Highlights
-
•
Two epilepsy patients did not have seizures during three weeks of intracranial EEG.
-
•
They were implanted with a device that enables chronic electrocorticography.
-
•
Seizures were localized by ictal recordings at long intervals after implantation.
Keywords: Epilepsy, RNS System, Chronic electrocorticography, Seizure localization
Abstract
Aims
To present two patients with medically-refractory focal epilepsy who, following non-diagnostic intracranial monitoring studies, had seizures localized by chronic ambulatory electrocorticography with an implanted neurostimulation device.
Methods
Case reports with clinical details and electrocorticograms showing seizures.
Results
Using electrodes placed at the suspected seizure onset zones, the neurostimulator recorded seizures in both patients at long intervals following implantation (49 days and 7.5 months).
Conclusions
Chronic ambulatory electrocorticography can provide valuable diagnostic information when there is a narrow hypothesis about seizure localization, though there are important caveats related to limited spatial sampling.
1. Introduction
One-third of patients with focal epilepsy fail to achieve seizure control with medication alone (Brodie et al., 2012). For these patients, surgical resection of epileptogenic brain tissue can be effective but requires precise seizure localization (Englot et al., 2017). In cases where non-invasive testing does not adequately localize seizures, localization can be achieved by implanting intracranial electrodes that record from regions suspected to generate seizures. Limitations of this approach include the unnatural measures required to provoke seizures, such as rapid medication taper and sleep deprivation, and intrinsic constraints of the inpatient environment where such recordings are performed. Furthermore, recordings to localize seizures seldom extend beyond two weeks due to risks of infection and bleeding, signal quality degradation, and patient intolerance of testing conditions (Shah and Mittal, 2014). If seizures are not recorded during invasive monitoring, resection is rarely offered based on interictal findings alone.
Recent advances allow for intracranial recordings over long timescales in natural ambulatory settings (King-Stephens et al., 2015, Rao et al., 2017). The NeuroPace RNS® System is an FDA-approved adjunctive therapy for adults with medically-refractory seizures arising from one or two foci. The RNS System involves a cranially-implanted neurostimulator connected to two four-contact leads placed in the brain at the known or presumed seizure onset zone(s) (Morrell and Halpern, 2016). The neurostimulator continuously monitors brain activity and responds to abnormal patterns of activity by delivering current pulses to abort incipient seizures. Electrocorticographic (ECoG) recordings of seizures are stored by the device for offline review. In addition to its therapeutic benefit by reducing seizure frequency (Bergey et al., 2015), the RNS System provides diagnostic information regarding seizure lateralization (King-Stephens et al., 2015) and can inform subsequent surgical treatments (DiLorenzo et al., 2014). Here, we describe use of the RNS System for seizure localization in two patients who did not have seizures recorded during inpatient intracranial monitoring.
2. Results – case reports
2.1. Patient 1
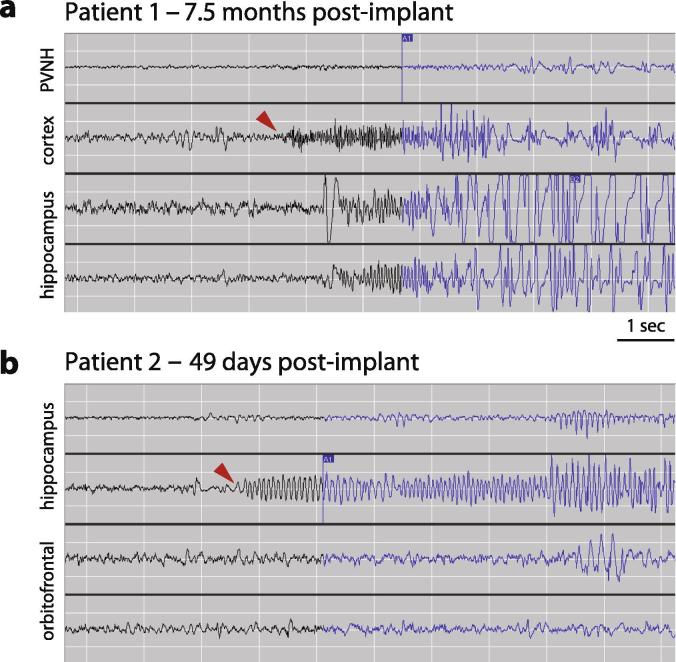
This 23-year-old right-handed female had epilepsy onset at age 18. Seizures occurred 1–5 times per month, and semiology involved behavioral arrest, lip-smacking automatisms, and rare secondary generalization. Brain magnetic resonance imaging (MRI) showed left periventricular heterotopic gray matter. Scalp video-electroencephalography seizure monitoring (VEEG) revealed a left lateralized, poorly localized ictal pattern. Positron emission tomography (PET) did not show focal hypometabolism, and magnetoencephalography (MEG) recorded no epileptiform discharges. She underwent invasive monitoring with depth electrodes sampling from the heterotopia (and overlying lateral temporal neocortex), and left mesial temporal structures. No seizures were captured in three weeks of recording. The RNS System was implanted with depth leads in the hippocampus and heterotopia, the structures with interictal spiking. Proximal electrode contacts of the heterotopia depth lead were located in overlying temporal neocortex. Stimulation function of the device was initially disabled. Her first seizure occurred 7.5 months later, and ECoG showed neocortical onset with rapid spread to the hippocampus (Fig. 1a). Numerous seizures with stereotyped ictal onset were subsequently recorded. Given cognitive risks associated with dominant temporal neocortical resection, the patient elected to proceed with neurostimulation for therapeutic purposes. At 15 months of follow-up, her clinical seizure frequency was 30% lower than pre-implant baseline.
Fig. 1.
Electrocorticograms with first seizures recorded by the RNS® System. (a) Patient 1 had her first seizure 7.5 months after implantation of the RNS System. Electrographic seizure onset (arrowhead) involves low-voltage fast activity arising from cortex overlying the periventricular nodular heterotopia (PVNH) with rapid spread to the hippocampus and then to the PVNH. (b) Patient 2 had his first seizure 49 days after implantation of the RNS System. Electrographic seizure onset (arrowhead) involves rhythmic alpha frequencies arising from the hippocampus without involvement of orbitofrontal cortex.
2.2. Patient 2
This 19-year-old right-handed male had epilepsy onset at age 18. He presented with focal seizures with impaired awareness occurring every two months. Semiology involved lightheadedness, nausea, oral automatisms, and occasional secondary generalization. Brain MRI was normal. VEEG recorded a right lateralized ictal pattern maximal over the temporal region.
PET did not show focal hypometabolism, and MEG recorded no epileptiform discharges. He underwent invasive monitoring with implantation of subdural electrodes extensively sampling temporal lobe neocortex and mesial temporal structures, the insula, orbitofrontal cortex (OFC), and cingulate gyrus. No seizures were captured after three weeks of recording, but abundant interictal spiking was observed from the hippocampus and OFC. Although interictal spikes are imperfect markers of the seizure onset zone (Marsh et al., 2010), the RNS System was implanted with electrodes in these structures because they were also potentially consistent with seizure semiology. His first seizure occurred 49 days later, and ECoG revealed hippocampal onset (Fig. 1b). Numerous seizures with an identical ictal pattern were subsequently recorded. Hippocampal resection was discussed, but he elected to proceed with neurostimulation. At 14 months of follow-up, his clinical seizure frequency was 50% lower than pre-implant baseline.
3. Discussion
Invasive monitoring with implanted electrodes detects seizures in 95% of epilepsy patients (Wellmer et al., 2012). In some of the remaining patients, we propose that the RNS System can be used for diagnostic purposes, provided there is a narrow hypothesis about seizure localization and a benefit to long-term monitoring.
Although the RNS System is FDA-approved for its therapeutic benefit in reducing seizure frequency (Morrell, 2011, Heck et al., 2014, Bergey et al., 2015), there is growing awareness of its clinical utility as diagnostic tool. Applications of the RNS System for diagnostic purposes include lateralization of mesial temporal lobe seizures (Spencer et al., 2011, Enatsu et al., 2012, Smart et al., 2013, King-Stephens et al., 2015) and further characterization of the seizure onset zone determined by short-term monitoring (DiLorenzo et al., 2014). Other investigators have used data from the RNS System to uncover temporal patterns of seizure timing (Duckrow and Tcheng, 2007, Spencer et al., 2011, Anderson et al., 2015, Spencer et al., 2016, Baud et al., 2018) and to reveal the effects of behavioral modifications (Mackow et al., 2016) and medication changes (Warner et al., 2016). However, to our knowledge, there are no reports of the RNS System being used to record seizures in patients who had non-diagnostic intracranial monitoring.
This report expands the diagnostic potential of the RNS System to include seizure localization in select cases. For the infrequent but vexing clinical scenario in which patients do not have seizures during intracranial monitoring, clinicians should be aware that the RNS System provides an option to obtain ictal recordings that can aid surgical decision-making. Ideally, ictal recordings should be obtained in patients before implanting RNS System electrodes, but the RNS System may provide localizing information in rare instances where, although intracranial recording does not provide ictal data, other findings strongly indicate the likely location of the epileptogenic zone.
With only eight electrode contacts, the RNS System allows limited spatial sampling and cannot supplant invasive inpatient monitoring. Indeed, it is possible that seizures recorded with the RNS System could represent a spread pattern from brain regions not sampled by electrodes. Both patients described here kept meticulous seizure diaries, and clinical seizures always had clear electrographic correlates in RNS System recordings. In cases where some clinical seizures are not reflected in RNS System recordings, however, localization information may be inadequate. In the future, if this approach is used in more patients, it may be helpful to compare patients in whom the RNS System provided localizing information with those in whom it did not to determine which factors are helpful in deciding RNS System electrode placement.
The long latency to the first electroclinical seizures recorded in our patients may relate to an effect of implanting RNS System hardware (Sun et al., 2018) and/or an effect of intracranial monitoring (Katariwala et al., 2001, Roth et al., 2012, Lane et al., 2017). When seizures are eventually recorded, some patients, like the two described here, may opt to explore the therapeutic benefit of responsive neurostimulation before pursuing an irreversible surgical treatment.
4. Summary
Chronic ambulatory electrocorticography with an implanted neurostimulation device can provide ictal recordings in patients who have had non-diagnostic intracranial monitoring evaluations. Lead placement strategy and limited spatial sampling are important considerations in obtaining and interpreting these data.
Acknowledgments
Acknowledgments
The authors are grateful to Emily Mirro for assistance with clinical management of the patients in this report.
Submitting author’s declaration
I acknowledge that all co-authors have been substantially involved in the study and/or preparation of the manuscript; no undisclosed groups or persons have had a primary role in the study and/or in the manuscript preparation; and all co-authors have seen and approved the submitted version of the paper and accept responsibility for its content.
Disclosure of conflict of interest
V.R.R. has served as a consultant for NeuroPace, Inc. but declares no targeted funding, compensation, or other financial incentive for this study.
References
- Anderson C.T., Tcheng T.K., Sun F.T., Morrell M.J. Day-night patterns of epileptiform activity in 65 patients with long-term ambulatory electrocorticography. J. Clin. Neurophysiol. 2015;32:406–412. doi: 10.1097/WNP.0000000000000183. [DOI] [PubMed] [Google Scholar]
- Baud M.O., Kleen J.K., Mirro E.A., Andrechak J.C., King-Stephens D., Chang E.F. Multi-day rhythms modulate seizure risk in epilepsy. Nat. Commun. 2018;9:88. doi: 10.1038/s41467-017-02577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey G.K., Morrell M.J., Mizrahi E.M., Goldman A., King-Stephens D., Nair D. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84:810–817. doi: 10.1212/WNL.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie M.J., Barry S.J., Bamagous G.A., Norrie J.D., Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–1554. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLorenzo D.J., Mangubat E.Z., Rossi M.A., Byrne R.W. Chronic unlimited recording electrocorticography-guided resective epilepsy surgery: technology-enabled enhanced fidelity in seizure focus localization with improved surgical efficacy. J. Neurosurg. 2014;120:1402–1414. doi: 10.3171/2014.1.JNS131592. [DOI] [PubMed] [Google Scholar]
- Duckrow R.B., Tcheng T.K. Daily variation in an intracranial EEG feature in humans detected by a responsive neurostimulator system. Epilepsia. 2007;48:1614–1620. doi: 10.1111/j.1528-1167.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- Enatsu R., Alexopoulos A., Bingaman W., Nair D. Complementary effect of surgical resection and responsive brain stimulation in the treatment of bitemporal lobe epilepsy: a case report. Epilepsy Behav. 2012;24:513–516. doi: 10.1016/j.yebeh.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Englot D.J., Birk H., Chang E.F. Seizure outcomes in nonresective epilepsy surgery: an update. Neurosurg. Rev. 2017;40:181–194. doi: 10.1007/s10143-016-0725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck C.N., King-Stephens D., Massey A.D., Nair D.R., Jobst B.C., Barkley G.L. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55:432–441. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katariwala N.M., Bakay R.A., Pennell P.B., Olson L.D., Henry T.R., Epstein C.M. Remission of intractable partial epilepsy following implantation of intracranial electrodes. Neurology. 2001;57:1505–1507. doi: 10.1212/wnl.57.8.1505. [DOI] [PubMed] [Google Scholar]
- King-Stephens D., Mirro E., Weber P.B., Laxer K.D., Van Ness P.C., Salanova V. Lateralization of mesial temporal lobe epilepsy with chronic ambulatory electrocorticography. Epilepsia. 2015;56:959–967. doi: 10.1111/epi.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane M.A., Kahlenberg C.A., Li Z., Kulandaival K., Secore K.L., Thadani V.M. The implantation effect: delay in seizure occurrence with implantation of intracranial electrodes. Acta Neurol Scand. 2017;135:115–121. doi: 10.1111/ane.12662. [DOI] [PubMed] [Google Scholar]
- Mackow M.J., Krishnan B., Bingaman W.E., Najm I.M., Alexopoulos A.V., Nair D.R. Increased caffeine intake leads to worsening of electrocorticographic epileptiform discharges as recorded with a responsive neurostimulation device. Clin Neurophysiol. 2016;127:2341–2342. doi: 10.1016/j.clinph.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Marsh E.D., Peltzer B., Brown M.W., 3rd, Wusthoff C., Storm P.B., Jr., Litt B. Interictal EEG spikes identify the region of electrographic seizure onset in some, but not all, pediatric epilepsy patients. Epilepsia. 2010;51:592–601. doi: 10.1111/j.1528-1167.2009.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell M.J., Group RNSSiES Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- Morrell M.J., Halpern C. Responsive direct brain stimulation for epilepsy. Neurosurg. Clin. N. Am. 2016;27:111–121. doi: 10.1016/j.nec.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Rao V.R., Leonard M.K., Kleen J.K., Lucas B.A., Mirro E.A., Chang E.F. Chronic ambulatory electrocorticography from human speech cortex. Neuroimage. 2017;153:273–282. doi: 10.1016/j.neuroimage.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Olasunkanmi A., Ma T.S., Carlson C., Devinsky O., Harter D.H. Epilepsy control following intracranial monitoring without resection in young children. Epilepsia. 2012;53:334–341. doi: 10.1111/j.1528-1167.2011.03380.x. [DOI] [PubMed] [Google Scholar]
- Shah A.K., Mittal S. Invasive electroencephalography monitoring: Indications and presurgical planning. Ann. Indian Acad. Neurol. 2014;17:S89–S94. doi: 10.4103/0972-2327.128668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart O., Rolston J.D., Epstein C.M., Gross R.E. Hippocampal seizure-onset laterality can change over long timescales: a same-patient observation over 500 days. Epilepsy Behav. Case Rep. 2013;1:56–61. doi: 10.1016/j.ebcr.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D., Gwinn R., Salinsky M., O'Malley J.P. Laterality and temporal distribution of seizures in patients with bitemporal independent seizures during a trial of responsive neurostimulation. Epilepsy Res. 2011;93:221–225. doi: 10.1016/j.eplepsyres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Spencer D.C., Sun F.T., Brown S.N., Jobst B.C., Fountain N.B., Wong V.S. Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia. 2016;57:1495–1502. doi: 10.1111/epi.13455. [DOI] [PubMed] [Google Scholar]
- Sun F.T., Arcot Desai S., Tcheng T.K., Morrell M.J. Changes in the electrocorticogram after implantation of intracranial electrodes in humans: the implant effect. Clin. Neurophysiol. 2018;129:676–686. doi: 10.1016/j.clinph.2017.10.036. [DOI] [PubMed] [Google Scholar]
- Warner N.M., Gwinn R.P., Doherty M.J. Individualizing therapies with responsive epilepsy neurostimulation - a mirtazapine case study of hippocampal excitability. Epilepsy Behav. Case Rep. 2016;6:70–72. doi: 10.1016/j.ebcr.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer J., von der Groeben F., Klarmann U., Weber C., Elger C.E., Urbach H. Risks and benefits of invasive epilepsy surgery workup with implanted subdural and depth electrodes. Epilepsia. 2012;53:1322–1332. doi: 10.1111/j.1528-1167.2012.03545.x. [DOI] [PubMed] [Google Scholar]