ABSTRACT
Background
Epidemiologic evidence on the association of a healthy Nordic diet and future type 2 diabetes (T2D) is limited. Exploring metabolites as biomarkers of healthy Nordic dietary patterns may facilitate investigation of associations between such patterns and T2D.
Objectives
We aimed to identify metabolites related to a priori-defined healthy Nordic dietary indexes, the Baltic Sea Diet Score (BSDS) and Healthy Nordic Food Index (HNFI), and evaluate associations with the T2D risk in a case-control study nested in a Swedish population-based prospective cohort.
Design
Plasma samples from 421 case-control pairs at baseline and samples from a subset of 151 healthy controls at a 10-y follow-up were analyzed with the use of untargeted liquid chromatography-mass spectrometry metabolomics. Index-related metabolites were identified through the use of random forest modelling followed by partial correlation analysis adjustment for lifestyle confounders. Metabolite patterns were derived via principal component analysis (PCA). ORs of T2D were estimated via conditional logistic regression. Reproducibility of metabolites was assessed by intraclass correlation (ICC) in healthy controls. Associations were also assessed for 10 metabolites previously identified as linking a healthy Nordic diet with T2D.
Results
In total, 31 metabolites were associated with BSDS and/or HNFI (−0.19 ≤ r ≤ 0.21, 0.10 ≤ ICC ≤ 0.59). Two PCs were determined from index-related metabolites: PC1 strongly correlated to the indexes (r = 0.27 for BSDS, r = 0.25 for HNFI, ICC = 0.45) but showed no association with T2D risk. PC2 was weakly associated with the indexes, but more strongly with foods not part of the indexes, e.g., pizza, sausages, and hamburgers. PC2 was also significantly associated with T2D risk. Predefined metabolites were confirmed to be reflective of consumption of whole grains, fish, or vegetables, but not related to T2D risk.
Conclusions
Our study did not support an association between healthy Nordic dietary indexes and T2D. However, foods such as hamburger, sausage, and pizza not covered by the indexes appeared to be more important for T2D risk in the current population.
Keywords: type 2 diabetes, healthy Nordic dietary pattern, HNFI, BSDS, metabolomics, biomarker, nested case-control study
INTRODUCTION
Diet plays an important role in the risk of developing type 2 diabetes (T2D) (1, 2). Observational studies have shown that higher consumption of individual food items such as whole grains (3, 4), fruits (5, 6), vegetables (6, 7), coffee (8), and fish (7, 9) is inversely associated with risk of future T2D, whereas red/processed meat intake is positively associated (7, 10). By taking the complexity and interrelations of multiple dietary exposures into account, dietary pattern analysis might be more informative about the role of diet for the etiology of diet-related diseases than single food item analysis (11, 12). Many studies have suggested that better adherence to healthy dietary patterns, as indicated by e.g., the Mediterranean Diet Score, Dietary Approaches to Stop Hypertension (DASH) score, and Healthy Eating Index, significantly reduces T2D risk (2, 13, 14).
A healthy Nordic diet has been defined as a dietary pattern that complies with current dietary guidelines and includes traditional Nordic food items, such as vegetables, fish, fruits, whole grains, and various seafoods (15). In randomized control trials (16–20), the healthy Nordic diet has been shown to have similarly beneficial effects on cardiometabolic risk factors as the Mediterranean diet (21). Moreover, higher reported adherence to healthy Nordic dietary indexes, e.g., the Healthy Nordic Food Index (HNFI) and the Baltic Sea Diet Score (BSDS), has been inversely associated with, e.g., total mortality (21, 22) and abdominal obesity (23) in observational studies. To our knowledge, only 2 observational studies have investigated the association of such dietary patterns with risk of developing T2D (1, 24). One of these studies (24) did not find any association in a Finnish population, whereas the other (1) found an inverse association between reported adherence to HNFI and risk of T2D in a Danish cohort.
Results from most observational studies on the role of diet and T2D rely on self-reported dietary assessment, most often food-frequency questionnaires (FFQs) (1, 7, 24). Such methods are known to suffer from relatively large systematic and random measurement errors, which may hamper discovery of potentially existing diet-disease relations or detect spurious associations (25, 26). Metabolomics has become a valuable tool for identifying metabolites that could objectively reflect specific food exposures (27–29) or dietary patterns (30–32). Thus, it may provide a complement to self-reported dietary assessments and help to improve understanding of disease-related metabolic processes influenced by diet (33–35). However, there are few objective measures of adherence to dietary patterns, derived either by data-driven methods or by a priori-defined patterns (30–32, 36). In addition, to the best of our knowledge, no prospective study has examined whether a specific metabolite related to a dietary pattern is associated with incident T2D.
Therefore, we conducted an untargeted liquid chromatography-mass spectrometry (LC-MS) metabolomics study based on a case-control study nested within the prospective Swedish Västerbotten Intervention Programme (VIP) (37). We aimed to identify plasma metabolites associated with 2 a priori-defined healthy Nordic dietary indexes, i.e., HNFI and BSDS, and to investigate whether such metabolites are associated with risk of developing T2D.
METHODS
Study cohort and sample collection
This case-control study was nested within the VIP (37) (Figure 1). Detailed information on the VIP and selection of participants is reported elsewhere (37, 38). Briefly, the inhabitants of Västerbotten County were invited to participate in a health survey at their local health care center at ages 30 (only recruited until 1996), 40, 50, and 60 y. Recruitment for the VIP started in 1985 and participants included in the present study were recruited from 1991 to 2005 (baseline).
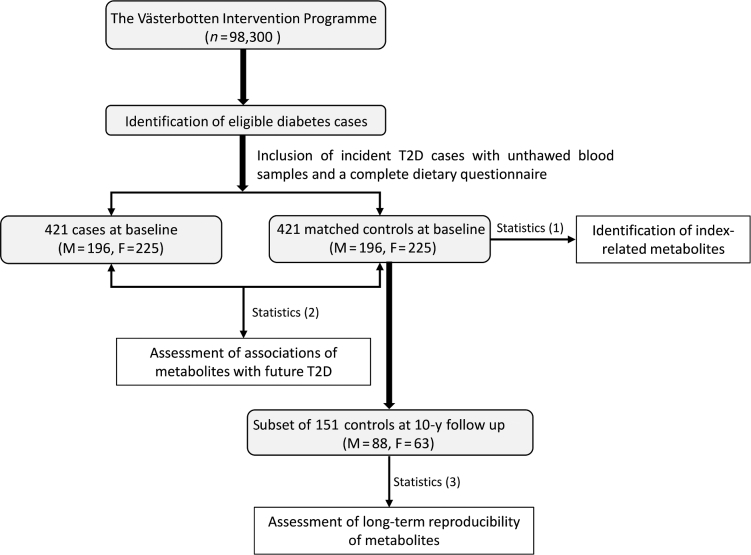
FIGURE 1.
Flowchart of participant selection from the Västerbotten Intervention Programme. Statistics (1): index-related metabolites were identified through the use of random forest modelling amongst healthy controls (n = 421) followed by partial correlation analysis adjustment for several lifestyle-related factors. Statistics (2): ORs of type 2 diabetes were estimated with the use of conditional logistic regression on case-control pairs (n = 842). Statistics (3): the long-term reproducibility of index-related metabolites was assessed by intraclass correlation amongst healthy controls. T2D, type 2 diabetes.
In the VIP, T2D cases were diagnosed according to the Diabetes Register in Northern Sweden (DiabNorth) (39). The diagnosis criteria are based on the WHO standard for diabetes diagnosis: a random fasting plasma glucose (FPG) ≥7 mmol/L or 2-h plasma glucose ≥11.1 mmol/L in a patient with diabetes symptoms. For people who had a single abnormal glucose test but no diabetic symptoms, repeated blood glucose measurement was required. Cases were ascertained by diabetes specialists. We here included the 421 participants (men: n = 196, women: n = 225) at baseline (a median time of 7 y before diagnosis) who had an unthawed fasting plasma sample in the biobank and a complete dietary questionnaire. Each case was individually matched to 1 nondiabetic participant according to age (±2 y), gender, sampling date (±90 d), and sample storage time. Among the 421 selected healthy controls, 151 had data collected and a follow-up sample drawn 10 y after baseline. These samples were also included in the present study in order to estimate the long-term reproducibility of potential dietary biomarkers. The entire study protocol was approved by the Regional Ethics Committee in Uppsala, Sweden (registration number 2014/011).
The present exploratory study aimed to explore plasma metabolites associated with a priori-defined dietary patterns and the risk of developing T2D among the Swedish population. No participants were prospectively assigned to an intervention. Thus, no clinical trial registration is required according to the recently updated NIH definition of a clinical trial. The primary outcome of the study was the incidence of T2D and it did not change during the analyses.
Healthy Nordic dietary indexes
Two modified and validated versions of the Northern Sweden FFQ were used: one with 84 food items and one with 64 items. Detailed information about the FFQs is published elsewhere (40–42). Food items were energy-adjusted by the density method for dietary indexes (31). We excluded 2 participants with an implausible caloric intake (<600 or >5000 kcal per day) and/or an incomplete FFQ (≥10% missing values).
The BSDS was derived based on the approach described in Kanerva et al. (43) and included 9 food components: 1) fruits (apples, pears, berries, oranges, mandarins, grapefruits); 2) vegetables (tomatoes, cucumbers, cabbage, legumes, carrot); 3) whole grains (whole-grain bread and rye/oat/barley porridge); 4) fish (perch, cod, Baltic herring, salmon, white fish, shellfish); 5) red and processed meat (beef, pork, processed meat); 6) low-fat milk products (milk with 0.5% fat, yogurt with ≤3% fat); 7) ratio of PUFAs to the sum of SFA and trans-fatty acids (fat ratio); 8) alcohol; and 9) total fat. All components except alcohol were scored according to gender-specific population consumption quartiles (Q1–Q4). For fruits, vegetables, whole grains, low-fat milk products, fish, and fat ratio, a score of 0, 1, 2, and 3 was given to questions Q1–Q4, respectively, whereas for red and processed meat and total fat, the score was given in reverse order. Alcohol scored 1 if intake was <20 g per day for men or <10 g per day for women, otherwise a score of 0 was given (43). The resulting BSDS ranged from 0 to 25 points, with higher scores representing stronger adherence to the Baltic Sea diet (43).
The HNFI was calculated according to Olsen et al. (21), based initially on 6 food items: fish, cabbage, rye bread, oatmeal, apples/pears, and root vegetables (carrots). However, because consumption of “rye bread”, “apples/pears”, and “oatmeal” was not assessed specifically by the FFQs used, we instead used “whole-grain bread”, “fruits (apples, pears, oranges, mandarins, grapefruits)”, and “oat/rye/barley porridge”, respectively. The index was calculated by summarizing scores, where each food item was scored 0 or 1 when intake was less than or greater than the sex-specific median intake of participants, respectively. The HNFI ranged from 0 to 6 and a higher score was indicative of stronger adherence (21).
Untargeted LC-MS metabolomics
Throughout this article, the term “feature” refers to a mass spectral peak, i.e., a molecular entity with a unique m/z and retention time as measured by an LC-MS instrument. The term “metabolite” refers to a compound, with or without identification.
The overall workflow of data processing is shown in Supplemental Figure 1. A detailed description of untargeted LC-MS metabolomics data acquisition and processing is published elsewhere (38, 44). In brief, de-proteinized fasting heparin plasma samples were analyzed by LC-qTOF-MS (Agilent Technologies), consisting of a 1290 LC system, a Jetstream electrospray ionization (ESI) source, and a 6540UHD accurate-mass qTOF spectrometer. Samples were analyzed on reverse phase (RP) and hydrophilic interaction chromatography (HILIC) columns, with the use of both positive (ESI+) and negative (ESI−) ionization modes. Data acquisition was performed with the MassHunter Acquisition B.04.00 software (Agilent Technologies). Plasma samples were analyzed in 8 batches and a constrained randomization was applied to keep sample pairs and follow-up samples within the same batch. Instrumental analyses were performed with ∼250 injections per batch, including study samples, and quality control samples constituted ∼16% of study samples to monitor the stability and functionality of the system (38, 44). Raw data acquired in each analytical batch were converted to mzXML format and deconvolution was performed with the open source R package “XCMS” (45). Two types of quality control samples were used to monitor the stability of the instrumental analysis, as described previously, and the R package “batchCorr” (44) was applied to correct for within- and between-batch measurement errors. Features potentially generated from a single metabolite were aggregated based on PUTMEDID-LCMS (46), to reduce the over-representation of a particular metabolite. Missing values were replaced with values randomly selected from a normal distribution between 0 and the lowest measured peak intensity within each feature. Poorly retained lipids in HILIC chromatography (retention time < 70 s) were removed from the data, because such lipids were better separated in RP chromatography.
Statistics
Identification of metabolites associated with healthy Nordic dietary indexes
Data obtained from RP and HILIC chromatography were processed independently. In brief, sparse partial least squares regression (R package “spls”) was applied as a prefilter to remove the majority of uninformative features per chromatographic mode (Supplemental Figure 1). The subset of features selected by “spls” was then modeled with a random forest algorithm incorporated into a repeated double crossvalidation framework with unbiased variable selection. This approach has been shown to effectively determine a parsimonious set of discriminative features ranked according to their importance with reduced risk of statistical overfitting (38, 47, 48). Model performance was confirmed by permutation analysis (n = 200, P < 10−7) (38, 49). The dietary index was calculated with the use of energy-adjusted FFQ data among healthy controls as the dependent variable in the model. In total, 84 and 73 features were selected to best predict the adherence to BSDS and HNFI, respectively.
For each feature selected by random forest, direct associations with each of the dietary indexes were assessed via partial Spearman correlation analysis, adjusting for case-control status, age at blood draw, gender, BMI (kg/m2), smoking status (smoker, former smoker, occasional smoker, non-smoker), education (elementary school, vocational school, secondary school, university education/college), and physical activity (inactive, moderately inactive, moderately active, active). The correlation analysis was repeated for individual food components of indexes. To compensate for multiple testing, Bonferroni-adjusted P values were calculated and the significance threshold was set at 0.05. In total, 29 and 16 features were independently associated with BSDS and HNFI, respectively, and thus were subjected to identification and further analyses (Supplemental Figure 1; see also “Metabolite identification” and Supplemental Table 1).
Associations between healthy Nordic dietary indexes and T2D
Associations between index-related metabolites at baseline and likelihood of developing T2D were investigated through the use of conditional logistic regression (R package “survival”). ORs were calculated for quartiles and per SD increment. Three models were constructed: Model 1: crude model with no adjustment; Model 2: adjustment for lifestyle-related factors, i.e., smoking status, education, physical activity at diet assessment, and daily energy intake (kcal per day); and Model 3: further adjustment for FPG (mmol/L). Sensitivity analyses were performed by adjusting further for BMI, total cholesterol (mmol/L), triglycerides (mmol/L), and systolic and diastolic blood pressure (mm Hg), because we regarded these variables as mediators rather than confounders (1). To compensate for multiple testing, false discovery rate–adjusted q values were calculated and the significance threshold was set at 0.05. In addition, we assessed the association between BSDS or HNFI and individual core food components of these indexes, measured by self-reported FFQ, and the risk of T2D.
Moreover, we performed principal component analysis (PCA) (R package “psych”) on index-related metabolites identified. We only considered components that had an eigenvalue >2 and that met the criteria of Very Simple Structure. The associations between PCA scores and risk of T2D were investigated through the use of conditional logistic regression, as mentioned earlier. Partial Spearman rank correlations between metabolites and energy-adjusted dietary variables, i.e., indexes and individual food categories reported in FFQs with <0.5% missing values, were calculated, adjusting for age, gender, case status, smoking status, education, and physical activity. A “triplot” was developed for multivariate risk modelling and visualization that combines dietary exposures, metabolome, and T2D risk. In addition, predictive ability of risk modeling for derived metabolite principal components (PCs) was assessed with the use of the area under the receiver operating characteristic curve (R package “pROC”).
Assessment of long-term reproducibility of metabolites
Reproducibility of metabolites was estimated by intraclass correlation (ICC) between the 2 sampling occasions over the 10-y period among the subset of healthy participants (n = 151). If the mean metabolite concentration between 2 sampling occasions differed significantly, indicating systematical differences over years, ICC was instead calculated on rank-transformed data. Predicted PC scores of the subset of controls were calculated by projecting metabolite concentrations from the subset of controls onto the initial PCA coordinate basis. ICCs of predicted PC scores were further estimated, reflecting the reproducibility of metabolite patterns.
Associations between previously identified dietary biomarkers of Nordic healthy foods and T2D
We also investigated associations between metabolites previously associated either with a healthy Nordic dietary pattern index, or with core foods of such a dietary pattern, and T2D risk. These metabolites were: trimethylamine N-oxide (associated with fish and/or meat) (50), 1-methylhistidine (meat and fish) (50), propionylcarnitine (meat) (50, 51), 2-methylbutyrylcarnitine (meat) (50, 51), threonate (fruits) (31), hippuric acid (fruits and vegetables) (30, 52, 53), indolepropionic acid [BSDS (31)], 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid (CMPF, fish) (54), 3-carboxy-4-methyl-5-pentyl-2-furanpropionic acid (fish, Mediterranean diet) (55, 56), and nonadecyl-benzenediol glucuronide (AR 19:0-gln, whole grains) (54).
All statistical analysis was conducted in R version 3.4.0, except for ICC, which was performed with the use of SAS macro “%ICC9” (SAS version 9.3, SAS Institute), available online from: https://www.hsph.harvard.edu/donna-spiegelman/software/icc9/.
Metabolite identification
For each of the features related to the 2 indexes (29 for BSDS and 16 for HNFI), identification was accomplished based on accurate mass (mass tolerance ≤15 ppm) and MS/MS fragmentation matched against online databases or the literature. The confidence level of annotation was categorized according to the Metabolomics Standard Initiative (MSI) (57). Annotated classes (MSI 3) were presented as “putative chemical class mass @retention time”, whereas unknown compounds (MSI 4) were presented as “analytical mode m/z @ retention time” only (Supplemental Table 1).
RESULTS
Baseline characteristics of the study population are shown in Table 1. Several known T2D risk factors were higher in participants who later developed T2D than among controls at baseline. Regardless of T2D development, intake of food components of the indexes increased with higher adherence to the corresponding indexes, and food components scored in reverse order were consumed to a lesser extent by participants who scored high (Supplemental Figures 2 and 3). The score distribution of the indexes did not differ between cases and controls (Supplemental Figure 4). No significant differences were observed in known T2D risk factors across low to high reported adherence to the indexes (Supplemental Figures 5 and 6).
TABLE 1.
Baseline characteristics of participants in the Swedish Västerbotten Intervention Programme who were included in the nested case-control study of incident type 2 diabetes1
| Characteristic | Cases (n = 421) | Controls (n = 421) | P |
|---|---|---|---|
| Men2, % | 46.5 | 46.5 | |
| Age2, y | 50.4 ± 7.9 | 50.4 ± 7.9 | |
| BMI, kg/m2 | 29.7 ± 4.72 | 25.5 ± 3.8 | <0.001 |
| Fasting plasma glucose, mmol/L | 6.1 ± 0.9 | 5.5 ± 1.2 | <0.001 |
| 2-h plasma glucose, mmol/L | 8.4 ± 2.8 | 6.5 ± 1.6 | <0.001 |
| Triglycerides, mmol/L | 2.0 ± 1.3 | 1.3 ± 0.7 | <0.001 |
| Total cholesterol, mmol/L | 5.9 ± 1.3 | 5.7 ± 1.1 | 0.005 |
| Systolic blood pressure, mm Hg | 138.9 ± 17.8 | 128.1 ± 16.7 | <0.001 |
| Diastolic blood pressure, mm Hg | 85.1 ± 10.4 | 79.4 ± 9.7 | <0.001 |
| Total caloric intake, kcal/d | 1742.5 ± 617.5 | 1745.8 ± 622.7 | 0.58 |
| Smoking status, % | 0.03 | ||
| Smoker | 21.7 | 18.2 | |
| Former smoker | 29.1 | 26.4 | |
| Occasional smoker | 0.7 | 3.8 | |
| Former occasional smoker | 9.5 | 6.9 | |
| Non-smoker | 39.0 | 44.2 | |
| Physical activity index, % | 0.08 | ||
| Inactive | 17.2 | 16.3 | |
| Moderately inactive | 36.4 | 35.0 | |
| Moderately active | 28.8 | 28.1 | |
| Active | 17.7 | 20.1 | |
| Education, % | 0.03 | ||
| Elementary school | 33.3 | 28.6 | |
| Vocational (training) school | 28.8 | 26.2 | |
| Secondary school | 21.5 | 21.1 | |
| University education/college | 16.3 | 23.6 | |
| BSDS and food components,3 g · d−1 · 1000 kcal−1 | |||
| BSDS | 13 (7, 19) | 13 (7, 19) | 0.74 |
| Fruits | 59.1 (10.9, 161.9) | 59.7 (12.5, 159.1) | 0.97 |
| Vegetables | 44.6 (10.1, 145.7) | 41.2 (10.4, 138.9) | 0.59 |
| Whole grains | 49.1 (15.3, 122) | 45.9 (15.3, 128.7) | 0.59 |
| Fish | 9.8 (0.5, 21.5) | 9.7 (2.2, 19.3) | 0.43 |
| Red/processed meat | 46.6 (27.3, 74.7) | 43.7 (24.6, 72.2) | 0.06 |
| Low-fat dairy products | 19.6 (0, 207.3) | 14.7 (0, 229.6) | 0.68 |
| Fat ratio | 0.3 (0.2, 0.5) | 0.3 (0.2, 0.5) | 0.28 |
| Total fat | 36.9 (28.1, 47.1) | 36.3 (28.3, 44.0) | 0.05 |
| Alcohol | 1.8 (0, 7.6) | 2.3 (0, 9.9) | 0.04 |
| HNFI and food components,4 g · d−1 · 1000 kcal−1 | |||
| HNFI | 3 (1, 5) | 3 (1, 5) | 0.91 |
| Fruits | 54.7 (8.1, 155.7) | 53.5 (10, 143.9) | 0.82 |
| Cabbage | 8.5 (0.8, 39.6) | 8.4 (0.8, 34.8) | 0.75 |
| Carrot | 12.4 (1.5, 56.6) | 10.2 (1.4, 50.7) | 0.34 |
| Whole-grain bread | 31.8 (10.7, 60.6) | 30.7 (10.9, 63.6) | 0.96 |
| Whole-grain porridge | 8.9 (0, 85.2) | 9.8 (0, 74.3) | 0.86 |
| Fish | 9.8 (0.5, 21.5) | 9.7 (2.2, 19.3) | 0.43 |
1Clinical parameters are means ± SDs or percentages. Index and corresponding food component values are median (10th, 90th percentile). P value indicates the significance between cases and controls. Differences in medians of dietary variables were tested by the use of Wilcoxon's rank-sum test, and differences in categorical variables were assessed by the use of a chi-square test. BSDS, Baltic Sea Diet Score; HNFI, Healthy Nordic Food Index.
2Matching factors.
3BSDS calculated based on 9 food components: fruits (apples, pears, oranges, mandarins, grapefruits, berries), vegetables (tomatoes, cucumbers, cabbage, legumes, carrots), whole grains (whole-grain bread and barley/oat/rye porridge), fish (perch, cod, Baltic herring, salmon, white fish, shellfish), red and processed meat (beef, pork, processed meats, sausages), low-fat milk products (milk with 0.5% fat, yogurt with ≤3% fat), ratio of PUFAs to the sum of SFA and trans-fatty acids (fat ratio), total fat, and alcohol.
4HNFI calculated based on 6 food components: fruits (apples, pears, oranges, mandarins, grapefruits), cabbage, carrot, whole-grain bread, whole-grain porridges (oat/rye/barley porridge), and fish (same species as BSDS).
We identified 24 individual metabolites associated with BSDS (r = −0.19 to 0.21), independent of case/control status, age, gender, BMI, and lifestyle-related factors, and 13 metabolites associated with HNFI (r = −0.17 to 0.16) (Table 2). Among these metabolites, 6 were associated with both indexes: docosahexaenoic acid (DHA), lysophatidylethanolamine (lysoPE 22:6), γ-tocopherol, and 3 unknown metabolites, i.e., RP383.1671@10.53, RP490.3516@8.99, and RP197.0926@3.17. Metabolites that were positively associated with the indexes were also positively correlated with whole grains, fruits, vegetables, and fish, and negatively correlated with red/processed meat and total fat. The opposite was observed for metabolites that were inversely associated with the indexes (Table 2).
TABLE 2.
Plasma metabolites identified in fasting samples at baseline and their associations with the BSDS and HNFI dietary indexes in a nested case-control study in the Västerbotten Intervention Programme1
| Metabolite2 | r With index3 | r With food components3 | ICC4 |
|---|---|---|---|
| BSDS and HNFI | |||
| DHA | 0.19, 0.17 | Fish (0.20), fruits (0.11), alcohol (0.12) | 0.46 (0.34, 0.58) |
| RP383.1671@10.53 | 0.16, 0.14 | Fish (0.18), alcohol (0.15) | 0.44 (0.31, 0.57) |
| RP490.3516@8.99 | 0.16, 0.15 | Fish (0.25), FR (0.12), alcohol (0.18) | 0.47 (0.35, 0.60) |
| lysoPE(22:6) | 0.14, 0.11 | Fish (0.11), whole grains (0.13) | 0.32 (0.19, 0.47) |
| γ-Tocopherol | −0.17, −0.12 | Meat (0.13), alcohol (−0.16), vegetables (−0.17) | 0.16 (0.09, 0.33) |
| RP197.0926@3.17 | −0.12, −0.12 | Alcohol (0.13), carrot (−0.15) | 0.27 (0.14, 0.44) |
| BSDS | |||
| RP303.6468@9.98 | 0.22 | Fish (0.28), low-fat milk products (0.13), FR (0.15), alcohol (0.14), fruits (0.13) | 0.47 (0.35, 0.59) |
| RP684.5545@13.41 | 0.20 | Fish (0.21), alcohol (0.13), FR (0.12) | 0.47 (0.42, 0.63) |
| lysoPC(22:6) | 0.18 | Fish (0.23), alcohol (0.13), vegetables (0.13) | 0.42 (0.30, 0.55) |
| RP826.5442@12.17 | 0.16 | Fish (0.17), fruits (0.14) | 0.33 (0.20, 0.48) |
| RN427.1641@10.59 | 0.16 | Fish (0.13), fruits (0.13), total fat (−0.12) | 0.35 (0.23, 0.50) |
| EPA | 0.15 | Fish (0.21) | 0.29 (0.17, 0.45) |
| RN153.0190@3.5 | 0.14 | Meat (−0.16), whole grains (0.20) | 0.59 (0.48, 0.69) |
| HP143.1179@1.55 | 0.14 | Fruits (0.12) | 0.40 (0.28, 0.54) |
| RN446.1541@10.59 | 0.14 | FR (0.12) | |
| RP827.5356@11.88 | 0.12 | Fish (0.13), total fat (−0.12) | 0.37 (0.24, 0.51) |
| Steroid glucuronide 659.3664@9.4 | 0.12 | Whole grains (0.22) | 0.49 (0.37, 0.61) |
| RP816.5684@12.52 | 0.12 | Vegetables (0.11) | 0.47 (0.35, 0.60) |
| RN201.1498@8.23 | −0.14 | FR (−0.23), low-fat milk products (−0.16), total fat (0.13), vegetables (−0.12) | 0.35 (0.22, 0.50) |
| RN830.5845@12.46 | −0.12 | Vegetables (−0.12) | 0.15 (0.05, 0.37) |
| lysoPC(18:0) | −0.16 | FR (−0.26), low-fat milk products (−0.16) | 0.25 (0.13, 0.43) |
| PC(18:2/15:0) | −0.14 | Fish (−0.16), alcohol (−0.15), FR (−0.23), low-fat milk products (−0.14) | 0.35 (0.22, 0.50) |
| RN814.5610@12.14 | −0.18 | Fish (−0.16), alcohol (−0.21), FR (−0.21), low-fat milk products (−0.13), vegetables (−0.16), fruits (−0.13) | 0.11 (0.02, 0.38) |
| RP431.3516@11.96 | −0.17 | Vegetables (−0.15), meat (0.13) | 0.23 (0.12, 0.39) |
| HNFI | |||
| RN472.1596@10.53 | 0.16 | Alcohol (0.14), FR (0.14), fish (0.15), cabbage (0.13) | 0.15 (0.05, 0.37) |
| HN151.0065@4.97 | 0.13 | Cabbage (0.13) | 0.37 (0.24, 0.51) |
| RN457.1742@10.50 | 0.13 | Cabbage (0.14), FR (0.14), vegetables (0.13) | 0.34 (0.22, 0.49) |
| 3,4,5-trimethoxycinnamic acid | 0.12 | Fish (0.14), whole grains (0.15) | 0.34 (0.22, 0.49) |
| RP225.1482@9.15 | 0.12 | Fruits (0.13), whole grains (0.16) | 0.44 (0.32, 0.57) |
| Pipecolic acid betaine | 0.11 | Whole grains (0.22) | 0.27 (0.15, 0.44) |
| RN203.0022@3.00 | 0.11 | 0.49 (0.37, 0.61) | |
1BSDS, Baltic Sea Diet Score; FR, fat ratio; HN, hydrophilic interaction chromatography ESI– mode; HNFI, Healthy Nordic Food Index; HP, hydrophilic interaction chromatography ESI+ mode; ICC, intraclass correlation coefficient; lysoPC, lysophosphatidylcholines; lysoPE, lysophatidylethanolamine; MSI, Metabolomics Standard Initiative; RN, reverse phase chromatography ESI− mode; RP, reverse phase chromatography ESI+ mode.
2Identified metabolites associated with the healthy Nordic dietary indexes. Metabolites denoted as classes (MSI 3) are presented as “putative chemical class mass @retention time”, whereas unknown compounds (MSI 4) are presented as “analytical mode m/z @ retention time”.
3Partial Spearman rank correlation coefficients between metabolites and dietary indexes (BSDS, HNFI) and energy-adjusted individual food components of indexes, controlling for age, gender, case/control status, BMI, smoking status, education, and physical activity index. Statistically significant with Bonferroni correction for multiple statistical testing at P < 0.05.
4Representing long-term reproducibility of metabolites among healthy controls (n = 151) over 10 y. ICC ≥ 0.4 denotes good reproducibility.
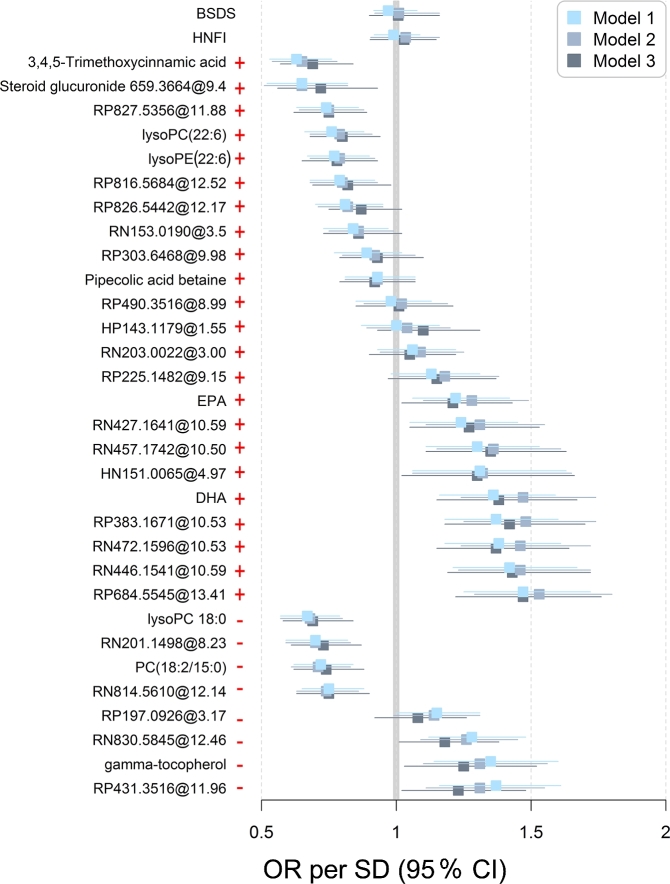
Among the 31 index-related metabolites, 12 were positively associated with risk of T2D and 10 were inversely associated independently of baseline lifestyle factors (Figure 2, Supplemental Table 2). Further adjustment for FPG did not substantially affect the results. However, no associations remained significant after further controlling for BMI, triglycerides, total cholesterol, and blood pressure. We did not find any association between the indexes, or individual foods included in the indexes derived from the FFQ, and future T2D (Supplemental Table 3).
FIGURE 2.
ORs per SD increment (95% CI) of type 2 diabetes calculated with conditional logistic regression for individual index-related metabolites. Model 1: crude model. Model 2: adjustment for lifestyle-related factors, i.e., smoking status, education, physical activity at diet assessment, and daily energy intake (kilocalories per day). Model 3: further adjustment for fasting plasma glucose (millimoles per liter). BSDS, Baltic Sea Diet Score; HNFI, Healthy Nordic Food Index; lysoPC, lysophosphatidylcholines; lysoPE, lysophatidylethanolamine; +, positive association with dietary indexes; –, inverse association with dietary indexes.
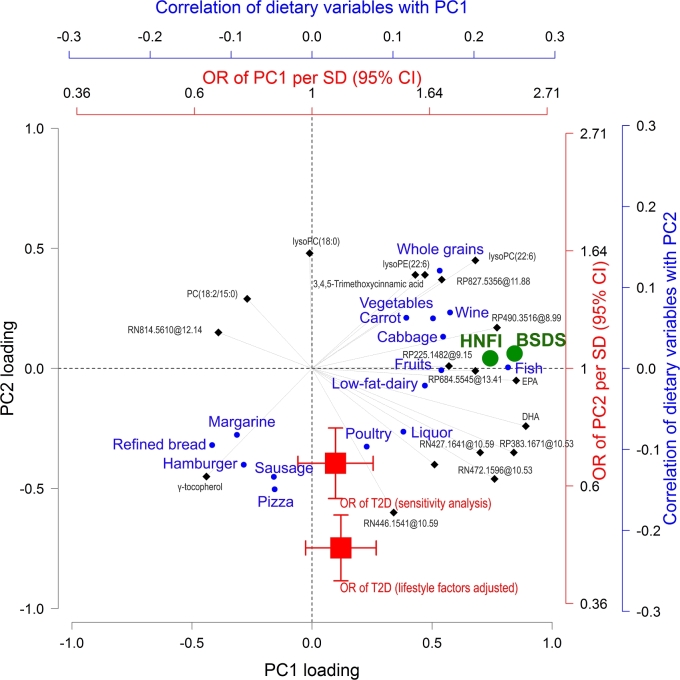
To account for the intercorrelations among the metabolites (−0.39 ≤ r ≤ 0.85, Supplemental Figure 7), 2 PCs were determined that accounted for 74% of the total variance in metabolite concentrations (Supplemental Table 4). PCs’ scores and individual metabolites were strongly correlated with the 2 dietary indexes, food components of the indexes, and to varying degrees with several food categories not captured by the indexes (Supplemental Figure 8). A “triplot” was developed to consider the intercorrelations between metabolites (PCA loadings), multiple dietary variables (correlation with PCA scores), and T2D risk (ORs of PCA score) simultaneously (Figure 3): PC1 was strongly associated with reported adherence to the indexes but was not associated with T2D risk. In contrast, PC2 related strongly in particular to consumption of, e.g., sausage, hamburger, or pizza, and was strongly associated with T2D risk, which yielded substantially better risk prediction than PC1 (Supplemental Figure 9).
FIGURE 3.
Associations between diet, index-related metabolites, and risk of developing T2D. The “triplot” represents the correlations between metabolites, dietary variables, and ORs of T2D. Correlations between PC scores and dietary variables (individual foods in blue circles and dietary indexes in green circles) and ORs per SD (95% CI) of T2D for scores (red squares) are superimposed. Correlations between PC scores and dietary variables are visualized if partial Spearman correlation at a Bonferroni-adjusted P < 0.05 controlled for age, gender, case/control status, BMI, smoking status, education, and physical activity. ORs were obtained from a lifestyle factors–adjusted conditional logistic regression model and a model further adjusted for fasting plasma glucose (millimoles per liter), BMI, total cholesterol (millimoles per liter), triglycerides (millimoles per liter), and blood pressure (mm Hg). For simple interpretation, metabolites, individual foods, and ORs pulling along the same axis in the plot are associated. Dietary indexes are orthogonal to ORs and therefore not associated with T2D risk. Metabolites denoted as classes (MSI 3) are presented as “putative chemical class mass @retention time,” whereas unknown compounds (MSI 4) are presented as “analytical mode m/z @ retention time.” HN, hydrophilic interaction chromatography ESI− mode; HP, hydrophilic interaction chromatography ESI+ mode; lysoPC, lysophosphatidylcholines; lysoPE, lysophatidylethanolamine; MSI, Metabolomics Standard Initiative; PC, principal component; RN, reverse phase chromatography ESI− mode; RP, reverse phase chromatography ESI+ mode; T2D, type 2 diabetes.
Among the 31 index-related metabolites, 15 showed good reproducibility among healthy controls (0.4 ≤ ICC ≤ 0.59) (Table 2). PC1 showed higher reproducibility (ICC 0.46) than PC2 (ICC 0.35).
In addition, among 10 a priori-selected metabolites previously known to be associated with a healthy Nordic diet, we confirmed associations for indolepropionic acid (BSDS), CMPF (fish), 3-carboxy-4-methyl-5-pentyl-2-furanpropionic acid (fish), threonate (vegetables), and AR 19:0-Gln (whole grains) (Supplemental Figure 10). No association between these metabolites and T2D risk was found, with the exception of indolepropionic acid, which was inversely associated (Supplemental Table 2).
DISCUSSION
We identified plasma metabolites associated with 2 healthy Nordic dietary indexes by applying untargeted metabolomics in combination with a robust data processing pipeline. For the first time, to our knowledge, we used both FFQ data and metabolomics to investigate the association between these indexes and T2D risk. Our study also provides information on the reproducibility of metabolites over 10 y and highlights the potential of some index-related metabolites as biomarkers representing a habitual dietary intake in free-living populations. Moreover, the metabolomics investigations provided complementary information to dietary indexes, and confirmed the lack of association between stronger adherence to a healthy Nordic dietary index and T2D risk. In contrast, foods such as pizza, hamburgers, and sausage were predominately associated with increased T2D risk in the current population.
Associations of plasma metabolites with healthy Nordic dietary indexes
Overall, the magnitude of correlations between indexes and metabolites was moderate and in the same range as previously reported for BSDS and other indexes, e.g., Healthy Eating Index 2010 and the WHO Healthy Diet Indicator (r = −0.14 to ∼0.25) (31). Only 6 metabolites were related to both BSDS and HNFI. The small overlap may be attributed to the different food items used to score adherence (31).
We identified metabolites with good reproducibility over 10 y (ICC ≥ 0.4) that have been suggested as biomarkers of particular foods that are key contributors to the indexes, i.e., EPA and DHA (fish) (58, 59), and pipecolic acid betaine (whole grains) (54, 60). However, the underlying causes of the associations between most individual metabolites and dietary indexes are not easily interpreted. The majority of index-related metabolites were associated with multiple food items included in the indexes, which may possibly reflect the co-consumption of foods in the study population (35, 61, 62), or endogenous formation of these metabolites in response to multiple dietary exposures (32). Of note, our data clearly suggest that the latent variable of index-related metabolites accounted for intercorrelations (PC1), could better reflect adherence to the indexes, compared to individual metabolites and showed good long-term reproducibility. This observation is in line with other studies supporting that multiple biomarkers may provide more accurate measures of dietary exposures. Moreover, a dietary index may have a metabolite profile that mainly reflects the underlying components used to score adherence, but the concentrations of index-related metabolites may be affected by other foods that are not part of the index (Figure 3, Supplemental Figure 7), in agreement with previous studies (30, 63). For instance, γ-tocopherol was inversely correlated with indexes, fruits, and vegetables and positively correlated with margarine, oil, and hamburgers. Collectively, our results suggest that index-related metabolites have great potential to be reflective of adherence to habitual dietary patterns and might provide information complementary to the FFQ-based indexes consisting of limited food components.
Associations between healthy Nordic dietary indexes and T2D
We found no association between healthy Nordic dietary indexes and the risk of developing T2D. This was surprising, given the beneficial cardiometabolic effects shown for healthy Nordic dietary patterns and/or food items in dietary intervention studies and observational studies (11, 19, 22, 64). In the present study, the metabolite pattern that was strongly correlated with healthy Nordic dietary indexes and their core food components (in particular, fish and fruits) was almost orthogonal to ORs of T2D direction, indicating a lack of association with T2D risk. Fish has been shown to have health-promoting effects on T2D prevention (65–68), although contradictory results exist (69, 70). It is regarded as a central healthy item in both indexes, with positive scores. We found no association between FFQ-measured fish intake and T2D; instead, positive associations between well-known biomarkers of fish consumption, i.e., EPA and DHA, were observed. This observation may relate to the presence of persistent organic pollutants known to occur in fish from the Baltic Sea (71–73), which might adversely affect the potential beneficial role of fish (9, 73). Moreover, although mechanisms remain unclear, our data suggest that fruit consumption was not associated with T2D risk, in line with previous studies (6, 74). In contrast, whole grains and some vegetables correlated with several index-related metabolites with high PC2 loadings that were inversely associated with T2D risk, supporting their beneficial role in T2D prevention (3, 7). Of note, the likelihood of developing T2D was predominantly associated with higher consumptions of foods that were not part of the indexes, such as pizza, hamburger, and sausages, in line with a previous study applying data-driven methods (75).
In addition to the metabolites identified through the use of the data-driven approach, we also assessed, to our knowledge for the first time, whether a priori–defined metabolites shown to be affected in response to core foods of a healthy Nordic diet in dietary interventions could be used as dietary exposure biomarkers in a free-living population and their associations with T2D risk. Our results highlighted the potential of AR 19:0-Gln, CMPF, 3-carboxy-4-methyl-5-pentyl-2-furanpropionic acid, and threonate as biomarkers of whole grains, fish, and vegetables. However, the null association of these biomarkers with T2D risk confirmed the limited effect of “healthy” foods in T2D prevention in the current population. Although BSDS-related indolepropionic acid was inversely associated with T2D, the poor reproducibility (ICC = 0.23) limits its use as a dietary biomarker and may affect the precision in risk estimates.
Strengths and limitations
This is the first study to our knowledge to investigate associations between adherence to a healthy Nordic diet, assessed both by FFQ and by biomarkers, and future T2D. Identifying metabolites related to the established dietary indexes, instead of patterns derived with data-driven approaches, allows for other studies conducted in different populations to reproduce and validate our findings. Moreover, we assessed the long-term reproducibility of index-related metabolites, suggesting whether they could be used for objective assessment of dietary habits, which has rarely been investigated before.
There are several limitations of the study. First, our dietary data were collected via FFQs, a method which is known to suffer from large measurement errors (25, 26, 76). In fact, under-reporting was indicated to a larger extent among cases (n = 61) than controls (n = 38), as measured by the number of individuals below the 10th percentile of the ratio between total caloric intake and estimated basal metabolic rate. However, exclusion of case-control pairs, based on indicated under-reporting, did not significantly affect the results (data not shown). Second, observed diet-metabolite associations are most likely weaker than the true associations owing to the measurement errors in self-reported dietary intake data and variations in metabolites (31). Our study may be subject to false-negative results, i.e., we may have missed some actual associations, which may hamper discovery of potential diet-disease relations. However, in the additional analysis, no association between potential biomarkers of key foods of healthy Nordic dietary indexes and T2D helped to confirm the limited effect of “healthy” foods in preventing T2D. Third, even with extensive efforts in metabolite identification, we did not manage to annotate all metabolites. However, unknown compounds did not affect the investigation and interpretation of our major primary focus in this study, i.e., the associations between studied dietary indexes and T2D risk. Furthermore, we cannot exclude the risk of residual confounding because of unmeasured confounders related to lifestyle habits in models. In addition, only 1 previous observational study has explored serum metabolites associated with established healthy dietary indexes, including BSDS, but metabolite profiles are difficult to compare with our data owing to a large number of unknown metabolites in both metabolite sets (31). Replication of our findings in other cohorts is necessary.
In conclusion, several fasting plasma metabolites were identified to be associated with predefined healthy Nordic dietary indexes. However, no association was observed between the 2 dietary indexes investigated and T2D risk, either for intake data derived from FFQs or for biomarkers of exposures. Foods not covered by the indexes, e.g., hamburgers, pizza, and sausages, appeared to be major contributors to increased risk of developing T2D in the current population.
Supplementary Material
Acknowledgements
We thank Västerbotten County Council and the organizers of the Västerbotten Intervention Programme and DiabNorth for making samples and data available.
The authors’ responsibilities were as follows—LS: participated in sample analyses, performed data processing and statistical analyses, interpreted the data, and drafted the manuscript; CB, IJ, IAB, BL, and RL: conceived and designed the study; CB: supervised statistical analyses and result interpretation, and revised the manuscript; IJ, IAB, KH, and BL: interpreted the data and provided critical intellectual input; KH: participated in the untargeted LC-MS analysis and metabolite identification; RL: supervised data analyses and interpretation, revised the manuscript, and had the overall responsibility for the project; and all authors: critically reviewed the manuscript and approved the final version. None of the authors reported a conflict of interest related to the study.
Notes
Supported by SLU (Sveriges Lantbruksuniversitet) through a young investigators’ quality grant (to RL). The China Scholarship Council provided scholarship funding to LS. A grant from the Swedish Research Council for Health, Working Life and Welfare (FORTE) supported the set-up of the case-control study. The LC-MS metabolomics unit at University of Eastern Finland is supported by Biocenter Finland, and KH holds an Academy of Finland Research Fellowship.
None of the funding bodies had any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental Tables 1–4 and Supplemental Figures 1–10 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- BSDS
Baltic Sea Diet Score
- CMPF
3-carboxy-4-methyl-5-propyl-2-furanpropionic acid
- ESI
electrospray ionization
- FFQ
food-frequency questionnaire
- FPG
fasting plasma glucose
- HILIC
hydrophilic interaction chromatography
- HNFI
Healthy Nordic Food Index
- ICC
intraclass correlation
- LC-MS
liquid chromatography-mass spectrometry
- MSI
Metabolomics Standard Initiative
- PC
principal component
- PCA
principal component analysis
- Q
quartile
- RP
reverse-phase chromatography
- T2D
type 2 diabetes
- VIP
Västerbotten Intervention Programme
REFERENCES
- 1. Lacoppidan S, Kyrø C, Loft S, Helnæs A, Christensen J, Hansen C, Dahm C, Overvad K, Tjønneland A, Olsen A. Adherence to a healthy Nordic food index is associated with a lower risk of type-2 diabetes—the Danish Diet, Cancer and Health cohort study. Nutrients 2015;7:8633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abiemo EE, Alonso A, Nettleton JA, Steffen LM, Bertoni AG, Jain A, Lutsey PL. Relationships of the Mediterranean dietary pattern with insulin resistance and diabetes incidence in the Multi-Ethnic Study of Atherosclerosis (MESA). Br J Nutr 2012;8:1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parker ED, Liu S, Van Horn L, Tinker LF, Shikany JM, Eaton CB, Margolis KL.. The association ofwhole grain consumptionwith incident type 2 diabetes: the Women's Health Initiative Observational Study. Ann Epidemiol 2013;23:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biskup I, Kyrø C, Marklund M, Olsen A, Van Dam RM, Tjonneland A, Overvad K, Lindahl B, Johansson I, Landberg R. Plasma alkylresorcinols, biomarkers of whole-grain wheat and rye intake, and risk of type 2 diabetes in Scandinavian men and women. Am J Clin Nutr 2016;104:88–96. [DOI] [PubMed] [Google Scholar]
- 5. Mursu J, Virtanen JK, Tuomainen T-P, Nurmi T, Voutilainen S. Intake of fruit, berries, and vegetables and risk of type 2 diabetes in Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 2014;99:328–33. [DOI] [PubMed] [Google Scholar]
- 6. Li M, Fan Y, Zhang X, Hou W, Tang Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: meta-analysis of prospective cohort studies. BMJ Open 2014;4:e005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwingshackl L, Hoffmann G, Lampousi A-M, Knüppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol 2017;32:363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ding M, Bhupathiraju SN, Chen M, Van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care 2014;37:569–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marushka L, Batal M, David W, Schwartz H, Ing A, Fediuk K, Sharp D, Black A, Tikhonov C, Chan HM. Association between fish consumption, dietary omega-3 fatty acids and persistent organic pollutants intake, and type 2 diabetes in 18 First Nations in Ontario, Canada. Environ Res 2017;156:725–37. [DOI] [PubMed] [Google Scholar]
- 10. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 2011;94:1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khakimov B, Poulsen SK, Savorani F, Acar E, Larsen TM, Astrup A, Dragsted LO, Engelsen SB, Lacoppidan SA, Kyrø C et al. Associations of adherence to the New Nordic Diet with risk of preeclampsia and preterm delivery in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutr 2014;17:920–7. [Google Scholar]
- 12. Alhazmi A, Stojanovski E, McEvoy M, Garg ML. The association between dietary patterns and type 2 diabetes: a systematic review and meta-analysis of cohort studies. J Hum Nutr Diet 2014;27:251–60. [DOI] [PubMed] [Google Scholar]
- 13. Jacobs S, Boushey CJ, Franke AA, Shvetsov YB, Monroe KR, Haiman CA, Kolonel LN, Le Marchand L, Maskarinec G. A priori-defined diet quality indices, biomarkers and risk for type 2 diabetes in five ethnic groups: the Multiethnic Cohort. Br J Nutr 2017;118:312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Koning L, Chiuve SE, Fung TT, Willett WC, Rimm EB, Hu FB. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care 2011;34:1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adamsson V, Reumark A, Cederholm T, Vessby B, Risérus U, Johansson G. What is a healthy Nordic diet? Foods and nutrients in the NORDIET study. Food Nutr Res 2012;56:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adamsson V, Reumark A, Fredriksson IB, Hammarström E, Vessby B, Johansson G, Risérus U. Effects of a healthy Nordic diet on cardiovascular risk factors in hypercholesterolaemic subjects: a randomized controlled trial (NORDIET). J Intern Med 2011;269:150–9. [DOI] [PubMed] [Google Scholar]
- 17. Adamsson V, Cederholm T, Vessby B, Risérus U. Influence of a healthy Nordic diet on serum fatty acid composition and associations with blood lipoproteins – results from the NORDIET study. Food Nutr Res 2014;58:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Damsgaard CT, Dalskov S-M, Petersen RA, Sørensen LB, Mølgaard C, Biltoft-Jensen A, Andersen R, Thorsen AV, Tetens I, Sjödin A et al. Design of the OPUS School Meal Study: a randomised controlled trial assessing the impact of serving school meals based on the New Nordic Diet. Scand J Public Health 2012;40:693–703. [DOI] [PubMed] [Google Scholar]
- 19. Uusitupa M, Hermansen K, Savolainen MJ, Schwab U, Kolehmainen M, Brader L, Mortensen LS, Cloetens L, Johansson-Persson A, Önning G et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome – a randomized study (SYSDIET). J Intern Med 2013;274:52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poulsen SK, Due A, Jordy AB, Kiens B, Stark KD, Stender S, Holst C, Astrup A, Larsen TM. Health effect of the New Nordic Diet in adults with increased waist circumference: a 6-mo randomized controlled trial. Am J Clin Nutr 2014;99:35–45. [DOI] [PubMed] [Google Scholar]
- 21. Olsen A, Egeberg R, Halkjaer J, Christensen J, Overvad K, Tjonneland A. Healthy aspects of the Nordic diet are related to lower total mortality. J Nutr 2011;141:639–44. [DOI] [PubMed] [Google Scholar]
- 22. Roswall N, Sandin S, Löf M, Skeie G, Olsen A, Adami HO, Weiderpass E. Adherence to the healthy Nordic food index and total and cause-specific mortality among Swedish women. Eur J Epidemiol 2015;30:509–17. [DOI] [PubMed] [Google Scholar]
- 23. Kanerva N, Kaartinen NE, Schwab U, Lahti-Koski M, Männistö S. Adherence to the Baltic Sea diet consumed in the Nordic countries is associated with lower abdominal obesity. Br J Nutr 2013;109:520–8. [DOI] [PubMed] [Google Scholar]
- 24. Kanerva N, Rissanen H, Knekt P, Havulinna AS, Eriksson JG, Männistö S. The healthy Nordic diet and incidence of type 2 diabetes—10-year follow-up. Diabetes Res Clin Pract 2014;106:34–7. [DOI] [PubMed] [Google Scholar]
- 25. Bennett DA, Landry D, Little J, Minelli C. Systematic review of statistical approaches to quantify, or correct for, measurement error in a continuous exposure in nutritional epidemiology. BMC Med Res Methodol 2017;17:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paeratakul S, Popkin BM, Kohlmeier L, Hertz-Picciotto I, Guo X, Edwards LJ. Measurement error in dietary data: implications for the epidemiologic study of the diet-disease relationship. Eur J Clin Nutr 1998;52:722–7. [DOI] [PubMed] [Google Scholar]
- 27. Scalbert A, Brennan L, Manach C, Andres-Lacueva C, Dragsted LO, Draper J, Rappaport SM, van der Hooft JJ, Wishart DS. The food metabolome: a window over dietary exposure. Am J Clin Nutr 2014;99:1286–308. [DOI] [PubMed] [Google Scholar]
- 28. Bordoni A, Capozzi F. The foodomics approach for discovering biomarkers of food consumption in nutrition studies. Curr Opin Food Sci 2015;4:124–8. [Google Scholar]
- 29. Brennan L, Hu FB. Metabolomics-based dietary biomarkers in nutritional epidemiology—current status and future opportunities. Mol Nutr Food Res 2018;Apr 24:1701064. doi: 10.1002/mnfr.201701064. [DOI] [PubMed] [Google Scholar]
- 30. Garcia-Perez I, Posma JM, Gibson R, Chambers ES, Hansen TH, Vestergaard H, Hansen T, Beckmann M, Pedersen O, Elliott P et al. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. LANCET Diabetes Endocrinol 2017;5:184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Playdon MC, Moore SC, Derkach A, Reedy J, Subar AF, Sampson JN, Albanes D, Gu F, Kontto J, Lassale C et al. Identifying biomarkers of dietary patterns by using metabolomics. Am J Clin Nutr 2017;105:450–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Esko T, Hirschhorn JN, Feldman HA, Hsu YHH, Deik AA, Clish CB, Ebbeling CB, Ludwig DS. Metabolomic profiles as reliable biomarkers of dietary composition. Am J Clin Nutr 2017;105:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Gorman A, Brennan L. Metabolomic applications in nutritional research: a perspective. J Sci Food Agric 2015;95:2567–70. [DOI] [PubMed] [Google Scholar]
- 34. Savolainen O, Lind MV, Bergström G, Fagerberg B, Sandberg A-S, Ross A. Biomarkers of food intake and nutrient status are associated with glucose tolerance status and development of type 2 diabetes in older Swedish women. Am J Clin Nutr 2017;31:1–9. [DOI] [PubMed] [Google Scholar]
- 35. Playdon MC, Ziegler RG, Sampson JN, Stolzenberg-Solomon R, Thompson HJ, Irwin ML, Mayne ST, Hoover RN, Moore SC. Nutritional metabolomics and breast cancer risk in a prospective study. Am J Clin Nutr 2017;106:637–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Floegel A, von Ruesten A, Drogan D, Schulze MB, Prehn C, Adamski J, Pischon T, Boeing H. Variation of serum metabolites related to habitual diet: a targeted metabolomic approach in EPIC-Potsdam. Eur J Clin Nutr 2013;67:1100–8. [DOI] [PubMed] [Google Scholar]
- 37. Norberg M, Wall S, Boman K, Weinehall L. The Västerbotten Intervention Programme: background, design and implications. Glob Health Action 2010;3:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi L, Brunius C, Lehtonen M, Auriola S, Bergdahl IA, Rolandsson O, Hanhineva K, Landberg R. Plasma metabolites associated with type 2 diabetes in a Swedish population: a case–control study nested in a prospective cohort. Diabetologia 2018;61:849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rolandsson O, Norberg M, Nyström L, Söderberg S, Svensson M, Lindahl B, Weinehall L. How to diagnose and classify diabetes in primary health care: lessons learned from the Diabetes Register in Northern Sweden (DiabNorth). Scand J Prim Health Care 2012;30:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johansson I, Hallmans G, Wikman A, Biessy C, Riboli E, Kaaks R. Validation and calibration of food-frequency questionnaire measurements in the Northern Sweden Health and Disease cohort. Public Health Nutr 2002;5:487–96. [DOI] [PubMed] [Google Scholar]
- 41. Johansson I, Van Guelpen B, Hultdin J, Johansson M, Hallmans G, Stattin P. Validity of food frequency questionnaire estimated intakes of folate and other B vitamins in a region without folic acid fortification. Eur J Clin Nutr 2010;64:905–13. [DOI] [PubMed] [Google Scholar]
- 42. Johansson I, Nilsson LM, Stegmayr B, Boman K, Hallmans G, Winkvist A. Associations among 25-year trends in diet, cholesterol and BMI from 140,000 observations in men and women in Northern Sweden. Nutr J 2012;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kanerva N, Kaartinen NE, Schwab U, Lahti-Koski M, Männistö S. The Baltic Sea Diet Score: a tool for assessing healthy eating in Nordic countries. Public Health Nutr 2014;17:1697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brunius C, Shi L, Landberg R. Large-scale untargeted LC-MS metabolomics data correction using between-batch feature alignment and cluster-based within-batch signal intensity drift correction. Metabolomics 2016;12:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith CA, Want EJ, O'Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem 2006;78:779–87. [DOI] [PubMed] [Google Scholar]
- 46. Brown M, Wedge DC, Goodacre R, Kell DB, Baker PN, Kenny LC, Mamas MA, Neyses L, Dunn WB. Automated workflows for accurate mass-based putative metabolite identification in LC/MS-derived metabolomic datasets. Bioinformatics 2011;27:1108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buck M, Nilsson LKJ, Brunius C, Dabiré RK, Hopkins R, Terenius O. Bacterial associations reveal spatial population dynamics in Anopheles gambiae mosquitoes. Sci Rep 2016;6:22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hanhineva K, Brunius C, Andersson A, Marklund M, Juvonen R, Keski-Rahkonen P, Auriola S, Landberg R. Discovery of urinary biomarkers of whole grain rye intake in free-living subjects using nontargeted LC-MS metabolite profiling. Mol Nutr Food Res 2015;59:2315–25. [DOI] [PubMed] [Google Scholar]
- 49. Lindgren F, Hansen B, Karcher W. Model validation by permutation tests. J Chemom 1996;10:521–32. [Google Scholar]
- 50. Cheung W, Keski-Rahkonen P, Assi N, Ferrari P, Freisling H, Rinaldi S, Slimani N, Zamora-Ros R, Rundle M, Frost G et al. A metabolomic study of biomarkers of meat and fish intake. Am J Clin Nutr 2017;105:600–8. [DOI] [PubMed] [Google Scholar]
- 51. Stella C, Beckwith-Hall B, Cloarec O, Holmes E, Lindon J, Powell J, van der Ouderaa F, Bingham S, Cross A, Nicholson J. Susceptibility of human metabolic phenotypes to dietary modulation. J Proteome Res 2006;5:2780–8. [DOI] [PubMed] [Google Scholar]
- 52. Beckmann M, Lloyd AJ, Haldar S, Favé G, Seal CJ, Brandt K, Mathers JC, Draper J. Dietary exposure biomarker-lead discovery based on metabolomics analysis of urine samples. Proc Nutr Soc 2013;72:352–61. [DOI] [PubMed] [Google Scholar]
- 53. de Mello VD, Lankinen MA, Lindström J, Puupponen-Pimiä R, Laaksonen DE, Pihlajamäki J, Lehtonen M, Uusitupa M, Tuomilehto J, Kolehmainen M et al. Fasting serum hippuric acid is elevated after bilberry (Vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol Nutr Food Res 2017;61:1700019. [DOI] [PubMed] [Google Scholar]
- 54. Hanhineva K, Lankinen MA, Pedret A, Schwab U, Kolehmainen M, Paananen J, de Mello V, Sola R, Lehtonen M, Poutanen K et al. Nontargeted metabolite profiling discriminates diet-specific biomarkers for consumption of whole grains, fatty fish, and bilberries in a randomized controlled trial. J Nutr 2015;145:7–17. [DOI] [PubMed] [Google Scholar]
- 55. Tovar J, de Mello VD, Nilsson A, Johansson M, Paananen J, Lehtonen M, Hanhineva K, Björck I. Reduction in cardiometabolic risk factors by a multifunctional diet is mediated via several branches of metabolism as evidenced by nontargeted metabolite profiling approach. Mol Nutr Food Res 2017;61:1–12. [DOI] [PubMed] [Google Scholar]
- 56. Bondia-Pons I, Martinez JA, de la Iglesia R, Lopez-Legarrea P, Poutanen K, Hanhineva K, Zulet M de los Á. Effects of short- and long-term Mediterranean-based dietary treatment on plasma LC-QTOF/MS metabolic profiling of subjects with metabolic syndrome features: the Metabolic Syndrome Reduction in Navarra (RESMENA) randomized controlled trial. Mol Nutr Food Res 2015;59:711–28. [DOI] [PubMed] [Google Scholar]
- 57. Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TW-M, Fiehn O, Goodacre R, Griffin JL et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007;3:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marckmann P, Lassen A, Haraldsdóttir J, Sandström B. Biomarkers of habitual fish intake in adipose tissue. Am J Clin Nutr 1995;62:956–9. [DOI] [PubMed] [Google Scholar]
- 59. Silva V, Barazzoni R, Singer P. Biomarkers of fish oil omega-3 polyunsaturated fatty acids intake in humans. Nutr Clin Pract 2014;29:63–72. [DOI] [PubMed] [Google Scholar]
- 60. Pekkinen J, Rosa-Sibakov N, Micard V, Keski-Rahkonen P, Lehtonen M, Poutanen K, Mykkänen H, Hanhineva K. Amino acid-derived betaines dominate as urinary markers for rye bran intake in mice fed high-fat diet—a nontargeted metabolomics study. Mol Nutr Food Res 2015;59:1550–62. [DOI] [PubMed] [Google Scholar]
- 61. Guertin KA, Moore SC, Sampson JN, Huang WY, Xiao Q, Stolzenberg-Solomon RZ, Sinha R, Cross AJ. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr 2014;100:208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lloyd AJ, Beckmann M, Haldar S, Seal C, Brandt K, Draper J. Data-driven strategy for the discovery of potential urinary biomarkers of habitual dietary exposure. Am J Clin Nutr 2013;97:377–89. [DOI] [PubMed] [Google Scholar]
- 63. Bhupathiraju SN, Hu FB. One (small) step towards precision nutrition by use of metabolomics. Lancet Diabetes Endocrinol 2017;5:154–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lankinen M, Kolehmainen M, Jääskeläinen T, Paananen J, Joukamo L, Kangas AJ, Soininen P, Poutanen K, Mykkänen H, Gylling H et al. Effects of whole grain, fish and bilberries on serum metabolic profile and lipid transfer protein activities: a randomized trial (Sysdimet). PLoS One 2014;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jacob JJ. Fish consumption and omega-3-fatty acids in prevention of diet-related noncommunicable diseases. J Soc Heal Diabetes 2016;4:115–20. [Google Scholar]
- 66. FAO/WHO. Report of the joint FAO / WHO expert consultation on the risks and benefits of fish consumption. FAO Fisheries and Aquaculture report. Rome, Italy: FAO; 2010. [Google Scholar]
- 67. Patel PS, Sharp SJ, Luben RN, Khaw K-T, Bingham SA, Wareham NJ, Forouhi NG. Association between type of dietary fish and seafood intake and the risk of incident type 2 diabetes: the European Prospective Investigation of Cancer (EPIC)-Norfolk cohort study. Diabetes Care 2009;32:1857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Villegas R, Xiang Y-B, Elasy T, Li H-L, Yang G, Cai H, Ye F, Gao Y-T, Shyr Y, Zheng W et al. Fish, shellfish, and long-chain n-3 fatty acid consumption and risk of incident type 2 diabetes in middle-aged Chinese men and women. Am J Clin Nutr 2011;94:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. van Woudenbergh GJ, van Ballegooijen AJ, Kuijsten A, Sijbrands EJG, van Rooij FJA, Geleijnse JM, Hofman A, Witteman J, Feskens EJM. Eating fish and risk of type 2 diabetes. Diabetes Care 2009;32:2021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xun P, He K. Fish consumption and incidence of diabetes: meta-analysis of data from 438,000 individuals in 12 independent prospective cohorts with an average 11-year follow-up. Diabetes Care 2012;35:930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. MacKenzie BR, Almesjö L, Hansson S. Fish, fishing, and pollutant reduction in the Baltic Sea. Environ Sci Technol 2004;38:1970–6. [DOI] [PubMed] [Google Scholar]
- 72. Järv L, Kiviranta H, Koponen J, Rantakokko P, Ruokojärvi P, Radin M, Raid T, Roots O, Simm M. Persistent organic pollutants in selected fishes of the Gulf of Finland. J Mar Syst 2017;171:129–33. [Google Scholar]
- 73. Wallin A, Di Giuseppe D, Orsini N, Åkesson A, Forouhi NG, Wolk A. Fish consumption and frying of fish in relation to type 2 diabetes incidence: a prospective cohort study of Swedish men. Eur J Nutr 2017;56:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Villegas R, Shu XO, Gao Y-T, Yang G, Elasy T, Li H, Zheng W. Vegetable but not fruit consumption reduces the risk of type 2 diabetes in Chinese women. J Nutr 2008;138:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr 2017;147:1174–82. [DOI] [PubMed] [Google Scholar]
- 76. Streppel MT, de Vries JHM, Meijboom S, Beekman M, de Craen AJM, Slagboom PE, Feskens EJM. Relative validity of the food frequency questionnaire used to assess dietary intake in the Leiden Longevity Study. Nutr J 2013;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.