Abstract
AIM
To investigate the role of peritoneal macrophage (PM) polarization in the therapeutic effect of abdominal paracentesis drainage (APD) on severe acute pancreatitis (SAP).
METHODS
SAP was induced by 5% Na-taurocholate retrograde injection in Sprague-Dawley rats. APD was performed by inserting a drainage tube with a vacuum ball into the lower right abdomen of the rats immediately after the induction of SAP. To verify the effect of APD on macrophages, PMs were isolated and cultured in an environment, with the peritoneal inflammatory environment simulated by the addition of peritoneal lavage in complete RPMI 1640 medium. Hematoxylin and eosin staining was performed. The levels of pancreatitis biomarkers amylase and lipase as well as the levels of inflammatory mediators in the blood and peritoneal lavage were determined. The polarization phenotypes of the PMs were identified by detecting the marker expression of M1/M2 macrophages via flow cytometry, qPCR and immunohistochemical staining. The protein expression in macrophages that had infiltrated the pancreas was determined by Western blot.
RESULTS
APD treatment significantly reduced the histopathological scores and levels of amylase, lipase, tumor necrosis factor-α and interleukin (IL)-1β, indicating that APD ameliorates the severity of SAP. Importantly, we found that APD treatment polarized PMs towards the M2 phenotype, as evidenced by the reduced number of M1 macrophages and the reduced levels of pro-inflammatory mediators, such as IL-1β and L-selectin, as well as the increased number of M2 macrophages and increased levels of anti-inflammatory mediators, such as IL-4 and IL-10. Furthermore, in an in vitro study wherein peritoneal lavage from the APD group was added to the cultured PMs to simulate the peritoneal inflammatory environment, PMs also exhibited a dominant M2 phenotype, resulting in a significantly lower level of inflammation. Finally, APD treatment increased the proportion of M2 macrophages and upregulated the expression of the anti-inflammatory protein Arg-1 in the pancreas of SAP model rats.
CONCLUSION
These findings suggest that APD treatment exerts anti-inflammatory effects by regulating the M2 polarization of PMs, providing novel insights into the mechanism underlying its therapeutic effect.
Keywords: Abdominal paracentesis drainage, Peritoneal macrophages, Polarization, Severe acute pancreatitis
Core tip: In the present study, we provided evidence for the first time that abdominal paracentesis drainage (APD) ameliorates inflammation in rats with severe acute pancreatitis (SAP) by regulating peritoneal macrophage M2 polarization. The important findings are that: (1) by removing pancreatitis-associated ascitic fluids, APD could improve the inflammatory environment of the peritoneal cavity; (2) the improved environment in the peritoneal cavity could polarize peritoneal macrophages towards the M2 phenotype; and (3) APD could promote M2 polarization of macrophages in the pancreas of SAP model rats. These findings provide new insights into the mechanisms underlying the effectiveness of APD, which may advance the clinical use of APD to benefit patients with SAP.
INTRODUCTION
Severe acute pancreatitis (SAP) is a lethal inflammatory condition that is frequently accompanied by many complications, such as systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS), which lead to a high risk of death in SAP patients. To date, the mortality rate for SAP remains high at 30%[1]. The key issue is that there is no effective strategy for controlling the activated inflammatory cascade and restoring immune homeostasis during SAP. Numerous studies have suggested that pancreatitis-associated ascitic fluids (PAAF) play an important role in the pathogenesis of SAP because they contain tumor necrosis factors, interleukins, endotoxins and other substances[2-4]. Our previous studies suggested that abdominal paracentesis drainage (APD) ameliorates SAP in patients safely and effectively by removing PAAF[5-7]. However, the mechanism underlying APD treatment remains poorly understood.
Macrophages are the main inflammatory cells implicated in the initiation and progression of the early stage of SAP[8,9]. They can be polarized into the following two different functional phenotypes in response to the microenvironment: classically activated macrophages (M1) or alternatively activated macrophages (M2). M1 macrophages produce pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, while the M2 phenotype produces anti-inflammatory cytokines, such as IL-10[10]. Macrophages can also transition between the M1 and M2 phenotypes in response to certain signals[11,12]. Therefore, the polarization of macrophages towards the M2 phenotype has emerged as an interesting strategy to control the progression of inflammation[13]. Among the macrophages associated with SAP, peritoneal macrophages (PMs) are known to play a crucial role in the progression from local to systemic inflammation. PMs widely interact with PAAF, and the function of PMs is regulated by PAAF[14]. Although the activation of macrophages is tightly associated with the severity of SAP, whether APD can change the polarization state of PMs by removing PAAF in rats with SAP is uncertain.
Based on the above considerations, in the present study, we sought to determine the polarization phenotypes of PMs and the corresponding inflammatory response in a rat model of SAP following APD treatment. Our study offers new insights into the mechanism by which APD treatment ameliorates SAP.
MATERIALS AND METHODS
Experimental animals
The animal protocol was designed to minimize pain or discomfort to the animals. Adult male Sprague-Dawley rats weighing 200-250 g were purchased from Chengdu Dossy Experimental Animals Co., Ltd. (Chengdu, China). All rats were housed and fed in a pathogen-free facility under 12-h day and night cycles throughout the experiment. Animal experiments were performed according to the guidelines of the Animal Welfare Committee of Chengdu Military General Hospital.
In vivo experiments
Eighteen rats were divided into the following three equal groups according to a random number table: an SAP group, an APD group and a sham operation group (SHAM). Pancreatitis was induced in the SAP and APD groups by retrograde injection of 5% Na-taurocholate (0.1 mL/100 g body weight, Sigma, United States)[15] via the pancreatic duct using a syringe pump. In the APD group, a drainage tube with a vacuum ball was inserted into the lower right abdomen immediately after pancreatitis was induced[16]. Rats in the sham group did not receive any operation except opening and closure of the abdomen. Anesthesia was performed with an anesthesia machine using isoflurane (RWD Life Science, Shenzhen, China). Rats were sacrificed 12 h after the model was established. Blood samples were collected, and pancreatic tissues were harvested. According to previously described methods[17], PMs were isolated by peritoneal lavage after the ascetic fluid of SAP rats was drained.
Pancreatic histological analysis
The pancreas was dissected along the pancreatic duct, and a 0.5 cm × 0.5 cm sample was fixed in 4% paraformaldehyde solution. After being embedded in paraffin, the samples were cut into 4-μm thick sections and stained with hematoxylin and eosin (HE). Then, the slides were observed under an optical microscope, and the histopathology was scored using a previously described scoring system[18]. The scores were averaged for five individual slides from every pancreas.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISAs) were performed with the serum samples from each group using commercial rat-specific kits for amylase, lipase, IL-1β and TNF-α (Dakewe Biotech Co., Ltd. Shenzhen, China) according to the supplier’s specifications.
Cell isolation
The ascetic fluid in the abdomen of SAP rats or the residual liquid in APD rats was drained followed by intraperitoneal injection of 20 mL of precooled PBS solution. After 5 minutes of abdominal kneading, 15 mL of lavage fluid containing peritoneal cells was transferred into the anticoagulant tube via a syringe. The cells were washed with PBS, resuspended in RPMI-1640 (Gibco, CA, United States) with 20% fetal bovine serum (Gibco, CA, United States) and cultured at 37 °C in 5% CO2 for 1 h. The adherent cells were used in the subsequent experiments.
Flow cytometry
To detect the polarization phenotype of the macrophages in the abdomen, the PMs were analyzed by flow cytometry. PMs were washed with staining buffer (1% BSA in PBS containing 0.01% NaN3, Thermo Fisher, United States) and incubated with 10% mouse serum for 20 min on ice. Subsequently, the cells were incubated with reagents from the LIVE/DEAD™ Fixable Dead Cell Stain Kit (Thermo Fisher, United States), FITC-conjugated anti-CD163 (Bio-Rad, United States), and PE-conjugated anti-CD86 (BD Biosciences, United States) at the manufacturer’s recommended dilution for 40 min on ice. For intracellular staining, the cells were fixed and permeabilized with fixation buffer from a Fixation/Permeabilization Solution Kit (BD Biosciences, United States) for 1 h at 4 °C in the dark, washed with permeabilization buffer and incubated with Alexa-Flour647-conjugated anti-CD68 (Bio-Rad, United States) antibody in permeabilization buffer for 1 h at 4 °C in the dark. The samples were then washed and resuspended in permeabilization buffer and analyzed with a FACS Canto II system (BD Biosciences). The results were analyzed with BD FACS DIVA software (BD Biosciences, United States).
RNA isolation and qPCR
Total RNA from the abovementioned PMs was extracted using a commercial RNA extraction kit (Axygen, United States). The RNA was quantified by measuring the absorbance at 260 nm and 280 nm using a spectrophotometer (NanoDrop Technologies, United States). cDNA was synthesized from a 100-ng RNA sample using the PrimescriptRT reagent kit with a gDNA eraser (Takara, Tokyo, Japan) and stored at -80 °C.
Real-time quantitative PCR was performed with a CFX96 Real-Time PCR Detection System using the SYBR Green Master Mix (Bio-Rad, United States). The primers used were as follows: INOS forward, 5’-CAGCCCTCAGAGTACAACGAT-3’ and reverse, 5’-CAGCAGGCACACGCAATGAT-3’; CD206 forward, 5’-ATTCCGGTCGCTGTTCAACT-3’ and reverse, 5’-AACGGAGATGGCGCTTAGAG-3’; TNF-α forward, 5’-CGTCGTAGCAAACCACCAAG-3’ and reverse, 5’-CACAGAGCAATGACTCCAAAG-3’; CD163 forward, 5’-CAACCGATGCTCAGGAAGAG-3’ and reverse, 5’-GATGGCACTTCCACATCCAA-3’. GAPDH was used as a reference gene, and the primers for GAPDH were the commercial Rat GAPDH Endogenous Reference Gene Primers (BBI Life Science, China).
Luminex assay
The concentrations of IL-1β, CXCL2, IL-4, IL-10 and TNF-α in the lavage fluid were determined by Luminex assays. A premixed commercial kit was used according to the manufacturer’s recommendation (RD, United States). The assays were performed using the Luminex X-200 System (Luminex Corp, United States).
Immunofluorescence staining
The distribution of the two phenotypes of macrophages in the pancreas was assayed by immunofluorescence staining. Pancreatic tissues were washed with PBS twice, fixed with 4% paraformaldehyde for 24 h and dehydrated in a 30% sucrose solution. Then, the tissues were embedded in Tissue Freezing Medium and cut into 7-μm thick sections. The slides were washed with PBS and permeabilized with 0.1% Triton X-100. Subsequently, the slides were incubated with goat serum at 37 °C for 30 min. The samples were stained by incubation with Alexa-Flour647-conjugated anti-CD68 (Bio-Rad, United States) and FITC-conjugated anti-CD163 (Bio-Rad, United States) or Alexa-Flour647-conjugated anti-CD68 and PE-conjugated anti-CD86 (BD Biosciences, United States) at the manufacturer’s recommended dilution at 4 °C overnight; then, the samples were stained with DAPI to visualize the nuclei. The distribution of the two phenotypes of macrophages in the pancreas was examined by laser scanning confocal microscopy.
Western blot analysis
Pancreatic tissues were dissociated in a commercial lysis buffer kit containing protease inhibitor and phenylmethylsulfonyl fluoride (PMSF) (Solarbio Science & Technology Co., Ltd, China) using a homogenizer. The tissue lysates were centrifuged at 12000 rpm for 30 min at 4 °C, and the supernatant was collected for analysis. The protein concentration was measured using a commercial BCA protein assay kit (Solarbio Science and Technology Co., Ltd, China) according to the manufacturer’s instructions. Proteins were mixed into the premixed protein sample buffer (Bio-Rad, United States) at equal concentrations and heated for 10 min at 100 °C to denature the protein. Electrophoresis was performed on 10% or 8% SDS-polyacrylamide gels followed by transferring the protein onto a PVDF membrane. After blocking with 5% nonfat milk in TBS solution for 1 h at room temperature, the blots were incubated in the following primary antibodies overnight at 4 °C: mouse anti-Arg1 (1:200, Santa Cruz, United States), mouse anti-CD163 (1:200, Bio-Rad, United States), mouse anti-CD86 (1:200, BD Biosciences, United States), mouse anti-NOS2 (Santa Cruz, United States), and mouse anti-GAPDH (Thermo Fisher, United States). Afterwards, the blots were incubated with goat anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody (1:5000, Abcam, United Kingdom) for 1 h at room temperature. Then, the blots were developed using the enhanced chemiluminescence (ECL) method (Merck Milipore, Germany) in a bioimaging system (UVP, United Kingdom).
In vitro experiments
Primary cultured PMs were isolated from an additional ten rats as described above, washed with PBS and resuspended in RPMI-1640 (Gibco, CA, United States) with 20% fetal bovine serum (Gibco, CA, United States). All cells were plated at 1 × 106 cells per well in a 6-well plate and then cultured at 37 °C in 5% CO2 for 3 d; adherent cells were identified as macrophages. Flow cytometry was used to identify the purity of the clones.
To test the effect of the abdominal inflammatory environment on PMs, primary cultured PMs were treated with medium containing 10% lavage fluid from SAP and APD rats separately for 12 h, and PBS was used as a control. The polarization phenotypes of the primary cultured PMs were determined by flow cytometry as mentioned above.
Statistical analysis
Statistical analyses were performed using SPSS 18.0 (SPSS Inc., United States), and the data are reported as the mean ± SD. Data were compared by Student’s t-tests or one-way ANOVA followed by the SNK test for multiple comparisons, and nonparametrically distributed variables were compared by the Mann-Whitney test. P < 0.05 was considered statistically significant.
RESULTS
Therapeutic effects of APD on SAP in rats
The SAP rat model induced by Na-taurocholate retrograde injection (Figure 1A) is a stable animal model that has many similarities to the clinical manifestations of SAP in humans. To simulate the APD treatment, a drainage tube was inserted into the lower right abdomen of SAP rats (Figure 1A). Histologically, apparent morphological damage in the form of acinar cell necrosis and inflammatory infiltration was observed in rats with SAP, while the tissue damage was significantly reduced in the APD treatment group (Figure 1B). This result was supported by the lower histopathological score in the APD group than in the SAP group (Figure 1C).
Figure 1.
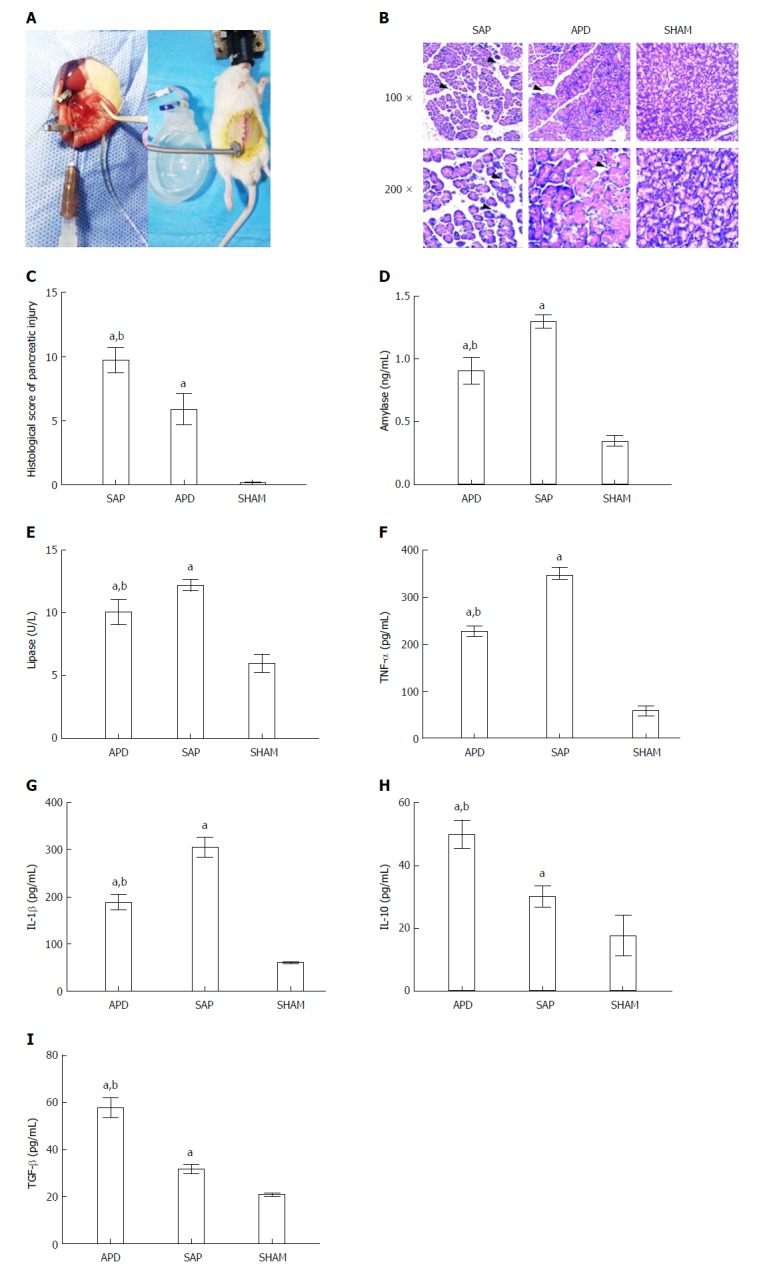
Abdominal paracentesis drainage ameliorates severe acute pancreatitis in a rat model. A: Model establishment. Retrograde injection of Na-taurocholate (left) and a rat after abdominal paracentesis drainage (APD) treatment (right); B and C: Histopathological analysis of the pancreas. Comprehensive disruption of the pancreatic structure with widespread infiltration of leukocytes, acinar cell vacuolization and necrosis was observed in severe acute pancreatitis (SAP) rats; localized leukocyte infiltration and relatively intact acinar structure were observed in APD rats; D-I: Plasma levels of amylase, lipase, tumor necrosis factor-α, interleukin (IL)-1β, IL-10 and transforming growth factor-β, respectively. Data indicate the mean ± SD of six mice (C-I). aP < 0.05 vs sham, bP < 0.05 vs SAP.
Subsequently, we measured the serum amylase and lipase levels, as they are known biomarkers of SAP. As shown in Figure 1, the APD group showed lower levels of amylase and lipase when compared with the SAP group (Figure 1D and E). In the APD and SAP groups, the concentrations of amylase and lipase were 0.906 ± 0.102 ng/mL vs 1.302 ± 0.052 ng/mL and 10.118 ± 1.019 U/L vs 12.251 ± 0.458 U/L, respectively.
Pro-inflammatory cytokines, such as TNF-α and IL-1β, are known to induce systemic inflammation in SAP. Compared with SAP rats, in APD rats, the levels of TNF-α and IL-1β were significantly lower, while the levels of the anti-inflammatory cytokines IL-10 and transforming growth factor-β were higher (Figure 1F-H), reflecting an amelioration of systematic inflammation in APD rats.
These results suggest that APD treatment could exert a protective role against the progression of SAP, as supported by the observed improved tissue damage and reduced systematic inflammation.
APD polarizes PMs towards the M2 phenotype in the peritoneal cavity
PMs play important roles in the progression of SAP. To assess the direct effect of APD on PMs, we first investigated the polarization phenotypes of the PMs in each group by flow cytometry. CD68 was used as a marker of all macrophages; CD68+CD86+ macrophages were identified as M1 macrophages, while CD68+CD163+ cells were identified as M2 macrophages. Flow cytometry plots revealed a significantly lower proportion of M1 PMs in APD rats compared to SAP rats, while a slight increase occurred in the proportion of M2 PMs (Figure 2B). Meanwhile, the M1/M2 ratio tended to be lower in the APD group than in the SAP group (Figure 2 B).
Figure 2.

Different polarized phenotypes of peritoneal macrophages in each group. A: Gating strategy for the peritoneal macrophage population; B-D: Representative dot plot (B) and the percentages (C) and M1/M2 ratio (D) of CD68+CD86+ (M1) cells and CD68+CD163+ (M2) cells in each group; E: Relative expression levels of CD206 and iNOS gene in peritoneal cells measured by real-time PCR and normalized to GAPDH mRNA. The data represent at least three independent experiments (A-B) or indicate the mean ± SD of six mice (E). aP < 0.05 vs abdominal paracentesis drainage.
To further confirm the polarization phenotype of PMs at the genetic level, the expression of the M1-associated gene iNOS and the M2-associated gene CD206 was determined by qPCR. As expected, the expression of CD206 gene increased in the APD group, while it was inhibited in the SAP group. Although the expression of the iNOS gene was upregulated in both groups, it increased markedly in the SAP group (Figure 2E). These data indicate that APD treatment polarized PMs towards the M2 phenotype in the peritoneal cavity of SAP model rats.
APD reduces the levels of pro-inflammatory cytokines and increases the levels of anti-inflammatory cytokines in the peritoneal cavity
APD treatment drained the PAAF from SAP rats, thus altering the inflammatory environment of the peritoneal cavity. To compare the inflammatory environments in SAP and APD rats, we measured the production of representative pro-inflammatory and anti-inflammatory cytokines in the peritoneal lavage of each group by Luminex (Figure 3). The protein levels of the pro-inflammatory mediators IL-1β and L-selectin were significantly lower in APD rats than in SAP rats, while the levels of the anti-inflammatory cytokines IL-4 and IL-10 were increased. Although there were no significant differences in the levels of the pro-inflammatory cytokines IL-6 and CXCL2, the mean levels of these cytokines were greater in SAP rats than in APD rats. These results indicate that APD treatment promoted anti-inflammatory cytokine production and inhibited pro-inflammatory cytokine production.
Figure 3.
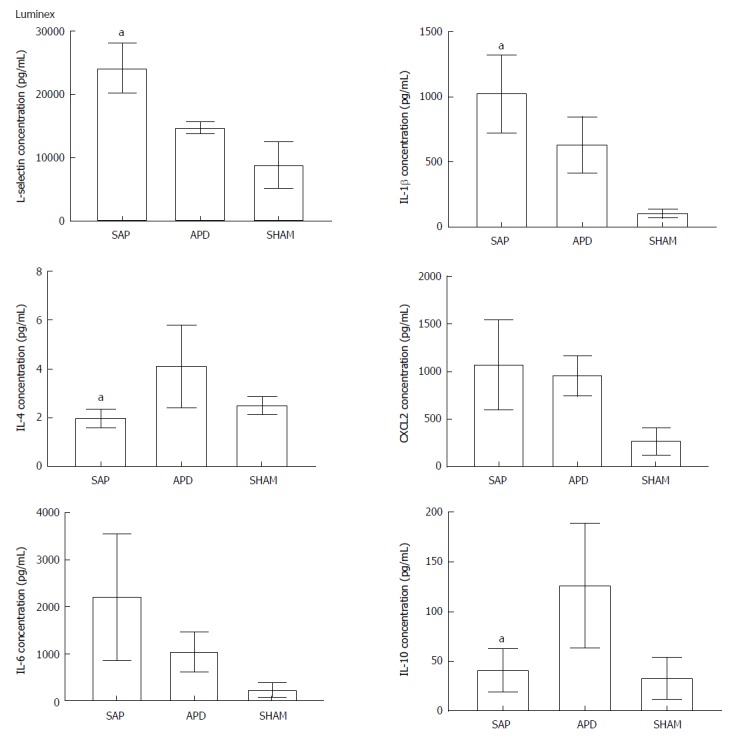
Abdominal paracentesis drainage alters the inflammatory environment in the peritoneal cavity. Protein levels in peritoneal lavage were measured by Luminex. The results reflect the mean ± SD obtained from six animals in each group. aP < 0.05 vs abdominal paracentesis drainage.
PMs are polarized to the M2 phenotype in the simulated peritoneal inflammatory environment of APD rats in vitro
The phenotypes of PMs depend on the inflammatory environment. To investigate whether the peritoneal inflammatory environment affects PMs polarization in vitro, we simulated this environment by adding peritoneal lavage from the SAP and APD groups to primary cultured PMs. PBS was used as a negative control. Then, the PMs were evaluated by flow cytometry. The dot plot of FCM (Figure 4) indicated that the number of M2 macrophages increased slightly in PMs cultured in the APD environment, while no increase was found in cells cultured in the SAP environment. Although the numbers of M1 macrophages rose in both groups, they increased dramatically in the SAP environment. These data indicate that the altered peritoneal inflammatory environment induced by APD treatment enhanced M2 macrophage polarization in PMs.
Figure 4.
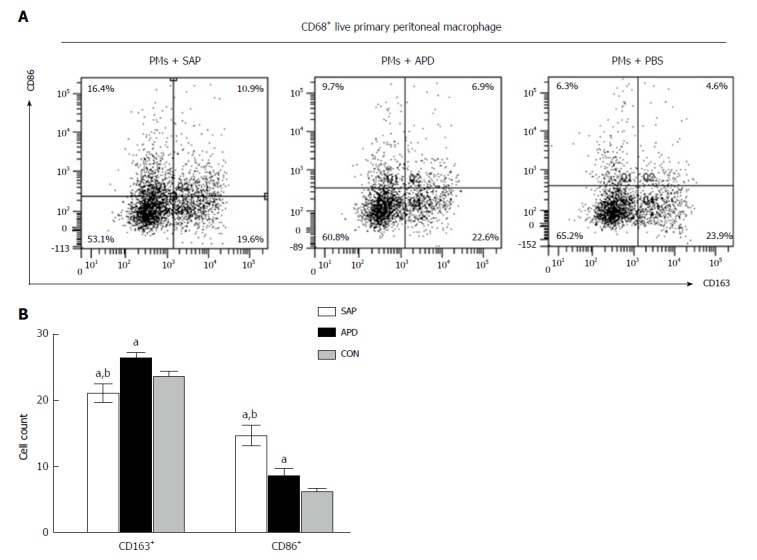
In vitro simulated peritoneal inflammatory environment of abdominal paracentesis drainage rats changes the polarized phenotype of peritoneal macrophages. Primary peritoneal macrophages were cultured in medium simulating different inflammatory environments. The percentages of CD86+ and CD163+ cells were measured by flow cytometry. The data are representative of at least three independent experiments. aP < 0.05 vs CON, bP < 0.05 vs abdominal paracentesis drainage.
APD promotes M2 polarization of macrophages in the pancreases of SAP rats
Given that macrophages are associated with intrapancreatic injury during SAP, we evaluated the effect of APD on the polarization response of macrophages in the pancreas by immunofluorescence staining and Western blot. As shown in Figure 5A, the number of M2 macrophages clearly increased, while the number of M1 macrophages decreased in the pancreas of APD rats compared with SAP rats. The morphological changes were confirmed by Western blot (Figure 5B). The pancreatic levels of M2-associated proteins Arg-1 and CD163 were higher in APD rats than in SAP rats. In contrast, the levels of M1-associated proteins iNOS and CD86 were lower in APD rats than in SAP rats. The data show that APD could promote M2 polarization of macrophages in the pancreas of SAP rats.
Figure 5.
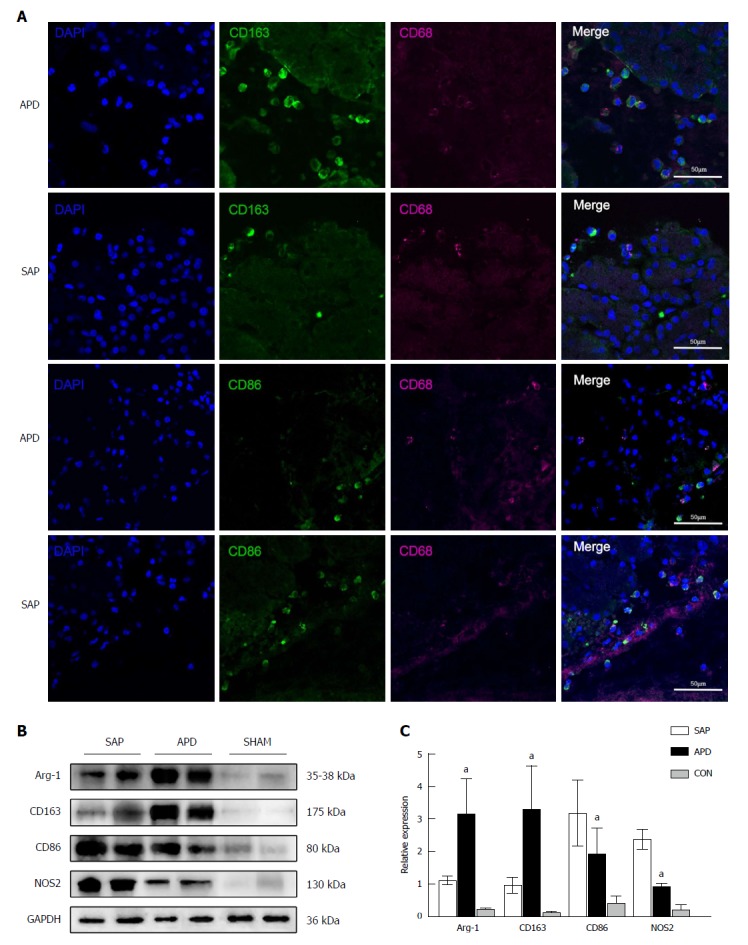
Number of M2 macrophages increases in the pancreas of abdominal paracentesis drainage rats and exerts anti-inflammatory effects. A: Pancreatic tissues from each group were stained with DAPI (blue), CD163 (green in upper two panels), CD86 (green in lower two panels) and CD68 (infrared represented by carmine). Representative images are shown; B and C: Protein levels of Arg-1, CD163, CD86 and iNOS in the pancreatic tissues of each group were measured by Western blot, and the relative expression of these proteins was normalized to GAPDH. Data are representative of at least three independent experiments. aP < 0.05 vs severe acute pancreatitis.
Based on the above findings, a schematic model was proposed to elucidate the possible mechanism responsible for the beneficial effects of APD on SAP (Figure 6). Once SAP occurs, the inflammatory cells are activated following acinar cell injury and exudate full of pro-inflammatory mediators collects in the peritoneal cavity, which can polarize the PMs towards the M1 phenotype and lead to the overexpression of pro-inflammatory mediators by PMs. By removing the PAAF, APD could improve the inflammatory environment of the peritoneal cavity and thus regulate M2 polarization of PMs in the peritoneal cavity. Meanwhile, APD could also promote M2 polarization of macrophages in pancreatic tissues. These events could upregulate the expression of anti-inflammatory cytokines, which ultimately ameliorate pancreatic injury.
Figure 6.
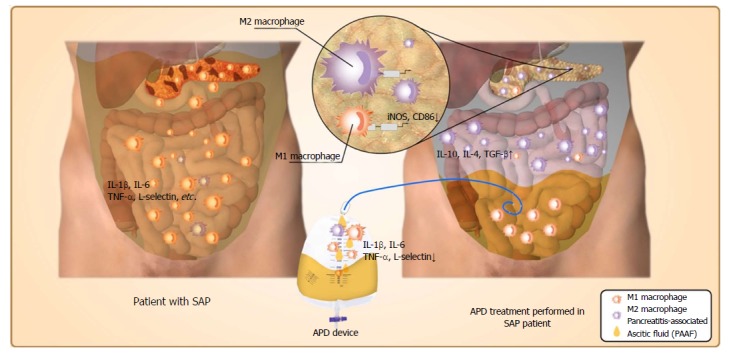
Possible mechanisms responsible for the beneficial effects of abdominal paracentesis drainage on severe acute pancreatitis. Once severe acute pancreatitis (SAP) occurs, the inflammatory cells are activated following acinar cell injuries and exudate full of pro-inflammatory mediators collects in the peritoneal cavity, which can polarize the peritoneal macrophages (PMs) towards the M1 phenotype and lead to the overexpression of pro-inflammatory mediators by PMs. By removing the pancreatitis-associated ascitic fluids, abdominal paracentesis drainage (APD) could improve the inflammatory environment of the peritoneal cavity, thus promoting M2 polarization of PMs in the peritoneal cavity. Meanwhile, APD could also promote M2 polarization of macrophages in pancreatic tissues. These events could upregulate the expression of anti-inflammatory cytokines, which ultimately ameliorate pancreatic injury. APD: Abdominal paracentesis drainage; SAP: Severe acute pancreatitis.
DISCUSSION
In the present study, we provided evidence for the first time that APD ameliorates inflammation in rats with SAP by regulating PM M2 polarization. The important findings are that: (1) by removing PAAF, APD can improve the inflammatory environment of the peritoneal cavity; (2) the improved environment in the peritoneal cavity can polarize PMs towards the M2 phenotype; and (3) APD can promote M2 polarization of macrophages in the pancreas of SAP rats. These findings provide new insight into the mechanisms underlying the effectiveness of APD in the treatment of SAP, which may advance the clinical use of APD to benefit patients with SAP.
APD treatment before percutaneous catheter drainage has been proven to exert a beneficial effect on patients with SAP in our serial reports. Here, we demonstrated that APD treatment ameliorates the levels of inflammatory factors, including TNF-α, IL-1β and IL-6, in the serum and ascitic fluid of patients and rats with SAP[16]. Consistent with these results, in this paper, we found that APD not only decreased the levels of pro-inflammatory cytokines, such as IL-1β, and the adhesion molecule L-selectin but also increased the levels of anti-inflammatory cytokines, such as IL-4 and IL-10. This is in line with the findings of a recent study by Zhu and colleagues[19] in which it was shown that the levels of pro-inflammatory cytokines in ascites, including IL-1β, IL-6, IL-8 and TNF-α, decreased after early-stage drainage of the PAAF, while the level of the anti-inflammatory cytokine IL-10 increased significantly. Similar results were also reported by Souza[20], and these two studies suppose M2 macrophages as causative factors.
As is known, macrophages will acquire distinct functional phenotypes when responding to environmental cues. Because APD improves the inflammatory environment of the peritoneal cavity, we speculated that APD may influence the activation state of PMs. In fact, our results indicate that the proportion of M1 PMs in APD rats decreased significantly while the proportion of M2 PMs increased slightly when compared with the proportions in SAP rats. From a recent point of view, it has been suggested that macrophages do not exist as distinct M1 or M2 phenotypes but rather as a continuum of overlapping functional states, and the transition between the M1 to M2 phenotype is a dynamic process[10]. Therefore, we calculated the M1/M2 ratio to reflect the dynamic balance of macrophage polarization. The results indicate that the M1/M2 ratios in SAP rats and APD rats were 7.286 ± 4.22 vs 1.278 ± 0.481, respectively. Although M1 polarization was dominant in both groups, there was a trend to skew the balance towards the M2 phenotype in the APD group.
The mechanism by which APD polarizes PMs towards an M2 phenotype in the peritoneal cavity remains unknown; however, based on the above findings, we infer that APD might eliminate the comprehensively inflammatory environment, which provides an environment suitable for the survival of M2 macrophages and enhances the M2 polarization of PMs. The increase in the number of M2 macrophages in turn leads to the production of anti-inflammatory cytokines. To validate the effect of APD on the polarization of macrophages, lavage from each group was used to treat the primary cultured PMs. Consistent with the in vivo experiments, the number of M2 PMs increased in the APD group.
Studies have shown that polarizing macrophages towards the M2 phenotype ameliorates many chronic inflammation and autoimmune diseases[21], such as SLE[22], rheumatoid arthritis[23] and colitis[24]. Many methods have been used to induce M2 polarization[25]. However, most of the reports are in vitro experiments, and the therapeutic effect of M2 macrophages on acute inflammatory disease[26] is rarely reported. Although Xu et al[27] induced M2 polarization in primary cultured liver macrophages from rats with acute pancreatitis, they did not report any therapeutic effect of these cells in SAP. One possible reason is that large amounts of pro-inflammatory cytokines are released when the inflammatory cascade is activated, which overwhelmingly skews the M1/M2 balance towards M1 polarization in vivo. Our successful induction of the M2 polarization of PMs in SAP rats in vivo is a new attempt to utilize the advantages of M2 macrophages to ameliorate acute inflammatory diseases.
In addition, our findings demonstrate that the number of M2 macrophages also increased in the pancreas of SAP rats; however, only trace amounts of macrophages in the peripheral blood and bone marrow were polarized (data not shown). The data imply that the increase in the number of M2 macrophages in the pancreas is independent of the number of CD68+CD163+ cells in the peripheral blood and bone marrow. In our analysis, M2 macrophages may have migrated from the peritoneal cavity to the pancreas and regulated the immune responses in the pancreas of SAP rats. PMs have been reported to selectively migrate to the specific sites of inflammation in a colitis rat model[21], indicating that PMs could directly migrate to the focal zone in the peritoneal cavity. Moreover, the protein level of Arg-1, which is a key modulator in regulating T-lymphocyte functions and maintaining immunological tolerance[28], also increased. However, whether the M2 macrophages in the pancreas are PMs migrating from the peritoneal cavity still needs to be proven.
In conclusion, our findings suggest that APD treatment exerts anti-inflammatory effects to ameliorate SAP by regulating PM M2 polarization, thereby increasing the number of M2 macrophages and Arg-1 protein levels in the pancreas; these findings provide novel insights into the mechanisms underlying the therapeutic effect of APD.
ARTICLE HIGHLIGHTS
Research background
Severe acute pancreatitis (SAP) is a highly lethal disease with limited therapeutic options and is characterized by a critical systemic inflammatory response. Pancreatitis-associated ascitic fluids (PAAF) play an important role in the pathogenesis of SAP because of the pro-inflammatory mediators the PAAF contain. Our previous studies suggested that APD ameliorates SAP by removing the PAAF. However, the mechanism underlying the success of APD treatment remains poorly understood. In the present study, we aimed to explore the possible mechanism by which APD ameliorates SAP.
Research motivation
The key issue in treating SAP is to control the activated inflammatory cascade and restore immune homeostasis. Peritoneal macrophages (PMs), crucial inflammatory cells in the abdominal cavity, are implicated in the initiation and progression of SAP in the early stage, and the function of PMs is regulated by the PAAF. In this study, we found that APD treatment exerts anti-inflammatory effects by regulating the M2 polarization of PMs, providing novel insights into the mechanisms underlying the therapeutic effect of APD.
Research objectives
The aim of this study was to determine the polarization phenotypes of PMs and the corresponding inflammatory responses in a rat model of SAP following APD treatment and to explore the possible mechanism by which APD treatment ameliorates SAP.
Research methods
The effect of APD on the polarization response of PMs was determined in an SAP rat model induced by 5% Na-taurocholate retrograde injection and in a peritoneal inflammatory environment simulated by adding peritoneal lavage to culture medium in vitro. HE staining and measurement of the levels of amylase, lipase, and inflammatory mediators were performed. The M1/M2 phenotype ratio of PMs was identified by flow cytometry and RT-PCR. The distribution of macrophages and their protein expression in the pancreas were determined by immunofluorescence staining and Western blot.
Research results
APD treatment ameliorates SAP by significantly reducing the pathological scores and the levels of amylase, lipase, tumor necrosis factor-α, and interleukin (IL)-1β. Importantly, APD treatment polarizes PMs towards the M2 phenotype and increases the anti-inflammatory mediators IL-4 and IL-10 in the peritoneal lavage. Furthermore, PMs exhibited a trend towards the M2 phenotype in a simulated peritoneal inflammatory environment in vitro. Finally, APD treatment increased the number of M2 macrophages and upregulated the expression of the anti-inflammatory protein Arg-1 in the pancreas of SAP rats.
Research conclusions
APD treatment exerts anti-inflammatory effects by regulating the M2 polarization of PMs, providing novel insights into the mechanisms underlying its therapeutic effect.
Research perspectives
Our study provided evidence for the first time that APD ameliorates inflammation in rats with SAP by regulating PM M2 polarization. However, solid evidence that APD polarizes PMs to the M2 phenotype and the underlying molecular mechanism still need to be explored. Furthermore, future research should focus on the effect of M2 macrophages on immune homeostasis restoration and tissue repair in the injured pancreas.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: All animal protocols were approved by the Animal Welfare Committee of Chengdu Military General Hospital, Chengdu, China (No. A20170312004).
Conflict-of-interest statement: The authors declare that there are no conflicts of interest related to this study.
Data sharing statement: No additional data are available.
ARRIVE guidelines statement: In the manuscript, the ARRIVE guidelines were adopted.
Peer-review started: September 27, 2018
First decision: October 14, 2018
Article in press: November 9, 2018
P- Reviewer: Isik AR, Smyrniotis V S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Huang Y
Contributor Information
Ruo-Hong Liu, PLA Center of General Surgery and Pancreatic Injury and Repair Key Laboratory of Sichuan Province, Chengdu Military General Hospital, Chengdu 610083, Sichuan Province, China; Third Military Medical University (Army Medical University), Chongqing 400037, China.
Yi Wen, PLA Center of General Surgery and Pancreatic Injury and Repair Key Laboratory of Sichuan Province, Chengdu Military General Hospital, Chengdu 610083, Sichuan Province, China; Third Military Medical University (Army Medical University), Chongqing 400037, China.
Hong-Yu Sun, PLA Center of General Surgery and Pancreatic Injury and Repair Key Laboratory of Sichuan Province, Chengdu Military General Hospital, Chengdu 610083, Sichuan Province, China.
Chun-Yu Liu, PLA Center of General Surgery and Pancreatic Injury and Repair Key Laboratory of Sichuan Province, Chengdu Military General Hospital, Chengdu 610083, Sichuan Province, China.
Yu-Fan Zhang, Jiaotong Hospital Affiliated with the Sichuan Provincial People’s Hospital, Chengdu 611730, Sichuan Province, China.
Yi Yang, PLA Center of General Surgery and Pancreatic Injury and Repair Key Laboratory of Sichuan Province, Chengdu Military General Hospital, Chengdu 610083, Sichuan Province, China.
Qi-Lin Huang, PLA Center of General Surgery and Pancreatic Injury and Repair Key Laboratory of Sichuan Province, Chengdu Military General Hospital, Chengdu 610083, Sichuan Province, China.
Jia-Jia Tang, Department of Ultrasound, Chinese Academy of Medical Sciences and Peking Union Medical College Hospital, Beijing 100032, China.
Can-Chen Huang, PLA Center of General Surgery and Pancreatic Injury and Repair Key Laboratory of Sichuan Province, Chengdu Military General Hospital, Chengdu 610083, Sichuan Province, China.
Li-Jun Tang, PLA Center of General Surgery and Pancreatic Injury and Repair Key Laboratory of Sichuan Province, Chengdu Military General Hospital, Chengdu 610083, Sichuan Province, China; Third Military Medical University (Army Medical University), Chongqing 400037, China. tanglj2016@163.com.
References
- 1.Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2016;375:1972–1981. doi: 10.1056/NEJMra1505202. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Xu P, Hou YQ, Xu K, Li QH, Huang L. Pancreatitis-associated ascitic fluid induces proinflammatory cytokine expression in THP-1 cells by inhibiting anti-inflammatory signaling. Pancreas. 2013;42:855–860. doi: 10.1097/MPA.0b013e318279fe5c. [DOI] [PubMed] [Google Scholar]
- 3.Takeyama Y, Nishikawa J, Ueda T, Hori Y, Yamamoto M, Kuroda Y. Involvement of peritoneal macrophage in the induction of cytotoxicity due to apoptosis in ascitic fluid associated with severe acute pancreatitis. J Surg Res. 1999;82:163–171. doi: 10.1006/jsre.1998.5535. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez PT, Folch-Puy E, Bulbena O, Closa D. Oxidised lipids present in ascitic fluid interfere with the regulation of the macrophages during acute pancreatitis, promoting an exacerbation of the inflammatory response. Gut. 2008;57:642–648. doi: 10.1136/gut.2007.127472. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Yan H, Liu W, Cui J, Wang T, Dai R, Liang H, Luo H, Tang L. Abdominal Paracentesis Drainage Does Not Increase Infection in Severe Acute Pancreatitis: A Prospective Study. J Clin Gastroenterol. 2015;49:757–763. doi: 10.1097/MCG.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 6.Liu WH, Ren LN, Chen T, Liu LY, Jiang JH, Wang T, Xu C, Yan HT, Zheng XB, Song FQ, et al. Abdominal paracentesis drainage ahead of percutaneous catheter drainage benefits patients attacked by acute pancreatitis with fluid collections: a retrospective clinical cohort study. Crit Care Med. 2015;43:109–119. doi: 10.1097/CCM.0000000000000606. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Liu W, Yan H, Cui J, Zhou J, Wang T, Tang L. Abdominal Paracentesis Drainage Does Not Bring Extra Risk to Patients With Severe Acute Pancreatitis. J Clin Gastroenterol. 2016;50:439. doi: 10.1097/MCG.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 8.Mikami Y, Takeda K, Shibuya K, Qiu-Feng H, Shimamura H, Yamauchi J, Egawa S, Sunamura M, Yagi H, Endo Y, et al. Do peritoneal macrophages play an essential role in the progression of acute pancreatitis in rats? Pancreas. 2003;27:253–260. doi: 10.1097/00006676-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Mayerle J, Dummer A, Sendler M, Malla SR, van den Brandt C, Teller S, Aghdassi A, Nitsche C, Lerch MM. Differential roles of inflammatory cells in pancreatitis. J Gastroenterol Hepatol. 2012;27 Suppl 2:47–51. doi: 10.1111/j.1440-1746.2011.07011.x. [DOI] [PubMed] [Google Scholar]
- 10.Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 11.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Labonte AC, Tosello-Trampont AC, Hahn YS. The role of macrophage polarization in infectious and inflammatory diseases. Mol Cells. 2014;37:275–285. doi: 10.14348/molcells.2014.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atri C, Guerfali FZ, Laouini D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int J Mol Sci. 2018:19. doi: 10.3390/ijms19061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh A, Shimosegawa T, Masamune A, Fujita M, Koizumi M, Toyota T. Ascitic fluid of experimental severe acute pancreatitis modulates the function of peritoneal macrophages. Pancreas. 1999;19:268–275. doi: 10.1097/00006676-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411–416. doi: 10.3109/00365528009181493. [DOI] [PubMed] [Google Scholar]
- 16.Chen GY, Dai RW, Luo H, Liu WH, Chen T, Lin N, Wang T, Luo GD, Tang LJ. Effect of percutaneous catheter drainage on pancreatic injury in rats with severe acute pancreatitis induced by sodium taurocholate. Pancreatology. 2015;15:71–77. doi: 10.1016/j.pan.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Pineda-Torra I, Gage M, de Juan A, Pello OM. Isolation, Culture, and Polarization of Murine Bone Marrow-Derived and Peritoneal Macrophages. Methods Mol Biol. 2015;1339:101–109. doi: 10.1007/978-1-4939-2929-0_6. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu L, Lu J, Yang J, Sun P. Early-phase peritoneal drainage and lavage in a rat model of severe acute pancreatitis. Surg Today. 2016;46:371–378. doi: 10.1007/s00595-015-1172-9. [DOI] [PubMed] [Google Scholar]
- 20.Souza LJ, Coelho AM, Sampietre SN, Martins JO, Cunha JE, Machado MC. Anti-inflammatory effects of peritoneal lavage in acute pancreatitis. Pancreas. 2010;39:1180–1184. doi: 10.1097/MPA.0b013e3181e664f2. [DOI] [PubMed] [Google Scholar]
- 21.Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154:186–195. doi: 10.1111/imm.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaper F, de Leeuw K, Horst G, Bootsma H, Limburg PC, Heeringa P, Bijl M, Westra J. High mobility group box 1 skews macrophage polarization and negatively influences phagocytosis of apoptotic cells. Rheumatology (Oxford) 2016;55:2260–2270. doi: 10.1093/rheumatology/kew324. [DOI] [PubMed] [Google Scholar]
- 23.Quero L, Hanser E, Manigold T, Tiaden AN, Kyburz D. TLR2 stimulation impairs anti-inflammatory activity of M2-like macrophages, generating a chimeric M1/M2 phenotype. Arthritis Res Ther. 2017;19:245. doi: 10.1186/s13075-017-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Ren J, Wang W, Wei XW, Shen GB, Liu YT, Luo M, Xu GC, Shao B, Deng SY, et al. Treatment of dextran sodium sulfate-induced experimental colitis by adoptive transfer of peritoneal cells. Sci Rep. 2015;5:16760. doi: 10.1038/srep16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran TH, Rastogi R, Shelke J, Amiji MM. Modulation of Macrophage Functional Polarity towards Anti-Inflammatory Phenotype with Plasmid DNA Delivery in CD44 Targeting Hyaluronic Acid Nanoparticles. Sci Rep. 2015;5:16632. doi: 10.1038/srep16632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Işik A, Firat D, İdiz UO. Nüks/komplike ve akut olgularda yaklaşımlar. Turkiye Klinikleri Journal of General Surgery Special Topics. 2018;11:112–114. [Google Scholar]
- 27.Xu L, Yang F, Lin R, Han C, Liu J, Ding Z. Induction of m2 polarization in primary culture liver macrophages from rats with acute pancreatitis. PLoS One. 2014;9:e108014. doi: 10.1371/journal.pone.0108014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–306. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]


