungkanuparph S, Phuphuakrat A. Cardiac implantable electronic device infection in the cardiac referral center in Thailand: incidence, microbiology, risk factors, and outcomes. J Arrhythmia. 2018;34:632–639. 10.1002/joa3.12123
References
- 1. Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter‐defibrillators: calendar year 2009–a World Society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011;34:1013–1027. [DOI] [PubMed] [Google Scholar]
- 2. Greenspon AJ, Patel JD, Lau E, et al. 16‐year trends in the infection burden for pacemakers and implantable cardioverter‐defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58:1001–1006. [DOI] [PubMed] [Google Scholar]
- 3. Sandoe JA, Barlow G, Chambers JB, et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother. 2015;70:325–359. [DOI] [PubMed] [Google Scholar]
- 4. Cunningham D, Charles R, Cunningham M, de Lange A. Cardiac rhythm management: UK national clinical audit 2010. Partnership HQI, editor, 2011.
- 5. Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemakers and cardioverter‐defibrillators: results of a large prospective study. Circulation. 2007;116:1349–1355. [DOI] [PubMed] [Google Scholar]
- 6. Romeyer‐Bouchard C, Da Costa A, Dauphinot V, et al. Prevalence and risk factors related to infections of cardiac resynchronization therapy devices. Eur Heart J. 2010;31:203–210. [DOI] [PubMed] [Google Scholar]
- 7. de Oliveira JC, Martinelli M, Nishioka SA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter‐defibrillators: results of a large, prospective, randomized, double‐blinded, placebo‐controlled trial. Circ Arrhythm Electrophysiol. 2009;2:29–34. [DOI] [PubMed] [Google Scholar]
- 8. Essebag V, Verma A, Healey JS, et al. Clinically significant pocket hematoma increases long‐term risk of device infection: BRUISE CONTROL INFECTION study. J Am Coll Cardiol. 2016;67:1300–1308. [DOI] [PubMed] [Google Scholar]
- 9. Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta‐analysis. Europace. 2015;17:767–777. [DOI] [PubMed] [Google Scholar]
- 10. Johansen JB, Jorgensen OD, Moller M, et al. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population‐based cohort study of 46299 consecutive patients. Eur Heart J. 2011;32:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poole JE, Gleva MJ, Mela T, et al. Complication rates associated with pacemaker or implantable cardioverter‐defibrillator generator replacements and upgrade procedures clinical perspective: results from the REPLACE registry. Circulation. 2010;122:1553–1561. [DOI] [PubMed] [Google Scholar]
- 12. Margey R, McCann H, Blake G, et al. Contemporary management of and outcomes from cardiac device related infections. Europace. 2010;12:64–70. [DOI] [PubMed] [Google Scholar]
- 13. Nery PB, Fernandes R, Nair GM, et al. Device‐related infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences. J Cardiovas Electrophysiol. 2010;21:786–790. [DOI] [PubMed] [Google Scholar]
- 14. Branch‐Elliman W. A Roadmap for reducing cardiac device infections: a review of epidemiology, pathogenesis, and actionable risk factors to guide the development of an infection prevention program for the electrophysiology laboratory. Curr Infect Dis Rep. 2017;19:34. [DOI] [PubMed] [Google Scholar]
- 15. Sohail MR, Hussain S, Le KY, et al. Risk factors associated with early‐ versus late‐onset implantable cardioverter‐defibrillator infections. J Interv Card Electrophysiol. 2011;31:171–183. [DOI] [PubMed] [Google Scholar]
- 16. Bloom H, Heeke B, Leon A, et al. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol. 2006;29:142–145. [DOI] [PubMed] [Google Scholar]
- 17. Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart. 2009;95:715–720. [DOI] [PubMed] [Google Scholar]
- 18. Phadke VK, Hirsh DS, Goswami ND. Patient report and review of rapidly growing mycobacterial infection after cardiac device implantation. Emerg Infect Dis. 2016;22:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parameswaran R, Sherchan JB, Varma DM, Mukhopadhyay C, Vidyasagar S. Intravascular catheter‐related infections in an Indian tertiary care hospital. J Infect Dev Ctries. 2011;5:452–458. [DOI] [PubMed] [Google Scholar]
- 20. Savetha P, Sreedharan SK. Study on the intravascular catheter‐related bloodstream infections in a tertiary care hospital of Tamil Nadu, India. Res J Pharm Biol Chem Sci. 2015;6:287–291. [Google Scholar]
- 21. Gaynes R, Edwards JR. National nosocomial infections surveillance system. Overview of nosocomial infections caused by gram‐negative bacilli. Clin Infect Dis. 2005;41:848–854. [DOI] [PubMed] [Google Scholar]
- 22. Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. [DOI] [PubMed] [Google Scholar]
- 23. Tsai JC, Sheng WH, Lo WY, Jiang CC, Chang SC. Clinical characteristics, microbiology, and outcomes of prosthetic joint infection in Taiwan. J Microbiol Immunol Infect. 2015;48:198–204. [DOI] [PubMed] [Google Scholar]
- 24. Lalremruata R. Prosthetic joint infection: a microbiological review. J Med Soc. 2015;29:120–128. [Google Scholar]
- 25. Sia IG, Berbari EF, Karchmer AW. Prosthetic joint infections. Infect Dis Clin North Am. 2005;19:885–914. [DOI] [PubMed] [Google Scholar]
- 26. Goya M, Nagashima M, Hiroshima K, et al. Lead extractions in patients with cardiac implantable electronic device infections: single center experience. J Arrhythm. 2016;32:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okada A, Shoda M, Tabata H, et al. Single‐center experience with percutaneous lead extraction of cardiac implantable electric devices. J Cardiol. 2018;71:192–196. [DOI] [PubMed] [Google Scholar]
- 28. Wang R, Li X, Wang Q, Zhang Y, Wang H. Microbiological characteristics and clinical features of cardiac implantable electronic device infections at a tertiary hospital in China. Front Microbiol. 2017;8:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. [DOI] [PubMed] [Google Scholar]
- 30. Baron EJ. Rapid identification of bacteria and yeast: summary of a National Committee for Clinical Laboratory Standards proposed guideline. Clin Infect Dis. 2001;33:220–225. [DOI] [PubMed] [Google Scholar]
- 31. Wayne P. Clinical and Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing: Twenty‐fourth informational supplement, M100‐S24, 2014.
- 32. Paek SC, Meemon N, Wan TTH. Thailand's universal coverage scheme and its impact on health‐seeking behavior. SpringerPlus. 2016;5:1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakajima H, Taki M. Incidence of cardiac implantable electronic device infections and migrations in Japan: Results from a 129 institute survey. J Arrhythm. 2016;32:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guan G, Liu Z, Zhang Y, et al. Application of an infection control protocol (ICP) reduced cardiac device infection (CDI) in low‐volume centers. Med Sci Monit. 2018;24:1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bongiorni MG, Tascini C, Tagliaferri E, et al. Microbiology of cardiac implantable electronic device infections. EP Europace. 2012;14:1334–1339. [DOI] [PubMed] [Google Scholar]
- 36. Jan E, Camou F, Texier‐Maugein J, et al. Microbiologic characteristics and in vitro susceptibility to antimicrobials in a large population of patients with cardiovascular implantable electronic device infection. J Cardiovasc Electrophysiol. 2012;23:375–381. [DOI] [PubMed] [Google Scholar]
- 37. Salas NM, Klein N. Mycobacterium goodii: an emerging nosocomial pathogen: a case report and review of the literature. Infect Dis Clin Pract (Baltim Md). 2017;25:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whitaker J, Rickaby W, Robson A, et al. Recurrent pocket infection due to Mycobacterium chelonae at the site of an explanted cardiac implantable electrical device in proximity to a long‐standing tattoo. HeartRhythm Case Rep. 2016;2:132–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fukunaga M, Goya M, Ogawa M, et al. Implantable cardioverter defibrillator infection due to Mycobacterium mageritense . J Infect Chemother. 2016;22:180–183. [DOI] [PubMed] [Google Scholar]
- 40. Jiangbo D, Xuebin L, Ping Z, et al. Reuse of infected cardiac rhythm management devices in the same individual. J Interv Card Electrophysiol. 2012;35:109–114. [DOI] [PubMed] [Google Scholar]
- 41. Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–e551. [DOI] [PubMed] [Google Scholar]
- 42. Boersma L, Burke MC, Neuzil P, et al. Infection and mortality after implantation of a subcutaneous ICD after transvenous ICD extraction. Heart Rhythm. 2016;13:157–164. [DOI] [PubMed] [Google Scholar]


