Abstract
Patients with gastrointestinal disease (GI) are at risk for osteopenia or osteoporosis, which can lead to fractures. Although these patients may be at risk from a young age, gastroenterologists often overlook this fact in practice. There are well-known GI diseases associated with osteopenia and osteoporosis, such as the post-gastrectomy state, inflammatory bowel disease (IBD), and celiac disease. As there is an increase in the prevalence of IBD patients, newly diagnosed celiac disease in adulthood, and gastric cancer survivors following gastrectomy, bone disease in these patients becomes an important issue. Here, we have discussed osteoporosis and fractures in GI disease, especially in the post-gastrectomy state, IBD, and celiac disease. Although the pathogenesis of bone loss in each disease has not been fully identified, we have confirmed that the prevalence of osteoporosis and fractures in each of these diseases is high. There are scarce studies comparing the prevalence of osteoporosis or osteoporotic fractures in GI disease patients with studies in postmenopausal women, and specific guidelines for their management in each disease have not been established. Intensive surveillance and management are needed to ensure that these patients attain peak bone mass for age and sex to prevent fractures.
Keywords: Celiac disease, Gastrectomy, Inflammatory bowel diseases, Osteoporosis, Osteoporotic fractures
INTRODUCTION
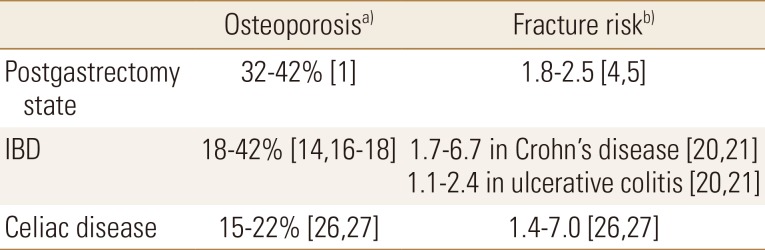
Gastrointestinal disease (GI) is known to be associated with osteopenia or osteoporosis. According to the World Health Organization, osteoporosis is defined as “systemic skeletal disease characterized by low bone mass and micro-architectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fractures”. Fractures significantly increase the morbidity as well as the social economic burden and decrease the quality of life. Patients with GI diseases, such as the postgastrectomy state, inflammatory bowel disease (IBD), and celiac disease, are reported to be at an increased risk of developing osteoporosis and fractures and are even affected at a younger age (Table 1). Nevertheless, osteoporosis or fractures in GI disease tend to be overlooked in practice. Herein, we discuss osteoporosis and fractures related to the postgastrectomy state, IBD, and celiac disease.
Table 1. The incidence of osteoporosis and the relative risk of fractures in the postgastrectomy state, inflammatory bowel disease and celiac disease.
a)Defined as T-score <−2.5 or Z-score <−2 at any site. b)Fracture risk compared to general population.
IBD, inflammatory bowel disease.
POSTGASTRECTOMY
Total or partial gastrectomy is a well-known risk factor for osteoporosis. The prevalence of osteoporosis following gastrectomy for peptic ulcer disease has been reported as 32% to 42%.[1] In recent times, although gastrectomy for peptic ulcer disease has decreased owing to improved medical therapy, such as the proton pump inhibitors, gastrectomy for gastric cancer has increased due to early detection. In addition, survival rate of gastric cancer patients after curative gastrectomy is as high as 90%, and bone health in gastric cancer survivors has become an important issue to avoid long-term complications. Several studies have been conducted in patients who underwent gastrectomy for gastric cancer, and the incidence of osteoporosis was found to be similar to that reported in previous studies in patients with peptic ulcer disease; a large population study using nationwide claims data reported 41.9 cases per 1,000 person-years,[2] and another large observation study reported 34.0%.[3]
Previous large population studies in patients with gastrectomy for peptic ulcer diseases reported a high incidence of fractures. In a study from Olmsted country, the cumulative incidence of fractures for a 30-year period was 72% in female patients and 48% in male. In this study, the incidence of hip fractures was 25% with a relative risk (RR) of 2.5 (95% confidence interval [CI], 1.9–3.3), and the incidence of vertebral fractures was 41% with an RR of 4.7 (95% CI, 3.8–5.7).[4] In another large population study in Sweden, the incidence of fractures in the postgastrectomy state was 35% with an RR of 1.83, compared to that in the control group.[5] Recent studies in patients with gastrectomy for gastric cancer also report an increased risk of fractures.[2,6] One single-center long-term large observation study reported the cumulative incidence of fractures as 40.6%.[6] In a national claims study in South Korea, the incidence of fractures was 27.6 per 1,000 person-years.[2] Interestingly, both studies reported that fractures occurred at an early period following the gastrectomy; the former study reported the occurrence of fractures at median 3.1 years after gastrectomy, and the latter reported that fractures rapidly increased until 5 years after gastrectomy.
The pathogenesis of osteoporosis and fractures in gastrectomy patients is uncertain. Several hypotheses have been suggested: malabsorption of calcium and vitamin D may play a role in postgastrectomy osteoporosis. Gastrectomy with anastomosis alters the physiology, which results in gastric dumping and formation of insoluble calcium soaps. However, many studies demonstrated normal calcium and vitamin D levels in postgastrectomy patients,[6,7,8,9] and it may contribute to elevated parathyroid hormone (PTH) levels and calcium homeostasis with dominant bone resorption.[10,11] One prospective longitudinal study showed elevated level of PTH at 1 year after gastrectomy, and higher PTH levels are associated with a greater decrease in the bone mineral density (BMD) in the spine as well as in the total hip.[10] In addition, only the bone resorption markers increased without concomitant increase in the bone formation markers within 1 year after gastrectomy, which indicated imbalance of bone resorption and formation due to hyperparathyroidism in the early phase. In another study, postgastrectomy patients with fractures had a significantly increased serum calcium level at 1-year postoperatively. Protein malnutrition may also attribute to postgastrectomy osteoporosis and fractures through the decreased formation of the collagen matrix in bone.[12] In animal data, a rat model confirmed the decrease in the number of trabeculae after gastrectomy.[13]
The American Gastroenterological Association (AGA) recommends BMD measurement or calcium or bisphosphonate supplementation after at least 10 years after gastrectomy.[14] Even the British Gastroenterology Society and other European gastroenterology societies have no specific guidelines on the monitoring and management of osteoporosis. However, according to studies, the changes in the bone turnover markers and the increased risk of osteoporosis or fractures occurred within the early period after gastrectomy.[2,3,6,10] Therefore, an early intensive surveillance program and a management plan is needed to prevent fractures following gastrectomy.
IBDs
The prevalence of osteoporosis in IBD patients has been reported to be from 18% to 42%.[15,16,17,18] In contrast to that in postmenopausal osteoporosis, in IBD the BMD was usually found to be lower or same in the hip than that in the vertebrae in many studies. Both male and female patients generally had similar BMD in Z or T scores. In most studies, disease diagnosis in IBD was not associated with the risk of osteoporosis.
Patients with ulcerative colitis (UC) or Crohn's disease (CD) are at an increased risk of developing fractures. There were several large population-based studies on fractures in IBD patients, and the overall incidence has been reported as 1/100 patient-years.[19] In a Danish study, although patients with UC had an overall fracture rate similar to that in the controls, patients with CD had an RR of 1.7 (95% CI, 1.7–2.3) for all fractures, 2.5 (95% CI, 1.7–3.6) for fractures in women, and 0.6 (95% CI, 0.3–1.3) in men. Both CD and UC patients have an increased risk for vertebrae fractures with RR 6.7 (95% CI, 2.1–21.7) and RR 2.4 (95% CI, 0.5–11.9), respectively.[20,21] Another two North American population-based studies also reported the small increased risk of fractures comparing to that in the control group, and the risk was greater in elderly patients.[19,22]
The main pathogenesis of osteoporosis in IBD patients is chronic inflammation. Receptor activator of nuclear factor-κB ligand (RANKL)-osteoprotegerin (OPG) system has a crucial role in bone metabolism. RANKL activates osteoclasts and stimulates bone resorption, while OPG blocks osteoclast formation. Proinflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-1, IL-6, IL-7, and IL-17, increase the ratio of RANKL to OPG, which leads to promotion of bone resorption. Mucosal inflammation and the underlying inflammatory process in IBD lead to bone loss.[23] Malabsorption due to IBD or poor diet is also another factor for osteoporosis in IBD patients. Other factors such as glucocorticoid therapy and hypogonadism in CD are also important in osteoporosis.
CELIAC DISEASE
Celiac disease is an immune-mediated enteropathy caused by gluten containing grains, in genetically susceptible people, and is more common in Europe and North America than in Asia. Celiac patients who were diagnosed at childhood had a lower BMD than that in the control group but showed improvement and reached to normal BMD following a gluten-free diet. However, many studies have confirmed the higher prevalence of low BMD compared to that in the general population in patients with celiac disease. The prevalence of osteoporosis in celiac disease differs in various sites; Valdimarsson reported the prevalence of osteoporosis to be 22% in the forearm, and 18% and 15% in the hip and lumber spine, respectively.[24] Meyer et al.[25] revealed low BMD in the hip 44% and in the lumbar spine 38%.
Celiac patients have an increased risk of fractures, up to 7 times higher than that in the general population of same age and gender.[26,27] According to the recent observation cohort study from Olmsted county, patients with celiac disease have an increased risk of fractures with an RR of 2.5 (95% CI, 1.1–5.6; P=0.030) greater than that in their matched controls. For axial fractures, patients with celiac disease had an increased risk, RR of 4.2 (95% CI, 1.0–17.6; P=0.050), compared to that in the controls.[28] As for other large studies from Europe, a Swedish study showed an RR of 2.1 (95% CI, 1.8–2.4) for hip fractures and an RR of 1.4 (95% CI, 1.3–1.5) for any fracture in celiac disease.[29] Another study from Spain showed a 3.5 times increased risk of fractures in patients with duodenal villous atrophy when compared to that in patients without villous atrophy.[30]
The pathogenesis of osteoporosis in celiac disease also might be strongly associated with malabsorption of calcium, vitamin D, zinc, and other nutritional factors due to intestinal villous atrophy. Reduced calcium intake and absorption by itself can lead to impaired bone mass, which also triggers secondary hyperparathyroidism. In addition, reduced insulin-like growth factor-1 levels may also lead to impaired bone mass. Moreover, decreased bone mass can be attributed to chronic inflammation with proinflammatory cytokines and neutralizing antibodies to OPG.
British Society of Gastroenterology recommended the measurement of calcium, alkaline phosphatase, vitamin D levels, and PTH for the diagnosis. Calcium intake should be maintained at or above 1,000 mg/day. BMD measurements should be made at intervals of 2 years in patients with ongoing villous atrophy, poor dietary adherence, and low BMD at the index measurement.[31] The AGA has recommended that dual energy X-ray absorptiometry scan should be performed in adult celiac disease 1 year after initiating the gluten-free diet.[14]
CONCLUSION
Osteoporosis is a well-known complication of GI disease. Although liver diseases, including liver transplantation state and chronic pancreatitis, are not discussed in this review, they are also known to be associated with an increased risk of bone loss and fractures. Gastroenterologists should pay attention to bone disease in these patients. Currently, most of these diseases do not have specific guidelines for a diagnostic and management algorithm, and the guidelines for primary osteoporosis is being followed. Further well-designed population-based prospective studies are required to create algorithms that would help in providing tailored therapy to individual patients with GI disease who are at risk for osteoporosis and fractures at a young age.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Zittel TT, Zeeb B, Maier GW, et al. High prevalence of bone disorders after gastrectomy. Am J Surg. 1997;174:431–438. doi: 10.1016/s0002-9610(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 2.Seo GH, Kang HY, Choe EK. Osteoporosis and fracture after gastrectomy for stomach cancer: a nationwide claims study. Medicine (Baltimore) 2018;97:e0532. doi: 10.1097/MD.0000000000010532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo SH, Lee JA, Kang SY, et al. Risk of osteoporosis after gastrectomy in long-term gastric cancer survivors. Gastric Cancer. 2018;21:720–727. doi: 10.1007/s10120-017-0777-7. [DOI] [PubMed] [Google Scholar]
- 4.Melton LJ, 3rd, Crowson CS, Khosla S, et al. Fracture risk after surgery for peptic ulcer disease: a population-based cohort study. Bone. 1999;25:61–67. doi: 10.1016/s8756-3282(99)00097-6. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson BE, Westlin NE. The fracture incidence after gastrectomy. Acta Chir Scand. 1971;137:533–534. [PubMed] [Google Scholar]
- 6.Oh HJ, Lim CH, Yoon BH, et al. Fracture after gastrectomy for gastric cancer: a long-term follow-up observational study. Eur J Cancer. 2017;72:28–36. doi: 10.1016/j.ejca.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Kwon SJ, Hahm JS, Cho YJ, et al. The influence of gastrectomy on the change of bone metabolism and bone density. Korean J Intern Med. 2000;15:25–31. doi: 10.3904/kjim.2000.15.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liedman B, Bosaeus I, Mellström D, et al. Osteoporosis after total gastrectomy. Results of a prospective, clinical study. Scand J Gastroenterol. 1997;32:1090–1095. doi: 10.3109/00365529709002986. [DOI] [PubMed] [Google Scholar]
- 9.Nilas L, Christiansen C. Influence of PTH and 1,25(OH)2D on calcium homeostasis and bone mineral content after gastric surgery. Calcif Tissue Int. 1985;37:461–466. doi: 10.1007/BF02557827. [DOI] [PubMed] [Google Scholar]
- 10.Baek KH, Jeon HM, Lee SS, et al. Short-term changes in bone and mineral metabolism following gastrectomy in gastric cancer patients. Bone. 2008;42:61–67. doi: 10.1016/j.bone.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Maier GW, Kreis ME, Zittel TT, et al. Calcium regulation and bone mass loss after total gastrectomy in pigs. Ann Surg. 1997;225:181–192. doi: 10.1097/00000658-199702000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tovey FI, Hall ML, Ell PJ, et al. A review of postgastrectomy bone disease. J Gastroenterol Hepatol. 1992;7:639–645. doi: 10.1111/j.1440-1746.1992.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 13.Stenström M, Olander B, Lehto-Axtelius D, et al. Bone mineral density and bone structure parameters as predictors of bone strength: an analysis using computerized microtomography and gastrectomy-induced osteopenia in the rat. J Biomech. 2000;33:289–297. doi: 10.1016/s0021-9290(99)00181-5. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124:795–841. doi: 10.1053/gast.2003.50106. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein CN, Seeger LL, Sayre JW, et al. Decreased bone density in inflammatory bowel disease is related to corticosteroid use and not disease diagnosis. J Bone Miner Res. 1995;10:250–256. doi: 10.1002/jbmr.5650100211. [DOI] [PubMed] [Google Scholar]
- 16.Bjarnason I, Macpherson A, Mackintosh C, et al. Reduced bone density in patients with inflammatory bowel disease. Gut. 1997;40:228–233. doi: 10.1136/gut.40.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollak RD, Karmeli F, Eliakim R, et al. Femoral neck osteopenia in patients with inflammatory bowel disease. Am J Gastroenterol. 1998;93:1483–1490. doi: 10.1111/j.1572-0241.1998.468_q.x. [DOI] [PubMed] [Google Scholar]
- 18.Roux C, Abitbol V, Chaussade S, et al. Bone loss in patients with inflammatory bowel disease: a prospective study. Osteoporos Int. 1995;5:156–160. doi: 10.1007/BF02106094. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein CN, Blanchard JF, Leslie W, et al. The incidence of fracture among patients with inflammatory bowel disease. A population-based cohort study. Ann Intern Med. 2000;133:795–799. doi: 10.7326/0003-4819-133-10-200011210-00012. [DOI] [PubMed] [Google Scholar]
- 20.Vestergaard P, Krogh K, Rejnmark L, et al. Fracture risk is increased in Crohn's disease, but not in ulcerative colitis. Gut. 2000;46:176–181. doi: 10.1136/gut.46.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vestergaard P, Mosekilde L. Fracture risk in patients with celiac Disease, Crohn's disease, and ulcerative colitis: a nationwide follow-up study of 16,416 patients in Denmark. Am J Epidemiol. 2002;156:1–10. doi: 10.1093/aje/kwf007. [DOI] [PubMed] [Google Scholar]
- 22.Loftus EV, Jr, Crowson CS, Sandborn WJ, et al. Long-term fracture risk in patients with Crohn's disease: a population-based study in Olmsted County, Minnesota. Gastroenterology. 2002;123:468–475. doi: 10.1053/gast.2002.34779. [DOI] [PubMed] [Google Scholar]
- 23.Tilg H, Moschen AR, Kaser A, et al. Gut, inflammation and osteoporosis: basic and clinical concepts. Gut. 2008;57:684–694. doi: 10.1136/gut.2006.117382. [DOI] [PubMed] [Google Scholar]
- 24.Valdimarsson T, Löfman O, Toss G, et al. Reversal of osteopenia with diet in adult coeliac disease. Gut. 1996;38:322–327. doi: 10.1136/gut.38.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer D, Stavropolous S, Diamond B, et al. Osteoporosis in a North American adult population with celiac disease. Am J Gastroenterol. 2001;96:112–119. doi: 10.1111/j.1572-0241.2001.03507.x. [DOI] [PubMed] [Google Scholar]
- 26.Fickling WE, McFarlane XA, Bhalla AK, et al. The clinical impact of metabolic bone disease in coeliac disease. Postgrad Med J. 2001;77:33–36. doi: 10.1136/pmj.77.903.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walters JR, Banks LM, Butcher GP, et al. Detection of low bone mineral density by dual energy x ray absorptiometry in unsuspected suboptimally treated coeliac disease. Gut. 1995;37:220–224. doi: 10.1136/gut.37.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jafri MR, Nordstrom CW, Murray JA, et al. Long-term fracture risk in patients with celiac disease: a population-based study in Olmsted County, Minnesota. Dig Dis Sci. 2008;53:964–971. doi: 10.1007/s10620-007-9976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludvigsson JF, Michaelsson K, Ekbom A, et al. Coeliac disease and the risk of fractures - a general population-based cohort study. Aliment Pharmacol Ther. 2007;25:273–285. doi: 10.1111/j.1365-2036.2006.03203.x. [DOI] [PubMed] [Google Scholar]
- 30.García-Manzanares A, Tenias JM, Lucendo AJ. Bone mineral density directly correlates with duodenal Marsh stage in newly diagnosed adult celiac patients. Scand J Gastroenterol. 2012;47:927–936. doi: 10.3109/00365521.2012.688217. [DOI] [PubMed] [Google Scholar]
- 31.Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210–1228. doi: 10.1136/gutjnl-2013-306578. [DOI] [PMC free article] [PubMed] [Google Scholar]