Abstract
A large number of liver transplants have been performed for hepatocellular carcinoma (HCC), and recurrence is increasingly encountered. The recurrence of HCC after liver transplantation is notoriously difficult to manage. We hereby propose multi-disciplinary management with a systematic approach. The patient is jointly managed by the transplant surgeon, physician, oncologist and radiologist. Immunosuppressants should be tapered to the lowest effective dose to protect against rejection. The combination of a mammalian target of rapamycin inhibitor with a reduced calcineurin inhibitor could be considered with close monitoring of graft function and toxicity. Comprehensive staging can be performed by dual-tracer positron emission tomography-computed tomography or the combination of contrast computed tomography and a bone scan. In patients with disseminated recurrence, sorafenib confers survival benefits but is associated with significant drug toxicity. Oligo-recurrence encompasses recurrent disease that is limited in number and location so that loco-regional treatments convey disease control and survival benefits. Intra-hepatic recurrence can be managed with graft resection, but significant operative morbidity is expected. Radiofrequency ablation and stereotactic body radiation therapy (SBRT) are effective alternative strategies. In patients with more advanced hepatic disease, regional treatment with trans-arterial chemoembolization or intra-arterial Yttrium-90 can be considered. For patients with extra-hepatic oligo-recurrence, loco-regional treatment can be considered if practical. Patients with more than one site of recurrence are not always contraindicated for curative treatments. Surgical resection is effective for patients with pulmonary oligo-recurrence, but adequate lung function is a pre-requisite. SBRT is a non-invasive and effective modality that conveys local control to pulmonary and skeletal oligo-recurrences.
Keywords: Hepatocellular carcinoma, Recurrence, Liver transplantation
Core tip: We propose a multi-disciplinary management algorithm for recurrent hepatocellular carcinoma after liver transplantation. The combination of a mammalian target of rapamycin inhibitor with a reduced calcineurin inhibitor can be considered. Staging is performed to differentiate between disseminated recurrence and oligo-recurrence. In patients with disseminated recurrence, sorafenib may confer survival benefits but is associated with significant toxicity. Oligo-recurrence encompasses recurrent disease that is limited in number and location so that loco-regional treatments convey disease control and survival benefits. Intra-hepatic and extra-hepatic oligo-recurrences can be managed with surgical resection, ablative therapy or regional treatments depending on the disease status.
INTRODUCTION
Despite stringent selection criteria, recurrence occurs in 6%-18% of patients transplanted for hepatocellular carcinoma (HCC)[1-4]. Since the implementation of the Model for End-Stage Liver Disease (MELD) system, patients waitlisted for HCC have been given increased priority for cadaveric grafts[5]. More liver transplants have been performed for HCC, and recurrence is more frequently encountered[6]. The recurrence of HCC after liver transplantation is notoriously difficult to manage. Experience is limited in the literature, and there is considerable debate concerning various systemic and local treatments. The objective of the present narrative review is to summarize the current available literature and propose a management algorithm for recurrence after liver transplantation.
A literature search was performed on PubMed (United States National Library of Medicine, National Institutes of Health, United States) for relevant English articles with a combination of keywords: “liver transplantation” with “hepatocellular carcinoma recurrence” or “HCC recurrence” and/or “immunosuppression” and/or “targeted therapy” and/or “immunotherapy” and/or “resection” and/or “ablation” and/or “stereotactic body radiotherapy” or “SBRT”. The references of the selected papers were reviewed for additional relevant articles.
UNIQUE PERSPECTIVES OF POST-TRANSPLANT RECURRENCE
Systemic disease
After liver transplantation, any recurrence is, by definition, metastasis from the native liver. The culprit is either the presence of undiagnosed distant metastasis before transplantation or spillage of tumour cells during transplantation. Even an isolated recurrence implicates solitary metastasis and represents a local phenomenon of the systemic event, which highlights the importance of systemic therapy and the input of oncology as a critical component of the therapeutic strategy.
Immuno-compromised state
Immunity is the primary defence against cancer[7]. The adaptive immune system recognizes and eliminates tumour cells based on their expression of tumour-specific antigens[8]. Termed concomitant immunity, the immune response induced by the primary tumour inhibits the growth of secondaries[9]. However, after liver transplantation, concomitant immunity is suppressed pharmacologically. Any microscopic tumour in vitro can progress without immune surveillance. It was observed that post-transplant HCC recurrence progresses significantly faster than in patients treated with hepatic resection[10].
Calcineurin inhibitors, e.g., tacrolimus and cyclosporine, form the cornerstones of maintenance immunosuppression in liver transplantation. In addition to host immune suppression, they promote tumour progression via non-immune-mediated pathways related to augmented transforming growth factor expression[11,12]. From a retrospective series of 70 HCC patients treated with liver transplantation, quantified cyclosporine exposure was identified as an independent risk factor for HCC recurrence[13]. Subsequently, Vivarelli et al[14] confirmed that high tacrolimus exposure independently predicted HCC recurrence. Immunosuppressive therapy affects the course of tumours in transplant patients and must be fully addressed in the comprehensive management of a recurrence.
Immuno-maintenance phase of the transplant
Throughout the course of treatment, the liver graft must be maintained. Reduction of immunosuppression increases the risk of graft rejection. Medical therapies potentially affect liver function. The use of immunotherapy is particularly concerned with immune-mediated graft injury. While formulating the treatment strategy, the benefits of the treatment must be balanced with the potential toxicities towards the liver graft.
PROPOSED TREATMENT ALGORITHM
The patient is jointly managed by the transplant surgeon, physician, oncologist and radiologist under a multidisciplinary approach.
IMMUNOSUPPRESSION
Whenever a recurrence is diagnosed, the immunosuppressant should be reviewed. Considering that immune failure contributes to cancer progression, immunosuppression should be tapered to the lowest effective dose protecting against rejection. Moreover, the regimen of immunosuppression warrants reconsideration.
Mammalian target of rapamycin inhibitor
Mammalian target of rapamycin (mTOR) is a protein involved in a signalling pathway that controls cellular growth and proliferation[15]. Rapamycin, more commonly known as sirolimus, inhibits the mTOR pathway to restrain regulatory T-cell proliferation[16]. Apart from immune modulation, mTOR is also involved in HCC pathogenesis and is associated with poor tumour biology[17-19]. Sirolimus has been investigated in a phase II trial showing promising efficacy against advanced HCC[20]. With the theoretical advantage over tumour control, sirolimus has been extensively investigated as immunosuppression therapy for patients engrafted for HCC[21-27] (Table 1).
Table 1.
Mammalian target of rapamycin inhibitors for patients engrafted for hepatocellular carcinoma
| No. (SRL/non-SRL) | 5-year OS (%) | 5-year DFS (%) | HAT (%) | ACR (%) | Discontinuation for toxicity (%) | |
| Prospective controlled trial | ||||||
| Geissler et al[21], 2016 | 261/264 | 79.4/70.33 | 72.6/68.4 | - | 23.4/17.0 | - |
| Meta-analysis | ||||||
| Liang et al[22], 2012 | 332/2615 | OR: 2.473 | 1 yr: OR 2.413 | OR: 1.32 | - | - |
| Zhang et al[23], 2018 | 7695 | OR: 1.683 | 1 yr: OR 2.133 | - | - | - |
| Case-control | ||||||
| Vivarelli et al[24],2010 | 31/31 | - | 3 yr 86/563 | 0/0 | 3.2/3.2 | - |
| Retrospective cohort | ||||||
| Zimmerman et al[25],2007 | 45/52 | 80/62 | 78.8/54 | 2.4/1.9 | 20/19.6 | - |
| Zhou et al[28], 20081 | 27/46 | 19.8 ± 1.2/16.0 ± 1.423 | 17.3 ± 1.4/15.9 ± 1.62 | 0/0 | 30.4/19.6 | 8.3 |
| Chinnakotla et al[26], 2009 | 121/106 | 80/503 | - | 1.9/2 | 62.8/54.7 | 0 |
| Toso et al[27], 2010 | 109/2382 | 83.1/68.73 | - | - | - | - |
All tumours were beyond Milan criteria;
Median survival in months;
Statistically significant. SRL: Sirolimus; OS: Overall survival; DFS: Disease-free survival; HAT: Hepatic artery thrombosis; ACR: Acute cellular rejection.
The highest level of evidence came from a prospective trial conducted by Geissler et al[21], where 525 patients were randomized to receive either a sirolimus-based or an mTOR inhibitor-free regimen. In the study group, sirolimus was incorporated 4-6 wk after transplantation, with or without a concomitant calcineurin inhibitor. The overall and recurrence free survival rates were improved up to 5 years (overall survival: 79.4% vs 70.3%; P = 0.048) and 3 years (disease-free survival: 80.6% vs 72.3%; P = 0.0499). The proportion of patients with acute rejection appeared to be higher in the sirolimus group (23.4% vs 17.0%, respectively; P = 0.07), but the difference did not reach statistical significance. The results were in concordance with an updated meta-analysis that demonstrated a survival benefit in patients receiving sirolimus-based immunosuppression therapy (OR = 1.68; CI = 1.21-2.33)[23]. From the pooled results of 11 studies, the risk of graft rejection or hepatic artery thrombosis was not increased. Sirolimus was generally well tolerated. In a small proportion of patients (0-8.3%), sirolimus was discontinued for drug toxicity, mostly due to oral ulcers[28].
Everolimus is a derivative of sirolimus with a shorter elimination half-life (30 h vs 60 h) and a quicker time to steady state (4 d vs 6 d)[29,30]. The clinical advantage is easier dose adjustment. Everolimus received evaluation in a phase III trial for its role in advanced primary HCC that progressed despite sorafenib therapy[31]. However, no further survival benefit was observed upon switching to everolimus (overall survival: 7.6 mo vs 7.3 mo). Everolimus has been evaluated in prospective trials for its efficacy in liver transplantation, although they were not focused on oncological outcomes. In a prospective multicentre study, everolimus with a reduced dose of tacrolimus was associated with better preserved renal function (estimated glomerular filtration rate decline over 36 mo: 7.0 mL/min/1.73 m2 vs 15.5 mL/min/1.73 m2; P = 0.005) compared with the standard dose of tacrolimus[32]. A similar regimen was studied in another prospective trial with a composite primary endpoint comprising rejection and graft loss[33]. Notably, in patients transplanted for HCC, recurrence was only observed in the control arm with a standard dose of tacrolimus (5/62 vs 0/62) after 12 mo of follow up. A direct comparison between everolimus and sirolimus was made in a meta-analysis[34]. Patients on everolimus had significantly fewer recurrences than those on sirolimus or calcineurin inhibitors (4.1% vs 10.5% vs 13.8%, respectively; P < 0.05). However, everolimus-treated recipients had a shorter follow-up time (13 mo vs 30 mo vs 43.2 mo, respectively) and fewer advanced tumours (HCC within Milan criteria: 84% vs 60.5% vs 74%, respectively; P < 0.05). The study did not compare survival, and no definite conclusions were drawn.
The data on mTOR inhibitor therapy for established recurrence after liver transplantation remain scarce. However, a combination of either sirolimus or everolimus with reduced-dose tacrolimus has been proven to be safe and effective in reducing recurrence[24,28,33]. There is inadequate evidence to recommend the optimal serum level of tacrolimus in this combination. In our experience, a sub-therapeutic level of tacrolimus might suffix. From Geissler’s prospective trial it appears that Siroliums monotherapy might be adequate for some patients[21]. From a registry database comprising 2491 patients transplanted for HCC, sirolimus was the only maintenance immunosuppressant affecting survival (5-year survival: 83.1% vs 68.7%, P < 0.05)[27]. Based on these findings, it appears sensible to incorporate an mTOR inhibitor with a reduced calcineurin inhibitor upon the diagnosis of recurrence. Overall, immunosuppression should be individualized and tapered to spare the remaining anti-tumour immunity. Patients should be closely monitored for liver function throughout the course of cancer treatment.
STAGING
Because post-transplant recurrence is essentially metastatic disease, complete staging is essential to guide subsequent management. Dual tracer positron emission tomography-computed tomography (PET-CT) has been validated for pre-transplant staging for HCC patients[35]. During the examination, a whole-body survey, both functional and structural, is performed for comprehensive staging. The two radioisotopes, namely C11-acetate and fludeoxyglucose (FDG), complement each other. C11-acetate is sensitive for well-differentiated HCC, but tumours with more unfavourable biology may have a predilection towards FDG[36]. Combining two tracers enhances sensitivity to detect occult metastasis. Dual tracer PET-CT is especially advantageous over computed tomography (CT) to diagnose bone metastasis (sensitivity 97% vs 72%, respectively; P < 0.05) and is not uncommon in patients with recurrence after liver transplantation[37].
Albeit effective, dual-tracer PET-CT may not be widely available. When contrast CT is performed as an alternative, it is better coupled with a skeletal survey using bone scan. Bone is the third most common site of recurrence after the lung and liver, affecting 20% of patients with recurrence[38]. The objective of radiological staging is to determine whether the recurrence is disseminated or limited, i.e., oligo-recurrence. While disseminated recurrence is managed primarily with systemic therapy, limited recurrence may be better controlled with additional loco-regional treatment. It has been observed that R0 resection conferred a survival benefit in selected candidates with isolated and resectable metastasis[39].
DISSEMINATED RECURRENCE
Disseminated recurrence is primarily managed with systemic treatment with the intention to prolong survival rather than to pursue cure.
Targeted therapy
Sorafenib is a multi-tyrosine kinase inhibitor with activity against vascular endothelial growth factor-2 and -3, platelet-derived growth factor receptor and Ras ligand[40]. It inhibits tumour signalling and angiogenesis pathways involved in HCC pathogenesis. In a randomised controlled trial, sorafenib was shown to prolong the median survival of patients with advanced HCC for 3 mo (10.7 mo vs 7.9 mo, P < 0.001)[41]. The major drawback was a poorly tolerated side effects profile. Hand-foot skin reaction and gastrointestinal disturbances were reported in 21% and 39% of the patients, respectively. Although mostly graded as 1 and 2 in severity, drug-related adverse events have led to discontinuation of sorafenib in 29% of the patients.
The efficacy of sorafenib in post-transplant HCC recurrence has been studied in numerous retrospective series, mostly in combination with an mTOR inhibitor (Table 2)[42-52]. Sorafenib and mTOR inhibition had synergistic effects on tumour growth in xenograft mice[53]. Ras blockade silenced the feedback signalling of mTOR inhibition, leading to upregulation of its anti-tumour activity[54]. A retrospective cohort reported by De’Angelis et al[45] provided insights into the use of sorafenib in patients with advanced recurrence. The outcomes of 15 patients treated with sorafenib were compared with those of 24 patients receiving best supportive care. Sorafenib was stared at 400 mg twice daily. More patients in the sorafenib group received an mTOR inhibitor due to the time effect, but the difference did not reach statistical significance (46.7% vs 16.7%, P = 0.13). Sorafenib conferred disease control (partial response or stable disease) in 11 of the 15 patients (73.4%), translating into a survival benefit (median OS: 41.4 mo vs 19.1 mo; P = 0.013). Notably, there was a high proportion of patients requiring dose reduction (53.3%) or discontinuation of treatment (13.3%) due to drug toxicity.
Table 2.
Sorafenib for recurrent hepatocellular carcinoma after liver transplantation
| No. (SFN/BSC) | Duration after LT (mo) | mTOR inhibitor (yes/no) | Response rate (% complete/partial/stable) | Median OS (mo) | Time to progression (mo) |
Drug toxicity leading to |
||
| Dose reduction (% patient) | Discontinuation (% patient) | |||||||
| Meta-analysis | ||||||||
| Mancuso et al[42],2015 | 113 | 13.6 | - | 0/4.8/44.4 | 10.5 | 5.6 | 42.8 | 31.9 |
| Retrospective cohort | ||||||||
| Sposito et al[43], 2013 | 15/24 | 38.1/15.72 | 7/8 | - | 21.3/11.82 | 8.8/10.2 | 53.3 | 4.1 |
| De'Angelis et al[45], 2016 | 15/18 | 18 | 7/8 | 0/26.6/46.8 | 41.4/19.12 | - | 53.3 | 13.3 |
| Pinero et al[46], 2016 | 10/10 | - | 7/3 | - | 20/12.5 | 5/32 | 90 | 20 |
| Case series | ||||||||
| Yoon et al[47], 2010 | 13 | 12.3 | 1/12 | 0/0/46 | 5.4 | 2.9 | 30.7 | 0 |
| Kim et al[48],2010 | 9 | 12.4 | 7/2 | 11/0/44 | -1 | - | - | 0 |
| Vitale et al[49], 2012 | 10 | 7 | 10/0 | 0/20/60 | 18 | 8 | 40 | 30 |
| Gomez-Martin et al[50], 2012 | 31 | 22.6 | 31/0 | 0/3.8/50 | 19.3 | 6.77 | 25.8 | - |
| Weinmann et al[51], 2012 | 11 | 37.5 | 9/2 | 0/0/36 | 20.1 | 4.1 | 73 | 18 |
| Sotiropoulos et al[52], 2012 | 14 | 8 | 14/0 | - | 25 | - | 33 | 17 |
| Zavaglia et al[44], 2013 | 11 | 12 | 7/4 | 0/18/9 | 5 | 17 | 90 | - |
Median survival not reached;
Statistically significant. SFN: Sorafenib; BSC: Best supportive care; LT: Liver transplant; mTOR: Mammalian target of rapamycin; OS: Overall survival.
Gomez-Martin et al[50] addressed the safety of combining sorafenib with an mTOR inhibitor in a post-transplant setting. In the multicentre cohort consisting of 31 patients with recurrent HCC, the immunosuppression was shifted to mTOR inhibitor therapy with initiation of sorafenib as systemic treatment. Most toxicities were grade 1 or 2. However, 2 episodes of gastric bleeding and 1 episode of cerebral haemorrhage were reported. The gastric bleedings were diffuse mucosal oozing unrelated to portal hypertension or ulcer disease. Thus, sorafenib appears to be effective to prolong survival after recurrence but at the cost of significant toxicity. Combination treatment with an mTOR inhibitor should be avoided in patients with potential bleeding complications.
Immunotherapy
Immunotherapy directs the host immunity towards the tumour[55]. The physiological immune response is regulated by immune checkpoints[56]. Immunotherapy consists of antibodies directed against these immune checkpoints on the T-cell surface to prompt reactions against tumour antigens. Examples include ipilimumab that targets cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and nivolumab and pembrolizumab that target programmed cell death protein 1 (PD-1). Nivolumab has been validated in a large phase II trial for its safety and efficacy against primary HCC[57]. Nivolumab 3 mg/kg was given every 2 wk to 214 patients with advanced HCC. Disease control was achieved in 64% of all patients, including 61% of patients who had previously failed sorafenib treatment. The overall survival was 83% at 6 mo. There was a favourable side effect profile compared with that of sorafenib. Only 2%-4% of patients discontinued nivolumab due to drug toxicity.
However, immune checkpoint modulation of cell-mediated immunity is implicated in transplant organ tolerance[58,59]. Downregulation of these pathways may inadvertently lead to transplant rejection[60]. In fact, clinical trials for immune checkpoint inhibitors often exclude solid organ transplant recipients due to the fear of graft injury[61,62]. Current experience in immunotherapy after liver transplantation is confined to case reports and small series[63-66] (Table 3). Limited survival (0.3 mo to 3 mo) was observed among the 10 patients treated with anti-PD-1. The salvage nature of immunotherapy must be considered while interpreting the results. Most patients had developed disease progression with sorafenib. Moreover, the clinical decision to employ immunotherapy for transplant patients is usually much delayed until treatment failure is evident. Although the therapeutic effect is considered rapid for immunotherapy, a 3-m interval is usually necessary before the treatment response can be evaluated57. In the reports, the rather limited survival interval after immunotherapy might not allow the efficacy of immunotherapy to be assessed.
Table 3.
Immunotherapy for recurrent hepatocellular carcinoma after liver transplantation
| Patient | Age | Ref. | Tumour | Agent | Years after LT | Immunosuppression | Prior sorafenib | Response | OS (mo) | Rejection |
| 1 | 41 | De Toni et al[63], 2017 | HCC | Nivolumab | 1 | Low dose tacrolimus | Yes | No | - | No |
| 21 | 20 | Friend et al[64], 2017 | HCC | Nivolumab | 4 | Sirolimus | - | - | 1 | Yes |
| 31 | 14 | Friend et al[64], 2017 | HCC | Nivolumab | 3 | Tacrolimus | - | - | 1 | Yes |
| 4 | 70 | Varkaris et al[65], 2017 | HCC | Pembrolizumab | 8 | Low dose tacrolimus | Yes | No | 3 | No |
| 5 | 57 | DeLeon et al[66], 2018 | HCC | Nivolumab | 2.7 | Tacrolimus | Yes | No | 1.2 | No |
| 6 | 56 | DeLeon et al[66], 2018 | HCC | Nivolumab | 7.8 | MMF/sirolimus | Yes | No | 1.1 | No |
| 7 | 35 | DeLeon et al[66], 2018 | HCC | Nivolumab | 3.7 | Tacrolimus | Yes | No | 1.3 | No |
| 8 | 64 | DeLeon et al[66], 2018 | HCC | Nivolumab | 1.2 | Tacrolimus | Yes | -2 | 0.3 | No |
| 9 | 68 | DeLeon et al[66], 2018 | HCC | Nivolumab | 1.1 | Sirolimus | Yes | - | 0.7 | Yes |
| 10 | 70 | Varkaris et al[65], 2017 | HCC | Pembrolizumab | 6 | Low dose tacrolimus | Yes | No | 3 | No |
| 11 | 59 | Ranganath et al[69], 2015 | Melanoma | Ipilimumab | 8 | Tacrolimus | - | - | - | No |
| 12 | 67 | Morales et al[70], 2015 | Melanoma | Ipilimumab | 8 | Sirolimus | - | - | - | No |
| 13 | 55 | DeLeon et al[66], 2018 | Melanoma | Pembrolizumab | 5.5 | MMF/everolimus | - | - | - | No |
| 14 | 63 | DeLeon et al[66], 2018 | Melanoma | Pembrolizumab | 3.1 | MMF/prednisolone | - | - | - | Yes |
| 15 | 62 | Kuo et al[71], 2018 | Melanoma | Ipilimumab and pembrolizumab | 6 | Sirolimus | - | - | - | Yes |
Fibrolamella hepatocellular carcinoma;
Multiorgan failure, unrelated to immunotherapy. HCC: Hepatocellular carcinoma; LT: Liver transplant; OS: Overall survival.
Acute rejection occurred in 3 of the 10 reported cases receiving anti-PD-1 treatment. Although a limited number of events precludes risk factor analysis, a hypothesis could be proposed. Two patients with rejection were relatively young, aged 14 and 20 years, respectively. A young age is a recognized risk factor for acute rejection after liver transplantation, and more aggressive immunosuppression is usually employed[67]. A long duration after transplantation is usually protective of acute rejection. However, the trend is not obvious from this series of observations. Practically, most recurrence occurs early after transplantation as well.
The differential effect of PD-1 and CTLA-4 blockade on rejection may also have implications[68]. Among the 5 reported liver transplant patients treated with immunotherapy for melanoma, rejection was observed only in patients receiving a PD-1 inhibitor[66,69-71] (Table 3). These clinical observations concurred with the findings from in vitro studies. Using a murine model it was demonstrated that the PD-1 pathway may play a stronger role in allograft tolerance than CTLA-4 and that PD-1 blockade could be associated with a higher risk of transplant rejection[72]. However, the effect of CTLA-4 blockade on HCC control has not been systematically investigated. The role of immunotherapy in treating HCC recurrence after liver transplantation remains largely unknown. The potential efficacy should not be overlooked but has to be balanced with its safety[73]. Further study in a large patient cohort is warranted to elucidate optimal patient selection.
MANAGEMENT OF OLIGO-RECURRENCE
Historically, distant recurrence is considered to be terminal. Post-transplant recurrence is, by definition, distant metastasis from the native liver and has been managed with palliative intent. However, the new notions of oligo-recurrence have led to a paradigm shift in the management of cancer recurrence or metastasis. First introduced by Hellman and Weichselbaum in 1995, the term described recurrent disease that was limited in number and location so that loco-regional treatments improved survival[74]. Oligo-recurrence represents a therapeutic opportunity that allows the patient to be treated with a curative strategy. Due to the improvement in systemic therapy, a durable cure is no longer a remote possibility in patients with limited disease. The concept has gained substantial popularity, and oligo-recurrences have been managed with a combination of systemic and loco-regional treatments with promising results[75]. A stringent definition for oligo-recurrence in terms of the number, size, or distribution of tumour is impractical. A pragmatic view to the concept is a rational use of loco-regional therapy in patients for whom disease burden is limited.
Role of surgery
The results for surgical resection have been retrospectively reported for patients with intra-hepatic or extrahepatic oligo-recurrence (Table 4)[4,39,76-79]. Patients eligible for surgical treatment ranged from 25% to 50%. The lung and liver were common sites for resection (Table 4). Survival benefits have been consistently demonstrated in patients treated with surgery, with a median survival of 28 mo to 65 mo observed for patients receiving surgery, compared with 5 mo to 15 mo in those receiving systemic treatment only[4,39,76-79].
Table 4.
Surgery for recurrent hepatocellular carcinoma after liver transplantation
| No. resection/total (%) | Site of resection |
Median OS in months |
Selection criteria | Resection: Independent predictor of survival | ||
| Overall | Resection/no resection | |||||
| Comparative study | ||||||
| Roayaie et al[4], 2004 | 18/57 (31.6) | Liver (n = 8), lung (n = 7), adrenal (n = 2), chest wall (n = 1)1 | 8.7 | - | Technical feasibility | Yes |
| Kornberg et al[76], 2010 | 7/16 (43.8) | Liver (n = 2), lung (n = 2), others (n = 3) | 10.5 | 65/55 | - | Yes |
| Valdivieso et al[39], 2010 | 11/23 (47.8) | Liver (n = 2), lung (n = 2), adrenal (n = 2), abdominal lymph node (n = 2) | - | 32.3 ± 21.5/11.9 ± 6.925 | Technical feasibility | - |
| Sapisochin et al[77], 2015 | 38/121 (31.4) | - | - | 31/12/535 | Technical feasibility | Yes |
| Bodzin et al[78], 2017 | 25/106 (23.6) | lung (n = 8), bone (n = 6), intra-abdominal (n = 4), liver (n = 3), brain (n = 2) | 10.6 | 27.8/10.6/3.735 | - | - |
| Fernandez-Sevilla et al[79], 2017 | 22/70 (31.4)4 | - | 19 | 35/155 | Technical feasibility. No progression with systemic treatment | Yes |
Include radiofrequency ablation of liver lesion (n = 2);
R0 resection vs no R0 resection;
Resection vs non-surgical treatment vs best supportive care;
R0/1 resection vs no R0/1 resection;
Statistically significant. OS: Overall survival.
Selection bias was inevitable because surgical candidates were invariably patients with localized disease and a better prognosis. In the most recent series, patient selection was further refined with an additional criterion being the absence of progression while on systemic treatment[79]. The genuine benefit conveyed by surgery could be questioned because the selected patients had a limited disease burden and more favourable tumour biology. However, a prospective randomized trial is unlikely under the current setting to be given ethical concern. A matched retrospective comparison is also difficult due to the intrinsic differences between the patients with oligo- and disseminated recurrence.
Reviewing the current literature, long-term survival after post-transplant recurrence has been achieved with surgical resection. Across numerous reported series, surgical treatment remained an independent predictor of superior survival after recurrence[4,76,77,79]. Surgery is supported as the treatment of choice in patients with resectable recurrence, especially when the tumour biology is favourable.
HEPATIC OLIGO-RECURRENCE
At this point, a differentiation must be made between intra-hepatic recurrence of the primary tumour and development of de novo hepatocellular carcinoma. The former occurs early after transplantation, usually within the first 2 years and represents metastatic deposits of the primary HCC into the liver graft. The latter develops late, years after transplantation, when the liver graft becomes cirrhotic secondary to chronic injury. Common culprits are the recurrence of primary liver diseases, diffuse ischaemic biliary injury (DIBI) or chronic rejection. De novo HCC resembles the usual situation in a non-transplant recipient where the disease could be presumed to be localized in the liver. Local treatments offer the opportunity of disease control before systemic dissemination. However, the graft function status must be considered when selecting the optimal therapeutic strategy.
Graft resection
This review focuses on genuine intra-hepatic recurrence. In the setting of primary HCC, surgical resection with partial hepatectomy confers favourable oncological outcomes[80]. Surgical resection is therefore given full consideration here when macroscopic disease is confined within the liver. Because recurrence usually occurs early, graft function is preserved and rarely precludes hepatectomy. Resectability is more determined by disease burden. A tumour-free future remnant with adequate volume is the prerequisite for graft hepatectomy. The main concern for graft resection is morbidity. Graft resection is technically challenging due to extensive hilar adhesions. Immunosuppressed patients are susceptible to infective complications. Graft resection for recurrence has been reported in several retrospective series in small numbers (Table 5)[81-83]. High morbidity (60%-80%), but no mortality, was reported. In the first series, infective complications occurred in 63% patients, including 5 Gram-negative bacteraemia requiring intravenous antibiotics[83]. Two series reported oncological outcomes[82,84], with 3-year overall survival rates ranging from 50% to 70%. Graft resection appears to be a feasible treatment for recurrent HCC, offering the chance of long-term survival. However, high morbidity, especially infection complications, is expected.
Table 5.
Graft resection for recurrent hepatocellular carcinoma after liver transplantation
| No. | Morbidity (%) | Mortality (%) | 3-yr OS (%) | |
| Case series | ||||
| Marangoni et al[83], 2008 | 111 | 812 | 0 | - |
| Sommacale et al[81], 2013 | 83 | 624 | 0 | - |
| Chok[82], 2015 | 3 | - | 0 | 66.7 |
Hepatocellular carcinoma (HCC) (n = 4), ischaemic cholangiopathy (n = 2), segmental hepatic artery thrombosis (HAT) (n = 2), others (n = 5);
Small bowel perforation (n = 1), bile leak (n = 1), intra-abdominal collection (n = 1), wound infection (n = 1), sepsis (n = 5);
HCC (n = 3), bile leak (n = 1), recurrent segmental cholangitis (n = 1), hydatid cyst (n = 1), segmental HAT (n = 1), biliary cyst (n = 1);
Statistically significant. OS: Overall survival.
Radiofrequency ablation
Due to the significant morbidity associated with graft resection, the role of ablative treatments has been investigated. Radiofrequency ablation (RFA) is a thermal ablative technique delivered via a needle electrode. For primary HCC, RFA offers equivalent local control as resection for small tumours less than 3 cm in size[85]. The advantage is that it can be performed percutaneously under radiological guidance. A favourable location would be away from the major vasculature and adjacent organs. RFA becomes more appealing when a small tumour is situated in the deep parenchyma, where major hepatectomy is necessitated for resection. In the setting of post-transplant recurrence, the additional benefit is the avoidance of morbidities associated with re-laparotomy in an immunocompromised patient.
One retrospective cohort compared RFA with resection for post-transplant HCC recurrence[84]. In the reported series, 15 patients were treated with surgery while 11 received RFA. The author reported similar 3-year (51% vs 51%, P = 0.88) and 5-year (35% vs 28%, P = 0.88) overall survivals in two groups. However, both hepatic and extra-hepatic recurrences were included, and the results represented the outcomes of heterogeneous procedures. Morbidity and mortality after graft resection were not reported.
Stereotactic body radiation therapy
Stereotactic body radiation therapy (SBRT) is a precise method of delivering ablative radiation by tomographic modulation. Intense and focused doses of radiation are given in a few or single fractions. SBRT for post-transplant HCC recurrence has several theoretical advantages. The radiation beam is focused on the tumour, sparing the adjacent normal liver parenchyma. A higher dose of radiation is delivered while the risk of collateral damage is minimized[86]. Moreover, SBRT is delivered over fewer treatment days than the 10-20 d for conventional radiotherapy, during which systemic therapy is usually deferred. SBRT is usually completed in 1-5 fractions, allowing systemic treatment to be commenced early.
Moreover, it is now established in pre-clinical models that stereotactic radiation upregulates anti-tumour immunity[87-89]. High-dose radiation stimulate antigen-presenting cells, leading to the activation and proliferation of tumour-specific cytotoxic T cells[89]. The abscopal response (from “ab scopus”, meaning away from target) denotes this systemic effect leading to the regression of metastatic lesions outside the irradiation field[90]. Interestingly, abscopal effect is synergistically enhanced when combined with immunotherapy-mediated PD-1 blockade[87], which potentially confers a further clinical advantage to SBRT for recurrent HCC because the role of systemic therapy is crucial.
SBRT has been investigated in several prospective studies for primary HCC[91-94]. In these series, the tumour size ranged from 2 cm to 7 cm. At 2 years after ablation, local control was achieved in 80% to 95% of patients. The figure compares favourably with that reported for RFA of small tumours[95,96]. In contrast to RFA, vascular invasion is not a contraindication[97,98]. In direct retrospective comparison, local control was found to be superior in the SBRT group for tumours more than 2 cm in size (HR: 3.35; P = 0.025). Grade III or above morbidity was similar (SBRT vs RFA: 5% vs 11%; P = 0.31). While RFA loses efficacy with increasing tumour size[99,100], SBRT seems to be as effective when treating larger tumours. To date, the role of SBRT for post-transplant HCC recurrence has yet to be systematically evaluated. While systemic control is of utmost importance, the potential of the SBRT and immunotherapy combination should be conscientiously explored.
Trans-arterial chemoembolization
In patients with multifocal intra-hepatic recurrence, trans-arterial chemoembolization (TACE) offers the opportunity of regional control. Ko et al[101] first reported the results of TACE for recurrent HCC after liver transplantation with 1- and 3-year survival rates of 47.9% and 6.0%, respectively. However, in their series, 64.3% of patients developed concomitant extra-hepatic metastasis, which could have affected the oncological outcome as well. Zhou et al[102] prospectively compared TACE versus systemic therapy in patients with unresectable intra-hepatic recurrence. Survival benefits were achieved in the TACE arm (P = 0.013), indicating that regional control could have contributed to the improvement in overall survival. Notably, both studies reported no major morbidity after graft liver TACE. In Zhou et al[102]’s series of 14 patients, no biliary complications were observed over a median follow up of 14.5 mo.
Trans-arterial radioembolization
Intra-arterial irradiation with Yttrium-90 (Y-90) microspheres has gained popularity in recent years to treat unresectable HCC. Injected through the feeding vessels, these microspheres emit high-dose radiation after entrapment at the pre-capillary level. In a large-scale longitudinal cohort comprising 291 patients, Y-90 achieved a 40%-60% response rate[103]. The median survival was 17.2 mo in patients with Child’s A cirrhosis. In contrast to TACE, portal vein thrombosis is not a contraindication. Considering the potential synergistic effect of irradiation and immunotherapy, clinical studies are ongoing to investigate the benefit of combining Y-90 and anti-PD1 therapy for primary HCC. Their results will shed light on further applications concerning post-transplant HCC recurrence.
EXTRA-HEPATIC OLIGO-RECURRENCE
The lung is the most common site for extra-hepatic recurrence, followed by the bone[4,38,103]. In the literature, the largest series of pulmonary metastatectomy after liver transplantation was reported by Hwang et al[104]. Among 43 patients with lung recurrence, 23 were selected for surgery based on the feasibility of complete resection with sufficient pulmonary function after surgery. Patients were resected for up to 3 tumours, regardless of laterality. Over a mean follow up of 33 mo, 4 patients (17.4%) remained disease-free. The resection group had a significantly greater 5-year survival rate (44.7% vs 12.8%; P = 0.017). There was no operative mortality or morbidity. The results from this retrospective study indicate that pulmonary resection for oligo-recurrence is safe and offers the chance for long-term survival.
Five patients in the resection group had prior extra-pulmonary recurrence successfully treated with loco-regional treatments (3 intrahepatic recurrences ablated with RFA, 1 adrenal and 1 diaphragmatic recurrence excised). Among the 19 patients who developed recurrences after pulmonary resection, 13 received further loco-regional therapy (pulmonary and extra-pulmonary) to enhance disease control. From this series, the notion of oligo-recurrence management was well demonstrated.
When pulmonary metastatectomy is precluded by inadequate lung function, SBRT is considered an alternative[105]. In a German multicentre cohort, 700 patients were treated with SBRT for inoperable pulmonary oligometastasis. The two-year local control and overall survival rates were reported as 82.1% and 54.4%, respectively. Grade 2 or higher pneumonitis occurred in 4.5%-6.5% of patients. SBRT has also been used to treat skeletal oligometastasis from visceral malignancies[106-109]. The 1-year local control rates were 83% and 91% in patients with and without prior radiotherapy, respectively[109]. Stereotactic irradiation was well tolerated with the most common toxicity reported as a transient pain flare[108]. SBRT has been evaluated to treat skeletal metastasis from HCC with a local control rate up to 79% to 88%[110,111]. With these promising results, the role of SBRT for skeletal oligo-recurrence after transplantation should be further explored.
CONCLUSION
To date, experience in managing post-transplant recurrence remains limited. Paucity of high level evidence renders a systematic review or meta-analysis difficult. We hereby propose a multi-disciplinary management algorithm with a systematic approach based on centre experience and best available evidence (Figure 1). The patient is jointly managed by the transplant surgeon, physician, oncologist and radiologist. Following a diagnosis of recurrence, immunosuppression is reviewed. Immunosuppressants should be tapered to the lowest effective dose protecting against rejection. mTOR inhibitors are associated with anti-tumour effects and are potentially beneficial to tumour control. The combination of an mTOR inhibitor with a reduced calcineurin inhibitor can be considered with close monitoring of graft function and toxicity.
Figure 1.
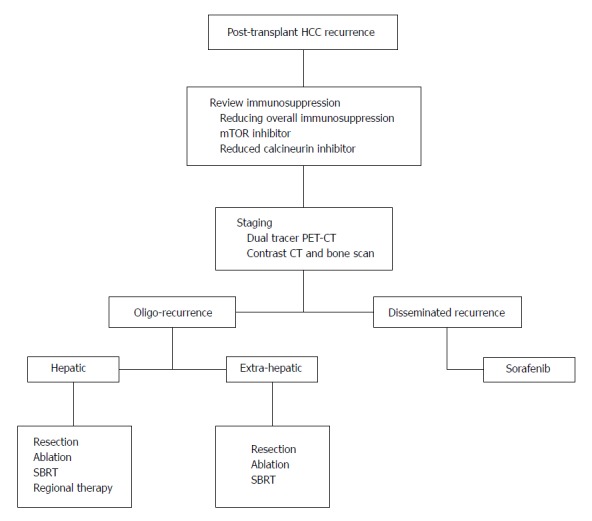
Multidisciplinary approach to manage post-transplant hepatocellular carcinoma recurrence. HCC: Hepatocellular carcinoma; PET-CT: Positron emission tomography-computed tomography; SBRT: Stereotactic body radiation therapy.
Comprehensive staging is mandatory due to the systemic disease nature. Dual-tracer PET-CT is an effective modality for staging. When contrast CT is used, it is better coupled with a bone scan. The essence of staging is to delineate the extent of disease. In patients presenting with disseminated recurrence, sorafenib may confer survival benefits but is associated with significant drug toxicity and is generally poorly tolerated. Dose reduction is frequently required. Patients at risk of bleeding complications should be avoided for the mTOR and sorafenib combination. In patients with poor tolerance to sorafenib, enrolment into a clinical trial may be beneficial. Disease progression is monitored biochemically with the serum level of AFP and radiologically with reassessment scans. Whenever disease regression is evident, the patients should be reviewed for the feasibility of loco-regional treatment. Additional local control may be beneficial to overall disease progression.
Oligo-recurrence encompasses recurrent disease limited in number and location so that loco-regional treatments convey disease control and survival benefits. Intra-hepatic recurrence can be managed with graft resection, but significant operative morbidity is expected. RFA and SBRT are effective alternative strategies. In patients with more advanced hepatic disease, regional treatment with TACE or intra-arterial Yttrium-90 can be considered. For patients with extra-hepatic oligo-recurrence, loco-regional treatment can be considered if practical. Patients with more than one site of recurrence are not always contraindicated for curative treatments. Surgical resection is effective for patients with pulmonary oligo-recurrence, but adequate lung function is a pre-requisite. SBRT is a non-invasive and effective modality that conveys local control to pulmonary and skeletal oligo-recurrences.
Recurrence of HCC after liver transplantation remains a deadly disease with rapid progression. However, with improved treatment modalities, long-term surviving patients are more frequently observed. More aggressive therapeutic strategies in selected patients with a limited disease burden appear to provide more favourable results than palliative measures. A multidisciplinary team is a comprehensive and coordinated approach to manage patients with post-transplant HCC recurrence.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
Conflict-of-interest statement: None of the authors has any conflict of interest.
Peer-review started: September 1, 2018
First decision: October 14, 2018
Article in press: November 7, 2018
P- Reviewer: Hoyos S, Kang KJ, Matsui K, Yao DF S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
Contributor Information
Kin Pan Au, Department of Surgery, Queen Mary Hospital, Hong Kong, China.
Kenneth Siu Ho Chok, Department of Surgery and State Key Laboratory for Liver Research, The University of Hong Kong, Hong Kong, China. chok6275@hku.hk.
References
- 1.Yoo HY, Patt CH, Geschwind JF, Thuluvath PJ. The outcome of liver transplantation in patients with hepatocellular carcinoma in the United States between 1988 and 2001: 5-year survival has improved significantly with time. J Clin Oncol. 2003;21:4329–4335. doi: 10.1200/JCO.2003.11.137. [DOI] [PubMed] [Google Scholar]
- 2.Zavaglia C, De Carlis L, Alberti AB, Minola E, Belli LS, Slim AO, Airoldi A, Giacomoni A, Rondinara G, Tinelli C, et al. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol. 2005;100:2708–2716. doi: 10.1111/j.1572-0241.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 3.Regalia E, Fassati LR, Valente U, Pulvirenti A, Damilano I, Dardano G, Montalto F, Coppa J, Mazzaferro V. Pattern and management of recurrent hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Surg. 1998;5:29–34. doi: 10.1007/pl00009947. [DOI] [PubMed] [Google Scholar]
- 4.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 5.Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127:S261–S267. doi: 10.1053/j.gastro.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 6.Yao FY, Bass NM, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: lessons from the first year under the Model of End-Stage Liver Disease (MELD) organ allocation policy. Liver Transpl. 2004;10:621–630. doi: 10.1002/lt.20159. [DOI] [PubMed] [Google Scholar]
- 7.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 9.Ruggiero RA, Bustuoabad OD, Bonfil RD, Meiss RP, Pasqualini CD. “Concomitant immunity” in murine tumours of non-detectable immunogenicity. Br J Cancer. 1985;51:37–48. doi: 10.1038/bjc.1985.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoyama I, Carr B, Saitsu H, Iwatsuki S, Starzl TE. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer. 1991;68:2095–2100. doi: 10.1002/1097-0142(19911115)68:10<2095::aid-cncr2820681002>3.0.co;2-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, Shimbo T, Suthanthiran M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 12.Maluccio M, Sharma V, Lagman M, Vyas S, Yang H, Li B, Suthanthiran M. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation. 2003;76:597–602. doi: 10.1097/01.TP.0000081399.75231.3B. [DOI] [PubMed] [Google Scholar]
- 13.Vivarelli M, Cucchetti A, Piscaglia F, La Barba G, Bolondi L, Cavallari A, Pinna AD. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl. 2005;11:497–503. doi: 10.1002/lt.20391. [DOI] [PubMed] [Google Scholar]
- 14.Vivarelli M, Cucchetti A, La Barba G, Ravaioli M, Del Gaudio M, Lauro A, Grazi GL, Pinna AD. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg. 2008;248:857–862. doi: 10.1097/SLA.0b013e3181896278. [DOI] [PubMed] [Google Scholar]
- 15.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 16.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Q, Lui VW, Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol. 2011;7:1149–1167. doi: 10.2217/fon.11.95. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Huang Y, Li J, Wang Z. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol. 2010;27:255–261. doi: 10.1007/s12032-009-9201-4. [DOI] [PubMed] [Google Scholar]
- 20.Decaens T, Luciani A, Itti E, Hulin A, Roudot-Thoraval F, Laurent A, Zafrani ES, Mallat A, Duvoux C. Phase II study of sirolimus in treatment-naive patients with advanced hepatocellular carcinoma. Dig Liver Dis. 2012;44:610–616. doi: 10.1016/j.dld.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Geissler EK, Schnitzbauer AA, Zülke C, Lamby PE, Proneth A, Duvoux C, Burra P, Jauch KW, Rentsch M, Ganten TM, et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016;100:116–125. doi: 10.1097/TP.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang W, Wang D, Ling X, Kao AA, Kong Y, Shang Y, Guo Z, He X. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18:62–69. doi: 10.1002/lt.22441. [DOI] [PubMed] [Google Scholar]
- 23.Zhang ZH, Li LX, Li P, Lv SC, Pan B, He Q. Sirolimus in Liver Transplant Recipients with Hepatocellular Carcinoma: An Updated Meta-Analysis. J Invest Surg. 2018:1–10. doi: 10.1080/08941939.2018.1447053. [DOI] [PubMed] [Google Scholar]
- 24.Vivarelli M, Dazzi A, Zanello M, Cucchetti A, Cescon M, Ravaioli M, Del Gaudio M, Lauro A, Grazi GL, Pinna AD. Effect of different immunosuppressive schedules on recurrence-free survival after liver transplantation for hepatocellular carcinoma. Transplantation. 2010;89:227–231. doi: 10.1097/TP.0b013e3181c3c540. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, Kam I. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl. 2008;14:633–638. doi: 10.1002/lt.21420. [DOI] [PubMed] [Google Scholar]
- 26.Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, Onaca N, Goldstein R, Levy M, Klintmalm GB. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15:1834–1842. doi: 10.1002/lt.21953. [DOI] [PubMed] [Google Scholar]
- 27.Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51:1237–1243. doi: 10.1002/hep.23437. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Wang Z, Wu ZQ, Qiu SJ, Yu Y, Huang XW, Tang ZY, Fan J. Sirolimus-based immunosuppression therapy in liver transplantation for patients with hepatocellular carcinoma exceeding the Milan criteria. Transplant Proc. 2008;40:3548–3553. doi: 10.1016/j.transproceed.2008.03.165. [DOI] [PubMed] [Google Scholar]
- 29.Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet. 2004;43:83–95. doi: 10.2165/00003088-200443020-00002. [DOI] [PubMed] [Google Scholar]
- 30.Shipkova M, Hesselink DA, Holt DW, Billaud EM, van Gelder T, Kunicki PK, Brunet M, Budde K, Barten MJ, De Simone P, et al. Therapeutic Drug Monitoring of Everolimus: A Consensus Report. Ther Drug Monit. 2016;38:143–169. doi: 10.1097/FTD.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 31.Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, Poon RT, Blanc JF, Vogel A, Chen CL, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 32.Fischer L, Saliba F, Kaiser GM, De Carlis L, Metselaar HJ, De Simone P, Duvoux C, Nevens F, Fung JJ, Dong G, et al. Three-year Outcomes in De Novo Liver Transplant Patients Receiving Everolimus With Reduced Tacrolimus: Follow-Up Results From a Randomized, Multicenter Study. Transplantation. 2015;99:1455–1462. doi: 10.1097/TP.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 33.Jeng LB, Lee SG, Soin AS, Lee WC, Suh KS, Joo DJ, Uemoto S, Joh J, Yoshizumi T, Yang HR, et al. Efficacy and safety of everolimus with reduced tacrolimus in living-donor liver transplant recipients: 12-month results of a randomized multicenter study. Am J Transplant. 2018;18:1435–1446. doi: 10.1111/ajt.14623. [DOI] [PubMed] [Google Scholar]
- 34.Cholongitas E, Mamou C, Rodríguez-Castro KI, Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int. 2014;27:1039–1049. doi: 10.1111/tri.12372. [DOI] [PubMed] [Google Scholar]
- 35.Cheung TT, Ho CL, Lo CM, Chen S, Chan SC, Chok KS, Fung JY, Yan Chan AC, Sharr W, Yau T, et al. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: surgeon’s perspective. J Nucl Med. 2013;54:192–200. doi: 10.2967/jnumed.112.107516. [DOI] [PubMed] [Google Scholar]
- 36.Cheung TT, Chan SC, Ho CL, Chok KS, Chan AC, Sharr WW, Ng KK, Poon RT, Lo CM, Fan ST. Can positron emission tomography with the dual tracers [11 C]acetate and [18 F]fludeoxyglucose predict microvascular invasion in hepatocellular carcinoma? Liver Transpl. 2011;17:1218–1225. doi: 10.1002/lt.22362. [DOI] [PubMed] [Google Scholar]
- 37.Ho CL, Chen S, Cheng TK, Leung YL. PET/CT characteristics of isolated bone metastases in hepatocellular carcinoma. Radiology. 2011;258:515–523. doi: 10.1148/radiol.10100672. [DOI] [PubMed] [Google Scholar]
- 38.Pecchi A, Besutti G, De Santis M, Del Giovane C, Nosseir S, Tarantino G, Di Benedetto F, Torricelli P. Post-transplantation hepatocellular carcinoma recurrence: Patterns and relation between vascularity and differentiation degree. World J Hepatol. 2015;7:276–284. doi: 10.4254/wjh.v7.i2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valdivieso A, Bustamante J, Gastaca M, Uriarte JG, Ventoso A, Ruiz P, Fernandez JR, Pijoan I, Testillano M, Suarez MJ, et al. Management of hepatocellular carcinoma recurrence after liver transplantation. Transplant Proc. 2010;42:660–662. doi: 10.1016/j.transproceed.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 41.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 42.Mancuso A, Mazzola A, Cabibbo G, Perricone G, Enea M, Galvano A, Zavaglia C, Belli L, Cammà C. Survival of patients treated with sorafenib for hepatocellular carcinoma recurrence after liver transplantation: a systematic review and meta-analysis. Dig Liver Dis. 2015;47:324–330. doi: 10.1016/j.dld.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59–66. doi: 10.1016/j.jhep.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 44.Zavaglia C, Airoldi A, Mancuso A, Vangeli M, Viganò R, Cordone G, Gentiluomo M, Belli LS. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: experience at a single center and review of the literature. Eur J Gastroenterol Hepatol. 2013;25:180–186. doi: 10.1097/MEG.0b013e328359e550. [DOI] [PubMed] [Google Scholar]
- 45.de’Angelis N, Landi F, Nencioni M, Palen A, Lahat E, Salloum C, Compagnon P, Lim C, Costentin C, Calderaro J, et al. Role of Sorafenib in Patients With Recurrent Hepatocellular Carcinoma After Liver Transplantation. Prog Transplant. 2016;26:348–355. doi: 10.1177/1526924816664083. [DOI] [PubMed] [Google Scholar]
- 46.PiñeroF, MarcianoS, AndersM Sorafenib for Recurrent Hepatocellular Carcinoma after Liver Transplantation: A South American Experience [Google Scholar]
- 47.Yoon DH, Ryoo BY, Ryu MH, Lee SG, Hwang S, Suh DJ, Lee HC, Kim TW, Ahn CS, Kim KH, et al. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010;40:768–773. doi: 10.1093/jjco/hyq055. [DOI] [PubMed] [Google Scholar]
- 48.Kim R, El-Gazzaz G, Tan A, Elson P, Byrne M, Chang YD, Aucejo F. Safety and feasibility of using sorafenib in recurrent hepatocellular carcinoma after orthotopic liver transplantation. Oncology. 2010;79:62–66. doi: 10.1159/000319548. [DOI] [PubMed] [Google Scholar]
- 49.Vitale A, Boccagni P, Kertusha X, Zanus G, D’Amico F, Lodo E, Pastorelli D, Ramirez Morales R, Lombardi G, Senzolo M, et al. Sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation? Transplant Proc. 2012;44:1989–1991. doi: 10.1016/j.transproceed.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 50.Gomez-Martin C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, Herrero I, Matilla A, Sangro B. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012;18:45–52. doi: 10.1002/lt.22434. [DOI] [PubMed] [Google Scholar]
- 51.Weinmann A, Niederle IM, Koch S, Hoppe-Lotichius M, Heise M, Düber C, Schuchmann M, Otto G, Galle PR, Wörns MA. Sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Dig Liver Dis. 2012;44:432–437. doi: 10.1016/j.dld.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Sotiropoulos GC, Nowak KW, Fouzas I, Vernadakis S, Kykalos S, Klein CG, Paul A. Sorafenib treatment for recurrent hepatocellular carcinoma after liver transplantation. Transplant Proc. 2012;44:2754–2756. doi: 10.1016/j.transproceed.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 53.Newell P, Toffanin S, Villanueva A, Chiang DY, Minguez B, Cabellos L, Savic R, Hoshida Y, Lim KH, Melgar-Lesmes P, et al. Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo. J Hepatol. 2009;51:725–733. doi: 10.1016/j.jhep.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carracedo A, Baselga J, Pandolfi PP. Deconstructing feedback-signaling networks to improve anticancer therapy with mTORC1 inhibitors. Cell Cycle. 2008;7:3805–3809. doi: 10.4161/cc.7.24.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Couzin-Frankel J. Immune therapy steps up the attack. Science. 2010;330:440–443. doi: 10.1126/science.330.6003.440. [DOI] [PubMed] [Google Scholar]
- 56.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12:2575–2587. doi: 10.1111/j.1600-6143.2012.04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, Grimm M, Waaga AM, Ueno T, Padera RF, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179:5204–5210. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang T, Fresnay S, Welty E, Sangrampurkar N, Rybak E, Zhou H, Cheng XF, Feng Q, Avon C, Laaris A, et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant. 2011;11:1599–1609. doi: 10.1111/j.1600-6143.2011.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 63.De Toni EN, Gerbes AL. Tapering of Immunosuppression and Sustained Treatment With Nivolumab in a Liver Transplant Recipient. Gastroenterology. 2017;152:1631–1633. doi: 10.1053/j.gastro.2017.01.063. [DOI] [PubMed] [Google Scholar]
- 64.Friend BD, Venick RS, McDiarmid SV, Zhou X, Naini B, Wang H, Farmer DG, Busuttil RW, Federman N. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr Blood Cancer. 2017:64 (12). doi: 10.1002/pbc.26682. [DOI] [PubMed] [Google Scholar]
- 65.Varkaris A, Lewis DW, Nugent FW. Preserved Liver Transplant After PD-1 Pathway Inhibitor for Hepatocellular Carcinoma. Am J Gastroenterol. 2017;112:1895–1896. doi: 10.1038/ajg.2017.387. [DOI] [PubMed] [Google Scholar]
- 66.DeleonTT, SalomaoMA, AqelBA Pilot evaluation of PD-1 inhibition in metastatic cancer patients with a history of liver transplantation: the Mayo Clinic experience. J Gastrointest Oncol. 2018:(suppl 4S). doi: 10.21037/jgo.2018.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang YC, Wu TJ, Wu TH, Lee CF, Chou HS, Chan KM, Lee WC. The risk factors to predict acute rejection in liver transplantation. Transplant Proc. 2012;44:526–528. doi: 10.1016/j.transproceed.2012.01.041. [DOI] [PubMed] [Google Scholar]
- 68.Liu M, Guo W, Zhang S. Cancer immunotherapy in patients with new or recurrent malignancies after liver transplantation. Int J Surg Oncol (NY) 2017;2:e49. doi: 10.1097/IJ9.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ranganath HA, Panella TJ. Administration of ipilimumab to a liver transplant recipient with unresectable metastatic melanoma. J Immunother. 2015;38:211. doi: 10.1097/CJI.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 70.Morales RE, Shoushtari AN, Walsh MM, Grewal P, Lipson EJ, Carvajal RD. Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J Immunother Cancer. 2015;3:22. doi: 10.1186/s40425-015-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuo JC, Lilly LB, Hogg D. Immune checkpoint inhibitor therapy in a liver transplant recipient with a rare subtype of melanoma: a case report and literature review. Melanoma Res. 2018;28:61–64. doi: 10.1097/CMR.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 72.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor PA. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 73.Kittai AS, Oldham H, Cetnar J, Taylor M. Immune Checkpoint Inhibitors in Organ Transplant Patients. J Immunother. 2017;40:277–281. doi: 10.1097/CJI.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 74.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 75.Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107–111. doi: 10.1093/jjco/hyp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kornberg A, Küpper B, Tannapfel A, Katenkamp K, Thrum K, Habrecht O, Wilberg J. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol. 2010;36:275–280. doi: 10.1016/j.ejso.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Sapisochin G, Goldaracena N, Astete S, Laurence JM, Davidson D, Rafael E, Castells L, Sandroussi C, Bilbao I, Dopazo C, et al. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann Surg Oncol. 2015;22:2286–2294. doi: 10.1245/s10434-014-4273-6. [DOI] [PubMed] [Google Scholar]
- 78.Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann Surg. 2017;266:118–125. doi: 10.1097/SLA.0000000000001894. [DOI] [PubMed] [Google Scholar]
- 79.Fernandez-Sevilla E, Allard MA, Selten J, Golse N, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Adam R. Recurrence of hepatocellular carcinoma after liver transplantation: Is there a place for resection? Liver Transpl. 2017;23:440–447. doi: 10.1002/lt.24742. [DOI] [PubMed] [Google Scholar]
- 80.Lee JG, Kang CM, Park JS, Kim KS, Yoon DS, Choi JS, Lee WJ, Kim BR. The actual five-year survival rate of hepatocellular carcinoma patients after curative resection. Yonsei Med J. 2006;47:105–112. doi: 10.3349/ymj.2006.47.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sommacale D, Dondero F, Sauvanet A, Francoz C, Durand F, Farges O, Kianmanesh R, Belghiti J. Liver resection in transplanted patients: a single-center Western experience. Transplant Proc. 2013;45:2726–2728. doi: 10.1016/j.transproceed.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 82.Chok KSh. Management of recurrent hepatocellular carcinoma after liver transplant. World J Hepatol. 2015;7:1142–1148. doi: 10.4254/wjh.v7.i8.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marangoni G, Faraj W, Sethi H, Rela M, Muiesan P, Heaton N. Liver resection in liver transplant recipients. Hepatobiliary Pancreat Dis Int. 2008;7:590–594. [PubMed] [Google Scholar]
- 84.Huang J, Yan L, Wu H, Yang J, Liao M, Zeng Y. Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation? J Surg Res. 2016;200:122–130. doi: 10.1016/j.jss.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 85.Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–107. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanuki N, Takeda A, Kunieda E. Role of stereotactic body radiation therapy for hepatocellular carcinoma. World J Gastroenterol. 2014;20:3100–3111. doi: 10.3748/wjg.v20.i12.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3:345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parker JJ, Jones JC, Strober S, Knox SJ. Characterization of direct radiation-induced immune function and molecular signaling changes in an antigen presenting cell line. Clin Immunol. 2013;148:44–55. doi: 10.1016/j.clim.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, Schwendener R, Pruschy M, Knuth A, van den Broek M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–566. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 90.Ng J, Dai T. Radiation therapy and the abscopal effect: a concept comes of age. Ann Transl Med. 2016;4:118. doi: 10.21037/atm.2016.01.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, Henderson MA, Schefter TE, Tudor K, Deluca J, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218–225. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 92.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HR. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 94.Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, Bae SH, Jung DH, Kim KB, Lee DH, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424–5431. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- 95.Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD 3rd, Dorfman GS, Eng C, Fong Y, Giusti AF, Lu D, Marsland TA, Michelson R, Poston GJ, Schrag D, Seidenfeld J, Benson AB 3rd. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- 96.Garrean S, Hering J, Saied A, Helton WS, Espat NJ. Radiofrequency ablation of primary and metastatic liver tumors: a critical review of the literature. Am J Surg. 2008;195:508–520. doi: 10.1016/j.amjsurg.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 97.Rim CH, Yang DS, Park YJ, Yoon WS, Lee JA, Kim CY. Effectiveness of high-dose three-dimensional conformal radiotherapy in hepatocellular carcinoma with portal vein thrombosis. Jpn J Clin Oncol. 2012;42:721–729. doi: 10.1093/jjco/hys082. [DOI] [PubMed] [Google Scholar]
- 98.Xi M, Zhang L, Zhao L, Li QQ, Guo SP, Feng ZZ, Deng XW, Huang XY, Liu MZ. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One. 2013;8:e63864. doi: 10.1371/journal.pone.0063864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pompili M, Mirante VG, Rondinara G, Fassati LR, Piscaglia F, Agnes S, Covino M, Ravaioli M, Fagiuoli S, Gasbarrini G, et al. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: Assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11:1117–1126. doi: 10.1002/lt.20469. [DOI] [PubMed] [Google Scholar]
- 100.Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, Sarli D, Schiavo M, Garbagnati F, Marchianò A, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900–909. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ko HK, Ko GY, Yoon HK, Sung KB. Tumor response to transcatheter arterial chemoembolization in recurrent hepatocellular carcinoma after living donor liver transplantation. Korean J Radiol. 2007;8:320–327. doi: 10.3348/kjr.2007.8.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou B, Shan H, Zhu KS, Jiang ZB, Guan SH, Meng XC, Zeng XC. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol. 2010;21:333–338. doi: 10.1016/j.jvir.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 103.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 104.Hwang S, Kim YH, Kim DK, Ahn CS, Moon DB, Kim KH, Ha TY, Song GW, Jung DH, Kim HR, et al. Resection of pulmonary metastases from hepatocellular carcinoma following liver transplantation. World J Surg. 2012;36:1592–1602. doi: 10.1007/s00268-012-1533-0. [DOI] [PubMed] [Google Scholar]
- 105.Aoki M, Hatayama Y, Kawaguchi H, Hirose K, Sato M, Akimoto H, Miura H, Ono S, Takai Y. Stereotactic body radiotherapy for lung metastases as oligo-recurrence: a single institutional study. J Radiat Res. 2016;57:55–61. doi: 10.1093/jrr/rrv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys. 2008;71:652–665. doi: 10.1016/j.ijrobp.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 107.Chang EL, Shiu AS, Mendel E, Mathews LA, Mahajan A, Allen PK, Weinberg JS, Brown BW, Wang XS, Woo SY, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151–160. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 108.Owen D, Laack NN, Mayo CS, Garces YI, Park SS, Bauer HJ, Nelson K, Miller RW, Brown PD, Olivier KR. Outcomes and toxicities of stereotactic body radiation therapy for non-spine bone oligometastases. Pract Radiat Oncol. 2014;4:e143–e149. doi: 10.1016/j.prro.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ahmed KA, Stauder MC, Miller RC, Bauer HJ, Rose PS, Olivier KR, Brown PD, Brinkmann DH, Laack NN. Stereotactic body radiation therapy in spinal metastases. Int J Radiat Oncol Biol Phys. 2012;82:e803–e809. doi: 10.1016/j.ijrobp.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 110.Lee E, Kim TG, Park HC, Yu JI, Lim DH, Nam H, Lee H, Lee JH. Clinical outcomes of stereotactic body radiotherapy for spinal metastases from hepatocellular carcinoma. Radiat Oncol J. 2015;33:217–225. doi: 10.3857/roj.2015.33.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoo GS, Park HC, Yu JI, Lim DH, Cho WK, Lee E, Jung SH, Han Y, Kim ES, Lee SH, et al. Stereotactic ablative body radiotherapy for spinal metastasis from hepatocellular carcinoma: its oncologic outcomes and risk of vertebral compression fracture. Oncotarget. 2017;8:72860–72871. doi: 10.18632/oncotarget.20529. [DOI] [PMC free article] [PubMed] [Google Scholar]


