Abstract
Background:
Comprehensive examinations of placental metal concentrations and correlations with infant parameters are under-investigated. Chattanooga, Tennessee’s consistently high incidence of low birth weight and potential for metal exposure provides an ideal opportunity to investigate potential correlations.
Objectives:
To investigate the associations between a wide variety of metals in placental tissue and multiple infant parameters.
Methods:
A total of 31 elements were screened via ICP-MS in 374 individual placental samples. Of those, 14 were quantifiable in >86% of the samples. We examined correlations between metal concentrations and infant parameters (birth weight, gestational age, birth weight centile, placental weight, birth length and head circumference). We fit multivariable regression models to estimate the covariate-adjusted associations of birth weight with ln-transformed concentrations of each of the 14 metals and used generalized additive models to examine nonlinear relationships.
Results:
Some of the strongest relationships with infant parameters came from several lesser-studied metals. Placental rhodium concentrations were negatively correlated with almost all infant parameters. In the fully adjusted regression model, birth weight was significantly associated with several metals. On an IQR (25th to the 75th percentile) basis, estimated changes in birthweight were: for cobalt (82.5 g, IQR=6.05 µg/kg, p = 0.006), iron (−51.5 g, IQR=171800 µg/kg, p = 0.030), manganese (−27.2 g, IQR=152.1 µg/kg, p = 0.017), lead (−72.7 g, IQR=16.55 µg/kg, p = 0.004) and rhodium (−1365.5 g, IQR=0.33 µg/kg, p < 0.001). Finally, a generalized additive model showed significant nonlinear relationships between birth weight and concentrations of Co and Rh.
Conclusions:
Our comprehensive examination of placental metals illustrate many strong associations between lesser-studied metals and infant parameters. These data, in combination with our correlations of well-studied metals, illustrate a need to consider in utero exposure to a broad array of metals when considering fetal development.
Keywords: birth weight, placenta, metals, rhodium, lead, manganese
Introduction
Singleton, full-term infants are classified as low birth weight (LBW) if weighing less than 2500 g and very low birth weight (VLBW) if weighing less than 1500 g. Birth weight is well known to correlate with infant mortality and myriad adult health indicators. Infants born on the lower end of the normal birth weight range also have an increased risk of adult chronic diseases, such as heart disease and type 2 diabetes (Barker et al. 1989; Yajnik et al. 1995). Hamilton County, TN, has a persistently high LBW rate. According to the Hamilton County Health Department (Fagerstedt et al. 2015), 27 of the 28 zip code areas in Hamilton County have an incidence of LBW infants that is consistently 50% higher than the national average. Race alone does not explain these observations. For example, white Hamilton County (HC) infants are consistently born LBW at a greater incidence than white United States (US) infants; similarly, black HC infants are consistently born LBW at a greater incidence than white or black US infants (Hamilton County Health Department 2015). While many factors are known to contribute to LBW (e.g., diet, smoking, access to health care, poverty) (Krans and Davis 2014; Lagiou et al. 2004; Magee et al. 2004; Miller and Korenman 1994), environmental pollution is another potential causative factor. Indeed, the potential for metal and metalloid intrauterine exposure must be understood for proper mitigation of LBW/VLBW.
Prior to environmental regulations, metal foundry sand was commonly used as fill dirt for Chattanooga area home construction which ultimately resulted in a large portion of Chattanooga residential soils being named a Superfund Site (EPA 2018). Foundry sand is often contaminated with multiple metals. For example, the EPA conducted a risk assessment of foundry sand from multiple types of metal smelting (Aluminum, Iron, Steel (EPA 2014)). That risk assessment focused on an array of metals and metalloids: Antimony (Sb), Arsenic (As), Beryllium (Be), Cadmium (Cd), Chromium (Cr), Cobalt (Co), Copper (Cu), Iron (Fe), Lead (Pb), Manganese (Mn) and Nickel (Ni), as these were the most commonly associated with all types of metal smelting. Because Hamilton County has a high incidence of LBW and because the majority resides in Chattanooga among widespread legacy foundry sand, exposure to co-occurring metals needs to be considered as potential contributors to LBW in Hamilton County, TN.
The placenta is a temporary organ that provides for the exchange of nutrients and wastes between fetus and mother. Along with this vital exchange, there is the potential for the passage of deleterious compounds from mother to fetus for intrauterine exposure. As such, placentae can serve as a non-invasive, long-term (gestational length) sample that can be studied for transplacental toxicant transfer (Iyengar and Rapp 2001). Several studies have addressed these questions of placental transfer by evaluating the ratio of metal concentration between fetal and maternal blood supply. The higher the ratio, the greater the passage of the metal through the placenta. Sakamoto (2010) found Cd had the lowest correlation coefficient of maternal and fetal blood supplies. Other metals measured had increased passage through the placenta (Se < Pb < As), with the greatest correlation between maternal and fetal blood samples being mercury. Similarly, Rudge et al. (2009) saw strong correlations between lead, cobalt, arsenic and selenium between maternal and fetal blood samples, while cadmium, manganese, copper and zinc have a decreased pathway through the placenta, suggesting tighter regulation.
Correlations of placental metal concentrations with birth weight are well documented. Osada (2002) quantified essential elements that are required for proper growth (magnesium (Mg), manganese, iron (Fe), copper (Cu), zinc (Zn), and selenium (Se)) and elements that are non-essential (rubidium (Rb), strontium (Sr), cadmium (Cd), cesium (Cs)) in placenta tissue of 30 appropriate for gestational age (AGA) infants and 21 intrauterine growth restricted (IUGR) infants in Japan. Se and Mg were significantly greater in the AGA infants compared to the IUGR infants. Zadrozna (2009) reported significantly greater placental concentrations of Zn and Cu in normal pregnancies compared to pregnancies complicated by IUGR. Both of these metals also had a significant, positive correlation with birth weight. In the same study however, Se was greater in placenta from IUGR cases compared to a control group. Llanos and Ronco (2009) reported lower birth weight as Cd, Pb, and As concentrations increased in the placenta. Ronco et al. (2009) also found that Cd exposure directly caused reduced birth weight in rats and depressed the transfer of Zn (a beneficial metal) across the placenta. Greater Pb concentrations were reported in placentae from associated with IUGR infants (Llanos and Ronco 2009). In addition, lead (Pb) has been found to readily pass through the placenta and previous work has shown that cord blood Pb concentrations were negatively correlated with birth weight, length and head circumference (Osman et al. 2000).
Several metals, including rhodium (Rh), barium (Ba), and aluminum (Al) are under reported in placenta tissue. Rh has been detected globally in urban environments, surface water, river sediment, freshwater isopods, well-water and many other environmental matrices (Gómez et al. 2001; Ravindra et al. 2004). To date, there are no published data on Rh in placental tissue. Sharma and Mishra (2006) studied the effects of Al on pregnant rats and showed that oral exposure to Al resulted in uptake to fetal and maternal brain tissues, as well as placental tissues. Subsequently, birth weight, placental weight and fetal growth were all significantly reduced compared to the control group. Al and Ba were detected in amniotic fluid (16–19 weeks gestation), at an average of 93.4 and 22.4 ug/L, respectively (Hall et al. 1983). Ba has also been detected in stillborn babies, which indicates passage through the placental barrier (IPCS 1990), as well as reduced birth weight in rats given an oral dose of Ba (ATSDR 2007).
To that end, the present study quantified and compared concentrations of 14 elements against birth weight (male and female births separately) as well as gestational age, birth weight centile (BWC), placental weight, birth length, and head circumference. In the absence of complete information about the effects of these 14 metals on birth outcomes, we consider this a hypothesis generating study.
Methods:
Placental collection was conducted at Erlanger Hospital (Baroness campus), Chattanooga, TN from singleton births of HIV and hepatitis negative mothers over 18 years of age. Infants born at or greater than 34 weeks, with no major morphological or chromosomal abnormalities, were included in the sample population. Over the study period, 690 women were asked to participate in the study. From those requests, 665 placentae were successfully collected across a large spectrum of pregnancies. When processing samples for elemental analysis, samples from normal weight births were a random subset of a larger population (n = 292). Because low birth weight samples were the minority of the births, all LBW placentae above the gestational date cutoff were processed (n= 82). Maternal and infant data were collected from questionnaire interviews and medical records. All specimen and data collection methods were approved by the UT College of Medicine Institutional Review Board (#05–031, FWA# 2301). As samples became available, consent was sought out and obtained within the day, or in the case of a night delivery, first thing the following morning. In the rare case the consent was not obtained or refused to be given, the samples were discarded. Post-delivery, placentae were refrigerated in a plastic container. Placentae were collected after no more than 12 hrs after delivery. After umbilical cord and amniotic sac were removed with ceramic scissors, the placenta was weighed, cut in half and placed in a sterile plastic bag and frozen at −20□ C until processing (as illustrated in Figure 1). The placental half was thawed and both fetal and maternal membranes (decidua basalis and chorionic plate, respectively) were removed, which allowed for sampling of the syncytiotrophoblast layers to be maximized, the area of fetal/maternal exchange (Sun et al. 1997). Because the spatial distribution of metals through the placenta is not uniform (Lagerkvist et al. 1996) the entire sample was homogenized and subsampled for metal analysis (Miller et al. 1988). Following tissue digestion, 14 elements were consistently quantified: aluminum (Al), arsenic (As), barium (Ba), cadmium (Cd), cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), lead (Pb), rhodium (Rh), selenium (Se), titanium (Ti), and zinc (Zn). Placental metal concentrations were compared between normal and low birth weight infants as well as between BWC groupings (small, average and large for gestation).
Figure 1.
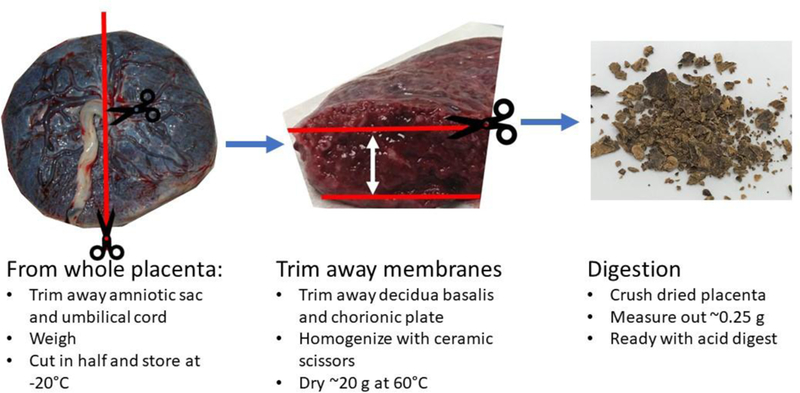
Placenta processing procedure.
Birth Weight Centile Calculation
An assessment based on infant weight alone ignores other important variables that influence birth weight, including gestational age at delivery, maternal height and weight, parity, ethnicity and infant sex (Gardosi et al. 1992). With the incorporation of these factors, infants can be grouped according to an individualized birth weight centile that can provide a more nuanced assessment of whether or not fetal growth met the expectations of the pregnancy. The BWC is grouped as small for gestational age (< 10th centile), average (11th-89th centile), or large for gestational age (>90th centile, SGA, AGA, LGA). GROW software was obtained for the United States coefficients (Gardosi and Francis 2009). Data for the gestational age adjustment (parity, maternal height/weight, ethnicity, infant sex, and weight) were obtained from the associated medical charts. Gestational age was based on best obstetric estimate that incorporated the use of first trimester ultrasound that was within +/− 3 days of last missed period (LMP) or a second trimester (< 20 weeks gestation) that was +/− 7 days of LMP. Although calculating a customizable birth weight centile has some limitations and adds complexity (Hutcheon 2014), we feel that the multi-ethnic population of Chattanooga merits these added measures of fetal growth and ultimately birth weight (Gardosi et al. 2018; Melamed et al. 2014).
Elemental Analysis
Placental concentration of each element was quantified at the Laboratorio Chimico Merceologico (Naples, Italy) by means of quadrupole inductively coupled plasma-mass spectrometry, ICP-MS (model 820-MS, Bruker Daltonics, Billerica, MA), equipped with a collision reaction interface (CRI) for suppression of polyatomic, isobaric interferences. The CRI utilized high purity He (99.9999%, SALDOGAS Srl, Italy) during analysis of Cr and high purity H2 (99.9999%, using DBS H2 generator PGH2–300) for all other elements. The plasma was generated using an RF power of 1400 W and the nebulizer gas flow rate was 0.98 L/min. All reagents were Optima grade (Fisher Scientific) and 18.2 MΩ·cm water was produced from a Direct Q3 Milli-Q system (Millipore, Bedford, MA). All 374 placentae samples were analyzed for Al, As, Ba, Be, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, Pb, Sb, Se, Tl, U, V, Zn in quantitative mode using external calibration. Multi-elemental standard stock solutions of 20 mg/L (Ultra Scientific, North Kingstown, USA) were used to prepare 10 calibration solutions ranging from 0.1 – 1000 µg/L for all elements except Hg (3-point calibration; range 0.1 – 10 µg/L). An internal standard (10 µg/L 89Y) was used for signal normalization and added via peristaltic pump to each standard and sample during analysis. Calibration curves used for quantitation were constructed by plotting internal standard-normalized signal intensities versus standard concentration and all had R2 values ≥ 0.999. After every 10 samples, a reagent blank and at least 2 standard samples were analyzed to verify instrument calibration. Analyses were stopped and the instrument re-calibrated if the blanks or standard samples differed by ±15% of the correct value. Additionally, Re, Ta, Rh, Ru, Ti, Ge, Pd, In, I, W, Os, Pt were measured in semi-quantitative mode using an average response factor instead of a calibration curve. Combined, a total of 31 elements were analyzed in placenta tissue.
Prior to and after each analytical session, bovine liver standard reference material (NIST SRM 1577c) was analyzed in order to assess the accuracy of analysis. The results were deemed to be reliable if the measured value was within the quoted uncertainty of the certified value for the SRM. Throughout the analytical campaign, a total of 23 SRM samples were digested, processed, and analyzed exactly as the samples. Concentrations determined with this method were in good agreement for all elements certified by NIST (As, Cd, Co, Cu, Fe, Mn, Ni, Pb, Se, V, Zn). Samples were prepared as follows. Before use, all glassware and plastic containers were cleaned by washing with 5% nitric acid for at least 24 h, and then copiously rinsed with ultra-pure water. The chorionic plate and decidua basalis were removed with acid-washed ceramic scissors, leaving the villous core tissue to homogenize. Samples were oven-dried at 60°C to constant weight and then powderized. As a result, all concentration data are reported on a dry-weight basis. Aliquots of dried placenta with nominal mass 0.25 g, determined to nearest 0.1 mg, were placed in acid-rinsed, 50 mL polypropylene digestion vessels. Hot plate digestion was carried out with a total of 5 mL of concentrated nitric acid and 3 mL of 30% hydrogen peroxide. Following digestion to a clear, yellow solution, samples were dried down and reconstituted to 10 mL with 2% nitric acid. Finally, samples were 0.2 um filtered, using a Teflon filter, prior to ICP-MS analysis.
Statistical Analysis
Statistical analyses were performed using R version 3.3.2. One infant had a birth weight of only 1275 g despite a gestational age of 275 days (39.3 weeks), which was very unlike all other subjects. This subject, the only infant of VLBW, was excluded from all statistical summaries and models, and consequently our model results only apply to normal or LBW infants. Correlations between birth weight, BWC, gestational age, head circumference, birth length and placental weight, and the log-transformed detectable metals were analyzed using Pearson’s correlation. Negative correlations indicate that the magnitude of a given outcome (e.g., birth weight) decreases as the element concentration increases, whereas positive correlations indicate that the outcome tends to increase as element concentration increases.
The distribution of the raw metal concentrations is summarized in Table 1, and median metal values within birth weight and birth weight centiles is summarized in Table 6. In all other analyses, 1 was added to the metal concentrations and then converted to the natural logarithm (Wicklin 2011). These values are referred to as the ln-transformed concentrations, for simplicity. The addition of 1 prior to the log-transform was necessary to reduce the influence of extremely small metal concentrations. To examine the relationship between birth weight and metal concentrations, separate multiple regression models were used for each metal, controlling for the following covariates: maternal pre-pregnancy BMI, maternal age, and gestational age as continuous variables, as well as three categorical covariates: race (white or minority), infant sex, and smoking (yes or no). Model assumptions were checked using standard methods (Weisberg 2005). Linearity was assessed by partial regression plots and by plots of studentized residuals against the predicted values, and in some cases nonlinearity was noted. There was no evidence of heteroscedastisticy, as assessed by plots of studentized residuals versus unstandardized predicted values. There was no evidence of multicollinearity, as assessed by small variance inflation factors (all were < 1.1). Q-Q plots suggested that the assumption of normality was reasonable. Unusual data points were evaluated for via Cook’s distance, and studentized residuals. After exclusion of the VLBW subject (noted above), there were no very unusual values (all studentized residuals were < 3.5 in absolute value, and all Cook’s distances were < 0.1). Due to the violation of linearity in some cases, generalized additive models (Hastie and Tibshirani 1990) were fit to evaluate possible nonlinearity in the relationships between birth weight and the ln-transformed metal concentrations, while controlling for the same covariates of the aforementioned multivariable regressions.
Table 1:
Dry-weight basis elemental concentrations (μg/kg) in placenta tissue collected from Hamilton County, TN (n=374).
| Element | Mean | Standard Deviation | Median | 25% | 75% | I.Q.R. | Min. | Max. | Detection Limit a | Detection Limit (ug/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Al | 1080 | 1990 | 385.3 | 120.9 | 1061 | 940.1 | 1.800 | 1474 0 |
3.6 | 0.09 |
| As | 43.01 | 15.03 | 42.14 | 33.66 | 52.79 | 19.13 | 1.800 | 105 | 3.6 | 0.09 |
| Ba | 91.62 | 146.0 | 47.41 | 26.67 | 93.08 | 66.41 | 1.200 | 1657 | 2.4 | 0.06 |
| Cd | 18.54 | 11.99 | 15.81 | 10.40 | 23.80 | 13.4 | 1.200 | 84.22 | 2.4 | 0.06 |
| Co | 8.890 | 12.72 | 6.600 | 3.600 | 9.650 | 6.05 | 1.400 | 158.3 | 2.8 | 0.07 |
| Cu | 3889 | 2480 | 3413 | 3001 | 3923 | 922 | 1356 | 2056 0 |
2 | 0.05 |
| Fe | 5032 00 |
232800 | 46350 0 |
3809 00 |
5527 00 |
1718 00 |
1751 00 |
1848 00 |
4.4 | 0.11 |
| Mn | 346.2 | 228.0 | 318.2 | 249.6 | 401.7 | 152.1 | 1.800 | 1774 | 3.6 | 0.09 |
| Ni | 111.0 | 219.5 | 60.50 | 23.79 | 117.5 | 93.71 | 1.600 | 2886 | 3.2 | 0.08 |
| Pb | 37.97 | 270.5 | 12.03 | 6.680 | 23.23 | 16.55 | 0.600 0 |
5073 | 1.2 | 0.03 |
| Rh | 0.390 | 0.190 | 0.380 | 0.220 | 0.550 | 0.33 | 0.060 0 |
0.780 | 0.0008 | 0.00002 |
| Se | 1504 | 383.0 | 1442 | 1329 | 1584 | 255 | 566.3 | 3773 | 2.4 | 0.06 |
| Ti | 381.7 | 77.21 | 380.7 | 315.4 | 448.4 | 133 | 238.0 | 532.7 | 0.0032 | 0.00008 |
| Zn | 5512 0 |
24120 | 50180 | 4587 0 |
5548 0 |
9610 | 1891 0 |
3325 00 |
5.6 | 0.14 |
Assuming 0.25 g of sample
Table 6:
Comparison of dry-weight basis placental element concentration to birth weight and birth weight centile groupings (LGA, AGA, SGA). Median values and interquartile range (in parentheses) for untransformed metal values are reported in µg/kg (n=374).
| Birth Weight | Birth Weight Centile | ||||
|---|---|---|---|---|---|
| Low | Normal | SGAa | AGA | LGA | |
| n | 82 | 292 | 89 | 253 | 32 |
| Al | 351.3 (970.2) | 393.4 (924.3) | 309.5 (726.4) | 409.2 (1029) | 540.8 (1085) |
| As | 43.5 (20) | 41.8 (19.3) | 41.9 (16.4) | 42.7 (20.5) | 37.7 (17.5) |
| Ba | 39.8 (47.1) | 49.6 (72.9) | 47.4 (66.6) | 47 (63.3) | 49.8 (104.1) |
| Cd | 16.3 (12.5) | 15.4 (14.1) | 18 (14.5) | 14.6 (13.4) | 13.8 (9.8)* |
| Co | 5 (5.5) | 6.8 (6.1)* | 4.9 (7.1) | 6.9 (5.9) | 8.3 (4.2)* |
| Cu | 3281 (625.3) | 3489 (955.8) | 3282 (821.5) | 3447 (923.8) | 3696 (1110) |
| Fe | 475800 (185000) | 460500 (160200) | 482400 (179600) |
453000 (165000) |
459800 (168200) |
| Mn | 332.9 (149.2) | 314.5 (145.7) | 335.7 (153) | 313.8 (143) | 329.1 (133.6) |
| Ni | 58.9 (108.9) | 60.7 (88.6) | 53.4 (90.3) | 62.2 (91.1) | 43.3 (55.3) |
| Pb | 11.5 (13.6) | 12.2 (17.2) | 15.1 (14.8) | 11.4 (16) | 12.7 (17.5) |
| Rh | 0.5 (0.4) | 0.4 (0.3) | 0.5 (0.3) | 0.4 (0.3) | 0.2 (0.1)*** |
| Se | 1396 (256.4) | 1445 (254.2) | 1391 (291.3) | 1449 (242.4) | 1447 (186.5) |
| Ti | 369.5 (121.4) | 382.5 (136.9) | 401.6 (116.8) | 372.3 (142.4) | 367.7 (92.5) |
| Zn | 49070 (10180) | 50690 (9008) |
48000 (10250) |
50550 (8617) | 51850 (11640)* |
Significance:
p<0.05
p<0.0001
LGA (large for gestational age, >90% BWC), AGA (average for gestational age, 11–89% BWC), SGA (small for gestational age, <10% BWC).
Results
Concentrations of metals/metalloids
A total of 31 elements were screened in 374 individual placental samples. Of those, 17 elements (Be, V, Ge, Pd, In, Sb, W, Os, Pt, Hg, Ru, Cr, U, Tl, Re, Ta, and I) were considered non-quantifiable (e.g., less than 40% of the samples contained quantifiable concentrations). Of the 14 quantifiable elements, 7 (Ti, Rh, Ba, Fe, Cu, Zn, and Se) were quantifiable in 100% of the samples while the other 9 were quantifiable in >86% of the samples (Al, As, Cd, Co, Mn, Ni, Pb,) (Table 1). For all placental samples containing one of the 14 metals at non-quantifiable concentrations, half of the detection limit was inserted as the concentration value (EPA 2000). This was done to provide a conservative estimate for those elements present at concentrations near the detection limit (EPA 2000). Descriptive statistics of the quantifiable metals present in placental samples are summarized in Table 1. Maternal descriptive statistics are summarized in Table 2; infant descriptive statistics are summarized in Table 3.
Table 2.
Descriptive statistics of maternal parameters associated with placentae from Hamilton County, TN (n=374).
|
Maternal characteristic |
N | % |
| Race | ||
| Caucasian | 225 | 60 |
| African Am. | 86 | 23 |
| Latina | 61 | 16 |
| Other | 2 | 1 |
| Maternal age (years) | ||
| 18–24 | 172 | 46 |
| 25–29 | 110 | 30 |
| 30–34 | 59 | 16 |
| 35+ | 33 | 9 |
| Cigarette/day | ||
| Non-smoking | 287 | 77 |
| Smoking | 87 | 23 |
| Delivery | ||
| C-section | 131 | 35 |
| SVD | 243 | 65 |
| Marital Status | ||
| Married | 194 | 52 |
| Single | 180 | 48 |
| Pre-pregnancy BMI | ||
| Underweight (≤18.5) | 16 | 4 |
| Average (18.5–24.9) | 168 | 45 |
| Overweight (25–29.9) | 101 | 27 |
| Obese (≥3 ) | 89 | 24 |
| Education | ||
| < high school | 105 | 28 |
| HS/GED | 104 | 28 |
| any college | 123 | 32 |
| Unknown | 42 | 11 |
| Parity | ||
| 0 | 115 | 30 |
| 1 | 138 | 37 |
| 2 | 67 | 18 |
| 3+ | 54 | 15 |
| Gestational Age | ||
| 40+ weeks | 43 | 11 |
| 40–37 weeks | 230 | 62 |
| 34–36 weeks | 101 | 27 |
Table 3.
Descriptive statistics of infant parameters associated with placentae from Hamilton County, TN.
| Mean | SD | Median | Minimum | Maximum | Range | n | |
|---|---|---|---|---|---|---|---|
| Birth Weight (g) |
2969 | 577.9 | 2942 | 1655 | 4472 | 2817 | 374 |
| Birth Weight Centile (%) |
38.4 | 30.8 | 30.8 | 0.1 | 100 | 99.9 | 374 |
| Placental Weight (g) |
576.6 | 135.7 | 562 | 306 | 1005 | 699 | 238a |
| Head Circumference (cm) |
34.6 | 17.4 | 33 | 13.5 | 337 | 324 | 317 a |
| Birth Length (cm) |
49.3 | 3.8 | 50 | 20 | 59.3 | 39.3 | 324 a |
| Gestational Age (days) |
266 | 13 | 268 | 236 | 299 | 63 | 374 |
| Maternal Age (years) |
25.9 | 5.5 | 25 | 18 | 47 | 29 | 374 |
Data missing from placental weight, head circumference and birth length compared to the original sample size due to incomplete clinical records.
Correlations Between Metal/Metalloid Concentrations and Infant Outcomes
A Pearson’s correlation matrix (Table 4) of transformed placental concentrations shows that most of the metal pairs are positively correlated. Only Rh and Zn were significantly negatively correlated. Metals quantified in placental tissue have been reported before with an accompanying correlation matrix, like those in Table 4 (Punshon et al. 2016). Pearson’s correlation were calculated between transformed placental metal concentrations and all infant outcomes (Table 5). Rh, Mn and Pb were all significantly negatively correlated with birth weight. Rh was significantly negatively correlated with BWC. Rh, Mn, and Cd were all significantly negatively correlated with birth length. Rh and Mn were all significantly negatively correlated with head circumference. Cu, Fe, Se, Rh, Mn, Pb, Al and Cd were all significantly negatively correlated with placental weight. The fetal/placental ratio, generally considered a measure of placental efficiency (larger placenta size is theorized to compensate for exchange inefficiencies/defects), was significantly positively correlated with Al, Cd, Co, Cu, Fe, Se, Zn. No metal concentrations significantly correlated with gestational age. The distribution of each metal/metalloid concentration was further grouped on the basis of birth weight and birth weight centile (Table 6). The median Co placental concentration was significantly smaller in LBW infants. Cd, Co, Rh, and Zn were significantly different across BWC groupings.
Table 4:
Pearson’s correlation matrix (r-values) between 14 ln-metals (defined as the natural logarithm of 1 + the metal concentration in placental tissues) (n = 374).
| Al | As | Ba | Cd | Co | Cu | Fe | Mn | Ni | Pb | Rh | Se | Ti | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | 1 | |||||||||||||
| As | 0.00 | 1 | ||||||||||||
| Ba | 0.10 | −0.03 | 1 | |||||||||||
| Cd | 0.15* | 0.03 | 0.06 | 1 | ||||||||||
| Co | 0.2* | −0.01 | 0.14* | 0.31* | 1 | |||||||||
| Cu | 0.45* | −0.04 | 0.14* | 0.32* | 0.43* | 1 | ||||||||
| Fe | 0.27* | 0.00 | 0.08 | 0.18* | 0.22* | 0.62* | 1 | |||||||
| Mn | 0.35* | −0.06 | 0.09 | 0.27* | 0.16* | 0.60* | 0.29* | 1 | ||||||
| Ni | 0.06 | −0.09 | 0.06 | 0.14* | 0.20* | 0.37* | 0.26* | 0.27* | 1 | |||||
| Pb | 0.26* | −0.08 | 0.19* | 0.21* | 0.12* | 0.41* | 0.29* | 0.43* | 0.07 | 1 | ||||
| Rh | −0.01 | −0.05 | −0.08 | 0.01 | −0.06 | −0.02 | 0.02 | 0.04 | −0.02 | 0.00 | 1 | |||
| Se | 0.24* | 0.01 | 0.12* | 0.27* | 0.40* | 0.70* | 0.43* | 0.23* | 0.31* | 0.08 | −0.02 | 1 | ||
| Ti | 0.00 | −0.04 | −0.01 | −0.02 | 0.00 | −0.02 | −0.05 | −0.02 | −0.09 | 0.05 | 0.09 | 0.00 | 1 | |
| Zn | 0.34* | −0.05 | 0.31* | 0.26* | 0.53* | 0.69* | 0.42* | 0.35* | 0.41* | 0.20* | −0.11* | 0.65* | −0.01 | 1 |
Significant correlations:
p<0.05
Table 5:
Pearson’s correlation coefficients (r-value) between ln-metal concentrations and birth weight, BWC, gestational age, length, head circumference and placental weight.
| Birth Weight (g) | Birth Weight Centile (%) | Gestational Age (days) | Birth Length (cm) | Head Circumference (cm) | Placental Weight (g) | Fetal/Placental Ratio | |
|---|---|---|---|---|---|---|---|
| n | 374 | 374 | 374 | 324a | 317a | 238a | 238a |
| Al | −0.005 | 0.006 | −0.006 | −0.006 | 0.033 | −0.195** | .183** |
| As | −0.019 | −0.006 | −0.009 | −0.024 | 0.061 | −0.081 | 0.055 |
| Ba | 0.058 | 0.002 | 0.091 | 0.09 | 0.023 | 0.011 | 0.095 |
| Cd | −0.069 | −0.096 | 0.048 | −0.111* | −0.102 | −0.195** | .134* |
| Co | 0.052 | 0.043 | 0.057 | 0.027 | 0.037 | −0.023 | .189** |
| Cu | −0.071 | −0.038 | 0.001 | −0.086 | −0.048 | −0.252*** | .237** |
| Fe | −0.081 | −0.052 | 0.014 | −0.077 | −0.047 | −0.228** | .196** |
| Mn | −0.172** | −0.096 | −0.096 | −0.173** | −0.093 | −0.281*** | −0.015 |
| Ni | 0.013 | 0.033 | −0.02 | −0.004 | 0.018 | 0.049 | −0.030 |
| Pb | −0.111* | −0.077 | −0.053 | 0.019 | −0.014 | −0.135* | 0.043 |
| Rh | − 0.329*** | − 0.463*** | 0.032 | − 0.144** | −0.141* | −0.156* | −0.086 |
| Se | 0.002 | −0.001 | 0.036 | −0.019 | −0.008 | −0.179** | .220** |
| Ti | −0.044 | −0.083 | 0 | −0.055 | −0.065 | 0.029 | −0.084 |
| Zn | 0.053 | 0.062 | 0.005 | 0.008 | 0.028 | −0.108 | .265** |
Significant correlations:
p<0.05
p<0.01
p<0.0001
Data missing from placental weight, head circumference and birth length compared to the original sample size due to incomplete clinical records.
Regression analysis
We fitted multivariable regression models that adjusted for confounding variables that affect birth weight (maternal pre-pregnancy BMI, maternal age, and gestational age as continuous variables, as well as three categorical covariates: race (white or minority), infant sex, and smoking (yes or no). Of the 14 metals, 5 metals were significantly associated with birth weight after adjusting for covariates (Table 7). Increasing levels of two metals were associated with a greater birth weight. Each unit increase in ln-Co was associated with an 83.7 g (95% CI: 23.9 to 143.5) birth weight increase. Increasing levels of four metals were associated with significant decreases in birth weight. Birth weight was predicted to decrease by 138.2 g (95% CI: −263.4 to −13) per ln-increase in Fe (Figure 2a), by 57.2 g (95% CI: −104.3 to −10.1) per ln-increase in Mn (Figure 2b), by 58.3 g (95% CI: −97.9 to −18.8) per ln-increase in Pb (Figure 2c), and by −1490.3 g (95% CI: −1767.1 to −1213.5) per ln-increase in Rh (Figure 2d). These same results can also be expressed as a percent change; a 1% increase in Rhodium would result in a 14.9 gram decrease in birth weight (p < 0.001). Lead, manganese and iron were strong predictors of birth weight, decreasing birth weight by 0.58, 0.57 and 1.38 g respectively with a 1% increase in placental concentration (p < 0.030). Cobalt was significantly positively associated with birth weight, increasing birth weight 0.84 g with a 1% increase in Co placental concentration (p = 0.006). Additionally, we calculated the change in birth weight between the 25 and 75 percentile of the ln-metal distribution (Table 7). Between these two percentiles, Co increased birth weight by 82.5 g. Additionally, birth weight decreased when comparing the 25th and 75th percentiles in Fe, Mn, Pb and Rh distribution (51.1, 27.2, 72.7, and 1365.5 g respectively).
Table 7:
Summary of ln-transformed metal coefficients in models fit for birth weight (grams), controlling for the following covariates: maternal pre-pregnancy BMI, maternal age and gestational age and three additional categorical variables: race (white or minority), infant sex and smoking while pregnant (yes or no) (n=374).
| Metal | Estimated Coefficient |
95% CI | p- value |
Estimated change in BW from 25th-75th percentile |
95% CI |
|---|---|---|---|---|---|
| Al | 2.7 | (−16.5, 21.9) |
0.783 | 5.9 | (− 35.8, 47.5) |
| As | −26 | (−110, 58.1) | 0.543 | −11.7 | (− 49.5, 26.1) |
| Ba | 16.3 | (−27.8, 60.3) |
0.468 | 20.4 | (− 34.7, 75.4) |
| Cd | −38.1 | (−114.7, 38.5) |
0.328 | −31.5 | (−95, 31.9) |
| Co | 83.7 |
(23.9, 143.5) |
0.006 | 82.5 |
(23.6, 141.5) |
| Cu | −17 | (−134.5, 100.6) |
0.776 | −4.6 | (−36, 26.9) |
| Fe | −138.2 |
(−263.4,− 13) |
0.030 | −51.5 |
(− 98.1,− 4.9) |
| Mn | −57.2 |
(−104.3,− 10.1) |
0.017 | −27.2 |
(− 49.6,− 4.8) |
| Ni | 12.2 | (−18.8, 43.3) |
0.438 | 19.5 | (−30, 69.1) |
| Pb | −58.3 |
(−97.9,− 18.8) |
0.004 | −72.7 |
(−122, −23.4) |
| Rh | −1490.3 |
(−1767,− 1214) |
0.000 | −1365.5 |
(− 1629, − 1119) |
| Se | 117.6 | (−88.2, 323.4) |
0.262 | 20.7 | (− 15.5, 56.8) |
| Ti | −104.6 | (−312.5, 103.3) |
0.323 | −36.8 | (− 109.9, 36.4) |
| Zn | 154.1 | (−4.5, 312.8) |
0.057 | 29.3 | (−0.9, 59.5) |
p<0.05
p<0.01
p<0.001
Figure 2.
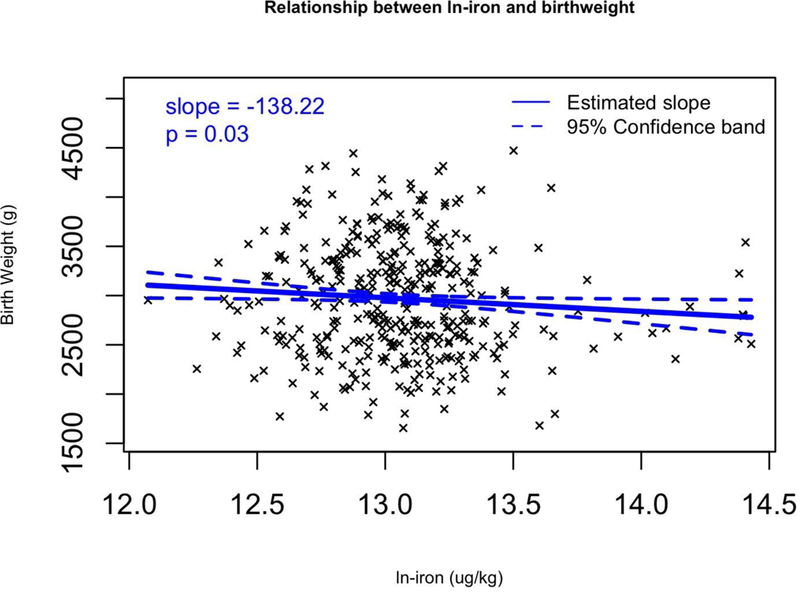
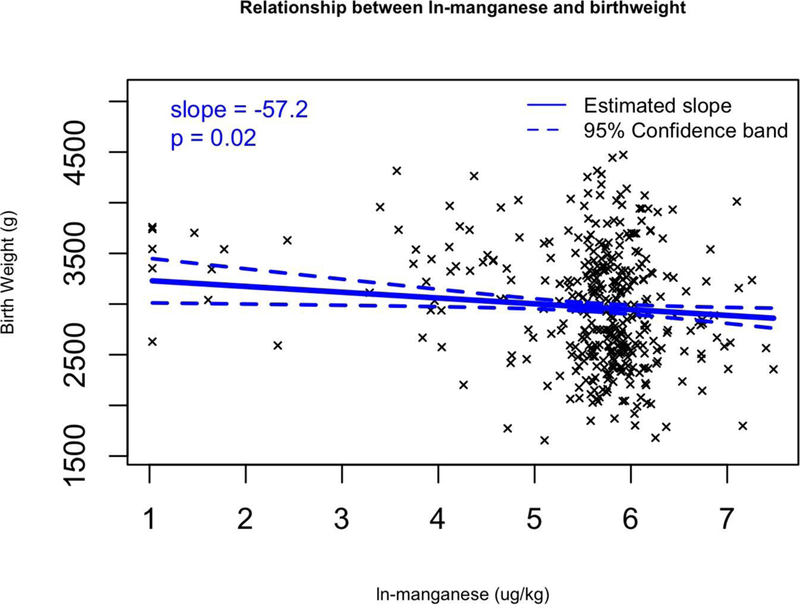
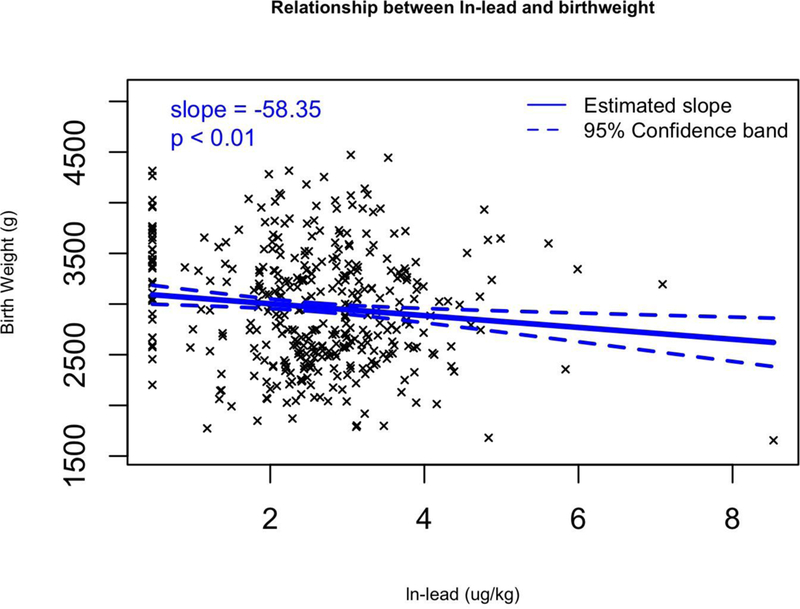
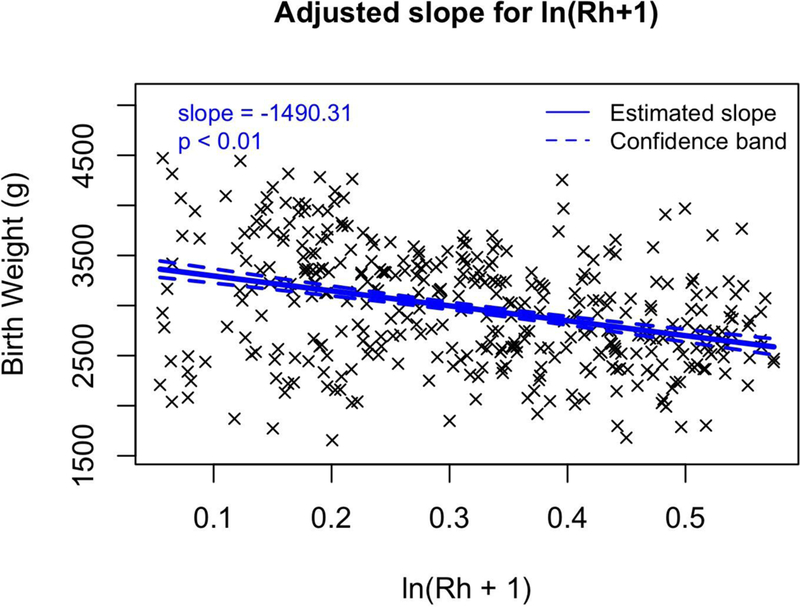
Multivariable regression models for birth weight (g) versus transformed (ln+1) metal concentrations for a) Fe, b) Mn, c) Pb, and d) Rh which all have significant slopes (β). Regression coefficients controlled for the following covariates: maternal pre-pregnancy BMI, maternal age, and gestational age as continuous variables, as well as three categorical covariates: race (white or minority), infant sex, and smoking (yes or no).
The same multivariable regression models were refit with the addition of a sex by ln-metal interaction, to understand whether the slope for metal on birthweight differed for female v. male infants. The interaction term was not significant for any of these models, suggesting that the effect of the ln-metals on birth weight was not different for female and male, and that the models that assume the same metal slope for both sexes are reasonable.
Results from generalized additive models showed that there were significant nonlinear relationships between two ln-metals and birth weight, after adjusting for covariates. For ln-Co, birth weight is predicted to increase at low levels until ln-Co is about 2.5 ug/kg (on the ln-transformed value, corresponding to an untransformed value of about 11 ug/kg), with a leveling off thereafter (df=3 for smoothing parameter; p=0.0008) for the non-linear effect) (Figure 3). For ln-Rh, birth weight was predicted to be relatively unaffected at low Rh levels, followed by a steep decrease in birth weight for ln-Rh above about 0.18 ug/kg (corresponding to an untransformed value of about 0.2 ug/kg, df=2 for smoothing parameter; p = 0.005) (Figure 4). In all models, gestational age showed a significant nonlinear relationship with birth weight. Birth weight is predicted to increase somewhat slowly for gestational age < 250 days, increase more quickly until gestational age of about 280 days, and then level off (Figure 5).
Figure 3.
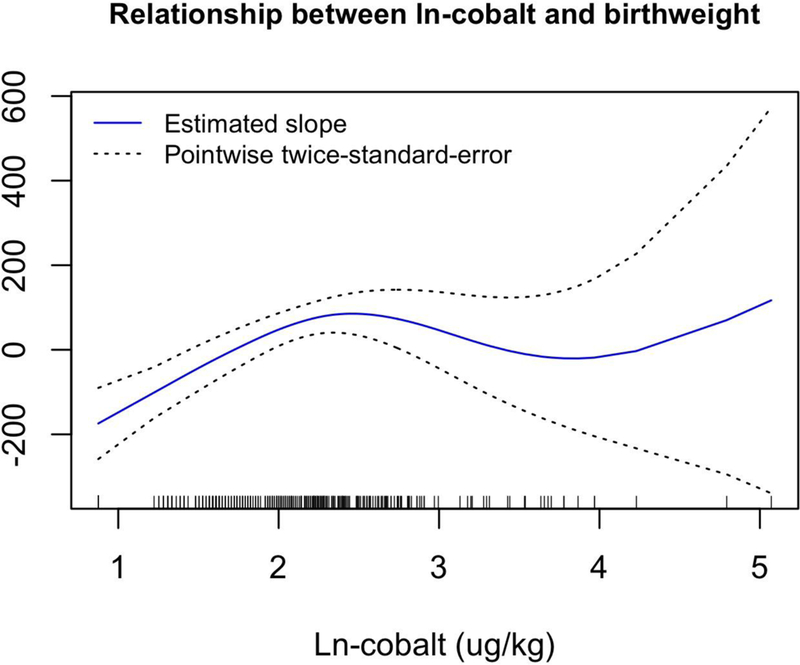
The estimated covariate-adjusted effect of log(cobalt + 1) on birthweight from a generalized additive model with 3 degrees of freedom for this relationship. Hash marks at the bottom of the plot show observed values for ln-cobalt. Regression coefficients controlled for the following covariates: maternal pre-pregnancy BMI, maternal age, and gestational age as continuous variables, as well as three categorical covariates: race (white or minority), infant sex, and smoking (yes or no).
Figure 4.
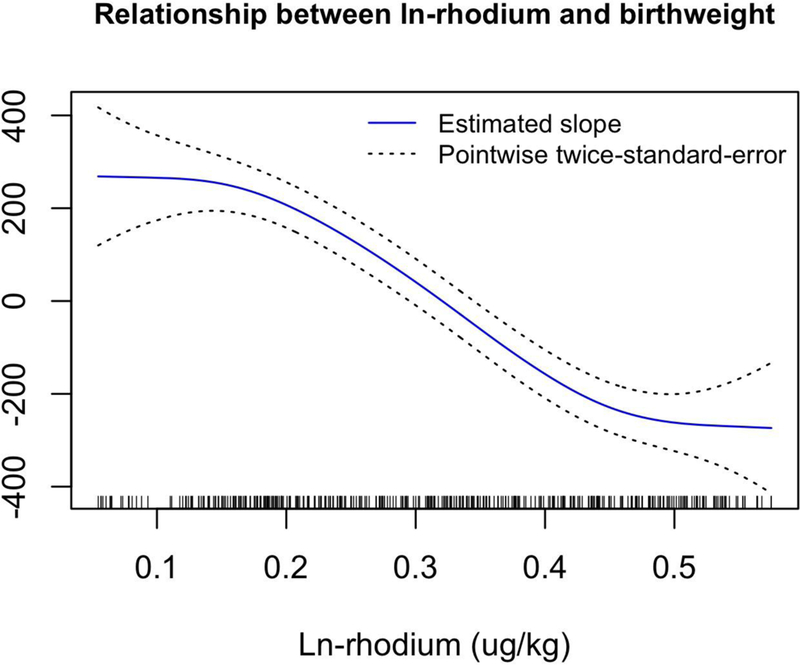
The estimated covariate-adjusted effect of log(rhodium + 1) on birthweight from a generalized additive model with 3 degrees of freedom for this relationship. Hash marks at the bottom of the plot show observed values for ln-rhodium. Regression coefficients controlled for the following covariates: maternal pre-pregnancy BMI, maternal age, and gestational age as continuous variables, as well as three categorical covariates: race (white or minority), infant sex, and smoking (yes or no).
Figure 5.
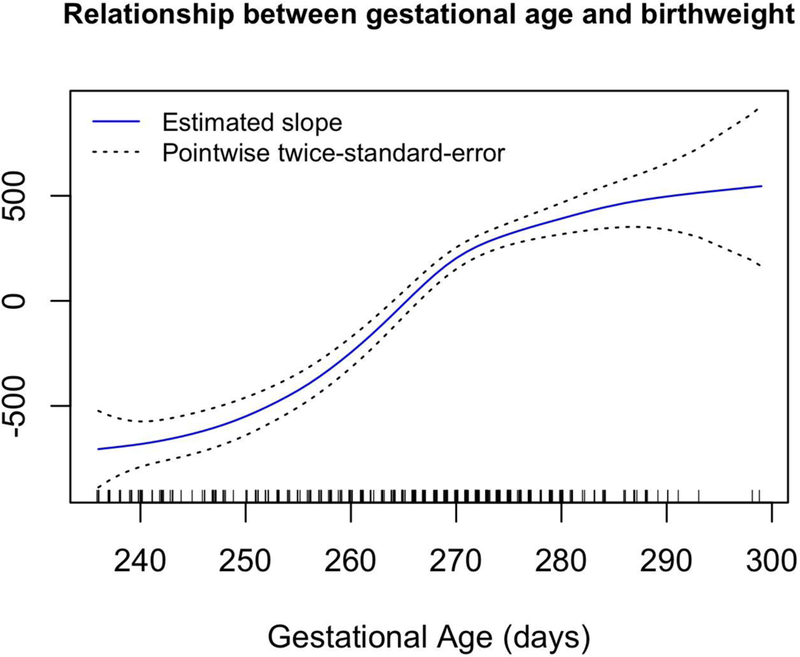
The estimated covariate-adjusted effect of gestational age on birthweight from a generalized additive model with 5 degrees of freedom for this relationship. The plot shown is for the model that adjusts for rhodium exposure; the relationship between gestational age and birthweight is extremely similar for all exposures. Hash marks at the bottom of the plot show observed gestational ages. Regression coefficients controlled for the following covariates: maternal pre-pregnancy BMI, maternal age, and gestational age as continuous variables, as well as three categorical covariates: race (white or minority), infant sex, and smoking (yes or no).
Discussion:
The present study quantified 14 metals/metalloids that were consistently detected in 374 placentae collected in Chattanooga, TN. Using Pearson’s correlation, we compared metal concentrations with a variety of fetal growth parameters. In addition, we used linear regression models to examine the relationships between birth weight and 14 placental metal concentrations in covariate-adjusted models. Because of the hypothesis-generating nature of this study and not wanting to rely solely on p-values for inference, we did not make any adjustment for multiple comparisons. Our aim with the hypothesis generating nature of our study was to underscore the complex nature of these elements, the placenta, and birth outcomes from the literature. To streamline this effort, metals that are discussed below are only those with significant associations (either essential or non-essential in the placenta or very under-reported in the placenta). Mn, Fe, Co and Zn serve essential functions, though at some concentrations, fetal growth can be impacted. Non-essential Cd and Pb are well documented to have deleterious effects on fetal growth. Several metals (Rh, Ti, Ba and Al) have limited to no published data on placental concentrations. As, Se, Cu and Ni have been previously studied in placental tissue and were not significantly associated with birth outcomes, and so are not extensively discussed below.
Essential metals significantly associated with fetal parameters
Manganese serves a vital role in the cell and is an essential nutrient (Leach and Harris 1997). In the present study, placental manganese concentrations were negatively correlated with birth weight, length and placental weight. A past study found that Mn concentrations in placental tissue was not significantly different between IUGR or low birth weight infants and normal weight infants (Osada et al. 2002). A few studies have investigated maternal blood Mn and infant parameters. When measuring maternal Mn in blood, Zota et al. (2009) reported an inverted U-shaped dose-response curve for birth weight; birth weights increased with increasing maternal whole blood Mn concentrations up to 3.1 μg/L, with a reduction of birth weight occurring past that threshold. However, Osada et al. (2002) did not find a significant difference in maternal blood Mn concentrations of normal and IUGR infants at concentrations that were at or below the 3.1 μg/L for both AGA and IUGR samples. Vigeh et al. (2008) reported responses at Mn concentrations well above 3.1 μg/L; maternal blood Mn concentrations for AGA infants were significantly higher than the IUGR infants (16.7 ± 4.8 μg/L for IUGR and 19.1 ± 5.9 μg/l for AGA infants). However, this trend was reversed in the infant cord blood samples (Mn = 44.7 ± 19.1 μg/L for IUGR and 38.2 ± 13.1 μg/l for AGA newborns). We found no evidence of non-linearity between ln-Mn and birth weight; however, we did find a significant decrease in birth weight with increasing ln-Mn levels. The results of the present study and other studies suggest that the relationship between Mn concentrations (in maternal tissues, in utero tissues, and transplacental transfer) and infant outcomes warrants further investigation.
Cobalt concentrations were greater in normal weight birth placentae and lower in SGA births compared to LGA births, and were a significant predictor of birth weight after adjusting for covariates. A generalized additive model allowing for nonlinearity in the effects of the continuous covariates and Co demonstrated a significant nonlinear relationship between Co and birth weight. Cobalt is an essential cofactor in Vitamin B-12, which plays a role in energy production (Banerjee and Ragsdale 2003). Data on Co and human pregnancy outcomes are limited. However, there have been reports linking Co deficiencies in sheep with high prenatal mortality. In one such study by Duncan et al. (1981), ewes were placed on a Co deficient diet for a period prior to conception. That Co deficiency lead to high perinatal mortality of the associated lambs. Cobalt appears to undergo transplacental transfer. Indeed, deSouza et al. (2012), examined women who had undergone hip replacement surgery (the metal of the replacement hip contains Co) and found that concentrations in the umbilical cord blood were approximately half that of maternal blood levels, demonstrating transplacental transport.
Zinc is an essential element in more than 1000 proteins required for biological functions ranging from antioxidants to cell signaling and gene expression. As such, it is extremely important for fetal growth (Maret 2009; Prasad 2008; Wilson et al. 2017). Zadrożna et al. (2009) found that Zn levels were significantly lesser in IUGR placentae than the control group, which mirrors the reduction of zinc in our population SGA infants (Table 6). Through the use of a Zn deficient mouse model, Wilson et al. (2017) found an 8% reduction in placental and birth weight, and a 32% reduction in weaning weight compared to mothers with a Zn supplementation.
Non-essential metals significantly associated with fetal parameters
Both lead and cadmium are well-documented toxic metals, even when considering the unique matrix such as the placenta (both of these metals specifically quantified in the placenta, have merited a review (Esteban-Vasallo et al. 2012)). In the present study, there was a significant negative correlation between placental cadmium concentration and birth length and placental weight. Guo et al. (2010) examined two cities in China, one of which is a major processor of e-waste (electronic waste). Placental Cd concentrations were not significantly different than the control city (where e-waste was not being processed), nor was there any significant correlations with placental Cd and birth weight. In the present study, no significant difference in Cd concentrations was found between LBW and normal weight infants, which is similar to past studies (Osada et al. 2002), although Cd has been found to be was significantly greater in IUGR/LBW placentae than normal placentae in other studies (Klapec et al. 2008; Llanos and Ronco 2009). Cadmium (Cd) is an endocrine disrupter (Henson and Chedrese 2004) that has been shown to inhibit 11β-HSD2 activity in the human placenta (Yang et al. 2006). Impaired fetal growth and intrauterine growth restriction (IUGR) are linked to reduced levels of 11β-HSD2 activity/expression in the placenta (Dy et al. 2008; McTernan et al. 2001; Mikelson et al. 2015). Further studies on the potential for cadmium to directly (transplacental transfer) or indirectly (myriad pathways) affect infant parameters is warranted.
Lead was significantly negatively correlated with birth weight and placental weight. Lead has been found to readily pass through the placenta (Rudge et al. 2009; Sakamoto et al. 2010). In a study of Swedish women, lead cord blood levels were negatively correlated to birth weight, length and head circumference (Osman et al. 2000), and higher Pb concentrations were reported in placenta from IUGR infants (Llanos and Ronco 2009), but this association has not always been found in other studies examining birth weight or IUGR infants (Al-Saleh et al. 2011; Falcón et al. 2003; Klapec et al. 2008). Guo et al. (2010) also did not find a significant correlation between placental lead and gestational age (similar to the present study), nor a significant correlation of lead with birth weight (dissimilar to the present study).
Underrepresented metals significantly associated with fetal parameters
The present study significantly contributed to a relatively extensive database of some placental metals (e.g., Pb and Zn) and the relationship each has with birth weight and other infant parameters. However, some of the strongest relationships that are presented herein came from several metals underrepresented in placental and birth outcome literature. For example, concentrations of Rh have not been reported in placental tissue, yet Rh was the strongest predictor of birth weight of all metals examined here. Indeed, placental Rh was consistently negatively correlated with many infant parameters. As noted earlier, the generalized additive model for ln-Rh demonstrated a significant nonlinear relationship between Rh and birth weight, after adjusting for covariates. In contrast to the other metals in the present study, Rh was not correlated with multiple metals; Rh correlated only with Zn, suggesting the source of exposure to Rh was different from exposure to most other metals. Rhodium is a relatively non-toxic metal (CDC 1994; Schertzinger et al. 2017); therefore, mechanisms for reduced birth weight are not established. Minor DNA damage has been observed at high concentrations (micromolar) in cell culture which may indicate a danger for those in industry (Iavicoli et al. 2012), but overall, Rh toxicity is low. When compared to more well-studied metals, cell culture LC50 values (tested on BEAS-2B, a human bronchial cell line) were significantly higher for Rh (1.2 mmol/L) as compared to Ni (0.8 mmol/L), and Cd salts (0.005 mmol/L) (Schmid et al. 2007). Through the use of catalytic converters, Rh release has been on the rise (Barbante et al. 2001), and with that, a concern for exposure from heavy, urban traffic environments (Kamala et al. 2015). Blood samples in police officers occupationally exposed to heavy traffic showed increased Rh blood levels with increasing experience and time on the force (Kamala et al. 2015). Thus, airborne concentration of Rh would be the most likely source of maternal exposure. Because Rh is not a Criteria Air Pollutant or considered to pose a threat to human health and the environment, the Clean Air Act does not require that it be regularly monitored or reported in the Toxic Release Inventory (E.P.A. 2006, 2017). Considering the increased abundance of automobiles in the US and the developing world, environmental monitoring of Rh and other platinum group elements has expanded, but is still very under-reported. (Ajmone-Marsan and Biasioli 2010; Barbante et al. 2001; Ravindra et al. 2004). Given our data and increased automobile emissions, Rh is a relevant metal to further investigate regarding fetal growth outcomes.
Aluminum negatively correlated with placental weight, but positively correlated with the fetal/placental ratio. Significant neurological development delays and decreased postnatal body weight gain were observed when dams were administered 300 and 600 mg/kg/day AlCl3 (Abu-Taweel et al. 2012; Bondy 2010). Sharma and Mishra (2006) studied the effects of Al on pregnant rats. Female rats were given 345 mg/kg day oral dose of aluminum chloride for 16 days pre- and post-partum. Al was quantified in fetal and maternal brain tissues, as well as placental tissues. Birth weight, placental weight, and fetal growth were all reduced compared to the control group. Neither Al transplacental passage nor related fetal outcomes have been extensively studied in humans. Al concentrations have been reported, but the focus was on distribution within the placenta rather than health outcomes (Kruger et al. 2010).
While the present study did not find any significant correlations of barium and titanium with birth outcomes, our data are important because reports of barium and titanium concentrations in placental tissues are very limited. A few studies have examined Ba concentrations in maternal tissues and associated birth outcomes, but none have examined correlations to birth weight (Han et al. 2011; Jain 2013; Odland et al. 2001). Titanium dioxide nanoparticles (TiO2 NP) are prominent sources which have increased in daily use and manufacturing (Rollerova et al. 2015). When administered to mice, a 1 mg/kg/day oral TiO2 NP treatment significantly reduced the ratio of placenta/body weight on gestational day 13; however, placental weight nor litter size was affected (Zhang et al. 2018).
Potential Mechanisms for Effects on Fetal Growth
The comprehensive group of metals and metalloids investigated herein likely involves many biochemical pathways associated with birth weight and fetal growth. Oxidative stress is one general mechanism that has been shown to affect birth weight and is related to maternal metal concentrations. Additionally, studies have examined how certain essential and non-essential metals affect placental morphology/function.
Oxidative Stress within the Placenta Due to Essential and Non-essential Metals
Oxidative stress in any cell or tissue is of great concern. Concentrations of free radical species in excess of antioxidant defenses causes damage to cells, mainly the cell membrane through lipid peroxidation (Betteridge 2000). A myriad of conditions including cardiovascular diseases, asthma, and various cancers, among many others, are thought to be related to oxidative stress (Phaniendra et al. 2015). Though some signaling pathways rely on reactive oxygen species in the placenta, if excess free radicals are not sequestered, oxidative stress can occur. Spontaneous abortion, preeclampsia, and IUGR are all associated with oxidative stress (Sultana et al. 2017; Wu et al. 2016).
The toxicity of iron-catalyzed reactive oxygen species (ROS) is well established. Shastri et al. (2016) measured hemoglobin (Hb) in the first trimester of pregnancy and grouped women with high Hb levels (≥120 g/l) and compared them to women with normal to low Hb levels (< 110 g/l). Both groups continued to take an iron supplement for the duration of the pregnancy. Measured during the first trimester, the high Hb mothers had a significant increase in plasma lipid peroxides and lower paraoxonase-1 (PON-1) activity (a common enzyme to mitigate lipid peroxidation) compared with women with low/normal Hb. Further, routine supplementation of Fe to these high Hb women for the duration of gestation resulted in persistent lower PON-1 activity in cord blood and lighter birth weights. Although these results were not measured in placental tissue, our correlations of Fe with birth weight, after covariate adjustments, is very similar to the relationship with birth weight reported by Shastri et al. (2016), which may be related to over exposure to iron and subsequent increase in oxidative stress. Cadmium and lead reduce total antioxidant potential by depleting glutathione and protein-bound sulfhydryl groups that serve to neutralize free radicles (Stohs and Bagchi 1995). Schmid et al. (2007) tested rhodium for ROS generation, but instead found that ROS decreased with Rh concentrations above 0.09 mol/L.
Several of the metals measured in the present study are important trace metals that serve a role as enzyme cofactors that help control oxidative stress in tissue. For example, superoxide dismutase, contains zinc and copper (Cu-Zn SOD) (Mistry and Williams 2011). Reactive oxygen species naturally generated in the mitochondria are captured with the antioxidant enzyme, manganese superoxide dismutase (MnSOD). Several other studies that examined metal concentrations in the placenta collected data on biomarkers of metals; such as metallothioneins (MT), delta-aminolevulinic acid dehydratase (ALAD), and lipid peroxidation (LPO) (Serafim et al. 2012), cytochrome c oxidase (CCO), glucose-6-phosphate dehydrogenase (G6PDH), Cu–Zn superoxide dismutase (Cu–Zn SOD), glutathione peroxidase (GSH-Px), and glutathione (GSH) (Zadrożna et al. 2 9). These biomarkers are related either to metal sequestration (MT), a measure of or abetment of oxidative stress (LPO, Cu–Zn SOD, GSH/GSH-Px). Or, are involved in a biochemical pathway or ATP production (e.g., CCO, G6PDH, ALAD), that have been shown to correlate with fetal health outcomes (Zadrożna et al. 2 9). Measuring such biomarkers and biochemical pathways was well beyond the scope of the present study. However, our data, particularly those on underrepresented metals, warrants future mechanistic investigations.
Effects of metals on placental morphology and function
Beyond their effects on biochemical pathways, metals may also affect placental morphology, and / or function. Zinc deficiencies are associated with significant decreases (16%) in trophoblast volume and trophoblast barrier thickness and increases in the volume density and surface area density of fetal capillaries in mice (Wilson et al. 2017). A 10 mg/kg/day dose of TiO2 NPs disrupted placental labyrinth vascularization and significantly inhibited placental cell proliferation and increased placental apoptosis (Zhang et al. 2018), suggesting a morphological change to the placenta. Cd is commonly detected in the placenta, especially in smokers (as seen in our data set). In addition to directly increasing placental oxidative stress, Cd in the placenta also alters the detoxifying mechanism of the placenta by downregulating a transport protein, ABCG2, in rats (Liu et al. 2016). ABCG2 is an ATP-binding cassette (ABC) transporter, that is well known to move heavy metals (e.g., Cd, Pb, Hg) from syncytiotrophoblast to maternal circulation, decreasing fetal exposure (Kummu et al. 2012). Further, Wier et al. (1990) reported decreased Zn transport into fetal circulation, altered ultrastructural changes and fetal vasculature with CdCl human placental perfusion.
Considerations for Infant Sex-specific effects of metals and Fetal/Placental Ratio
Several studies have reported sex-specific differences in metal toxicity. Karagas et al. (2012) cited two studies (Gao et al. 2007; Philibert et al. 2008) in a review of methyl mercury toxicity that examined sex-specific effects and called for an increased analysis of this important variable. Cd showed a sex-specific mechanism in a study conducted by Kippler et al. (2012), who measured maternal urine Cd concentrations and found a significant negative correlation with birth weight and head circumference in female infants only. A review by Clifton (2010) highlights several broad areas of fetal health that have shown a sex-biased difference (different growth rates in response to environmental stress, cytokine expression, insulin-like growth factor pathways, placental response to cortisol, preterm delivery placental function). Additionally, Clifton states, “ignor[ing] the sex of the placenta is no longer sound scientific practice.” We did not find any evidence that the effects of ln-metals on birth weight differed by sex in this study. However, we concur that sex-specific effects on infant parameters should be considered in future studies.
Another approach to assessing fetal growth is calculating the ratio of fetal weight to placental weight, or how much fetal growth can be supported per gram of placenta (Matsuda et al. 2015). The fetal/placental ratio is one based on the theory that a relatively large placenta represents an inefficiency in nutrient transfer across the placenta (i.e., more tissue is needed to exchange molecules); thus, placental growth is stimulated to compensate for exchange inefficiencies (Salafia et al. 2006). As normal fetal growth proceeds and birth weight increases, the fetal/placental ratio value increases (which is to imply that proper fetal growth outpaces the growth of the placenta) (Lurie et al. 1999; Matsuda et al. 2015). When correlated with ln transformed metals, Al, Cd, Co, Cu, Fe, Se and Zn all had positive correlations (e.g., as Al increased, so did the fetal/placenta ratio). This was the only birth outcome with positive correlations for those metals, meaning that as metal concentrations increased so did the fetal/placental ratios, indicating improved placental function. This is interesting considering the well-known deleterious and non-essential functions of Al and Cd. Little research in this area has been conducted, but more investigation is warranted.
Study Limitations:
Limitations in the present study must be taken into consideration. The maternal records lacked complete information on maternal income and dietary habits. Dietary habits and vitamin supplementation are well documented as being significant influences on the metal status of the mother and fetus (Hovdenak and Haram 2012; Mistry and Williams 2011; Scholl 2005). After an analysis of six metals and several biomarkers, Serafim et al. (2012) found that diet was a significant influence on the metal status of the placenta (as well as tobacco use). Additionally, examination of individual metals does not address potentially additive or synergetic effects, but an analysis of the effects of metal mixtures is beyond the scope of the current manuscript. Like all observational studies, we can only assess association, but not causation. The results of this study cannot be construed as showing that exposure to high levels of these metals causes changes in birth outcomes. Additionally, we can only review and consider potential mechanisms or biomarkers that would directly link fetal growth to these placental concentrations. Finally, though our sample size is relatively large, and we made great efforts to eliminate any selection bias from our sample population, it is not guaranteed that our selection process would affect all metals in the same way. Although the relationship between a factor that affects participation and birth weight will remain constant across variables, the relationship between these factors and the metals may vary.
The main objective of the present study was to quantify the placental concentrations of a wide spectrum of metals and describe correlations with fetal parameters – regardless of the source or associated mechanisms of action. To that end, the present study expands the database on underrepresented placental metals and provides one of the most comprehensive examinations of placental metals and correlations with fetal parameters to date.
Supplementary Material
Highlights:
Placental Rh concentrations are negatively correlated with many infant parameters.
Each unit increase in ln-Rh was associated with a 1490 gram decrease in birth weight
Pb and Mn were significantly negatively correlated with many of the infant parameters
Cobalt was significantly positively associated with birth weight.
Significant nonlinear relationships between birth weight and placental Co and Rh.
Acknowledgements
Regional Obstetrical Consultants for the generous funding, uses of equipment and facilities, and providing personnel and expertise to facilitate placental collection. Dr. Dawn Misra (Wayne State University) for her help in study design, advice, and funding.
Laboratorio Chimico Merceologico for the generous donation of employee time, equipment and supplies. Lorrie Mason at Regional Obstetrical Consultants for her time and help in placental collection. The many students at UTC who helped with a large variety of tasks. Research reported in this publication was supported by U.S. Department of Health and Human Services (DHHS/HRSA 1H1SMC10654-01-00 RICHARDS 08-) and the National Institute of Environmental Health Sciences of the National Institutes of Health (NIH) under award number T32ES007271 and P30 ES01247. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Placental collection was conducted at Erlanger Hospital (Baroness campus), Chattanooga. All specimen and data collection methods were approved by the UT College of Medicine Institutional Review Board (#05-031, FWA# 2301).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Abu-Taweel GM, Ajarem JS, Ahmad M. 2012. Neurobehavioral toxic effects of perinatal oral exposure to aluminum on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol Biochem Behav 101:49–56. [DOI] [PubMed] [Google Scholar]
- Ajmone-Marsan F, Biasioli M. 2010. Trace elements in soils of urban areas. Water Air Soil Pollut 213:121–143. [Google Scholar]
- Al-Saleh I, Shinwari N, Mashhour A, Mohamed GED, Rabah A. 2011. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. International Journal of Hygiene and Environmental Health 214:79–101. [DOI] [PubMed] [Google Scholar]
- ATSDR. 2007. Toxicological profile for barium Atlanta, GA:Center for Disease Control, Division of Toxicology and Human Health Sciences. [Google Scholar]
- Banerjee R, Ragsdale SW. 2003. The many faces of vitamin b-12: Catalysis by cobalamin-dependent enzymes. Annu Rev Biochem 72:209–247. [DOI] [PubMed] [Google Scholar]
- Barbante C, Veysseyre A, Ferrari C, Van De Velde K, Morel C, Capodaglio G, et al. 2001. Greenland snow evidence of large scale atmospheric contamination for platinum, palladium, and rhodium. Environmental Science & Technology 35:835–839. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Osmond C, Winter PD, Margetts B, Simmonds SJ. 1989. Weight in infancy and death from ischaemic heart disease The Lancet 334:577–580. [DOI] [PubMed] [Google Scholar]
- Betteridge DJ. 2000. What is oxidative stress? Metabolism - Clinical and Experimental 49:3–8. [DOI] [PubMed] [Google Scholar]
- Bondy SC. 2010. The neurotoxicity of environmental aluminum is still an issue. Neurotoxicology 31:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Rhodium. 1994.
- Clifton VL. 2010. Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta 31, Supplement:S33–S39. [DOI] [PubMed] [Google Scholar]
- Davis MA, Higgins J, Li Z, Gilbert-Diamond D, Baker ER, Das A, et al. 2015. Preliminary analysis of in utero low-level arsenic exposure and fetal growth using biometric measurements extracted from fetal ultrasound reports. Environmental Health 14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deSouza R-M, Wallace D, Costa ML, Krikler SJ. 2012. Transplacental passage of metal ions in women with hip resurfacing: No teratogenic effects observed. Hip International 22:96–99. [DOI] [PubMed] [Google Scholar]
- Duncan WRH, Morrison ER, Garton GA. 1981. Effects of cobalt deficiency in pregnant and post-parturient ewes and their lambs. British Journal of Nutrition 46:337–344. [DOI] [PubMed] [Google Scholar]
- Dy J, Guan H, Sampath-Kumar R, Richardson BS, Yang K. 2008. Placental 11β-hydroxysteroid dehydrogenase type 2 is reduced in pregnancies complicated with idiopathic intrauterine growth restriction: Evidence that this is associated with an attenuated ratio of cortisone to cortisol in the umbilical artery. Placenta 29:193–200. [DOI] [PubMed] [Google Scholar]
- E.P.A. 2006. National ambient air quality standards for particulate matter; final rule 40 CFR Part 50.
- E.P.A. 2017. Toxic chemical release reporting: Community right-to-know 40 CFR PART 372.
- EPA. 2014. Risk assessment of spent foundry sands in soil-related applications. Evaluating silica-based spent foundry sand from iron, steel, and aluminum foundries EPA-530-R-14–003.
- EPA. National priorities list. Southside chattanooga lead site. 2018.
- EPA US. Assigning values to nondetected/non-quantified pesticide residue in human health food exposure assessments. Washington: 2000. [Google Scholar]
- Esteban-Vasallo MD, Aragonés N, Pollan M, López-Abente G, Perez-Gomez B. 2012. Mercury, cadmium, and lead levels in human placenta: A systematic review. Environmental Health Perspectives 120:1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstedt S, Kippler M, Scheynius A, Gutzeit C, Mie A, Alm J, et al. 2015. Anthroposophic lifestyle influences the concentration of metals in placenta and cord blood. Environmental Research 136:88–96. [DOI] [PubMed] [Google Scholar]
- Falcón M, Viñas P, Luna A. 2003. Placental lead and outcome of pregnancy. Toxicology 185:59–66. [DOI] [PubMed] [Google Scholar]
- Gao Y, Yan C-H, Tian Y, Wang Y, Xie H-F, Zhou X, et al. 2007. Prenatal exposure to mercury and neurobehavioral development of neonates in zhoushan city, china. Environmental research 105:390–399. [DOI] [PubMed] [Google Scholar]
- Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. 1992. Customised antenatal growth charts. The Lancet 339:283–287. [DOI] [PubMed] [Google Scholar]
- Gardosi J, Francis A. 2009. Customised weight centile calculator – grow-centile Part 6.2 Birmingham, UK:Gestation Network. [Google Scholar]
- Gardosi J, Francis A, Turner S, Williams M. 2018. Customized growth charts: Rationale, validation and clinical benefits. American Journal of Obstetrics and Gynecology 218:S609–S618. [DOI] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Emond JA, Baker ER, Korrick SA, Karagas MR. 2016. Relation between in utero arsenic exposure and birth outcomes in a cohort of mothers and their newborns from new hampshire. Environmental Health Perspectives 124:1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez B, Gómez M, Sanchez JL, Fernández R, Palacios MA. 2001. Platinum and rhodium distribution in airborne particulate matter and road dust. Science of The Total Environment 269:131–144. [DOI] [PubMed] [Google Scholar]
- Guo YY, Huo X, Li Y, Wu KS, Liu JX, Huang JR, et al. 2010. Monitoring of lead, cadmium, chromium and nickel in placenta from an e-waste recycling town in china. Science of The Total Environment 408:3113–3117. [DOI] [PubMed] [Google Scholar]
- Hall GS, Carr MJ, Cummings E, Lee M. 1983. Aluminum, barium, silicon, and strontium in amniotic fluid by emission spectrometry. Clinical Chemistry 29:1318. [PubMed] [Google Scholar]
- Hamilton County Health Department, G. 2015. Picture of our health, hamilton county, tennessee Chattanooga, TN. [Google Scholar]
- Han BH, Lee KS, Han JY, Choi JS, Ahn HK, Ryu HM, et al. 2011. Pregnancy outcome after 1st-trimester inadvertent exposure to barium sulphate as a contrast media for upper gastrointestinal tract radiography. J Obstet Gynaecol 31:586–588. [DOI] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ. 1990. Generalized additive models Roca Raton:Chapman and Hall/CRC [Google Scholar]
- Henson MC, Chedrese PJ. 2004. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Experimental Biology and Medicine 229:383–392. [DOI] [PubMed] [Google Scholar]
- Hovdenak N, Haram K. 2012. Influence of mineral and vitamin supplements on pregnancy outcome. European Journal of Obstetrics & Gynecology and Reproductive Biology 164:127–132. [DOI] [PubMed] [Google Scholar]
- Hu X, Zheng T, Cheng Y, Holford T, Lin S, Leaderer B, et al. 2015. Distributions of heavy metals in maternal and cord blood and the association with infant birth weight in china. The Journal of reproductive medicine 60:21–29. [PMC free article] [PubMed] [Google Scholar]
- Hutcheon J 2014. Do customized birth weight charts add anything but complexity to the assessment of fetal growth? Journal of Obstetrics and Gynaecology Canada 36:107–109. [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Cufino V, Corbi M, Goracci M, Caredda E, Cittadini A, et al. 2012. Rhodium and iridium salts inhibit proliferation and induce DNA damage in rat fibroblasts in vitro. Toxicology in Vitro 26:963–969. [DOI] [PubMed] [Google Scholar]
- IPCS IPOCS. 1990. Environmental health criteria 107: Barium Geneva. [Google Scholar]
- Iyengar GV, Rapp A. 2001. Human placenta as a ‘dual’ biomarker for monitoring fetal and maternal environment with special reference to potentially toxic trace elements.: Part 2: Essential minor, trace and other (non-essential) elements in human placenta. Science of The Total Environment 280:207–219. [DOI] [PubMed] [Google Scholar]
- Jain RB. 2013. Effect of pregnancy on the levels of urinary metals for females aged 17–39 years old: Data from national health and nutrition examination survey 2003–2010. Journal of Toxicology and Environmental Health, Part A 76:86–97. [DOI] [PubMed] [Google Scholar]
- Kamala CT, Balaram V, Satyanarayanan M, Kumar AK, Subramanyam KSV. 2015. Biomonitoring of airborne platinum group elements in urban traffic police officers. Arch Environ Contam Toxicol 68:421–431. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, et al. 2012. Evidence on the human health effects of low-level methylmercury exposure. Environmental Health Perspectives 120:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippler M, Tofail F, Gardner R, Rahman A, Hamadani JD, Bottai M, et al. 2012. Maternal cadmium exposure during pregnancy and size at birth: A prospective cohort study. Environmental Health Perspectives 120:284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapec T, Ćavar S, Kasač Z, Ručević S, Popinjač A. 2008. Selenium in placenta predicts birth weight in normal but not intrauterine growth restriction pregnancy. Journal of Trace Elements in Medicine and Biology 22:54–58. [DOI] [PubMed] [Google Scholar]
- Krans EE, Davis MM. 2014. Strong start for mothers and newborns: Implications for prenatal care delivery. Curr Opin Obstet Gynecol 26:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger PC, Schell LM, Stark AD, Parsons PJ. 2010. A study of the distribution of aluminum in human placental tissues based on alkaline solubilization with determination by electrothermal atomic absorption spectrometry. Metallomics 2:621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummu M, Sieppi E, Wallin K, Rautio A, Vähäkangas K, Myllynen P. 2012. Cadmium inhibits abcg2 transporter function in bewo choriocarcinoma cells and mcf-7 cells overexpressing abcg2. Placenta 33:859–865. [DOI] [PubMed] [Google Scholar]
- Lagerkvist B, Sandberg S, Frech W, Jin T, Nordberg G. 1996. Is placenta a good indicator of cadmium and lead exposure?. Archives of Environmental Health 51:389–394. [DOI] [PubMed] [Google Scholar]
- Lagiou P, Tamimi RM, Mucci LA, Adami HO, Hsieh CC, Trichopoulos D. 2004. Diet during pregnancy in relation to maternal weight gain and birth size. Eur J Clin Nutr 58:231–237. [DOI] [PubMed] [Google Scholar]
- Leach R, Harris E. 1997. Handbook of nutritionally essential minerals New York:Marcel Dekker, Inc. [Google Scholar]
- Liu L, Zhou L, Hu S, Zhou S, Deng Y, Dong M, et al. 2016. Down-regulation of abcg2 and abcb4 transporters in the placenta of rats exposed to cadmium. Oncotarget 7:38154–38163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos MN, Ronco AM. 2009. Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities. Reproductive Toxicology 27:88–92. [DOI] [PubMed] [Google Scholar]
- Lurie S, Feinstein M, Mamet Y. 1999. Human fetal-placental weight ratio in normal singleton near-term pregnancies. Gynecologic and Obstetric Investigation 48:155–157. [DOI] [PubMed] [Google Scholar]
- Magee BD, Hattis D, Kivel NM. 2004. Role of smoking in low birth weight. Journal of Reproductive Medicine 49:23–27. [PubMed] [Google Scholar]
- Maret W 2009. Molecular aspects of human cellular zinc homeostasis: Redox control of zinc potentials and zinc signals. Biometals 22:149–157. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Ogawa M, Nakai A, Hayashi M, Satoh S, Matsubara S. 2015. Fetal/placental weight ratio in term japanese pregnancy: Its difference among gender, parity, and infant growth. International Journal of Medical Sciences 12:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTernan CL, Draper N, Nicholson H, Chalder SM, Driver P, Hewison M, et al. 2001. Reduced placental 11β-hydroxysteroid dehydrogenase type 2 mrna levels in human pregnancies complicated by intrauterine growth restriction: An analysis of possible mechanisms. The Journal of Clinical Endocrinology & Metabolism 86:4979–4983. [DOI] [PubMed] [Google Scholar]
- Melamed N, Ray JG, Shah PS, Berger H, Kingdom JC. 2014. Should we use customized fetal growth percentiles in urban canada? Journal of Obstetrics and Gynaecology Canada 36:164–170. [DOI] [PubMed] [Google Scholar]
- Mikelson C, Kovach MJ, Troisi J, Symes S, Adair D, Miller RK, et al. 2015. Placental 11β-hydroxysteroid dehydrogenase type 2 expression: Correlations with birth weight and placental metal concentrations. Placenta 36:1212–1217. [DOI] [PubMed] [Google Scholar]
- Miller JE, Korenman S. 1994. Poverty and childrens nutritional-status in the united-states. American Journal of Epidemiology 140:233–243. [DOI] [PubMed] [Google Scholar]
- Miller RK, Mattison DR, Plowchalk D. 1988. Biological monitoring of the human placenta. In: Biological monitoring of toxic metals, (Clarkson TW, Friberg L, Nordberg GF, Sager PR, eds). Boston, MA:Springer US, 567–602. [Google Scholar]
- Mistry HD, Williams PJ. 2011. The importance of antioxidant micronutrients in pregnancy. Oxidative Medicine and Cellular Longevity 2011. [DOI] [PMC free article] [PubMed]
- Odland J, Nieboer E, Romanova N, Thomassen Y, Hofoss D, Lund E. 2001. Factor analysis of essential and toxic elements in human placentas from deliveries in arctic and subarctic areas of russia and norway. J Environ Monit 3:177–184. [DOI] [PubMed] [Google Scholar]
- Osada H, Watanabe Y, Nishimura Y, Yukawa M, Seki K, Sekiya S. 2002. Profile of trace element concentrations in the feto-placental unit in relation to fetal growth. Acta Obstet Gynecol Scand 81:931–937. [DOI] [PubMed] [Google Scholar]
- Osman K, Åkesson A, Berglund M, Bremme K, Schütz A, Ask K, et al. 2000. Toxic and essential elements in placentas of swedish women. Clinical Biochemistry 33:131–138. [DOI] [PubMed] [Google Scholar]
- Phaniendra A, Jestadi DB, Periyasamy L. 2015. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian Journal of Clinical Biochemistry 30:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert A, Bouchard M, Mergler D. 2008. Neuropsychiatric symptoms, omega-3, and mercury exposure in freshwater fish-eaters. Archives of Environmental & Occupational Health 63:143–153. [DOI] [PubMed] [Google Scholar]
- Prasad AS. 2008. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Experimental gerontology 43:370–377. [DOI] [PubMed] [Google Scholar]
- Punshon T, Li ZG, Marsit CJ, Jackson BP, Baker ER, Karagas MR. 2016. Placental metal concentrations in relation to maternal and infant toenails in a us cohort. Environmental Science & Technology 50:1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindra K, Bencs L, Van Grieken R. 2004. Platinum group elements in the environment and their health risk. Science of The Total Environment 318:1–43. [DOI] [PubMed] [Google Scholar]
- Rollerova E, Tulinska J, Liskova A, Kuricova M, Kovriznych J, Mlynarcikova A, et al. 2015. Titanium dioxide nanoparticles: Some aspects of toxicity/focus on the development. Endocrine regulations 49:97–112. [DOI] [PubMed] [Google Scholar]
- Ronco AM, Urrutia M, Montenegro M, Llanos MN. 2009. Cadmium exposure during pregnancy reduces birth weight and increases maternal and foetal glucocorticoids. Toxicology Letters 188:186–191. [DOI] [PubMed] [Google Scholar]
- Rudge CV, Rollin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JO. 2009. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of south african delivering women. Journal of Environmental Monitoring 11:1322–1330. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Murata K, Kubota M, Nakai K, Satoh H. 2010. Mercury and heavy metal profiles of maternal and umbilical cord rbcs in japanese population. Ecotox Environ Safe 73:1–6. [DOI] [PubMed] [Google Scholar]
- Salafia CM, Charles AK, Maas EM. 2006. Placenta and fetal growth restriction. Clin Obstet Gynecol 49:236–256. [DOI] [PubMed] [Google Scholar]
- Schertzinger G, Zimmermann S, Grabner D, Sures B. 2017. Assessment of of sublethal endpoints after chronic exposure of the nematode caenorhabditis elegans to palladium, platinum and rhodium. Environmental Pollution 230:31–39. [DOI] [PubMed] [Google Scholar]
- Schmid M, Zimmermann S, Krug HF, Sures B. 2007. Influence of platinum, palladium and rhodium as compared with cadmium, nickel and chromium on cell viability and oxidative stress in human bronchial epithelial cells. Environment International 33:385–390. [DOI] [PubMed] [Google Scholar]
- Scholl TO. 2005. Iron status during pregnancy: Setting the stage for mother and infant. The American journal of clinical nutrition 81:1218S–1222S. [DOI] [PubMed] [Google Scholar]
- Serafim A, Company R, Lopes B, Rosa J, Cavaco A, Castela G, et al. 2012. Assessment of essential and nonessential metals and different metal exposure biomarkers in the human placenta in a population from the south of portugal. J Toxicol Env Health Part A 75:867–877. [DOI] [PubMed] [Google Scholar]
- Sharma P, Mishra KP. 2006. Aluminum-induced maternal and developmental toxicity and oxidative stress in rat brain: Response to combined administration of tiron and glutathione. Reproductive Toxicology 21:313–321. [DOI] [PubMed] [Google Scholar]
- Shastri L, Pammal RS, Mani I, Thomas T, Kurpad AV. 2016. Oxidative stress during early pregnancy and birth outcomes. Public Health Nutr 19:3210–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D. 1995. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biology and Medicine 18:321–336. [DOI] [PubMed] [Google Scholar]
- Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R. 2017. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. American Journal of Reproductive Immunology 77:10. [DOI] [PubMed] [Google Scholar]
- Sun K, Yang K, Challis JRG. 1997. Differential expression of 11β-hydroxysteroid dehydrogenase types 1 and 2 in human placenta and fetal membranes. Journal of Clinical Endocrinology & Metabolism 82:300–305. [DOI] [PubMed] [Google Scholar]
- Thomas S, Arbuckle TE, Fisher M, Fraser WD, Ettinger A, King W. 2015. Metals exposure and risk of small-for-gestational age birth in a canadian birth cohort: The mirec study. Environmental research 140:430–439. [DOI] [PubMed] [Google Scholar]
- Vigeh M, Yokoyama K, Ramezanzadeh F, Dahaghin M, Fakhriazad E, Seyedaghamiri Z, et al. 2008. Blood manganese concentrations and intrauterine growth restriction. Reproductive Toxicology 25:219–223. [DOI] [PubMed] [Google Scholar]
- Weisberg S 2005. Applied linear regression 3 ed. Hoboken, NJ:Wiley. [Google Scholar]
- Wicklin R 2011. Log transformations: How to handle negative data values? Available: https://blogs.sas.com/content/iml/2011/04/27/log-transformations-how-to-handle-negative-data-values.html [accessed 4/16/18.
- Wier PJ, Miller RK, Maulik D, DiSant’Agnese PA. 1990. Toxicity of cadmium in the perfused human placenta. Toxicol Appl Pharmacol 105:156–171. [DOI] [PubMed] [Google Scholar]
- Wilson RL, Leemaqz SY, Goh Z, McAninch D, Jankovic-Karasoulos T, Leghi GE, et al. 2017. Zinc is a critical regulator of placental morphogenesis and maternal hemodynamics during pregnancy in mice. Sci Rep 7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Tian FJ, Lin Y, Xu WM. 2016. Oxidative stress: Placenta function and dysfunction. American Journal of Reproductive Immunology 76:258–271. [DOI] [PubMed] [Google Scholar]
- Yajnik CS, Fall CHD, Vaidya U, Pandit AN, Bavdekar A, Bhat DS, et al. 1995. Fetal growth and glucose and insulin metabolism in four-year-old indian children. Diabetic Medicine 12:330–336. [DOI] [PubMed] [Google Scholar]
- Yang K, Julan L, Rubio F, Sharma A, Guan H. 2006. Cadmium reduces 11β-hydroxysteroid dehydrogenase type 2 activity and expression in human placental trophoblast cells. American Journal of Physiology - Endocrinology And Metabolism 290:E135–E142. [DOI] [PubMed] [Google Scholar]
- Zadrożna M, Gawlik M, Nowak B, Marcinek A, Mrowiec H, Walas S, et al. 2009. Antioxidants activities and concentration of selenium, zinc and copper in preterm and iugr human placentas. Journal of Trace Elements in Medicine and Biology 23:144–148. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xie X, Zhou Y, Yu D, Deng Y, Ouyang J, et al. 2018. Gestational exposure to titanium dioxide nanoparticles impairs the placentation through dysregulation of vascularization, proliferation and apoptosis in mice. International journal of nanomedicine 13:777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota A, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, et al. 2009. Maternal blood manganese levels and infant birth weight. Epidemiology 20:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


