Abstract
As a calcium sensitizer and inodilator that augments cardiac contractility without increasing myocardial oxygen demand or exacerbating ischaemia, levosimendan may be well configured to deliver inotropic support in cases of acute heart failure (AHF). Other factors favouring levosimendan in this setting include its extended duration of action due to the formation of an active metabolite and the lack of any attenuation of effect in patients treated with beta-blockers. Effects of levosimendan on systemic haemodynamics include its significant, dose-dependent increases in cardiac output, stroke volume and heart rate, and decreases in right and left ventricular filling and total peripheral resistance. Rapid and sustained reduction in levels of natriuretic peptides is a consistent effect of levosimendan use and potentially favourable effects on other neurohormonal indicators of cardiac distress are also observed. Levosimendan has repeatedly been shown to be effective in relief of symptoms of AHF, notably dyspnoea and fatigue, while mortality data from clinical trials and registries suggest that levosimendan is markedly less likely than catecholaminergic inotropes to worsen prognosis. The vasodilator pharmacology of levosimendan is also pertinent to the drug’s use in AHF, in which setting organ under-perfusion is often a key pathology. These considerations suggest that levosimendan may have a more favourable impact on the circumstances of the majority of AHF patients than adrenergic agents that act only or primarily as cardiac stimulants. They also suggest that levosimendan may advantageously be integrated into a comprehensive strategy of early intervention designed and intended to prevent cardiac destabilization worsening to the point where hospitalization is necessary. Levosimendan should be used with caution and with tightened haemodynamic monitoring in patients who have low baseline blood pressure (systolic blood pressure <100 mmHg; diastolic blood pressure <60 mmHg), or who are at risk of a hypotensive episode.
Keywords: Inotrope, Inodilator, Cardioactive, Vasoactive, Treatment, Efficacy, Safety
Introduction
Acute heart failure (AHF) is characterized in the 2016 European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic heart failure (HF)1 as ‘rapid onset or worsening of symptoms and/or signs of HF. It is a life-threatening medical condition requiring urgent evaluation and treatment, typically leading to urgent hospital admission’.
The ESC guidelines acknowledge immediately that this umbrella definition of AHF encompasses a condition with an extensive array of possible causes: while AHF may be a de novo clinical incident, it is more often a consequence of acute decompensation of chronic HF. Similarly, it may originate from primary cardiac dysfunction (most usually acute myocardial dysfunction, which itself may arise from a range of causes, as well as acute valve insufficiency) or it may, especially in patients with chronic HF, be initiated by the operation of external precipitating factors. An extensive range of such factors is identified in the 2016 ESC guidelines.1
Identification (or exclusion) and correction of all such precipitants is an urgent priority in the first-phase response to a presentation of presumed or suspected AHF. In this context, it should be noted that AHF precipitated by either an episode of acute coronary syndrome (ACS) or an infection is attended by exceptionally high short-term mortality.2 When ACS is attended by cardiogenic shock a validated risk prediction instrument such as the CardShock risk score may be used to aid clinical decision making.3
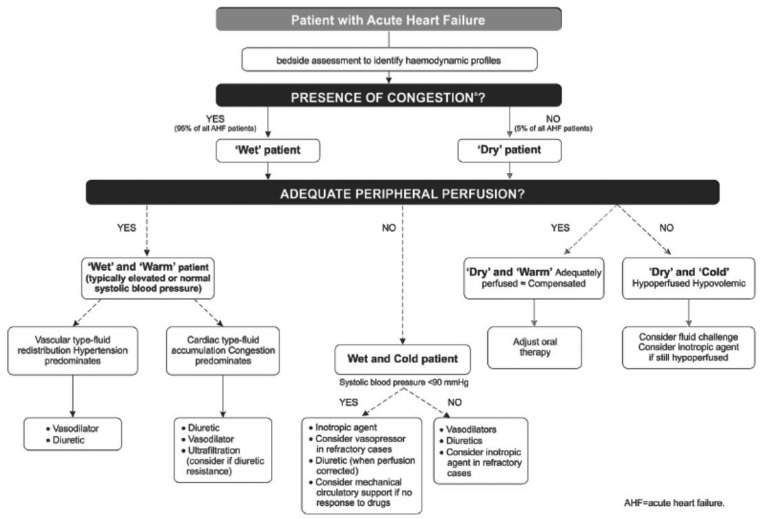
Clinical classification, as endorsed by the ESC guidelines, emphasizes the significance of congestion (pulmonary, jugular, peripheral or paroxysmal nocturnal dyspnoea, ascites, intestinal congestion, and hepatic congestion of the hepato-jugular reflex) and hypoperfusion, indicated by oliguria, dizziness, mental confusion and cold, sweated extremities. (It should be emphasized that hypoperfusion is not a synonym for hypotension, although these conditions coexist in the majority of cases.) After the urgent and immediate phases of stabilization and a diagnostic work-up including natriuretic peptide determinations (primarily as an aid to excluding AHF), this wet/cold/dry/warm classification can be used to profile patients and outline their therapeutic trajectory (Figure 1). This stratification identifies the ‘wet and cold’ patient as the type most likely to be a candidate for inotropic therapy, as one of a range of therapeutic interventions.
Figure 1.
European Society of Cardiology-advised criteria for classifying acute heart failure based on the presence/absence of congestion and/or hypoperfusion. From Ponikowski et al.1
These extracts from expert guidance provide a framework within which to assess the potential contribution of levosimendan to the management of AHF.
As a cardiac myofilament calcium sensitizer, levosimendan increases cardiac contractility by increasing calcium sensitivity rather than by increasing intracellular ionic free calcium.4 Consequently, it is not associated with increases in myocardial oxygen demand5,6 or ischaemia.7,8 Levosimendan is further distinguished by its pharmacokinetics, in particular the formation via a reduction–acetylation pathway in the intestines of a long-acting active metabolite designated OR-1896. This compound shares the pharmacologic and haemodynamic properties of the parent drug while having a greatly extended plasma half-life.9 The particular kinetics of this metabolite contribute to the persistence of therapeutic effects after a single infusion of the parent drug.
More than 3000 patients have been involved in primary and regulatory clinical trials of levosimendan in left sided HF. Narrative summaries of key studies appear in Table 1. Consolidated findings from these studies are discussed below.
Table 1.
Narrative summaries of principal controlled trials of levosimendan in left-sided acute heart failure
| In the LIDO study, levosimendan was compared with dobutamine in 203 patients with low-output heart failure who required right heart catheterization and treatment with an intravenous inotropic drug. Levosimendan was administered as a 24-h intravenous infusion at a rate of 0.1–0.2 µg/kg/min.16 |
| The RUSSLAN study (N = 504) was primarily a safety evaluation of levosimendan in patients with left ventricular failure complicating an acute myocardial infarction.17 Patients randomized to levosimendan were treated with a 6-h intravenous infusion at a rate of 0.1–0.4 µg/kg/min. Invasive haemodynamics were not performed. |
| The SURVIVE study (N = 1327) compared the effects of levosimendan or dobutamine on mortality in patients with severe systolic heart failure. Levosimendan was infused at rates of 0.1–0.2 µg/kg/min for 24 h.18 |
| The REVIVE studies (REVIVE I, n = 100; REVIVE II, n = 600) evaluated the efficacy of levosimendan on symptoms of acute heart failure based on a novel composite endpoint of clinically relevant measures expressed as ‘improved’, ‘unchanged’, or ‘worse’ during 5 days of observation. Levosimendan was administered as a 24-h intravenous infusion at a rate of 0.1–0.2 µg/kg/min. Both REVIVE studies were conducted mainly in the USA.12 |
Haemodynamic effects
Levosimendan produces significant, dose-dependent increases in cardiac output (CO) and stroke volume, and decreases in pulmonary capillary wedge pressure (PCWP), mean blood pressure (BP), mean pulmonary artery pressure (PAP), mean right atrial pressure, and total peripheral resistance.10 These effects are registered rapidly (within a few minutes of starting infusion). There is no evidence of the development of tolerance or attenuation of effect even after infusions up to 48 h in duration.11 The presence of the long-acting metabolite designated OR-1896 means that these core haemodynamic effects persist for several days after termination of levosimendan infusion and for much longer than with dobutamine.9
The increase in CO evoked by levosimendan is similar to that achieved with dobutamine at comparable doses but the reduction in PCWP produced by levosimendan is considerably greater. Moreover, and in contrast to dobutamine, the haemodynamic effects of levosimendan are not attenuated by concomitant beta-blocker use. This difference contributes to the position of the 2016 ESC HF guidelines that levosimendan should be the preferred agent when inotropy is indicated for an HF patient pretreated with a beta-blocker.1
Use of an initial bolus of levosimendan is nowadays generally avoided in order to minimize the risk of hypotension. Infusion is most often commenced initially at a dose of 0.1 µg/kg/min [or 0.05 µg/kg/min when systolic blood pressure (SBP) is marginal] and titrated to 0.2 µg/kg/min if BP remains stable after the first 2–3 h. The recommended duration of infusion in AHF is 24 h.
Levosimendan should be used with caution—and with no bolus dose—in patients with low baseline SBP (<100 mmHg) or diastolic blood pressure (DBP, <60 mmHg), or those at risk of a hypotensive episode. Patients who might be considered ineligible for levosimendan therapy on these grounds account for <10% of the AHF population according to the 2016 ESC guidelines.1 Hypovolaemia should be corrected prior to levosimendan infusion, as a precautionary measure.
Effects on neurohormones
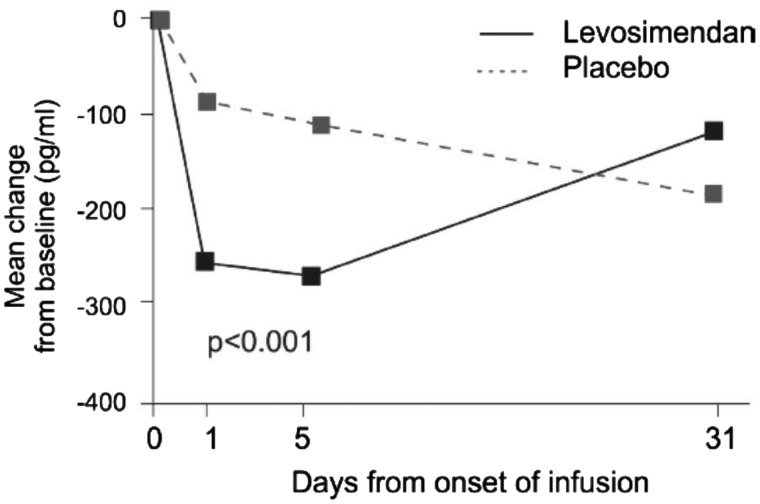
Rapid and sustained reduction in levels of natriuretic peptides is a hallmark of levosimendan use in clinical trials. The data depicted in Figure 2, from the REVIVE II study,12 may be regarded as exemplary of findings in other studies.
Figure 2.
Levosimendan infusion in the REVIVE II study was associated with a marked and sustained reduction in circulating brain natriuretic peptide levels. From Packer et al.12
Correlations between discharge brain natriuretic peptide (BNP) and longer-term clinical prognosis are not always resilient or persistent,13,14 and it would be imprudent to assume from its effect on BNP alone that levosimendan is certain to have an enduring impact on prognosis in all cases.
Impact on signs and symptoms of acute heart failure
In patients with clear signs of pulmonary oedema and elevated PCWP who do not respond adequately to vasodilators, selected inotropes are acknowledged for their ability to reduce left ventricular filling pressures and decongest the lungs. Within the broad class of inotropes, the reductions in PCWP and right atrial pressure produced by levosimendan are greater than those for agents such as dobutamine.15
Factors necessitating the use of rescue medications in the REVIVE programme are summarized in Table 2 and show consistent advantages with levosimendan, including in the major categories of ‘worsening dyspnoea or tachypnoea’ and ‘persistent/unresponsive symptoms’.12 It should be noted that many patients in both REVIVE studies were in receipt of extensive and aggressive polypharmacy before the introduction of levosimendan and most patients who received levosimendan were infused at the maximum permitted rate of 0.2 µg/kg/min.
Table 2.
Summary of worsening clinical status leading to use of rescue therapy in the REVIVE programme
| REVIVE I |
REVIVE II |
|||
|---|---|---|---|---|
| Levosimendan (n = 51) | Placebo (n = 49) | Levosimendan (n = 299) | Placebo (n = 301) | |
| Proportion requiring rescue therapy | 16% | 29% | 15% | 26% |
| Worsening dyspnoea or tachypnoea | 10% | 12% | 7% | 13% |
| Increased pulmonary oedema | 0% | 2% | 3% | 6% |
| Diaphoresis | 0% | 2% | 1% | 1% |
| Cool extremities and cyanosis | 2% | 2% | 0% | 2% |
| Worsening renal function | 6% | 2% | 3% | 5% |
| Decreased mental status | 0% | 0% | 1% | 2% |
| Persistent/unresponsive symptoms | 10% | 18% | 6% | 11% |
From Packer et al.12
Dyspnoea and fatigue symptoms also responded better to levosimendan than to control therapies in the LIDO trial but, in that instance, the treatment effect of levosimendan was numerically larger but not statistically significant (dyspnoea improvement 68% vs. 59%, P = 0.865; fatigue improvement 63% vs. 47%, P = 0.155).16
Clinical outcomes
Hospitalization data
Length of hospital stay and time alive and out of hospital offer a perspective on the effectiveness of therapy in AHF. Patients in the levosimendan group of the LIDO study spent significantly more days alive and out of hospital than dobutamine-treated patients in a retrospective 180-day follow-up analysis (median 157 vs. 133 days; P = 0.027).16 In RUSSLAN, the combined risk of death and worsening HF was significantly lower in patients treated with levosimendan than in the control group during the infusion period (2% vs. 6%; P = 0.033) and at 24 h (4% vs. 9%; P = 0.044)17 while, in the REVIVE II study, a greater percentage of patients treated with levosimendan were released within 5 days (46% vs. 37%) and the mean duration of the initial hospitalization was almost 2 days shorter in the levosimendan group than the placebo group (7.0 vs. 8.9 days).12 In SURVIVE, the mean number of days alive and out of hospital during 189 days of follow-up was 120.2 in the levosimendan group vs. 116.6 in the dobutamine group (P = 0.3).18
Mortality data
Thirty-one-day mortality was examined as a secondary endpoint in LIDO and revealed a survival advantage from levosimendan treatment [mortality rate 8% vs. 17% with dobutamine, hazard ratio (HR) 0.43, P = 0.049].16 This initial gain was corroborated in a retrospective extension of follow-up to 180 days (mortality rate 26% vs. 38% with dobutamine, HR 0.57, P = 0.029). Similar findings emerged from the RUSSLAN study, in which a survival benefit from levosimendan persisted in a retrospective follow-up to 180 days (23% vs. 31%; P = 0.053).17
In the SURVIVE and REVIVE studies, in contrast, there was no significant difference in survival between the study groups.12,18
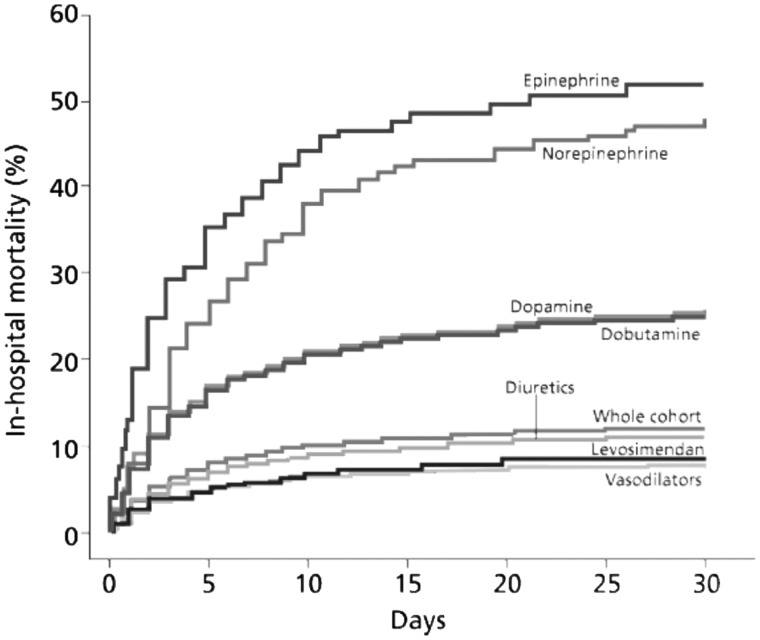
Evidence from the ALARM-HF registry suggests that the mixed inodilator profile of levosimendan may be associated in routine clinical practice with a mortality rate much closer to that associated with vasodilators than those seen with calcium-mobilizing inotropes (Figure 3).19 Complementary data on this theme have recently emerged from the ESC Heart Failure Long-Term Registry, which revealed that ≈12% of patients undergoing unscheduled hospitalizations for AHF received intravenous (i.v.) inotropes and/or vasopressors.20
Figure 3.
Levosimendan, a calcium-sensitizing inotrope and vasodilator, was associated with a substantially lower mortality rate in the population of the ALARM-HF registry than was seen with traditional adrenergic, calcium-mobilizing inotropes such as dobutamine. From Mebazaa et al.19
A survival effect of levosimendan approximating to a 5% absolute risk reduction has been documented by Landoni et al. in a meta-analysis of 45 randomized controlled studies that published mortality data involving 5480 patients (levosimendan n = 2915; control n = 2565).21 There was statistically robust evidence of a survival advantage of levosimendan vs. either dobutamine (odds ratio 0.68; P = 0.003) or placebo (odds ratio 0.82; P = 0.02) and consistency of effect was observed across an extensive range of sub-analyses. There was also evidence of reduced length of hospital stay [weighted mean difference (WMD) 1.31; P = 0.007].
Subgroup analyses from REVIVE and SURVIVE data
Subgroup analysis of these two large trials of levosimendan12,18 produced a range of interesting insights.
SURVIVE
Although no significant difference was demonstrated between levosimendan and placebo for the primary endpoint of 180-day mortality, the survival rate in the levosimendan-treated patients was significantly higher in the subgroup of patients with a history of HF (1171 patients, 88.2% of the study population), with a net benefit of 19 fewer deaths up to 31 days (P = 0.05). Separately, randomization to levosimendan was associated with significantly lower early-phase (Days 0–5) mortality in the subgroup of patients exposed to concomitant beta-blocker therapy (P = 0.03).22 It is of note that only half of patients in the SURVIVE trial were in receipt of beta-blockers before the introduction of study medication, a much lower percentage than is encountered in many current randomized clinical trials, in which the usage rate for beta-blockers is often ≥90%. Prima facie, therefore, clinical circumstances favouring the use of levosimendan may be more prevalent now than when SURVIVE was conducted.
REVIVE
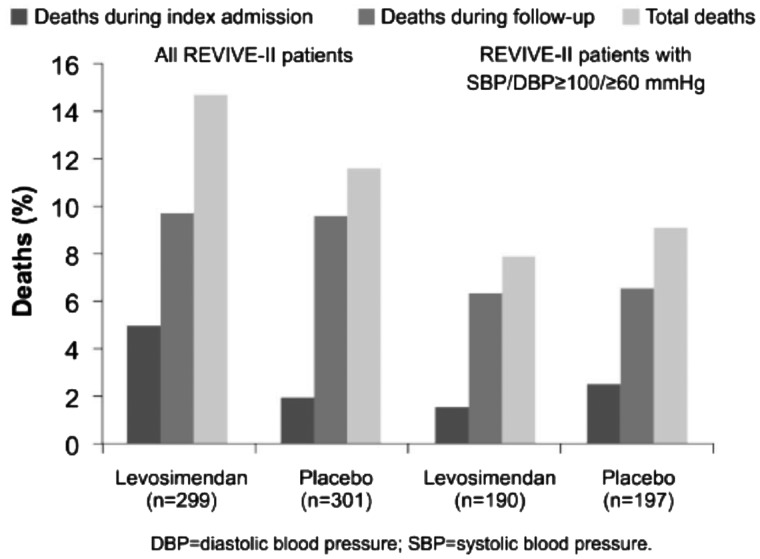
Post hoc analyses of the REVIVE II dataset identified baseline SBP <100 mmHg or DBP <60 mmHg at baseline as factors associated with increased mortality risk and revealed that, in patients with initial low BP, mortality was 27% for levosimendan vs. 16% for placebo. Of note, the excess mortality in the whole levosimendan cohort occurred during the index admission (15 deaths vs. 6); deaths during follow-up were identical in both groups (n = 29).
Re-analysis of the mortality data excluding patients with low baseline arterial BP eliminated the early excess of mortality in the levosimendan cohort (three deaths vs. five with placebo); deaths during follow-up remained very similar (levosimendan, n = 13; placebo, n = 12) so that overall mortality in patients with well-supported BP at baseline was similar between groups (HR 0.92, P = 0.81; Figure 4). The study’s composite primary endpoint, which was positive for levosimendan in the overall study population, was likewise positive in the subset of patients with higher baseline BP.
Figure 4.
Analysis of deaths in the REVIVE II study according to patients’ baseline blood pressure revealed an association between higher blood pressures and improved outcomes with levosimendan use. From Packer et al.12
These analyses identified low BP at baseline as a possible risk factor for the use of levosimendan and the current summary of product characteristics reflects that verdict.
Safety synopsis
An integrated safety data summary prepared by the sponsor of the placebo-controlled studies of i.v. levosimendan in AHF revealed no difference in the proportion of patients experiencing reduction in arterial BP in response to treatment (23.1% vs. 23.1%). REVIVE II (N = 600), as noted above, was an outlier to this aggregate trend, with 52.6% of levosimendan-treated patients experiencing a reduction in BP, compared with 37.9% in the placebo group (P < 0.001).
The integrated summary also identified a greater likelihood of atrial arrhythmias with levosimendan than placebo (8.2% vs. 5.4%; P = 0.024). This difference was slightly more marked than the average in the REVIVE II study (9% vs. 2%; P < 0.001), which also recorded a statistically significant difference in the incidences of ventricular tachycardia (levosimendan 25%, placebo 17%, P = 0.031). However, that difference was not replicated in the integrated summary (levosimendan 10.0%, placebo 11.3%, P = 0.371).
The incidences of worsening HF and renal function disturbances, recorded as adverse events, were significantly lower in the levosimendan group than the placebo group (HF events, 15.6% vs. 28.4%, P < 0.001; renal events, 6.9% vs. 10.4%, P = 0.007).
There was no statistically significant difference between the levosimendan and placebo groups in respect of cardiac ischaemia (7.3% vs. 8.9%, P = 0.233); decreased haemoglobin (2.3% vs. 3.8%, P = 0.058); decreased potassium (4.9% vs. 7.0%, P = 0.059); or increased blood glucose (1.6% vs. 2.6%, P = 0.117). It should be noted in this context that, while the numerical differences in this analysis favour levosimendan, reductions in serum potassium and haemoglobin were reported in early small-scale studies of i.v. levosimendan, and that correction of hypokalaemia is recommended before administering levosimendan.
The safety of levosimendan in AHF has also been the subject of a meta-analysis by Gong et al.23 From 5349 patients in 25 randomized controlled studies including LIDO, SURVIVE, and the REVIVE programme, but excluding RUSSLAN, these investigators concluded that levosimendan therapy increased the risk of recurrence of extrasystoles [risk ratio (RR) 1.88, 95% confidence interval (CI) 1.26–2.81, P = 0.002], headache or migraine (RR 1.94, 95% CI 1.54–2.43; P < 0.00001) and hypotension (RR 1.33, 95% CI 1.15–1.53; P = 0.0001) in patients with HF, compared with combined control therapy comprising placebo or dobutamine. There were non-statistically significant differences in the RRs, several of which favoured levosimendan, for ventricular tachycardia, constipation, diarrhoea, hypokalaemia, nausea, vomiting, urinary tract infection, dizziness and angina pectoris, chest pain, or myocardial ischaemia.
Effects on BP were recorded in this meta-analysis as efficacy variables. Compared with dobutamine, levosimendan was associated with reductions in SBP (WMD −7.08 mmHg, P = 0.02), DBP (WMD −4.75 mmHg, P < 0.00001), and mean arterial pressure (WMD −0.25 mmHg, P = 0.02), whereas stroke volume was enhanced (WMD +9.02, P = 0.02). Levosimendan also lowered SBP significantly more than placebo (WMD −4.76 mmHg, P = 0.003).
Levosimendan in acute heart failure complicating acute coronary syndrome
The incidence of AHF as a complication of ACS has decreased over recent years, but it is still a significant complication of ACS, affecting a substantial proportion of patients and being associated with worse outcomes.24
RUSSLAN17 is the largest placebo-controlled study of levosimendan in this scenario, and was primarily a safety study, but decreased incidence of worsening HF and improvements in short- and long-term mortality were noted. Corroboration of that effect was not forthcoming from the dobutamine-controlled SURVIVE study,18 in which 178 patients (≈13% of the study cohort) were classified as having acute myocardial infarction (AMI), but it is of note that, in that subset of patients, 31-day mortality was 4% lower in the levosimendan arm than with dobutamine [23/83 (28%) vs. 30/95 (32%), RR 0.83 (95% CI 0.48–1.43)], whereas in the non-AMI contingent (n = 1149), death rates at 31 days were very similar in both treatment assignments [56/581 (10%) vs. 61/568 (11%), RR 0.89 (95% CI 0.62–1.28)].
A series of smaller studies have added to the database of experience with levosimendan in this setting.25–31 Individually, these studies do not provide adequately powered insights into any effect of levosimendan on survival in this situation but they have produced repeated indications of favourable effects on systemic haemodynamics and on left ventricular function and wall motion. In conjunction with the latter, several reports have documented accelerated recovery from Takotsubo cardiomyopathy (transient left ventricular apical ballooning syndrome) with levosimendan, accompanied by favourable haemodynamic responses and more rapid restoration of pre-incident troponin levels.32,33
Expert opinion on the use of levosimendan in AHF complicating ACS/AMI currently advises that it may be considered as an alternative to adrenergic agents in all patients who have received chronic beta-blocker therapy or in those whose urinary output is insufficient after diuretics. It may also be relevant to the needs of patients whose SBP is in the range 85–100 mmHg (equivalent to Killip Class III, with acute pulmonary oedema) and may be a preferred intervention (in combination with noradrenaline or other vasopressors) in patients with cardiogenic shock (approximating to Killip Class IV and SBP <85 mmHg, with indications of peripheral vasoconstriction34).
Renal effects of levosimendan
The emphasis on preservation of renal function in clinical management protocols reflects appreciation of the relation between worsening kidney function and deterioration of prognosis in AHF.
Haemodynamics are not the sole contributors to end-organ damage, but an increase in CO and a reduction in central venous pressure (CVP) are nonetheless important therapeutic targets for the preservation or recovery of renal function. Levosimendan has been actively evaluated for its effects on renal function in HF. The balance of the evidence, as summarized in a recent review,35 suggests a modest but sustained advantageous effect mediated primarily through changes in CO and CVP. Separately, direct observation of human renal arteries after administration of levosimendan has documented renal arterial vasodilatation and augmented renal blood flow with increase in glomerular filtration rate (GFR) and promotion of diuresis without compromise of renal oxygenation,36 and theoretically advantageous effects on various markers of kidney function have been reported in levosimendan-treated patients with acutely decompensated AHF and renal impairment.37
Further recent insights into renal effects of levosimendan have been reported by Lannemyr et al.38 As part of an elective cardiac work-up, 32 adult patients with chronic HF (mean baseline left ventricular ejection fraction ≈27%) and impaired renal function (mean GFR <80 mL/min/1.73 m2) were randomly assigned to short-term (75 min) treatments with either levosimendan (loading dose of 12 µg/kg over 10 min, then infusion at 0.1 µg/kg/min for 65 min; n = 16) or dobutamine (continuous infusion started at 5.0 µg/kg/min for 10 min, then 7.5 µg/kg/min for 65 min; n = 16).
Both treatments were associated with quantitatively and qualitatively very similar alterations in major systemic haemodynamic indices and significant (P < 0.05) enhancement of renal blood flow but only levosimendan therapy was associated with a significant increase in GFR (P < 0.05 vs. baseline).
In aggregate, these observations are compatible with a scenario in which enhancement of CO by levosimendan initiates a virtuous circle in which improved GFR and diuresis lead to decongestion and a lower CVP, which in turn promotes further improvement in both cardiac and renal function. In addition to these clinical findings, experimental observations suggest that, in some situations, levosimendan may exert renal-protective qualities arising from antioxidant, anti-apoptotic, and cytoprotective actions.39
Zangrillo et al.40 have recently published the findings of a subgroup analysis of the CHEETAH study linking levosimendan with a lower rate of post-operative acute kidney injury vis-à-vis placebo (30% vs. 52%, P = 0.035) in patients undergoing mitral valve surgery who developed post-operative myocardial dysfunction/low CO syndrome. These findings are consistent with other meta-analyses that have suggested a renal-protective benefit of levosimendan in a range of clinical situations41,42 but, particularly because the REVIVE programme—to date the largest randomized study of levosimendan in AHF—produced no indications of significant impact on renal function,12 such post hoc exercises must be regarded as in need of validation in prospective, randomized, controlled trials.
These reservations notwithstanding, however, it is reasonable to conclude that inotropes or inodilators may be indicated for short-term management in AHF with renal dysfunction—mostly in cases of low-output HF that can provoke renal hypoperfusion—and to assert that levosimendan is probably currently underutilized in clinical practice, especially in ‘wet’ patients (Figure 1) who do not respond to diuretics.
Right heart failure and pulmonary hypertension
Decisive randomized trials of levosimendan are lacking in right ventricular failure with or without pulmonary hypertension (PH) but two proof-of-concept trials by Jiang et al.43 and of Kleber et al.44 suggest some benefits.
The first of these studies was an open-label uncontrolled trial in 45 hospitalized patients with precapillary PH and right HF.43 Levosimendan was administered at a rate of 0.05–0.1 µg/kg/min, up to a total dose of 12.5 mg.
On Day 7 after levosimendan infusion, 7 of 13 patients initially in World Health Organization functional Class IV had improved their functional status by at least one class (P = 0.008). The second of the primary endpoints, Borg dyspnoea score, was also significantly improved (P < 0.001), as were the secondary outcomes of 6-min walk test distance and N-terminal pro-BNP (P < 0.001 for both).
Diastolic blood pressure decreased significantly in response to levosimendan infusion (68.4 ± 11.6 vs. 63.7 ± 10.7 mmHg, P = 0.01) but only one patient required dopamine as a precaution against hypotension.
Kleber et al.44 evaluated levosimendan in a placebo-controlled trial of 28 patients with PH (n = 8), pulmonary venous hypertension resulting from left ventricular failure (n = 17) or chronic thrombo-embolic PH (n = 3). Patients were randomized in a 1:2 ratio to placebo or levosimendan, which was initiated at a loading dose of 12 µg/kg (infused over 10 min) followed by a continuous infusion of 0.1 µg/kg/min for 50 min. The infusion rate was thereafter doubled and maintained for 23 h if the initial dose was well tolerated. Levosimendan was then re-administered at 2-week intervals as a continuous infusion (0.2 µg/kg/min for 6 h) for a total of five cycles of treatment.
A total of 24 of the 28 randomized patients (levosimendan, n = 16; placebo, n = 8) completed the study as planned. One levosimendan and two placebo patients discontinued due to an adverse event (hypotension in the levosimendan patient) and one levosimendan patient withdrew consent.
Levosimendan reduced pulmonary vascular resistance (PVR) following the initial 24-h infusion by 12 ± 9% (vs. an increase of 25 ± 11% in the placebo group, P = 0.009). Pulmonary vascular resistance at 8 weeks remained lower in patients treated with levosimendan but the differences, while substantial, were not statistically significant (levosimendan –21 ± 83%, n = 13; placebo, +35 ± 73%, n = 7; P = 0.253).
A Phase II double-blind, randomized, placebo-controlled study of levosimendan in pulmonary hypertension in patients with heart failure with preserved left ventricular ejection fraction (PH-HFpEF) is ongoing in the USA (NCT03541603).
Conclusions
The applications of levosimendan in the management of AHF may be summarized as follows.
All cases of AHF in patients who are on beta-blockers.
In cardiogenic shock: to improve cardiac function and reduce catecholamine requirements.
In ACS: for its cardioprotective qualities, to limit myocyte loss and improve heart function.
In cardio-renal syndrome: to relieve renal congestion and improve kidney function.
In right HF: to improve right heart function, reduce venous congestion, reduce PAP, and improve kidney function.
Levosimendan should be considered more often as a preferable alternative to conventional adrenergic inotropes. We base this conclusion on assessment of the drug’s haemodynamic effects and its highly reassuring safety profile in clinically unstable patients. The question of mortality/survival benefit remains contentious but the striking lack of any increase in mortality with levosimendan in the ALARM-HF registry (Figure 3), along with direct comparison with dobutamine in randomized clinical trials and in meta-analysis,21 persuades us that, among available inotropes, levosimendan is least likely to worsen prognosis. Given the scale of the mortality effect recorded with other agents in the ALARM-HF dataset this is a potentially major gain, separate from any wider consideration of the ancillary features and benefits of levosimendan. It may also be argued that levosimendan (along with numerous other drugs) has suffered from shifting expectations in this area. The SURVIVE investigators noted that, in AHF, ‘there is a need for agents that at least improve haemodynamics and relieve symptoms without adversely affecting survival’.18 Levosimendan has consistently met that standard for being a useful addition to the options for AHF treatment.
The vasodilator pharmacology of levosimendan is pertinent to the drug’s use in low-output states such as AHF (and also cardiogenic shock). To characterize such conditions solely as ‘low-output states’ may be misleading because, in many cases, a key pathology is organ hypoperfusion and a preoccupation with raising SBP at the expense of restoring appropriate organ perfusion may be detrimental to some patients. The use of a drug such as levosimendan that delivers both augmentation of CO plus venodilatation may therefore be expected to have a more favourable impact on the circumstances of some patients than an agent that acts only as a cardiac stimulant or pressor,45 while also identifying it as an option for patients who are normally treated with vasodilators.
Acknowledgements
The authors thank Hughes associates, Oxford, UK, for assistance in the preparation of this article.
Conflict of interest: P.P. is a full-time employee of Orion Pharma. The other authors report no conflicts of interest apart from lecture honoraria relating to the practical tutorial lectures on “Inodilators in Acute and Advanced heart failure” held at the annual meeting of the ESC in Munich, Germany on 26–28 August 2018 (which were covered by an unrestricted educational grant from Orion Pharma), and various other national or international educational events on levosimendan sponsored by Orion Pharma. The lecturers and programme were approved by the ESC programme committee. Orion Pharma follows the EFPIA HCP Code.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 2. Arrigo M, Gayat E, Parenica J, Ishihara S, Zhang J, Choi D-J, Park JJ, Alhabib KF, Sato N, Miro O, Maggioni AP, Zhang Y, Spinar J, Cohen-Solal A, Iwashyna TJ, Mebazaa A.. Precipitating factors and 90-day outcome of acute heart failure: a report from the intercontinental GREAT registry. Eur J Heart Fail 2017;19:201–208. [DOI] [PubMed] [Google Scholar]
- 3. Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, Parissis J, Banaszewski M, Silva-Cardoso J, Carubelli V, Di Somma S, Tolppanen H, Zeymer U, Thiele H, Nieminen MS, Mebazaa A;. CardShock Study Investigators; GREAT network. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail 2015;17:501–509. [DOI] [PubMed] [Google Scholar]
- 4. Hasenfuss G, Pieske B, Castell M, Kretschmann B, Maier LS, Just H.. Influence of the novel inotropic agent levosimendan on isometric tension and calcium cycling in failing human myocardium. Circulation 1998;98:2141–2147. [DOI] [PubMed] [Google Scholar]
- 5. Kaheinen P, Pollesello P, Levijoki J, Haikala H.. Effects of levosimendan and milrinone on oxygen consumption in isolated guinea-pig heart. J Cardiovasc Pharmacol 2004;43:555–561. [DOI] [PubMed] [Google Scholar]
- 6. Eriksson O, Pollesello P, Haikala H.. Effect of levosimendan on balance between ATP production and consumption in isolated perfused guinea-pig heart before ischemia or after reperfusion. J Cardiovasc Pharmacol 2004;44:316–321. [DOI] [PubMed] [Google Scholar]
- 7. Levijoki J, Pollesello P, Kaheinen P, Haikala H.. Improved survival with simendan after experimental myocardial infarction in rats. Eur J Pharmacol 2001;419:243–248. [DOI] [PubMed] [Google Scholar]
- 8. Papp JG, Pollesello P, Varro AF, Vegh AS.. Effect of levosimendan and milrinone on regional myocardial ischemia/reperfusion-induced arrhythmias in dogs. J Card Pharmacol Therapeut 2006;11:129–135. [DOI] [PubMed] [Google Scholar]
- 9. Kivikko M, Antila S, Eha J, Lehtonen L, Pentikainen PJ.. Pharmacodynamics and safety of a new calcium sensitizer, levosimendan, and its metabolites during an extended infusion in patients with severe heart failure. J Clin Pharmacol 2002;42:43–51. [DOI] [PubMed] [Google Scholar]
- 10. Nieminen MS, Akkila J, Hasenfuss G, Kleber FX, Lehtonen LA, Mitrovic V, Nyquist O, Remme WJ.. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol 2000;36:1903–1912. [DOI] [PubMed] [Google Scholar]
- 11. Kivikko M, Lehtonen L, Colucci WS.. Sustained hemodynamic effects of intravenous levosimendan. Circulation 2003;107:81–86. [DOI] [PubMed] [Google Scholar]
- 12. Packer M, Colucci W, Fisher L, Massie BM, Teerlink JR, Young J, Padley RJ, Thakkar R, Delgado-Herrera L, Salon J, Garratt C, Huang B, Sarapohja T.. REVIVE Heart Failure Study Group. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail 2013;1:103–111. [DOI] [PubMed] [Google Scholar]
- 13. Sudharshan S, Novak E, Hock K, Scott MG, Geltman EM.. Use of biomarkers to predict readmission for congestive heart failure. Am J Cardiol 2017;119:445–451. [DOI] [PubMed] [Google Scholar]
- 14. Torrado H, Lopez-Delgado JC, Farrero E, Rodríguez-Castro D, Castro MJ, Periche E, Carriò ML, Toscano JE, Pinseau A, Javierre C, Ventura JL.. Five-year mortality in cardiac surgery patients with low cardiac output syndrome treated with levosimendan: prognostic evaluation of NT-proBNP and C-reactive protein. Minerva Cardioangiol 2016;64:101–113. [PubMed] [Google Scholar]
- 15. Ishihara S, Gayat E, Sato N, Arrigo M, Laribi S, Legrand M, Placido R, Manivet P, Cohen-Solal A, Abraham WT, Jessup M, Mebazaa A.. Similar hemodynamic decongestion with vasodilators and inotropes: systematic review, meta-analysis, and meta-regression of 35 studies on acute heart failure. Clin Res Cardiol 2016;105:971–980. [DOI] [PubMed] [Google Scholar]
- 16. Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L.. Steering Committee and Investigators of the Levosimendan Infusion versus Dobutamine (LIDO) Study Efficacy and safety of intravenous levosimendan, a novel calcium sensitiser, in severe low output heart failure: results of a randomised, double-blind comparison with dobutamine (LIDO Study). Lancet 2002;360:196–202. [DOI] [PubMed] [Google Scholar]
- 17. Moiseyev VS, Põder P, Andrejevs N, Ruda MY, Golikov AP, Lazebnik LB, Kobalava ZD, Lehtonen LA, Laine T, Nieminen MS, Lie KI; RUSSLAN Study Investigators. Safety and efficacy of a novel calcium sensitiser, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction: a randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J 2002;23:1422–1432. [DOI] [PubMed] [Google Scholar]
- 18. Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Põder P, Kivikko M; SURVIVE Investigators. Levosimendan vs dobutamine for patients with acute decompensated heart failure. The SURVIVE Randomized Trial. JAMA 2007;297:1883–1891. [DOI] [PubMed] [Google Scholar]
- 19. Mebazaa A, Parissis J, Porcher R, Gayat E, Nikolaou M, Boas FV, Delgado JF, Follath F.. Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med 2011;37:290–301. [DOI] [PubMed] [Google Scholar]
- 20. Mebazaa A, Motiejunaite J, Gayat E, Crespo-Leiro MG, Lund LH, Maggioni AP, Chioncel O, Akiyama E, Harjola VP, Seferovic P, Laroche C, Julve MS, Roig E, Ruschitzka F, Filippatos G; ESC Heart Failure Long-Term Registry Investigators. Long-term safety of intravenous cardiovascular agents in acute heart failure: results from the European Society of Cardiology Heart Failure Long-Term Registry. Eur J Heart Fail 2018;20:332–341. [DOI] [PubMed] [Google Scholar]
- 21. Landoni G, Biondi-Zoccai G, Greco M, Greco T, Bignami E, Morelli A, Guarracino F, Zangrillo A.. Effects of levosimendan on mortality and hospitalization. A meta-analysis of randomized controlled studies. Crit Care Med 2012;40:634–646. [DOI] [PubMed] [Google Scholar]
- 22. Mebazaa A, Nieminen MS, Filippatos GS, Cleland JG, Salon JE, Thakkar R, Padley RJ, Huang B, Cohen-Solal A.. Levosimendan vs. dobutamine: outcomes for acute heart failure patients on beta-blockers in SURVIVE. Eur J Heart Fail 2009;11:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gong B, Li Z, Yat Wong PC.. Levosimendan treatment for heart failure: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2015;29:1415–1425. [DOI] [PubMed] [Google Scholar]
- 24. Desta T, Jernberg I, Löfman C, Hofman-Bang I, Hagerman J, Spaak J, Persson H.. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART Registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended therapies): a study of 199, 851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail 2015;3:234–242. [DOI] [PubMed] [Google Scholar]
- 25. Sonntag S, Sundberg S, Lehtonen LA, Kleber FX.. The calcium sensitizer levosimendan improves the function of stunned myocardium after percutaneous transluminal coronary angioplasty in acute myocardial ischemia. J Am Coll Cardiol 2004;43:2177–2182. [DOI] [PubMed] [Google Scholar]
- 26. De Luca L, Proietti P, Celotto A, Bucciarelli-Ducci C, Benedetti G, Di Roma A, Sardella G, Genuini I, Fedele F.. Levosimendan improves hemodynamics and coronary flow reserve after percutaneous coronary intervention in patients with acute myocardial infarction and left ventricular dysfunction. Am Heart J 2005;150:563–568. [DOI] [PubMed] [Google Scholar]
- 27. De Luca L, Sardella G, Proietti P, Battagliese A, Benedetti G, Di Roma A, Fedele F.. Effects of levosimendan on left ventricular diastolic function after primary angioplasty for acute anterior myocardial infarction: a Doppler echocardiographic study. J Am Soc Echocardiogr 2006;19:172–177. [DOI] [PubMed] [Google Scholar]
- 28. Husebye T, Eritsland J, Müller C, Sandvik L, Arnesen H, Seljeflot I, Mangschau A, Bjørnerheim R, Andersen GØ.. Levosimendan in acute heart failure following primary percutaneous coronary intervention-treated acute ST-elevation myocardial infarction. Results from the LEAF trial: a randomized, placebo-controlled study. Eur J Heart Fail 2013;15:565–572. [DOI] [PubMed] [Google Scholar]
- 29. Wu X, Wu J, Yan X, Zhang Y.. Enhancement of myocardial function and reduction of injury with levosimendan after percutaneous coronary intervention for acute myocardial infarction: a pilot study. Cardiology 2014;128:202–208. [DOI] [PubMed] [Google Scholar]
- 30. Jia Z, Guo M, Zhang YQ, Liang HQ, Zhang LY, Song Y.. Efficacy of intravenous levosimendan in patients with heart failure complicated by acute myocardial infarction. Cardiology 2014;128:195–201. [DOI] [PubMed] [Google Scholar]
- 31. Bergh CH, Andersson B, Dahlström U, Forfang K, Kivikko M, Sarapohja T, Ullman B, Wikström G.. Intravenous levosimendan vs. dobutamine in acute decompensated heart failure patients on beta-blockers. Eur J Heart Fail 2010;12:404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santoro F, Ieva R, Ferraretti A, Ienco V, Carpagnano G, Lodispoto M, Di Biase L, Di Biase M, Brunetti ND.. Safety and feasibility of levosimendan administration in takotsubo cardiomyopathy: a case series. Cardiovasc Ther 2013;31:e133–e137. [DOI] [PubMed] [Google Scholar]
- 33. Yaman M, Arslan U, Kaya A, Akyol A, Ozturk F, Okudan YE, Bayramoglu A, Bektas O.. Levosimendan accelerates recovery in patients with takotsubo cardiomyopathy. Cardiol J 2016;23:610–615. [DOI] [PubMed] [Google Scholar]
- 34. Nieminen MS, Buerke M, Cohen-Solál A, Costa S, Édes I, Erlikh A, Franco F, Gibson C, Gorjup V, Guarracino F, Gustafsson F, Harjola VP, Husebye T, Karason K, Katsytadze I, Kaul S, Kivikko M, Marenzi G, Masip J, Matskeplishvili S, Mebazaa A, Møller JE, Nessler J, Nessler B, Ntalianis A, Oliva F, Pichler-Cetin E, Põder P, Recio-Mayoral A, Rex S, Rokyta R, Strasser RH, Zima E, Pollesello P.. The role of levosimendan in acute heart failure complicating acute coronary syndrome: a review and expert consensus opinion. Int J Cardiol 2016;218:150–157. [DOI] [PubMed] [Google Scholar]
- 35. Yilmaz MB, Grossini E, Silva Cardoso JC, Édes I, Fedele F, Pollesello P, Kivikko M, Harjola VP, Hasslacher J, Mebazaa A, Morelli A, Le Noble J, Oldner A, Oulego Erroz I, Parissis JT, Parkhomenko A, Poelzl G, Rehberg S, Ricksten SE, Rodríguez Fernández LM, Salmenperä M, Singer M, Treskatsch S, Vrtovec B, Wikström G.. Renal effects of levosimendan: a consensus report. Cardiovasc Drugs Ther 2013;27:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fedele F, Bruno N, Brasolin B, Caira C, D’Ambrosi A, Mancone M.. Levosimendan improves renal function in acute decompensated heart failure: possible underlying mechanisms. Eur J Heart Fail 2014;16:281–288. [DOI] [PubMed] [Google Scholar]
- 37. Rafouli-Stergiou P, Parissis JT, Farmakis D, Bistola V, Frogoudaki A, Vasiliadis K, Ikonomidis I, Paraskevaidis I, Kremastinos D, Filippatos G, Lekakis J.. Effects of levosimendan on markers of kidney function in patients with acutely decompensated heart failure and renal impairment. J Cardiovasc Med (Hagerstown) 2017;18:771–773. [DOI] [PubMed] [Google Scholar]
- 38. Lannemyr L, Ricksten S-E, Rundqvist B, Andersson B, Bartfay S-E, Ljungman C, Dahlberg P, Bergh N, Hjalmarsson C, Gilljam T, Bollano E, Karason K.. Differential effects of levosimendan and dobutamine on glomerular filtration rate in patients with heart failure and renal impairment: a randomized double-blind controlled trial. J Am Heart Assoc 2018;7:e008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grossini E, Molinari C, Pollesello P, Bellomo G, Valente G, Mary D, Vacca G, Caimmi P.. Levosimendan protection against kidney ischemia/reperfusion injuries in anesthetized pigs. J Pharmacol Exp Ther 2012;342:376–388. [DOI] [PubMed] [Google Scholar]
- 40. Zangrillo A, Alvaro G, Belletti A, Pisano A, Brazzi L, Calabrò MG, Guarracino F, Bove T, Grigoryev EV, Monaco F, Boboshko VA, Likhvantsev VV, Scandroglio AM, Paternoster G, Lembo R, Frassoni S, Comis M, Pasyuga VV, Navalesi P, Lomivorotov VV; CHEETAH Study Group. Effect of levosimendan on renal outcome in cardiac surgery patients with chronic kidney disease and perioperative cardiovascular dysfunction: a substudy of a multicenter randomized trial. J Cardiothorac Vasc Anesth 2018; doi: 10.1053/j.jvca.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 41. Bove T, Matteazzi A, Belletti A, Paternoster G, Saleh O, Taddeo D, Dossi R, Greco T, Bradic N, Husedzinovic I, Nigro Neto C, Lomivorotov VV, Calabrò MG.. Beneficial impact of levosimendan in critically ill patients with or at risk for acute renal failure: a meta-analysis of randomized clinical trials. Heart Lung Vessel 2015;7:35–46. [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B.. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016;67:408–416. [DOI] [PubMed] [Google Scholar]
- 43. Jiang R, Zhao QH, Wu WH, Zhang R, Yuan P, Gong SG, He J, Luo CJ, Qiu HL, Wang L, Liu JM.. Efficacy and safety of a calcium sensitizer, levosimendan, in patients with right heart failure due to pulmonary hypertension. Clin Respir J 2018;12:1518–1525. [DOI] [PubMed] [Google Scholar]
- 44. Kleber FX, Bollmann T, Borst MM, Costard-Jäckle A, Ewert R, Kivikko M, Petterson T, Pohjanjousi P, Sonntag S, Wikström G.. Repetitive dosing of intravenous levosimendan improves pulmonary hemodynamics in patients with pulmonary hypertension: results of a pilot study. J Clin Pharmacol 2009;49:109–115. [DOI] [PubMed] [Google Scholar]
- 45. Pollesello P, Papp Z, Papp JG.. Calcium sensitizers: what have we learned over the last 25 years? Int J Cardiol 2016;203:543–548. [DOI] [PubMed] [Google Scholar]