Abstract
Inotropes may be an appropriate treatment for patients with advanced heart failure (AdHF) who remain highly symptomatic despite optimized standard therapies. Objectives for inotrope use in these situations include relief of symptoms and improvement of quality of life, and reduction in unplanned hospitalizations and the costs associated with such episodes. All of these goals must be attained without compromising survival. Encouraging findings with intermittent cycles of intravenous levosimendan have emerged from a range of exploratory studies and from three larger controlled trials (LevoRep, LION-HEART, and LAICA) which offered some evidence of clinical advantage. In these settings, however, obtaining statistically robust data may prove elusive due to the difficulties of endpoint assessment in a complex medical condition with varying presentation and trajectory. Adoption of a composite clinical endpoint evaluated in a hierarchical manner may offer a workable solution to this problem. Such an instrument can explore the proposition that repetitive administration of levosimendan early in the period after discharge from an acute episode of worsening heart failure may be associated with greater subsequent clinical stability vis-à-vis standard therapy. The use of this methodology to develop a ‘stability score’ for each patient means that all participants in such a trial contribute to the overall outcome analysis through one or more of the hierarchical endpoints; this has helpful practical implications for the number of patients needed and the length of follow-up required to generate endpoint data. The LeoDOR study (NCT03437226), outlined in this review, has been designed to explore this new approach to outcome assessment in AdHF.
Keywords: Intermittent treatment, Repetitive inotrope, Quality of life, Rehospitalization, Outcome
Introduction
Patients with advanced heart failure (AdHF) are a relatively small but important contingent of the wider heart failure (HF) population, who face substantial morbidity through continued deterioration of symptoms, frequent hospitalization, and eventual high mortality.
AdHF presents a major challenge to patients, physicians, and healthcare systems. Patients experience a progressive deterioration in their quality of life (QoL), with worsening clinical status that requires frequent and sometimes prolonged hospitalizations. Progression of HF leads to prolongation of hospital stays,1 with consequences for the cost of care,2 while hospitalizations for worsening HF are significant predictors of increased mortality risk per se. Each time a patient is hospitalized for acute decompensated HF (ADHF) there is a risk of further worsening of myocardial function, leading to further episodes of hospitalization. The growing complexities of case management as this cycle repeats itself add further to the cost of care.
Present standard-of-care medical interventions relevant to AdHF include the full repertoire of established therapies (diuretics, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, angiotensin-receptor/neprilysin inhibitors, mineralocorticoid receptor antagonists, beta-adrenergic blockers, and ivabradine).3 Use of conventional adrenergic inotropes has been characterized by worse mortality and no strong impact on hospitalization, despite some favourable effects on haemodynamics and symptomatic improvements in small clinical studies.4
The issuance in 2018 of a position paper on AdHF by the Heart Failure Association of the European Society of Cardiology (HFA-ESC) identifying new and revised concepts in the management of AdHF has contextualized the use of levosimendan in this situation.5
Fresh criteria for a diagnosis of AdHF (Table 1) supersede previous European6 and US7 guidance. A diagnosis of AdHF should not be based narrowly on left ventricular ejection fraction (LVEF), but on the patient’s symptoms, prognostic markers, the presence of end-organ damage, and goals for therapy. (This advice is designed to give due emphasis to the phenomenon of progressing HF despite preserved LVEF.)
AdHF is considered an unstable condition where standard treatment is, by definition, insufficient, and additional interventions must be considered.
Use of vasopressors (specifically dopamine, noradrenaline, and adrenaline) receives only limited recommendation from the HFA-ESC, which notes that these agents have been associated with worse outcomes in observational studies and advises that their use should be restricted to instances of cardiogenic shock [i.e. low systolic blood pressure (SBP) with evidence of organ hypoperfusion] and then only if the low blood pressure is considered a reversible condition or if progression to mechanical circulatory support (MCS) or transplant is planned. Dosage should be limited to the lowest that delivers the desired clinical goals.
The intermittent use of levosimendan for long-term symptomatic improvement or palliation has gained in popularity.5 Evidence for clinical benefit in terms of survival and reduced hospitalizations from meta-analysis and from the LION-HEART study is regarded as suggestive and encouraging, and prima facie better than experience with vasopressors and adrenergic inotropes. These data—examined in fuller detail later in this review—are in need of further corroboration. It is also suggested by the HFA-ESC that studies should be performed to ascertain if use of levosimendan in this way may benefit patients for whom long-term MCS or transplantation is contraindicated or infeasible.
Table 1.
Criteria for a diagnosis of advanced heart failure as advised in the 2018 Position Paper of the Heart Failure Association of the European Society of Cardiology
| All the following must be present despite optimal guideline-directed treatment: |
|
|
|
|
| [In addition to the above, extra-cardiac organ dysfunction due to heart failure (e.g. cardiac cachexia, kidney, or liver dysfunction) or type 2 pulmonary hypertension may be present, but are not required.] |
Reproduced with permission from Crespo-Leiro et al.5
LVEF, left ventricular ejection fraction; ARVC, arrhythmogenic right ventricular cardiomyopathy; BNP, brain natriuretic peptide; NT-proBNP, N-terminal-pro-brain natriuretic peptide; LV, left ventricular; ESC, European Society of Cardiology; HFpEF, heart failure with preserved ejection fraction; HFmrEF, heart failure with mid-range ejection fraction; 6MWT, 6-min walk test; PVO2, peak exercise oxygen consumption; RV, right ventricle/ventricular.
As a calcium sensitizer that promotes inotropy through non-adrenergic mechanisms, levosimendan may represent a way to realize the potential benefits of inotropic support in AdHF with fewer of the adverse effects imputed to conventional inotropes. Use of levosimendan in this way may be regarded as a step towards the ‘decatecholaminization’ of patients with AdHF, in much the same way that has been advocated for cardiogenic shock.8,9 Levosimendan exerts a range of cardiovascular effects, including ventriculo-arterial recoupling, decongestion, and cardiac protection against ischaemia–reperfusion injury, as well as potentially advantageous ancillary effects on kidney function, all of which may be relevant in AdHF10,11; its effects are not impaired by beta-adrenergic blockade (which is likely to be widely implemented in these patients)12; and its use and effectiveness as an intermittent therapy are likely to be enhanced by the existence of a long-acting active metabolite designated OR-1896.13
Clinical studies on the intermittent use of levosimendan in advanced heart failure
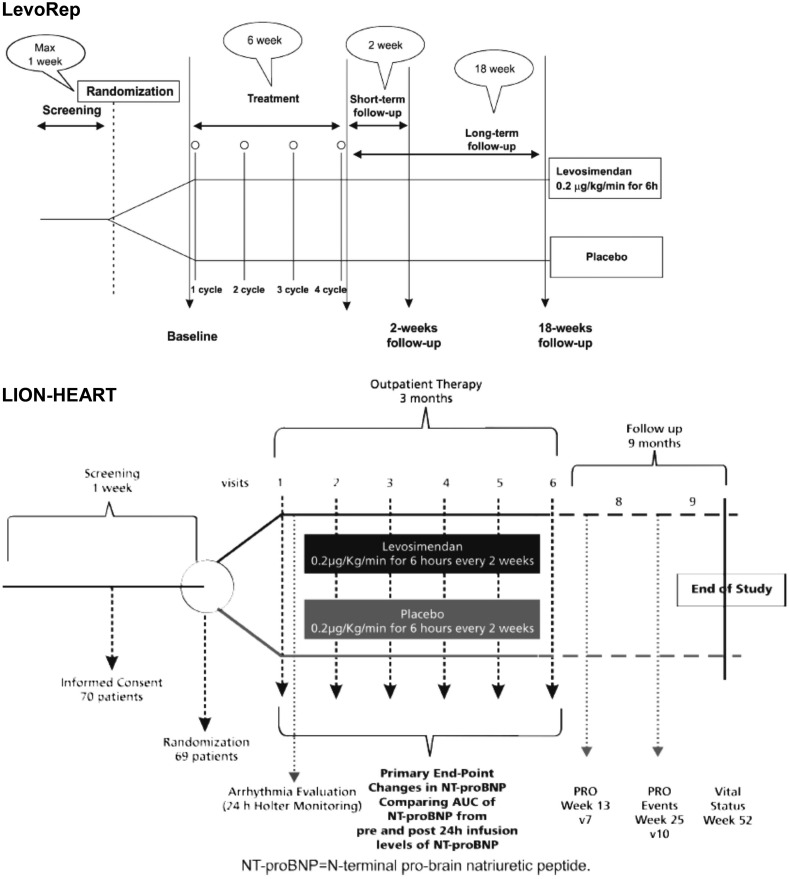
Indications of benefit from intermittent intravenous (i.v.) levosimendan in AdHF in a series of preliminary studies (see Poelzl et al.14 and Table 2 for a synopsis) stimulated the development of a series of larger randomized trials: LevoRep, LION-HEART, and LAICA. These three prospective, randomized, double-blind, placebo-controlled, multicentre, parallel-group trials investigated repetitive therapy (cycles every 2–4 weeks) in similar patient populations (Table 3)22–24 but slightly differing dose schedules in order to cover a range of treatment possibilities. The protocol for LevoRep specified four cycles of i.v. levosimendan therapy. For LION-HEART, the protocol specified two additional cycles of levosimendan therapy in order to assess the effect of a larger cumulative dose of levosimendan (Figure 1). The study dose per cycle was identical in LevoRep and LION-HEART (0.2 µg/kg/min for 6 h at 2-week intervals) while the LAICA study examined a lower dose administered for longer (0.1 µg/kg/min intravenously for 24 h at 30-day intervals for up to 12 months; median treatment duration 6 months).
Table 2.
Summary particulars of preliminary studies of intermittent levosimendan in advanced heart failure
| References | No. of patients enrolled | Levosimendan dose | Infusion duration (h) | Infusion frequency | Design comparator | Endpoints |
|---|---|---|---|---|---|---|
| Nanas15 | 36 | Bolus 6 μg then 0.2 μg/kg/min | 24 | 2 weeks |
|
Haemodynamics; 45-day survival |
| Parissis16 | 25 | Bolus 6 μg then 0.1 μg/kg/min to max. 0.4 μg/kg/min | 24 | 3 weeks |
|
LVEF, LV dimensions and volumes; levels of NT-proBNP, troponin T, CRP, IL-6 |
| Mavrogeni17 | 50 | Bolus 6 μg then 0.1 μg/kg/min to max. 0.2 μg/kg/min | 24 | 30 days |
|
LVEF, LV dimensions and volumes; symptoms and QoL, RV systolic pressure, mitral regurgitation |
| Berger18 | 75 | Bolus 12 μg if BP >95 mmHg. then 0.1 μg/kg/min. Infusion-only if BP <95 mmHg | 24 | 4 weeks |
|
LVEF, BNP, up-titration of beta-blockers |
| Papadopoulou19 | 20 | 0.1 μg/kg/min (no loading dose) | 24 | 30 days |
|
LVEF and QoL |
| Bonios20 | 63 | 0.2 μg/kg/min if given with dobutamine; 0.3 μg/kg/min if given alone | 6 | Weekly |
|
Haemodynamics, survival/freedom from LVAD at 3 and 6 months |
| Malfatto21 | 33 | 0.1 μg/kg/min with hourly 0.1 μg/kg/min increments to max. 0.4 μg/kg/min | 24 | 30 days |
|
Haemodynamics, echocardiography, BNP |
From Poelzl et al.14
LVEF, left ventricular ejection fraction; LV, left ventricular; NT-pro-BNP, pro-N-terminal brain natriuretic peptide; QoL, quality of life; RV, right ventricular; BNP, brain natriuretic peptide.
Table 3.
Summary of patient populations and study protocols in the LevoRep, LION-HEART, and LAICA studies of intermittent levosimendan infusion in advanced heart failure patients
| LevoRep | LION-HEART | LAICA | |
|---|---|---|---|
| (n = 120) | (n = 69) | (n = 97) | |
| Baseline clinical status | NYHA Class III or IV for >3 months | NYHA Class III or IV for >4 weeks | NYHA Class III or IV |
| Enrolment criteria | LVEF <35% | LVEF <35%Episode of pulmonary or systemic congestion requiring i.v. vasoactive drugs within previous 12 months | One of:
|
| Levosimendan schedule | 0.2 μg/kg/min for 6 h every 2 weeks (total of 4 cycles) | 0.2 μg/kg/min for 6 h every 2 weeks (total of 6 cycles) | 0.1 μg/kg/min for 24 h every 30 days |
| Duration | 24 weeks | 52 weeks | Median 24 weeks, maximum 52 weeks |
Derived from references 22–24.
CVP, central venous pressure; HF, heart failure; i.v., intravenous; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PCWP, pulmonary capillary wedge pressure.
Figure 1.
Illustration of the complementary designs of the LevoRep and LION-HEART studies.
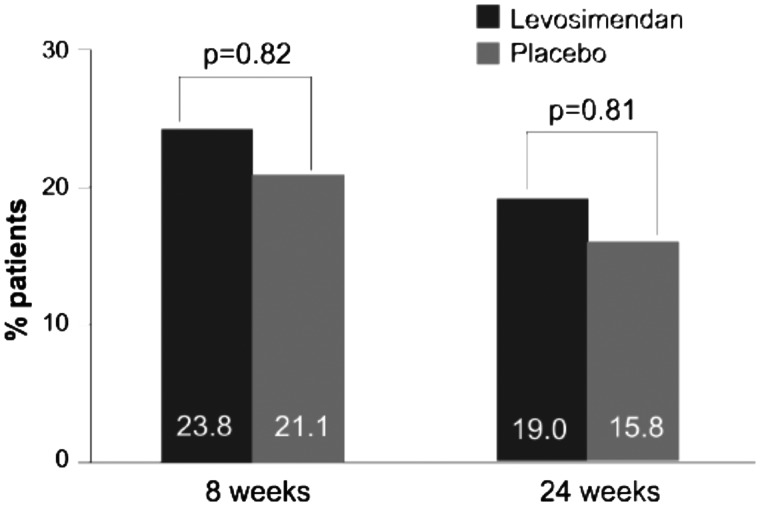
The LevoRep study (NCT01065194) randomized 120 outpatients with AdHF [EF ≤35%, New York Heart Association (NYHA) Class III–IV].22 The total study period comprised a 6-week treatment period and an 18-week follow-up period. Follow-up visits were scheduled at 2 and 18 weeks after the treatment period. The primary outcome was a combination of the proportion of patients with a ≥20% improvement in the 6-min walk test and a ≥15% score increase on the Kansas City Cardiomyopathy Questionnaire (KCCQ)25 at the end of the 24-week study period. That endpoint was reached in 19% of patients receiving levosimendan and in 15.8% receiving placebo [odds ratio 1.25, 95% confidence interval (95% CI) 0.44–3.59, P = 0.810] (Figure 2).
Figure 2.
Principal endpoint results from LevoRep (composite of percentage of patients with improvement in the 6-min walk test of ≥20% and Kansas City Cardiomyopathy Questionnaire clinical summary score ≥15%) at 2 and 18 weeks after completion of four cycles of levosimendan treatment during a 6-week interval. The percentages of patients who reached the primary endpoint did not differ between the two groups (Fisher’s exact test). From Altenberger et al.22
Secondary outcomes of LevoRep included event-free survival after 24 weeks. The net gain with levosimendan on this endpoint was not statistically significant [hazard ratio (HR) 0.50, 95% CI 0.24–1.05, P = 0.069 (log-rank test)].
The LION-HEART study (NCT01536132) was designed to test the efficacy and safety of i.v. administration of repetitive doses of levosimendan in outpatients with AdHF (LVEF <35%).23 A total of 69 patients were randomized to either levosimendan (n = 48) or placebo (n = 21) administered in an ambulatory setting. Patients were followed for clinical outcomes for 6 months (1 year for vital status as a safety endpoint).
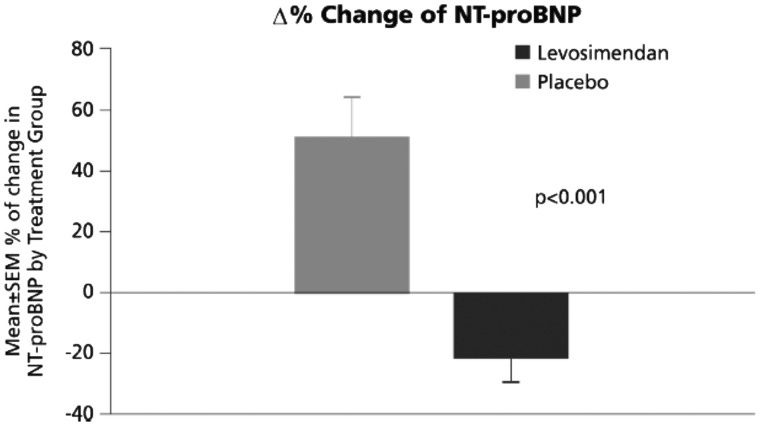
The study’s primary endpoint of change in N-terminal pro-brain natriuretic peptide (NT-proBNP) from baseline was significantly in favour of levosimendan when examined as either baseline-adjusted area under the curve (intergroup pre- vs. post-mean difference ≈1766 pg/mL; P < 0.001) or as percent change from baseline (P < 0.001) (Figure 3). Among clinical outcomes specified as secondary endpoints, there was a significant reduction in the combined incidence of all-cause mortality and hospitalization for HF in patients receiving levosimendan [81% vs. 45.8% at 100 days, P = 0.015 (log-rank test)]. The reduction in hospitalizations for HF was significant (22.9% vs. 66.7%, HR 0.25, 95% CI 0.11–0.55; P = 0.001).23
Figure 3.
LION-HEART: comparison of mean NT-proBNP by visit (analysis of covariance for repeated measures). NT-proBNP, N-terminal pro-brain natriuretic peptide; SEM, standard error of the mean.
LAICA (NCT00988806) evaluated the efficacy and safety of long-term intermittent administration of levosimendan to reduce the incidence of hospital admissions for acute HF episodes.24 Randomized patients had diagnostic criteria of AdHF together with at least one hospital admission for acute decompensation or HF worsening within 6 months.
A total of 97 patients (levosimendan, n = 70; placebo, n = 27) were randomly assigned to receive infusions once every 30 days in addition to optimal standard HF therapy. The primary endpoint was the incidence of admission for ADHF or HF worsening, defined as admission to the emergency department or a hospital ward for >12 h due to worsening of signs and symptoms of HF. No significant difference was observed in the primary endpoint but the findings of LAICA favoured levosimendan in terms of both fewer admissions for ADHF and lower mortality rates: levosimendan, 6.6%; placebo, 22.2% (P = 0.0439 by log-rank test).
Adverse events and safety profile
In aggregate, experience in these three larger trials of intermittent levosimendan therapy indicates that this approach was well tolerated, despite the parlous clinical status of many patients and the widespread use of complex polypharmacy.
In the LevoRep trial, levosimendan infusions were associated with a greater median reduction in SBP than with placebo, but the overall adverse event profiles of the two interventions were otherwise similar.22 The median drop in SBP was more pronounced in the levosimendan group than with placebo [−6.7 mmHg, interquartile range (IQR) −16.6 to −2.7 mmHg, vs. –1.0 mmHg, IQR −4.5 to +3.5 mmHg; P = 0.01]. Active measures for symptomatic hypotension (e.g. fluid administration or vasopressor application) were reported in ≈9% of patients in both groups. The frequency of side effects was comparable and low (<3%) in both groups with respect to tachycardia, new-onset atrial fibrillation, and non-sustained ventricular tachycardia. Most doses in both groups were completed without any adverse events: levosimendan, 212 courses (91%); placebo, 201 courses (94%).
Levosimendan was regarded as well tolerated in LION-HEART, with comparable frequencies of patients experiencing an adverse event (levosimendan, 77.1%; placebo, 90.9%).23 A greater proportion of levosimendan-treated patients needed dose reduction or interruption due to significant hypotension (SBP <80 mmHg; levosimendan, 15%; placebo, 9%), although this difference did not reach statistical significance.
The rate of adverse events in the LAICA study was comparable between the levosimendan and placebo groups.24 This included similar incidences of patients with an adverse event leading to discontinuation: levosimendan, n = 22 (31.4%) vs. placebo, n = 9 (33.3%).
Of these studies, LION-HEART exerted a particularly strong influence on the content of the 2018 HFA-ESC Position Paper on AdHF,5 with the authors noting that ‘NT-proBNP over time, the primary endpoint, was significantly lower in the levosimendan group compared to the placebo group. Patients randomized to levosimendan were also less likely to be hospitalized for heart failure or experience a decline in health-related quality of life compared to placebo. Adverse events were similar between groups’.
Registry experience with intravenous levosimendan
Important additional insights into the impact of intermittent levosimendan in AdHF have recently emerged from the multicentre RELEVANT-HF registry.26 This initiative enrolled 185 ambulatory AdHF patients (148 men) who had systolic dysfunction and who had experienced two or more HF-related hospitalizations/emergency visits in the previous 6 months despite optimal medical management. Most of these patients (n = 124) were treated in a hospital or outpatient setting, with specifically tailored intermittent levosimendan therapy in the dose range 0.05–0.2 μg/kg/min (with no preliminary bolus) at 3–4-week intervals.
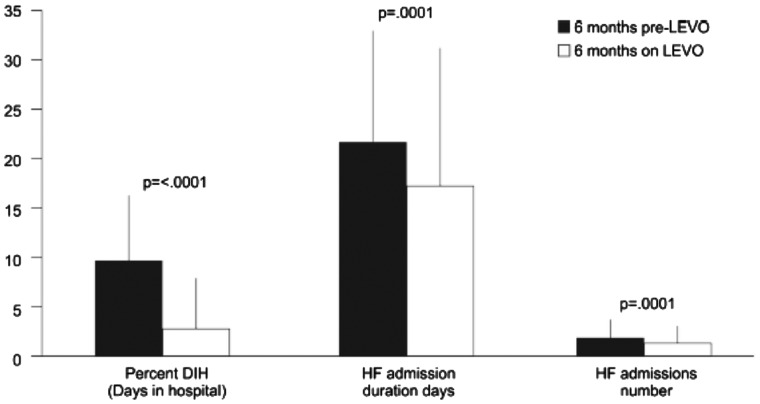
Clinical benefits were recorded in the 6 months after starting levosimendan therapy, with statistically significant reductions in the number and duration of HF-related hospitalizations and in total days in hospital (Figure 4). Overall event-free 1-year survival was 76%, though lower (62%) in those (n = 37) who required adjustment of their levosimendan schedule due to loss of effect. Six-month direct costs were estimated to be reduced by an average of €1157 per capita, with indications that savings of up to almost €4000 might be achievable in that time. Given the comparison with historic data, however, these results should be interpreted with caution. (See Nieminen et al.27 for an additional recent favourable, but more conservative, perspective on the impact of levosimendan on savings in cost of care in 7 European countries.)
Figure 4.
Changes in days in hospital (DIH) and number and duration of hospital admissions for heart failure (HF) in the 6 months after start of levosimendan treatment compared to the preceding 6 months in the RELEVANT-HF registry. Data are mean ± standard deviation and non-parametric P-values are shown. From Oliva et al.26
The impact of levosimendan on hospitalizations in RELEVANT-HF conforms to the findings of a recent meta-analysis by Silvetti et al.,28 which documented a risk ratio of 0.4 (95% CI 0.27–0.59) for rehospitalization in the 3 months following conclusion of treatment in patients who received intermittent levosimendan vs. treatment with a range of comparator interventions.
Taken overall, experience in these large trials has confirmed that repetitive application of levosimendan is feasible and safe even in an outpatient setting. Among the randomized studies, however, only LION-HEART met its primary endpoint, but there were strong trends towards reduction in HF-related hospital re-admission and mortality and clear evidence that repeat use of levosimendan reduces NT-proBNP levels.
Delivering repetitive levosimendan therapy
The protocol provisions and experiences of the LevoRep, LION-HEART, and LAICA studies and the RELEVANT-HF registry have helped to frame a pragmatic schedule for the delivery of intermittent levosimendan therapy in AdHF. The process starts with the identification of candidate patients and the goals of therapy.
For this purpose, patients with AdHF may be identified by the following criteria: (i) LVEF <35%; (ii) NYHA Class IV or IIIb, or NYHA Class IIIa with frequent decompensation despite optimal standard therapies; and (iii) mean SBP >90 mmHg, or 80–90 mmHg if the patient has previously tolerated levosimendan. Many candidate patients will conform to stages 4–7 of the classification of the Interagency Registry for Mechanically Assisted Circulatory Support: final selections should be guided by the criteria of controlled trials such as LAICA and LION-HEART (Table 3).
The first objectives of therapy are to prevent disease progression, reduce hospitalizations, and improve QoL. These aims should be clearly explained to the patient, but discussion of improved survival may not be appropriate and a decision on that point needs to be shaped by assessment of the patient’s temperament, mood, and expectations. This is an arena of HF where the adoption of a ‘Goals of Care’ programme (see e.g. Dougherty et al.29) may be highly advantageous to all concerned in furthering a proper appreciation of the patient’s needs and goals. Subject to the usual caveats about resource availability, a steadily strengthening case can be made for such programmes becoming part of the standard of care in the management of AdHF.
Blood tests should be performed before treatment is started. Patients should not be treated if at this stage there are signs of:
severe hypotension [SBP <90 mmHg (<80 mmHg in repeat treatment if previous infusion was well tolerated)] or tachycardia
severe renal or hepatic impairment
hypokalaemia
significant mechanical obstructions affecting ventricular filling or outflow
a history of torsades de pointes.
A bolus dose of levosimendan should not be used. As patient characteristics and responses to treatment are variable, the initial dosing schedule needs to be flexible. The first-time infusion should be initiated at a rate of 0.1 μg/kg/min. If that dose is well tolerated during the first 1–2 h it may be increased to 0.2 μg/kg/min. If the initial dose is not well tolerated (as evidenced by hypotension) it should be halved to 0.05 μg/kg/min and re-evaluated. If that lower dose is also not tolerated then treatment should be stopped.
Determination of the interval between courses of therapy (2–4 weeks) should thereafter be shaped by the trajectory of symptoms in individual patients. Weight-specific infusion rates for levosimendan are to be used.
Intermittent levosimendan therapy must take place in the context of ongoing advice and support that includes counselling on diet and exercise/rest schedules, as well as QoL evaluation. Ideally, specialist nurses will perform these tasks as part of a comprehensive HF management programme.
Future developments: the LeoDOR study
Symptom relief is a major clinical target in the management of AdHF but reliance on symptoms as an objective endpoint in HF trials can be problematic: responses cannot always be measured consistently and reliably and may not always be an ideal primary indicator of the efficacy of an intervention. A composite endpoint combining death and rehospitalizations might be widely supported but presents several interrelated drawbacks which may complicate both the conduct of any study and the interpretation of its findings (see e.g. Allen et al.30).
A solution to this challenge may be to use a composite clinical endpoint evaluated in a hierarchical manner as in the FIGHT trial.31 Those investigators proposed, as the primary endpoint, a Global Rank Score (GRS) where each patient is assigned a numerical value corresponding to their stability index, which mirrors their respective clinical conditions. Each patient is given a score value based on outcome during follow-up, and then analysed in hierarchical categories of (i) time to death; (ii) time to HF hospitalization; and (iii) time-averaged proportional change in NT-proBNP. In this way, deaths are the most important events, followed by rehospitalizations and finally by NT-proBNP elevation.
The GRS is easy to interpret: the higher the value, the more stable a patient’s clinical condition. Such an instrument may make it feasible to examine the hypothesis that, compared with placebo, repetitive administration of levosimendan early in the period after discharge from an acute episode of worsening HF may be associated with greater clinical stability over the course of subsequent weeks. All the individual components of the GRS could be explored as secondary endpoints. This approach would create an opportunity to recruit an advanced but fairly stable HF population that could be enrolled within a manageable span of time and should have a low dropout rate by virtue of the shorter treatment time per patient.
The LeoDOR study (NCT03437226) has been designed to explore this proposition. As a multicentre, randomized, double-blind, placebo-controlled, three-arm trial, LeoDOR will evaluate the efficacy and safety of repetitive levosimendan therapy given for 12 weeks, either as a 6-h continuous infusion at a rate of 0.2 μg/kg/min every 2 weeks (for 7 cycles) or as a 24-h continuous infusion at a rate of 0.1 μg/kg/min every 3 weeks (for 5 cycles). The treatment arm with a 24-h continuous infusion of levosimendan has been included to meet the infrastructural and economic requirements of countries and centres currently unable to support ambulatory therapy in AdHF. After the cycles of treatment (i.e. at Day 84), study medication will be stopped for all patients. Follow-up visits will then be conducted 14 ± 2 and 180 ± 14 days after completion of treatment.
The primary efficacy assessment in LeoDOR is based on a composite combined outcome metric in which all participants are ranked across three hierarchical groups in order of importance. At the head of the ranking comes (i) time to death or urgent heart transplantation or implantation of a ventricular assist device, followed by (ii) time to non-fatal HF hospitalization requiring i.v. vasoactive therapy, and (iii) time-averaged proportional change in NT-proBNP.
Enrolment in the LeoDOR study commenced in March 2018 and is scheduled to continue until mid-2019, by which time it is planned to have enrolled 264 patients at 28 centres in 9 European countries. Detailed particulars of the rationale, design, and conduct of the LeoDOR trial, plus regular progress reports, are available via www.leodortrial.com.
Another noteworthy initiative in this area is research by one of the authors (F. Fedele) into improved methods for identifying patients most likely to derive benefit from intermittent levosimendan therapy. This centres on the development and validation of a novel heart failure classification, the HLM methodology. This is based on staging the heart (H), lung (L), and multiple other organs (M). The purpose of this new instrument is to differentiate between patients with AdHF (in whom cardiac and systemic dysfunction and symptoms are potentially reversible) and those with end-stage HF (in whom they are not) as part of the wider goal of introducing the right treatment to the right patient at the right time.
In a development of this research, the HLM instrument has been used to evaluate outcomes in 300 patients who received intermittent levosimendan therapy. HLM criteria allowed for differentiation between a subset of patients with a good response to levosimendan and a group with limited or no response. These groups were indistinguishable on the basis of baseline characteristics, HF risk factors, and aetiology, but the difference in risk of death or rehospitalization up to 12 months after completion of levosimendan therapy was very large and statistically robust (P ≈ 0.001). These data are in need of corroboration but suggest that the HLM classification might aid the identification of AdHF patients who could benefit from intermittent levosimendan.
Prevention of destabilization as a strategic goal
An episode of HF-related hospitalization is recognized as an opportunity to implement or revise guideline-directed medical therapy recommended by the ESC and other professional societies as well as to manage cardiac and non-cardiac comorbidities with the aim of bringing the now-stabilized patient onto a long-term plateau of clinical stability. (See Cheema et al.32 for a recent commentary on this subject.) In addition, however, it should be noted (Figure 5) that destabilization is not invariably immediate: it may be possible to identify intervals during which timely recognition of (and intervention for) signs and symptoms of decompensation may avoid unplanned/urgent hospitalizations due to haemodynamic crises. In particular, as noted by Cheema et al.,32 ‘Haemodynamic congestion, evidenced by elevated filling pressures and/or [natriuretic peptide] elevation, may exist with or without overt signs and symptoms of clinical congestion… subclinical haemodynamic congestion may progress to clinical congestion requiring hospital admission… intervention [at the] subclinical stage may prevent this’.
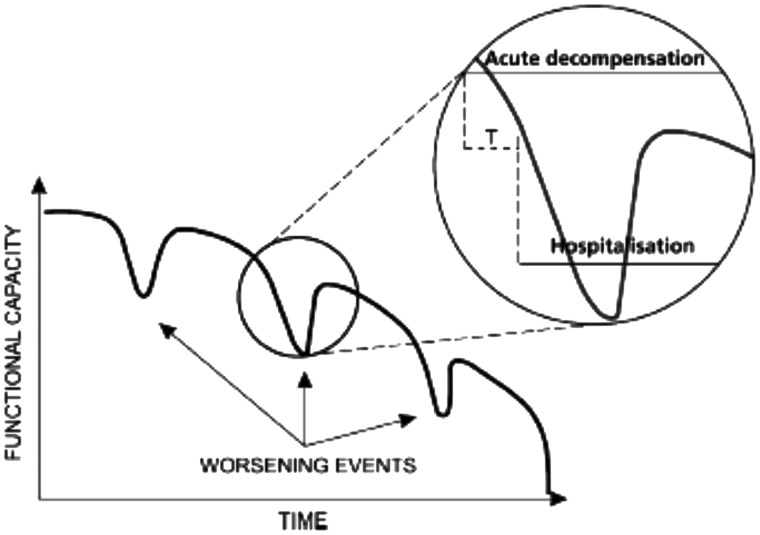
Figure 5.
The variable course of decompensation in advanced heart failure means that it should be possible in many instances to identify intervals during which the timely recognition of the signs and symptoms of decompensation permit interventions that can avert unplanned hospitalizations due to haemodynamic deterioration. T: Interval during which the timely recognition of signs and symptoms of decompensation and its timely treatment can avoid unplanned hospitalization due to haemodynamic crisis, which usually accompanies loss of myocardial tissue. See text for further discussion.
This possibility may be more attainable for decompensation of primarily cardiac origin or for cases of non-adherence to medications or fluid restriction than for cases associated with external factors such as infection, but recent and ongoing developments in the technology and science of telemedicine and telemonitoring identify this as an area where significant progress may be anticipated within a short span of years. Levosimendan may be an appropriate intervention in this ‘acute but non-hospitalized’ situation, depending on the clinical circumstances of an individual patient.33 The successful use of intermittent cycles of oral or parenteral levosimendan, delivered in the outpatient setting, speaks to the feasibility of this intervention.
In this context, a full evaluation of the impact of using levosimendan in combination with agents such as dobutamine may be instructive: useful insights on that matter should be obtainable from post hoc analysis of data from completed randomized trials supplementing the exploratory work of Nanas et al.,15 who observed survival advantages from the use of levosimendan with dobutamine (45-day survival rate 61%, vs. 6% with dobutamine only, P = 0.0002 in log-rank test) in patients with AdHF. Reports of the successful use of levosimendan in combination with nesiritide for the relief of symptoms and reduction in 3-month rates of re-admission or death in 120 acute heart failure patients34 are a further intimation of the utility and potential of levosimendan as an element in such a strategy. Experiences in the ongoing Early Management Strategies of Acute Heart Failure for Patients with NSTEMI (EMSAHF) study (NCT03189901) may provide insights into this premise.
Conclusions
We concur with the view of the HFA-ESC5 that AdHF patients are inherently unstable. Their situation often requires inotropic therapy. Our opinion is that such therapy should not be limited to critical care treatment or the later phases of rehospitalization. Advanced heart failure patients decompensate well before being hospitalized and preventing their rehospitalization could often mean saving myocardial tissue viability and contractility reserve. As we illustrate in Figure 5, there is often a crucial interval during which the timely recognition of signs and symptoms of decompensation can avoid unplanned hospitalization due to haemodynamic crisis which usually accompanies loss of myocardial tissue. In this window of opportunity, levosimendan can be a viable option to avoid the loss of contractile reserve, and the burden of rehospitalization.
Acknowledgements
The authors thank Hughes associates, Oxford, UK, for assistance in the preparation of this article.
Conflict of interest: M.K. is a full-time employee of Orion Pharma. The other authors report no conflicts of interest apart from lecture honoraria relating to the practical tutorials lectures on “Inodilators in Acute and Advanced heart failure” held at the annual meeting of the ESC in Munich, Germany on 26–28 August 2018 (which were covered by an unrestricted educational grant from Orion Pharma), and various other national or international educational events on levosimendan sponsored by Orion Pharma. The lecturers and programme were approved by the ESC programme committee. Orion Pharma follows the EFPIA HCP Code.
References
- 1. Cotter G, Metra M, Davison BA, Senger S, Bourge RC, Cleland JG, Jondeau G, Krum H, O'Connor CM, Parker JD, Torre-Amione G, van Veldhuisen DJ, Milo O, Kobrin I, Rainisio M, McMurray JJ, Teerlink JR; VERITAS Investigators. Worsening heart failure, a critical event during hospital admission for acute heart failure: results from the VERITAS study. Eur J Heart Fail 2014;16:1362–1371. [DOI] [PubMed] [Google Scholar]
- 2. Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlström U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A; Heart Failure Association (HFA) of the European Society of Cardiology (ESC). European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail 2016;18:613–625. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 4. Upadya S, Lee FA, Saldarriaga C, Verma S, Sedrakyan A, Nystrom K, Katz SD.. Home continuous positive inotropic infusion as a bridge to cardiac transplantation in patients with end-stage heart failure. J Heart Lung Transplant 2004;23:466–472. [DOI] [PubMed] [Google Scholar]
- 5. Crespo-Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge-Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hülsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska-Migaj E, McDonagh T, Seferovic P, Ruschitzka F.. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; doi: 10.1002/ejhf.1236. [DOI] [PubMed] [Google Scholar]
- 6. Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, Böhm M, Anker S, Dargie H, Brutsaert D, Komajda M; Heart Failure Association of the European Society of Cardiology. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2007;9:684–694. [DOI] [PubMed] [Google Scholar]
- 7. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 8. Singer M. Catecholamine treatment for shock—equally good or bad? Lancet 2007;370:636–637. [DOI] [PubMed] [Google Scholar]
- 9. Andreis DT, Singer M.. Catecholamines for inflammatory shock: a Jekyll-and-Hyde conundrum. Intensive Care Med 2016;42:1387–1397. [DOI] [PubMed] [Google Scholar]
- 10. Altenberger J, Gustafsson F, Harjola V-P, Karason K, Kindgen-Milles D, Kivikko M, Malfatto G, Papp Z, Parissis J, Pollesello P, Pölzl G, Tschöpe C.. Levosimendan in acute and advanced heart failure: an appraisal of the clinical database and evaluation of its therapeutic applications. J Cardiovasc Pharmacol 2018;71:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farmakis D, Alvarez J, Gal TB, Brito D, Fedele F, Fonseca C, Gordon AC, Gotsman I, Grossini E, Guarracino F, Harjola VP, Hellman Y, Heunks L, Ivancan V, Karavidas A, Kivikko M, Lomivorotov V, Longrois D, Masip J, Metra M, Morelli A, Nikolaou M, Papp Z, Parkhomenko A, Poelzl G, Pollesello P, Ravn HB, Rex S, Riha H, Ricksten SE, Schwinger RHG, Vrtovec B, Yilmaz MB, Zielinska M, Parissis J.. Levosimendan beyond inotropy and acute heart failure: evidence of pleiotropic effects on the heart and other organs: an expert panel position paper. Int J Cardiol 2016;222:303–312. [DOI] [PubMed] [Google Scholar]
- 12. Mebazaa A, Nieminen MS, Filippatos GS, Cleland JG, Salon JE, Thakkar R, Padley RJ, Huang B, Cohen-Solal A.. Levosimendan vs. dobutamine: outcomes for acute heart failure patients on beta-blockers in SURVIVE. Eur J Heart Fail 2009;11:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kivikko M, Antila S, Eha J, Lehtonen L, Pentikäinen PJ.. Pharmacokinetics of levosimendan and its metabolites during and after a 24-hour continuous infusion in patients with severe heart failure. Int J Clin Pharmacol Ther 2002;40:465–471. [DOI] [PubMed] [Google Scholar]
- 14. Pölzl G, Altenberger J, Baholli L, Beltrán P, Borbély A, Comin-Colet J, Delgado JF, Fedele F, Fontana A, Fruhwald F, Giamouzis G, Giannakoulas G, Garcia-González MJ, Gustafsson F, Kaikkonen K, Kivikko M, Kubica J, von Lewinski D, Löfman I, Malfatto G, Manito N, Martínez-Sellés M, Masip J, Merkely B, Morandi F, Mølgaard H, Oliva F, Pantev E, Papp Z, Perna GP, Pfister R, Piazza V, Bover R, Rangel-Sousa D, Recio-Mayoral A, Reinecke A, Rieth A, Sarapohja T, Schmidt G, Seidel M, Störk S, Vrtovec B, Wikström G, Yerly P, Pollesello P.. Repetitive use of levosimendan in advanced heart failure: need for stronger evidence in a field in dire need of a useful therapy. Int J Cardiol 2017;243:389–395. [DOI] [PubMed] [Google Scholar]
- 15. Nanas JN, Papazoglou P, Tsagalou EP, Ntalianis A, Tsolakis E, Terrovitis JV, Kanakakis J, Nanas SN, Alexopoulos GP, Anastasiou-Nana MI.. Efficacy and safety of intermittent, long-term, concomitant dobutamine and levosimendan infusions in severe heart failure refractory to dobutamine alone. Am J Cardiol 2005;95:768–771. [DOI] [PubMed] [Google Scholar]
- 16. Parissis JT, Adamopoulos S, Farmakis D, Filippatos G, Paraskevaidis I, Panou F, Iliodromitis E, Kremastinos DT.. Effects of serial levosimendan infusions on left ventricular performance and plasma biomarkers of myocardial injury and neurohormonal and immune activation in patients with advanced heart failure. Heart 2006;92:1768–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mavrogeni S, Giamouzis G, Papadopoulou E, Thomopoulou S, Dritsas A, Athanasopoulos G, Adreanides E, Vassiliadis I, Spargias K, Panagiotakos D, Cokkinos DV.. A 6-month follow-up of intermittent levosimendan administration effect on systolic function, specific activity questionnaire, and arrhythmia in advanced heart failure. J Card Fail 2007;13:556–559. [DOI] [PubMed] [Google Scholar]
- 18. Berger R, Moertl D, Huelsmann D, Bojic A, Ahmadi R, Heissenberger I, Pacher R.. Levosimendan and prostaglandin E1 for uptitration of beta-blockade in patients with refractory, advanced chronic heart failure. Eur J Heart Fail 2007;9:202–208. [DOI] [PubMed] [Google Scholar]
- 19. Papadopoulou EF, Mavrogeni SI, Dritsas A, Cokkinos DV.. Assessment of quality of life using three different activity questionnaires in heart failure patients after monthly, intermittent administration of levosimendan during a six-month period. Hell J Cardiol 2009;50:269–274. [PubMed] [Google Scholar]
- 20. Bonios MJ, Terrovitis JV, Drakos SG, Katsaros F, Pantsios C, Nanas SN, Kanakakis J, Alexopoulos G, Toumanidis S, Anastasiou-Nana M, Nanas JN.. Comparison of three different regimens of intermittent inotrope infusions for end stage heart failure. Int J Cardiol 2012;159:225–229. [DOI] [PubMed] [Google Scholar]
- 21. Malfatto G, Della Rosa F, Villani A, Rella V, Branzi G, Facchini M, Parati G.. Intermittent levosimendan infusions in advanced heart failure: favourable effects on left ventricular function, neurohormonal balance, and one-year survival. J Cardiovasc Pharmacol 2012;60:450–455. [DOI] [PubMed] [Google Scholar]
- 22. Altenberger J, Parissis JT, Costard-Jaeckle A, Winter A, Ebner C, Karavidas A, Sihorsch K, Avgeropoulou E, Weber T, Dimopoulos L, Ulmer H, Poelzl G.. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial. Eur J Heart Fail 2014;16:898–906. [DOI] [PubMed] [Google Scholar]
- 23. Comín-Colet J, Manito N, Segovia-Cubero J, Delgado J, García Pinilla JM, Almenar L, Crespo-Leiro MG, Sionis A, Blasco T, Pascual-Figal D, Gonzalez-Vilchez F, Lambert-Rodríguez JL, Grau M, Bruguera J; LION-HEART Study Investigators. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION‐HEART multicentre randomised trial. Eur J Heart Fail 2018; doi: 10.1002/ejhf.1145. [DOI] [PubMed] [Google Scholar]
- 24. García-González MJ. Efficacy and security of intermittent repeated levosimendan administration in patients with advanced heart failure: a randomized, double-blind, placebo controlled multicenter trial: LAICA study. Oral Presentation at the HEART FAILURE Congress, 21 May, 2016, Florence, Italy. https://esc365.escardio.org/Congress/HEART-FAILURE-2016/Late-Breaking-Trials-I-Focus-on-acute-heart-failure/136186-efficacy-and-security-of-intermittent-repeated-levosimendan-administration-in-patients-with-advanced-heart-failure-a-randomized-double-blind-placebo-controlled-multicenter-trial-laica-study#slide. (Last accessed 11 November 2018.) [Google Scholar]
- 25. Green CP, Porter CB, Bresnahan DR, Spertus JA.. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 26. Oliva F, Perna E, Marini M, Nassiacos D, Cirò A, Malfatto G, Morandi F, Caico I, Perna G, Meloni S, Vincenzi A, Villani A, Vecchi AL, Minoia C, Verde A, De Maria R; on behalf of the RELEVANT-HF Study Group. Scheduled intermittent inotropes for ambulatory advanced heart failure. The RELEVANT-HF multicentre collaboration. Int J Cardiol 2018; doi:10.1016/j.ijcard.2018.08.048. [DOI] [PubMed] [Google Scholar]
- 27. Nieminen MS, Buerke M, Parissis J, Ben-Gal T, Pollesello P, Kivikko M, Karavidas A, Severino P, Comín-Colet J, Wikström G, Fedele F.. Pharmaco-economics of levosimendan in cardiology: a European perspective. Int J Cardiol 2015;199:337–341. [DOI] [PubMed] [Google Scholar]
- 28. Silvetti S, Belletti A, Fontana A, Pollesello P.. Rehospitalization after intermittent levosimendan treatment in advanced heart failure patients: a meta-analysis of randomized trials. ESC Heart Fail 2017;4:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dougherty CM, Coats HL, Randall Curtis J, Doorenbos AZ.. Development and testing of a goals of care intervention in advanced heart failure. Appl Nurs Res 2017;38:99–106. [DOI] [PubMed] [Google Scholar]
- 30. Allen LA, Metra M, Milo-Cotter O, Filippatos G, Reisin LH, Bensimhon DR, Gronda EG, Colombo P, Felker GM, Cas LD, Kremastinos DT, O'connor CM, Cotter G, Davison BA, Dittrich HC, Velazquez EJ.. Improvements in signs and symptoms during hospitalization for acute heart failure follow different patterns and depend on the measurement scales used: an international, prospective registry to evaluate the evolution of measures of disease severity in acute heart failure (MEASURE-AHF). J Card Fail 2008;14:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, Mann DL, Whellan DJ, Kiernan MS, Felker GM, McNulty SE, Anstrom KJ, Shah MR, Braunwald E, Cappola TP; NHLBI Heart Failure Clinical Research Network. Effects of Liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2016;316:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheema B, Ambrosy AP, Kaplan RM, Senni M, Fonarow GC, Chioncel O, Butler J, Gheorghiade M.. Lessons learned in acute heart failure. Eur J Heart Fail 2018;20:630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schumann J, Henrich EC, Strobl H, Prondzinsky R, Weiche S, Thiele H, Werdan K, Frantz S, Unverzagt S.. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev 2018;1:CD009669.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jia Z, Guo M, Zhang LY, Zhang YQ, Liang HQ, Song Y.. Levosimendan and nesiritide as a combination therapy in patients with acute heart failure. Am J Med Sci 2015;349:398–405. [DOI] [PubMed] [Google Scholar]