Rapid systemic signaling is accompanied by coordinated metabolic changes in local, transport, and systemic tissues.
Abstract
Plants evolved multiple signaling pathways that transduce light-related signals between leaves. These are thought to improve light stress acclimation in a process termed systemic acquired acclimation. Although responses to light stress have been studied extensively in local leaves, and to a lesser degree in systemic leaves, little is known about the responses that occur in the different tissues that connect the local to the systemic leaves. These could be important in defining the specificity of the systemic response as well as in supporting the generation of different systemic signals. Here, we report that local application of light stress to one rosette leaf of bolting Arabidopsis (Arabidopsis thaliana) plants resulted in a metabolic response that encompassed local, systemic and transport tissues (stem tissues that connect the local to the systemic tissues), demonstrating a high degree of physical and metabolic continuity between different tissues throughout the plant. Our results further indicate that the response of many of the systemically altered metabolites is associated with the function of the reactive oxygen species wave and that the levels of eight different metabolites are altered in a similar manner in all tissues tested (local, systemic, and transport). These compounds could define a core metabolic signature for light stress that propagates from the local to the systemic leaves. Our findings suggest that metabolic changes occurring in cells that connect the local and systemic tissues play an important role in systemic acquired acclimation and could convey specificity to the rapid systemic response of plants to light stress.
Light regulates many different aspects of plant development, growth, and responses to biotic and abiotic conditions. Because in nature not all leaves belonging to the same plant are subjected to the same light intensity or quality, plants evolved multiple signaling pathways to transduce light-related signals between different leaves (Karpinski et al., 1999; Rossel et al., 2007; Kangasjärvi et al., 2009; Szechyńska-Hebda et al., 2010, 2017; Gorecka et al., 2014; Gilroy et al., 2016; Devireddy et al., 2018). These are thought to improve light stress acclimation in a process termed systemic acquired acclimation (SAA; Karpinski et al., 1999). The process of SAA is thought to optimize the overall light perception of the plant and prevent photoinhibition in leaves that are not yet acclimated to high light (HL) intensities (Suzuki et al., 2013; Devireddy et al., 2018). Therefore, a local leaf that is subjected to an HL stress could send a systemic signal(s) to a systemic leaf or the floral tissue that has not yet been subjected to HL intensities, activating different acclimation pathways in these systemic tissues and preparing them for the possibility of being subjected to light stress (Miller et al., 2009; Mittler et al., 2011; Gilroy et al., 2014; Choi et al., 2017; Kollist et al., 2018).
Local responses to light stress have been studied at the physiological, transcriptional, proteomic/phosphoproteomic, and metabolomic levels (Davis et al., 2013; Suzuki et al., 2013, 2015; Chen and Hoehenwarter, 2015; Bode et al., 2016; Crisp et al., 2017; du Plessis et al., 2017; Miller et al., 2017). They were shown to include the activation of photorespiration and different mechanisms to remove reactive oxygen species (ROS), such as the ascorbate-glutathione cycle, the water-water cycle, superoxide dismutases, 2-Cys peroxiredoxins, and catalases (Mullineaux et al., 2000; Oelze et al., 2012; Cui et al., 2016; Kerchev et al., 2016; Müller et al., 2017); mechanisms for singlet oxygen removal and nonphotochemical quenching (Ramel et al., 2012; Kulasek et al., 2016); retrograde signaling from the chloroplast and mitochondria and the function of mitochondrial alternative oxidases (Oelze et al., 2012; Gordon et al., 2013; Karpiński et al., 2013; Szechyńska-Hebda and Karpiński, 2013; Florez-Sarasa et al., 2016a); cyclic electron flow and the synthesis of different flavonoid compounds (Florez-Sarasa et al., 2016b; Alric and Johnson, 2017); abscisic acid (ABA), nitric oxide, and jasmonic acid (JA)/12-oxophytodienoic acid signaling (Gorecka et al., 2014; Trotta et al., 2014; Müller et al., 2017; Devireddy et al., 2018); stomatal responses (Devireddy et al., 2018); and leaf morphological alterations (Munekage et al., 2015). In addition, responses to light stress at the local leaf level were divided into rapid and long-term acclimation responses (Suzuki et al., 2015).
The different systemic signals activated in response to the perception of light stress by a local leaf are thought to include electric signals, systemic ROS and calcium waves, systemic redox changes, volatile and transported hormones such as JA, ABA, and auxin (indole-3-acetic acid [IAA]), and hydraulic pressure waves (Karpinski et al., 1999; Rossel et al., 2007; Kangasjärvi et al., 2009; Miller et al., 2009; Szechyńska-Hebda et al., 2010, 2017; Mittler et al., 2011; Christmann et al., 2013; Suzuki et al., 2013; Gilroy et al., 2014, 2016; Gorecka et al., 2014; Matsuo and Oelmüller, 2015; Guo et al., 2016; Choi et al., 2017; Devireddy et al., 2018). The triggering of these signals is thought to require the production of singlet oxygen at the local leaf, the function of the PsbS (PSII 22-kD protein; CP22; NONPHOTOCHEMICAL QUENCHING4) protein involved in nonphotochemical quenching, alterations in calcium and ROS production, and the function of the plant hormone ABA (Miller et al., 2009; Ciszak et al., 2015; Carmody et al., 2016; Devireddy et al., 2018). The transduction of the systemic signal from the local to the systemic leaf is thought to involve the function of the respiratory burst oxidase homolog D (RBOHD) protein and the function of calcium channels and calcium-derived protein kinases such as two-pore channel 1 and CDPKs that drive the ROS/Ca2+ wave, the plant hormone IAA, and electric and hydraulic signals mediated by yet unknown proteins and channels (Miller et al., 2009; Drerup et al., 2013; Dubiella et al., 2013; Choi et al., 2014, 2017; Guo et al., 2016; Devireddy et al., 2018). In addition, the transmission of the systemic signal was shown to be most efficient among leaves that have a direct vascular connection between them, suggesting that at least some of the components involved are transmitted through the phloem or other vascular companion cells such as bundle sheath cells (Fryer et al., 2003; Rossel et al., 2007; Kangasjärvi et al., 2009; Carmody et al., 2016; Guo et al., 2016).
Compared with the many studies conducted on local leaves subjected to light stress, little is known about the physiological, metabolic, and molecular alterations that accompany SAA in systemic leaves. A recent study has shown that systemic responses to light stress involved rapid stomatal responses in leaves that were not subjected directly to the HL stress (Devireddy et al., 2018). In addition, certain stress-specific metabolic and transcriptomic changes were shown to occur in systemic leaves in response to HL stress of a local leaf (Suzuki et al., 2013). These include metabolites involved in photorespiration and certain HL-response transcripts such as Zat10 and Zat12 (Davletova et al., 2005; Rossel et al., 2007). In contrast to studies conducted on local or systemic tissues, less is known about the metabolic responses that occur in the tissues that transmit different signals from the local to the systemic leaf. Because, in addition to light stress, systemic signals such as the ROS/Ca2+/hydraulic/electric waves are triggered by many other stimuli such as wounding, salinity, or biotic stimuli, the exact metabolic changes that occur in the tissues transporting the systemic signal could be responsible for the specificity of the transmitted response (Suzuki et al., 2013; Gilroy et al., 2014; Choi et al., 2017). In addition, they could be required to support the active production of ROS during the propagation of the ROS wave (Miller et al., 2009). To begin addressing these important questions and to identify key metabolites associated with the SAA response of plants to light stress, we conducted time-course metabolic profiling analyses of local leaves, systemic tissues, and transport tissues (tissues connecting the local and systemic leaves; Fig. 1A) in response to light stress. We hypothesized that some of the metabolic changes that occur in the local leaf in response to light stress would be propagated or transported throughout the plant, reaching all the way to the systemic tissues, inducing in them a heightened state of acclimation to light stress, and that the mediation of this response would be dependent upon the function of the RBOHD protein and the ROS wave. Here, we report that the metabolic response of systemic tissues is highly similar to that of local leaves subjected to HL stress. In addition, we report that the responses of many of the systemically altered metabolites are dependent on the function of the RBOHD protein and that the levels of eight different metabolites are altered in a similar way in all tissues tested (local, systemic, and stem tissues that connect the local and systemic tissues) in response to local HL application. These compounds could define a core metabolic signature that propagates from the local to the systemic leaves during light stress. We further propose that NADPH produced due to the function of cytosolic NADP-dependent isocitrate dehydrogenase (cICDH) supports the initiation/propagation of the systemic signal and is linked to ROS production in systemic tissues. Taken together, our findings suggest that rapid metabolic changes occurring in cells that connect the local and systemic tissues could play an important role in mediating the rapid systemic response of plants to light stress. In addition, they demonstrate a high degree of physical and metabolic continuity between different tissues throughout the plant.
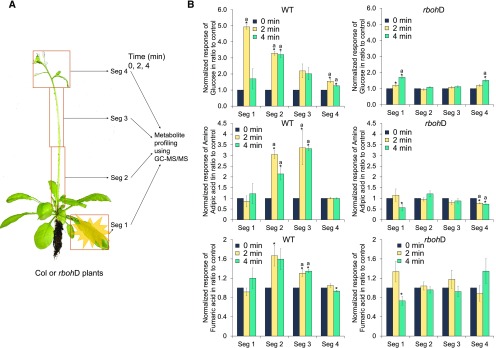
Figure 1.
Rapid metabolic responses of local, connecting, and systemic tissues to locally applied light stress. A, Outline of the experimental procedure used to measure metabolic responses to locally applied light stress. GC-MS/MS, Gas chromatography-tandem mass spectrometry. B, Bar graphs showing changes in the levels of different metabolites in response to light stress applied to a local rosette leaf (segment 1 [Seg 1]). Additional compounds are shown in Table 1, Supplemental Figures S1 to S3, and Supplemental Table S1. Results are presented as means ± se of three biological replicates each with 40 plants per time point. Significant changes (P < 0.05) from time zero (0 min) in each segment were calculated using an ANOVA followed by Dunnett’s test (indicated with letter a) or a two-tailed Student’s t test (indicated with an asterisk).
RESULTS
Rapid Metabolic Responses of Local, Connecting, and Systemic Tissues to Locally Applied Light Stress
To study metabolic responses associated with light stress-induced SAA, we subjected a single Arabidopsis (Arabidopsis thaliana) rosette leaf to HL stress (1,500 μmol m−2 s−1) and sampled the local (HL-treated) leaf, fragments of the inflorescence stem (tissues connecting the local to the systemic tissues; herein referred to as transport tissues), and systemic tissues (the top 2-cm part of the inflorescence with one cauline leaf) at 0, 1, 2, 4, 8, and 12 min following HL stress application (Fig. 1A; for a more detailed protocol, see “Materials and Methods”; the total height of the inflorescence stem of all plants used was about 12 cm). The rationale for choosing this experimental system is that a stress-induced signal or metabolic state originating in the vegetative tissues of the plant (i.e. a local rosette leaf) could be transmitted all the way to the systemic leaves and/or floral tissues of the plant (i.e. the inflorescence tip), inducing in them a state of heightened acclimation to stress. This process would be especially important due to the high vulnerability of reproductive plant tissues to stress (Gray and Brady, 2016) and in line with the proposed role of SAA to facilitate the acclimation of tissues that were not yet subjected to stress (Karpinski et al., 1999; Rossel et al., 2007; Kangasjärvi et al., 2009; Szechyńska-Hebda et al., 2010, 2017; Gorecka et al., 2014; Gilroy et al., 2016; Devireddy et al., 2018). All tissues sampled were subjected to GC-MS/MS metabolic profiling analysis using ribitol as an internal standard (Fig. 1; Table 1; Supplemental Figs. S1–S3; Suzuki et al., 2013; Dias et al., 2015). In total, we detected and measured relative changes in the levels of 60 different compounds. Of these, 13 compounds were found to respond in local leaves, 17 in tissues connecting the local and systemic tissues (transport tissue), and 16 in systemic tissues (Table 1). The earliest significant changes we detected in almost all tissue types sampled initiated at 1 or 2 min post HL stress application to the local leaf (Fig. 1; Supplemental Figs. S1–S3). These could be divided into five different types: (1) metabolites that changed primarily in transport and systemic tissues but not in local tissues (e.g. Glu and JA); (2) metabolites that responded primarily in local and systemic tissues and to a lesser degree in transport tissues (Gly and glycolic acid); (3) metabolites that altered primarily in all tissues (e.g. 2-oxoglutaric acid, Suc, shikimic acid, and malic acid); (4) metabolites that changed primarily in transport tissues (e.g. aminoadipic acid); and (5) metabolites that responded primarily in local and transport tissues and not in systemic tissues (IAA). The results are presented in Figure 1, Table 1, and Supplemental Figures S1 to S3 and demonstrate that systemic metabolic responses to a local application of HL stress are rapid and can be detected in local, transport, and systemic tissues as early as 1 to 2 min. In addition, they demonstrate that the responses of several different metabolites are similar in local, transport, and systemic tissues, suggesting that these metabolites could be associated with the transduction of systemic signals and/or SAA.
Table 1. Summary of metabolic changes occurring during light-induced rapid systemic signaling in Arabidopsis.
Metabolites significantly altered at P < 0.05 (ANOVA followed by Dunnett’s test and/or Student’s t test) are shown. The metabolic changes are divided based on their overall response patterns. Response patterns in rbohD mutants are indicated in the right column.
| Metabolite Name | Response in Local Tissue | Response in Transport Tissue | Response in Systemic Tissue | Response in rbohD |
|---|---|---|---|---|
| Metabolites that respond in transport and systemic tissue | ||||
| Glu | No change | Increase | Increase | Delayed increase |
| JA | No change | Increase | Increase | No change |
| Trp | No change | Increase | Increase | No change |
| Glyceraldehyde-3-phosphate | No change | Decrease | Increase | Increase |
| Metabolites that respond primarily in local and systemic tissue | ||||
| Gly | Increase | No change | Increase | No change |
| Glycolic acid | Increase | Increase/decrease | Increase | Increase |
| Metabolites that respond in local, transport, and systemic tissue | ||||
| Glc | Increase | Increase | Increase | Delayed increase |
| Suc | Increase | Increase | Increase | No change |
| Citric acid | Increase | Decrease | Increase | Decrease |
| Lactic acid | Decrease | Increase | Increase | No change |
| 2-Oxoglutaric acid | Increase | Increase | Increase | Delayed increase |
| Shikimic acid | Increase | Increase | Increase | Increase |
| Maleic acid | Increase | Increase | Increase | No change |
| Fumaric acid | Increase | Increase | Increase | No change |
| Malic acid | Increase | Increase | Increase | No change |
| Inositol | Increase | Increase | Increase | Increase |
| Metabolites that respond in transport tissue only | ||||
| Aminoadipic acid | No change | Increase | No change | No change |
| Linolenic acid | No change | Decrease | No change | No change |
| Metabolites that respond in local and transport tissue only | ||||
| IAA | Increase | Increase | No change | Delayed increase |
Accumulation of Hydrogen Peroxide along the Path of the Systemic Signal
The rate of the systemic metabolic response to local application of HL stress was relatively rapid compared with the rate of phloem transport in Arabidopsis (estimated to be about 3 cm min−1; Ruan, 2017). Thus, the levels of different compounds began to change in systemic tissues that were 12 cm away from the local leaf in about 1 to 2 min, suggesting a rate of about 6 cm min−1 or higher. Prior studies have shown that the ROS wave autopropagates throughout the plant at about 8 cm min−1 in Arabidopsis (Miller et al., 2009; Devireddy et al., 2018). These studies prompted us to measure hydrogen peroxide (H2O2) accumulation in the different tissues shown in Figure 1A. The goal of this analysis was to determine whether the initial changes in metabolite levels (Fig. 1; Supplemental Figs. S1–S3) coincided with the activation of the ROS wave (Miller et al., 2009; Suzuki et al., 2013; Devireddy et al., 2018). As shown in Figure 2, H2O2 levels accumulated in local leaves at 1, 2, 4, 8, and 12 min post HL application. This finding was expected, since HL stress was shown previously to induce H2O2 accumulation (Szechyńska-Hebda et al., 2010; Devireddy et al., 2018). Interestingly, the accumulation of H2O2 also was found at 1 and 2 min in segments 2, 3, and 5 of the transport tissues, and at 2 min in segment 4, post HL application to the local leaf (Fig. 2). In systemic tissues, significant H2O2 accumulation was detected at 4 min post HL application to the local leaf. These findings suggest that, at least in transport tissues, detectable H2O2 accumulation coincides with rapid change in metabolic levels (Fig. 1; Supplemental Figs. S1–S3). The results shown in Figure 2 also demonstrate that the accumulation of detectable H2O2 levels by our assay is transient during the course of the SAA to HL stress, suggesting that a single wave of ROS production could potentially be triggered following the local application of HL stress (Miller et al., 2009; Mittler et al., 2011).
Figure 2.
Accumulation of H2O2 along the path of the systemic signal. A, Outline of the experimental procedure used to measure the systemic accumulation of H2O2 in response to locally applied light stress. B, Bar graph showing changes in the levels of H2O2 in response to light stress applied to a local rosette leaf (segment 1 [Seg 1]). Results are presented as means ± se of three biological replicates each with 40 plants per time point. Significant changes (P < 0.05) from time zero (0 min) in each segment were calculated using an ANOVA followed by Dunnett’s test (indicated with letter a) or a two-tailed Student’s t test (indicated with an asterisk).
Dependency of the Systemic Metabolic Response on the Function of the RBOHD Protein
The finding that H2O2 accumulated in transport tissues at 1 and/or 2 min post HL treatment of the local leaf (Fig. 2) suggests that the ROS wave could be involved in the initiation/propagation of at least some of the systemic metabolic responses shown in Figure 1 and Supplemental Figures S1 to S3. Because the ROS wave is dependent on the function of the RBOHD protein in Arabidopsis (Miller et al., 2009; Mittler et al., 2011; Suzuki et al., 2013; Devireddy et al., 2018), we compared the HL-induced systemic metabolic response of wild-type plants with that of the rbohD mutant plants. As shown in Figure 3 and Table 1, the accumulation of 14 out of the 19 compounds that showed increases in their relative concentrations in transport and/or systemic tissues in response to local application of HL was suppressed in rbohD plants. Of particular interest to this analysis is the fact that JA accumulation was suppressed in rbohD plants, whereas the accumulation of IAA was delayed. In addition, the enhanced accumulation of aminoadipic acid, a marker for oxidative stress (Requena et al., 2001), in transport tissues was suppressed in rbohD plants. As expected (Miller et al., 2009; Devireddy et al., 2018), compared with wild-type plants (Fig. 2), transient accumulation in H2O2 levels in the different fragments was not found in rbohD mutants (Supplemental Fig. S4). Taken together, these findings (Figs. 1–3; Table 1; Supplemental Figs. S1–S4; Supplemental Tables S1 and S2) suggest that the systemic metabolic response to light stress could involve both ROS wave-dependent and ROS wave-independent compounds, demonstrating that more than one type of systemic signal is activated during SAA.
Figure 3.
Dependency of the metabolic response on the function of the RBOHD protein. A, Outline of the experimental procedure used to measure metabolic responses to locally applied light stress in wild-type (Columbia [Col]) and rbohD plants. B, Bar graphs showing changes in the levels of different metabolites in response to light stress applied to a local rosette leaf (segment 1 [Seg 1]) in wild-type (WT) and rbohD plants. Additional compounds are shown in Table 1 and Supplemental Table S1. Results are presented as means ± se of three biological replicates each with 40 plants per time point. Significant changes (P < 0.05) from time zero (0 min) in each segment were calculated using an ANOVA followed by Dunnett’s test (indicated with letter a) or a two-tailed Student’s t test (indicated with an asterisk).
Propagation of the Metabolic Response Is Mediated Primarily in the Upward Direction along the Stem of Arabidopsis
To determine how similar is the locally applied HL-induced metabolic response of systemic leaves (SAA) to that of systemic leaves subjected directly to HL stress, as well as to determine whether the systemic signal could propagate in the downward direction along the Arabidopsis inflorescence, we subjected the upper part of the inflorescence having one cauline leaf (referred to above as the systemic tissue) to HL stress and measured changes in metabolic levels in the systemic, transport, and local tissues (Fig. 4). The rationale for choosing this experimental system was as follows. (1) The ROS wave was shown to propagate in both the upward and downward directions along the Arabidopsis inflorescence stem (Miller et al., 2009), and if this signal is mediating the metabolic responses reported here, they should be propagating in both directions. (2) Phloem connections and mobility are directed primarily from the vegetative to the reproductive tissues, and if the responses reported here are mediated through the phloem, they should be moving primarily in the upward direction. (3) Responses to light, temperature, wind, and relative air content might be first sensed at the tip of the plant and only then propagated in the downward direction to induce a successful SAA of the entire plant. Direct application of HL stress to the systemic tissue resulted in the accumulation of eight different compounds in systemic tissues (citric acid, lactic acid, 2-oxoglutaric acid, Gly, glycolic acid, maleic acid, malic acid, and glyceraldehyde-3-phosphate; Supplemental Table S1), all of which also accumulated in systemic tissues in response to local application of HL stress (SAA; Fig. 4; Table 1; Supplemental Table S1). Similarly, direct application of HL stress to the systemic tissue resulted in changes in the levels of six different compounds in transport tissues (Glc, citric acid, lactic acid, 2-oxoglutaric acid, JA, and fumaric acid; Supplemental Table S1), all of which also were altered in transport tissues in response to local application of HL stress (SAA; Fig. 4; Table 1; Supplemental Table S1). In contrast, direct application of HL stress to the systemic tissue resulted in changes in the levels of only three different compounds in local leaves (Glu, shikimic acid, and fumaric acid; Supplemental Table S1), only two of which (shikimic acid and fumaric acid) also were altered in local tissues in response to the local application of HL stress (Fig. 4; Table 1; Supplemental Table S1). These findings reveal a high degree of overlap between the metabolic response of systemic tissues to HL stress applied to the local or the systemic tissues, demonstrating that the metabolic SAA response is very similar to the real HL stress response of the systemic tissue. In addition, the findings presented in Figure 4 suggest that, although the systemic metabolic response can travel in both the upward and downward directions along the Arabidopsis inflorescence, it propagates faster or more efficiently in the upward direction.
Figure 4.
Propagation of the metabolic response is mediated primarily in the upward direction along the stem of Arabidopsis. A, Outline of the experimental procedure used to measure metabolic responses to light stress applied to a single rosette leaf (local tissue). B, Venn diagrams showing the overlap between changes in the levels of different metabolites in response to light stress applied to a single rosette leaf (local tissue) or to the top 2 cm of the inflorescence stem (systemic tissue). See text and Supplemental Table S1 for the identities of the different compounds. C, Outline of the experimental procedure used to measure metabolic responses to light stress applied to the top 2 cm of the inflorescence stem (systemic tissue). Results are presented for three biological replicates each with 40 plants per time point. Significant changes (P < 0.05) from time zero (0 min) in each segment were calculated using an ANOVA followed by Dunnett’s test or a two-tailed Student’s t test.
Possible Involvement of cICDH in Supporting the Initiation/Propagation of Systemic Responses to HL Stress
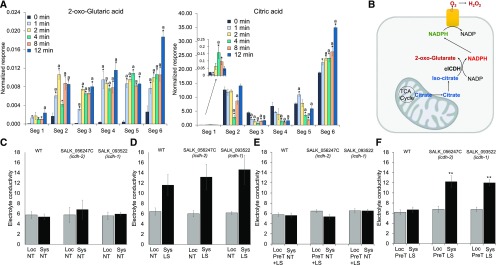
The rapid rate of the systemic metabolic response (Fig. 1), the systemic accumulation of H2O2 (Fig. 2), the dependency of many metabolites involved in this response on the function of RBOHD (Table 1; Fig. 3), and the partial dual directionality of this response along the inflorescence stem of Arabidopsis (Fig. 4) suggest that the ROS wave could be involved in facilitating at least some aspects of this response. Further supporting this possibility are our previous studies showing that the ROS wave is required for HL-induced SAA (Suzuki et al., 2013; Devireddy et al., 2018). Producing a burst of H2O2 via the function of the NADPH oxidase enzyme RBOHD requires a supply of NADPH. Therefore, we examined what pathways within the plant could provide this NADPH during the HL-induced metabolic response. The changes in metabolite levels in the different tissues studied suggested a possible involvement of at least two different pathways. An increase in Glc coupled with an increase in lactic acid could suggest the activation of anaerobic glycolysis in transport and systemic tissues (Table 1). Although this pathway will provide ATP and NADH, it does not directly produce NADPH. In contrast, an increase in 2-oxoglutaric acid coupled with a decrease in citric acid (Fig. 5A) could suggest that citrate, potentially originating in the mitochondria via the tricarboxylic acid cycle, could be converted to isocitrate first via aconitase and then to 2-oxoglutaric acid via cICDH, directly yielding NADPH that could be used for RBOHD function (Fig. 5B). The increase in Glc also could support this possibility, since it will feed sugars into the tricarboxylic acid cycle. This possibility also is supported by previous studies that suggested a role for cICDH in providing NADPH for different reactions involved in ROS metabolism in Arabidopsis (Mhamdi et al., 2010). To test the possibility that cICDH is involved in generating NADPH to support the HL-induced SAA process, we tested the local and systemic acclimation of two mutants impaired in cICDH function (SALK_056247C and SALK_093522). As shown in Figure 5C, similar to wild-type plants, the local leaves of the two cICDH mutants could acclimate to HL stress following a brief local HL stress treatment. In contrast, unlike wild-type plants, the systemic leaves of the two cICDH mutants failed to acclimate to HL stress following a brief local application of HL stress (SAA; Fig. 5, C–F; enhanced ion leakage from cells, measured as increased conductivity following light stress, is a measure of tissue injury). Furthermore, the two cICDH mutants failed to accumulate H2O2 in their local and systemic leaves in response to the local application of light stress (Supplemental Fig. S5), supporting our hypothesis that cICDH plays a key role in supporting the production of H2O2 during the initiation/propagation of the ROS wave. The findings shown in Figure 5 and Supplemental Figure S5 suggest that, although cICDH is not required for HL stress acclimation per se, it could be required for H2O2 production that supports the systemic signal that mediates SAA to light stress.
Figure 5.
Possible involvement of cICDH in supporting the initiation/propagation of systemic signals during SAA to light stress. A, Bar graphs showing changes in the levels of 2-oxoglutaric acid and citric acid in response to light stress applied to a local rosette leaf. See Figure 1A for the experimental design. Results are presented as means ± se of three biological replicates each with 40 plants per time point. Significant changes (P < 0.05) from time zero (0 min) in each segment (Seg) were calculated using an ANOVA followed by Dunnett’s test (indicated with letter a) or a two-tailed Student’s t test (indicated with an asterisk). B, Hypothetical model showing the conversion of citrate to isocitrate first via aconitase and then to 2-oxoglutaric acid via cICDH, directly yielding NADPH that could be used during systemic signaling (e.g. RBOHD function). TCA, Tricarboxylic acid. C to F, Local (Loc) and systemic (Sys) acclimation of wild-type (WT) plants and mutants deficient in cICDH function (SALK_056247C and SALK_093522) to light stress. C, Conductivity measurements of local and systemic leaves in the absence of any light stress or pretreatment of leaves with light stress (NT, nontreated). D, Conductivity measurements of local and systemic leaves following the application of light stress (LS) to systemic leaves. E, Conductivity measurements of local and systemic leaves following pretreatment of local leaves (PreT) and the application of light stress to local leaves (+LS). F, Conductivity measurements of local and systemic leaves following pretreatment of local leaves and the application of HL stress to systemic leaves. Results are presented as means ± se of three biological replicates each with 30 plants per time point; **, P < 0.01 (Student’s two-tailed t test).
DISCUSSION
Our study unraveled several different metabolic responses associated with HL-induced rapid systemic signaling in Arabidopsis. These occurred in local, transport, and systemic tissues and displayed a complex pattern evident in the rate and overall accumulation of the different metabolites in the different tissues. Of particular interest are our findings that many of the metabolic changes that occur in systemic tissues during SAA also occur in systemic tissues directly subjected to the HL stress (Fig. 4) and the finding that many of these metabolic changes also occur in the transport tissues that connect the local and the systemic tissues (Figs. 1, 3, and 4; Table 1). These findings reveal that the process of SAA is accompanied by rapid systemic changes in the levels of many different metabolites, relevant to light stress acclimation, and that some of these changes occur in all tissues of the plant in response to local application of HL stress, potentially reflecting a high degree of physical and metabolic continuity among different tissues. At least two of the metabolites we identified as rapidly accumulating during the SAA of Arabidopsis to light stress (Suc and Gly; Table 1) were found previously to accumulate in systemic tissues of Arabidopsis in response to light stress, supporting the validity of our analysis (Suzuki et al., 2013).
Several different factors should be kept in mind when considering the overall levels and rates of accumulation of the different compounds measured (Figs. 1 and 3; Supplemental Figs. S1–S3), as well as ROS accumulation (Fig. 2), in the different tissues sampled in response to local application of HL stress. These include the different developmental programs occurring in each tissue type (rosette leaf, inflorescence stem, and systemic tissues) and the restrictions they could impose on gene and metabolite accumulation; the different physiological state of each tissue (e.g. sink versus source versus transport tissue); the basal hormone level in each fragment; and the ratio between different cell types composing each of the different tissues (e.g. the ratio between phloem, xylem vessels, companion cells, and bundle sheath cells as well as the ratio between mesophyll and epidermal cells). These could affect the overall accumulation and/or rate of accumulation of ROS and different metabolites and hormones as well as the function of RBOHD. The complex overall responses observed in our study in the different tissues sampled, therefore, could be the result of integrating many different signals and programs occurring within these tissues.
The different systemic responses observed during HL-induced SAA could be divided into RBOHD-dependent and RBOHD-independent responses, suggesting that at least some of the responses observed are dependent on the function of the ROS wave. This possibility was supported by measurements of H2O2 levels in the different tissues that suggested that a single wave of ROS could potentially travel throughout the plant in response to a local stimulus (Fig. 2; Supplemental Fig. S4; Mittler et al., 2011). This finding is important since prior studies of the ROS wave using promoter-luciferase constructs (Zat12 or WRKY40; Miller et al., 2009; Mittler et al., 2011; Gilroy et al., 2014; Devireddy et al., 2018) could not distinguish between the occurrence of a single or multiple ROS waves, due to the fact that the promoter-reporter constructs stayed at the on position for a long time following their initial activation. It is also possible, however, that more than one ROS wave occurs and that many of the ROS waves that occur are at levels that are below our detection limit. The development of new and more sensitive and dynamic imaging methods for H2O2 should be able to address the existence of these in future studies (Noctor et al., 2016).
The ROS wave was shown to be activated in response to many different stimuli, including HL, wounding, heat stress, and salinity, suggesting that, although it is required for SAA to some of these stresses, it does not convey specificity to the systemic response itself (Miller et al., 2009; Mittler et al., 2011; Suzuki et al., 2013). Thus, other factors, such as calcium waves, electric signals, hormones, and other metabolic responses, could be involved in mediating specificity to the SAA response (Gilroy et al., 2014; Choi et al., 2017). Our analysis here of metabolic changes during HL-induced SAA provides further support to this possibility, demonstrating that the process of SAA to HL stress is complex and involves ROS wave-dependent and ROS wave-independent responses, hormones such as JA and IAA, and many different metabolites with different accumulation patterns. It would be interesting in future studies to study how the systemic metabolic responses to HL stress, analyzed in this study, are different from those to other stresses such as heat or wounding (Suzuki et al., 2013). Such studies could provide a more complete picture of the signals involved in the SAA responses to different abiotic stresses. In addition, a more careful analysis of hormone levels, in particular ABA, which is absent from our current analysis, should shed further light on the complex relationships between the ROS/Ca2+/electric/hydraulic waves and hormones such as ABA and JA (Devireddy et al., 2018).
Because the rosette leaves subjected to HL stress in our study are connected with the inflorescence stem via vascular tissues such as phloem transport and companion cells, it is possible that some of the systemic metabolic changes observed in our study propagated through these tissues. In this context, it is important to note that bundle sheath cells of Arabidopsis were proposed to be involved in mediating systemic signals during HL-induced SAA (Kangasjärvi et al., 2009) and were shown to play a key role in ABA signaling and gene expression during this response (Fryer et al., 2003; Gorecka et al., 2014). Although bundle sheath cells, as well as phloem transport vessels, can be used to translocate many of the metabolites studied in our experiments from the local to the systemic tissues, the speed of the metabolic response observed (about 6 cm min−1 or more) appears to be too fast to be explained by such transports. At least two different models could account for such speed. The local application of HL stress could have triggered a mechanism that facilitated phloem and/or bundle sheath transport of metabolites from the local to the systemic tissues. This mechanism could be triggered by different components of the ROS/Ca2+/electric/hydraulic wave (Mittler et al., 2011; Christmann et al., 2013; Choi et al., 2017) that travel at a faster rate than 6 cm min−1 and could trigger faster translocation of metabolites through these tissues. Another possibility is that each cell along the path of the systemic signal actively synthesizes the different metabolites (similar to the manner in which it produces ROS during the autopropagation of the ROS wave; Miller et al., 2009; Mittler et al., 2011) and that this process autopropagates a metabolic signature from the local to the systemic tissue. Of course, further studies are needed to address these and other mechanisms that could mediate rapid changes in metabolic activity during SAA. In particular, these should focus on comparing the different local and systemic metabolic responses of plants to different stresses such as light, heat, and mechanical injury (Suzuki et al., 2013) and on studying what plant tissues mediate the different systemic signals (e.g. epidermis, phloem, and companion cells; Kangasjärvi et al., 2009). In addition, a better mechanistic understanding of how apoplastic changes in ROS levels are translated into changes in different metabolic levels or phloem transport is needed.
The identification of a core set of metabolites that change in a similar manner in the local leaf, stem, and systemic tissues in response to local application of light stress could suggest that the rapid metabolic response to HL stress links the local and systemic leaves and prepares the systemic tissues for the possibility of being subjected to HL stress. This response could be a result of the active transport of metabolites from local to systemic tissues (Shah et al., 2014) or a result of an autopropagating process (Mittler et al., 2011). Taken together, our findings suggest that metabolic changes occurring in cells that connect the local and systemic tissues play an important role in SAA and could convey specificity to the rapid systemic response of plants to light stress.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia wild-type, rbohD knockout (Torres et al., 2002; Miller et al., 2009), and icdh-1 (SALK_093522) and icdh-2 (SALK_056247C; Mhamdi et al., 2010) mutant plants (Arabidopsis Biological Resource Center; Supplemental Fig. S6) were grown in peat pellets (Jiffy-7; Jiffy) at 23°C. Plants were grown under constant low light (50 μmol m−2 s−1) for bolting and metabolic analysis or under an 8-h light period (short day; low light, 50 μmol m−2 s−1) for acclimation studies, as described previously (Suzuki et al., 2013, 2015).
Light Stress Treatment
For metabolite profiling, 21- to 30-d-old approximately 12-cm-tall bolting plants were used, and a single rosette leaf was exposed to a light intensity of 1,500 µmol m−2 s−1 for periods of 0, 1, 2, 4, 8, and 12 min as described by Devireddy et al. (2018). As a control, similar age and developmental plants grown side by side with the plants used for the experiments were sampled at 0, 6, and 12 min without being exposed to light stress. At each of the time points indicated above, whole plants were immediately flash frozen in liquid nitrogen and cut into six different segments: segment 1 being the treated local rosette leaf, segments 2 to 5 being the inflorescence stem (without any cauline leaves), and segment 6 being the top 2 cm of the inflorescence with one cauline leaf (Fig. 1A). For the comparison between wild-type Columbia and rbohD plants, a rosette leaf was treated with a similar light stress for 0, 2, and 4 min and plants were sampled and cut into four different segments: segment 1 being the local leaf, segments 2 and 3 being the inflorescence stem (without any cauline leaves), and the top 2 cm of the inflorescence with one cauline leaf being segment 4 (Fig. 3A). To compare between light stress applied to the local or the systemic tissue, light stress was applied to a local leaf as described above (local tissue) or to the top 2 cm of the inflorescence stem with one cauline leaf (systemic tissue) and the plant was cut into three segments: rosette leaf, inflorescence stem without cauline leaves, and the top of the inflorescence stem with one cauline leaf. Forty different plants were used for each time point in each experiment, and each experiment was repeated in three biological repeats. Steady-state transcript levels in response to light stress were determined according to Devireddy et al. (2018).
Metabolome Analysis
Metabolite profiling was performed using GC-MS/MS. Metabolite extraction and derivatization were performed as described previously with slight modifications (Shuman et al., 2011; Suzuki et al., 2013; Dias et al., 2015). In brief, plant samples were ground in liquid nitrogen with mortar and pestle and lyophilized for 72 h in a freeze drier (Labconco). Ten milligrams of lyophilized samples was taken per replicate. For metabolite extraction, samples were ground in an MM 300 mixer mill (Retsch) using 2.3-mm stainless steel beads. Samples then were extracted with 250 µL of an ice-cold methanol:water (3:1, v/v) solution containing ribitol internal standard at 12 µg mL−1, ground again in 250 µL of an ice-cold methanol:water (1:3, v/v) solution, and centrifuged for 10 min at 13,000g at 4°C. The aqueous fraction was transferred to a 1.1-mL glass Chromacol vial and extracted again with 250 µL of chloroform. The upper aqueous fraction was transferred to a clean vial and dried at room temperature in the Centrivap centrifugal concentrator (Labconco). The dried polar phase was derivatized by adding 80 µL of methoxamine reagent (Thermo Fisher Scientific) for 135 min at 45°C followed by 80 µL of N-methyl-N-trimethylsilyltrifluoroacetamide + 1% (v/v) trimethylchlorosilane silylation reagent (Thermo Fisher Scientific) for 30 min at 37°C.
A 1-µL derivatized sample was injected into the GC-MS/MS system consisting of Triplus RSH autosampler, Trace 1300 gas chromatograph, and TSQ8000 electron ionization triple quadrupole mass spectrometer (Thermo Fisher Scientific). Chromatography was performed using a 30-m × 0.25-mm Rxi-5Sil MS column with film thickness of 0.25 µm (Restek). Samples were run in both split (10:1) and splitless mode, and the injector temperature was 230°C. The oven temperature program was 5 min isothermal at 70°C followed by a 5°C min−1 ramp to 310°C and hold for 1 min at 310°C. Carrier gas was helium at a constant flow of 1 mL min−1. To monitor instrument sensitivity, 1 pg µL−1 octafluoronaphthalene was injected after every batch of 20 samples. The transfer line and ion source were kept at 250°C. An in-house MS/MS compound database was built using AutoSRM software (Thermo Fisher Scientific). Pure standard compound (50 µg) was derivatized as described above and run in full scan mode. The retention time was recorded, and three to four precursor ions were chosen and run in selected ion monitoring mode for product ion screening. Most abundant product ions were selected for collision energy optimization. After applying a range of collision energy from 0 to 50 V, the optimized collision energy for each precursor-to-product ion transition was then exported into the compound database. The compound database was created using Trace Finder software (Thermo Fisher Scientific) with the retention time and unique transitions with optimized collision energy for each compound. The compound database was used to create a multiple reaction monitoring method targeting 60 Arabidopsis metabolites. The samples were run using targeted multiple reaction monitoring. Data analysis was performed using Trace Finder software (Thermo Fisher Scientific). Peak area was integrated using the Genesis algorithm with constrained peak width, and the level of each metabolite was expressed relative to the internal standard ribitol (Rizhsky et al., 2004).
Acclimation Assays
Acclimation assays were performed as described previously by Suzuki et al. (2013) with a few modifications. For local acclimation, 40- to 45-d-old plants grown in short days were used. A single fully expanded leaf was pretreated for 10 min with 1,500 µmol m−2 s−1, and plants were incubated for 50 min under controlled conditions. After the recovery period, the same leaf was exposed again to 1,500 µmol m−2 s−1 for 45 min. For systemic acclimation, pretreatment was applied to a local leaf for 10 min at 1,500 µmol m−2 s−1, and plants were incubated for 50 min under controlled conditions. After the recovery period, a systemic leaf was exposed to 1,500 µmol m−2 s−1 for 45 min. For controls, plants were untreated and not subjected to any light stress treatment (untreated local or systemic tissues) or subjected to the light stress treatment (1,500 µmol m−2 s−1 for 50 min) on their local or systemic tissues without being pretreated (nonacclimated local and systemic leaves subjected to light stress). In all cases, electrolyte leakage was assayed for local and systemic tissues as described by Suzuki et al. (2015). Briefly, leaves were immersed in 10 mL of distilled, deionized water in a 50-mL Falcon tube. Samples were shaken for 1 h at room temperature, and the conductivity of the water was measured using a conductivity meter. Leaves were then returned to the incubation water and heated to 95°C for 20 min using a heat block, shaken for 1 h at room temperature, and the conductivity of the water was measured again. The electrolyte leakage was calculated as the percentage of the conductivity before heating (stress-induced ion leaking) over that after heating (total ion leakage).
H2O2 Assay
The accumulation of H2O2 in the different samples was measured using Amplex Red (Molecular Probes, Invitrogen) according to the protocol described by Suzuki et al. (2015). Briefly, 500 µL of 50 mm sodium phosphate buffer (pH 7.4) containing 50 µm Amplex Red and 0.05 units mL−1 horseradish peroxidase was mixed with ground tissues, and samples were centrifuged at 12,000g for 12 min at 4°C. Following centrifugation, 450 µL of supernatant was transferred into fresh tubes and incubated for 30 min at room temperature in the dark. Absorbance at 560 nm was then measured, and the concentration of H2O2 in each sample was determined from a standard curve consisting of 0, 0.5, 1, 3, 6, 9, 12, and 15 µm H2O2. Following the measurement of absorbance, tissue samples were dried completely using a speed vacuum concentrator at 30°C, and H2O2 accumulation per mg dry weight of tissue was calculated.
Statistical Analysis
An ANOVA followed by Dunnett’s posthoc test was performed to compare metabolic changes at different time points and time 0 min within each segment using SPSS software (Hughes et al., 2017). The significance of differences between mean values of two experimental groups was assessed by Student’s two-tailed t test (Suzuki et al., 2015). P ≤ 0.05 was considered significant.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Bar graphs showing changes in the levels of different metabolites in response to light stress applied to a local rosette leaf.
Supplemental Figure S2. Bar graphs showing changes in the levels of different metabolites in response to light stress applied to a local rosette leaf.
Supplemental Figure S3. Bar graphs showing changes in the levels of different metabolites in response to light stress applied to a local rosette leaf.
Supplemental Figure S4. Accumulation of H2O2 along the path of the systemic signal in rbohD plants.
Supplemental Figure S5. Accumulation of H2O2 in local and systemic leaves of the wild type and two independent cICDH mutants in response to local application of light stress.
Supplemental Figure S6. PCR analysis of the two cICDH mutants.
Supplemental Table S1. Fold change in the levels of the different metabolites detected.
Supplemental Table S2. Fold change in the levels of the different metabolites detected.
Footnotes
Supported by funding from the National Science Foundation (IOS-1353886, IOS-1063287, IOS-1557787), and the University of North Texas, College of Arts and Sciences.
Articles can be viewed without a subscription.
References
- Alric J, Johnson X (2017) Alternative electron transport pathways in photosynthesis: a confluence of regulation. Curr Opin Plant Biol 37: 78–86 [DOI] [PubMed] [Google Scholar]
- Bode R, Ivanov AG, Hüner NP (2016) Global transcriptome analyses provide evidence that chloroplast redox state contributes to intracellular as well as long-distance signalling in response to stress and acclimation in Arabidopsis. Photosynth Res 128: 287–312 [DOI] [PubMed] [Google Scholar]
- Carmody M, Crisp PA, d’Alessandro S, Ganguly D, Gordon M, Havaux M, Albrecht-Borth V, Pogson BJ (2016) Uncoupling high light responses from singlet oxygen retrograde signaling and spatial-temporal systemic acquired acclimation. Plant Physiol 171: 1734–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hoehenwarter W (2015) Changes in the phosphoproteome and metabolome link early signaling events to rearrangement of photosynthesis and central metabolism in salinity and oxidative stress response in Arabidopsis. Plant Physiol 169: 3021–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S (2014) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111: 6497–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WG, Miller G, Wallace I, Harper J, Mittler R, Gilroy S (2017) Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J 90: 698–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmann A, Grill E, Huang J (2013) Hydraulic signals in long-distance signaling. Curr Opin Plant Biol 16: 293–300 [DOI] [PubMed] [Google Scholar]
- Ciszak K, Kulasek M, Barczak A, Grzelak J, Maćkowski S, Karpiński S (2015) PsbS is required for systemic acquired acclimation and post-excess-light-stress optimization of chlorophyll fluorescence decay times in Arabidopsis. Plant Signal Behav 10: e982018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp PA, Ganguly DR, Smith AB, Murray KD, Estavillo GM, Searle I, Ford E, Bogdanović O, Lister R, Borevitz JO, et al. (2017) Rapid recovery gene downregulation during excess-light stress and recovery in Arabidopsis. Plant Cell 29: 1836–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui LL, Lu YS, Li Y, Yang C, Peng XX (2016) Overexpression of glycolate oxidase confers improved photosynthesis under high light and high temperature in rice. Front Plant Sci 7: 1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Fiehn O, Durnford DG (2013) Metabolic acclimation to excess light intensity in Chlamydomonas reinhardtii. Plant Cell Environ 36: 1391–1405 [DOI] [PubMed] [Google Scholar]
- Davletova S, Schlauch K, Coutu J, Mittler R (2005) The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol 139: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy AR, Zandalinas SI, Gómez-Cadenas A, Blumwald E, Mittler R (2018) Coordinating the overall stomatal response of plants: rapid leaf-to-leaf communication during light stress. Sci Signal 11: eaam9514. [DOI] [PubMed] [Google Scholar]
- Dias DA, Hill CB, Jayasinghe NS, Atieno J, Sutton T, Roessner U (2015) Quantitative profiling of polar primary metabolites of two chickpea cultivars with contrasting responses to salinity. J Chromatogr B Analyt Technol Biomed Life Sci 1000: 1–13 [DOI] [PubMed] [Google Scholar]
- Drerup MM, Schlücking K, Hashimoto K, Manishankar P, Steinhorst L, Kuchitsu K, Kudla J (2013) The calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol Plant 6: 559–569 [DOI] [PubMed] [Google Scholar]
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte CP, Schulze WX, Romeis T (2013) Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci USA 110: 8744–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis K, Young PR, Eyéghé-Bickong HA, Vivier MA (2017) The transcriptional responses and metabolic consequences of acclimation to elevated light exposure in grapevine berries. Front Plant Sci 8: 1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez-Sarasa I, Noguchi K, Araújo WL, Garcia-Nogales A, Fernie AR, Flexas J, Ribas-Carbo M (2016a) Impaired cyclic electron flow around photosystem I disturbs high-light respiratory metabolism. Plant Physiol 172: 2176–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez-Sarasa I, Ribas-Carbo M, Del-Saz NF, Schwahn K, Nikoloski Z, Fernie AR, Flexas J (2016b) Unravelling the in vivo regulation and metabolic role of the alternative oxidase pathway in C3 species under photoinhibitory conditions. New Phytol 212: 66–79 [DOI] [PubMed] [Google Scholar]
- Fryer MJ, Ball L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR (2003) Control of Ascorbate Peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J 33: 691–705 [DOI] [PubMed] [Google Scholar]
- Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR, Mittler R (2014) A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci 19: 623–630 [DOI] [PubMed] [Google Scholar]
- Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpiński S, Mittler R (2016) ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol 171: 1606–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MJ, Carmody M, Albrecht V, Pogson B (2013) Systemic and local responses to repeated HL stress-induced retrograde signaling in Arabidopsis. Front Plant Sci 3: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorecka M, Alvarez-Fernandez R, Slattery K, McAusland L, Davey PA, Karpinski S, Lawson T, Mullineaux PM (2014) Abscisic acid signalling determines susceptibility of bundle sheath cells to photoinhibition in high light-exposed Arabidopsis leaves. Philos Trans R Soc Lond B Biol Sci 369: 20130234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SB, Brady SM (2016) Plant developmental responses to climate change. Dev Biol 419: 64–77 [DOI] [PubMed] [Google Scholar]
- Guo Z, Wang F, Xiang X, Ahammed GJ, Wang M, Onac E, Zhou J, Xia X, Shi K, Yin X, et al. (2016) Systemic induction of photosynthesis via illumination of the shoot apex is mediated sequentially by phytochrome B, auxin and hydrogen peroxide in tomato. Plant Physiol 172: 1259–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J, Hepworth C, Dutton C, Dunn JA, Hunt L, Stephens J, Waugh R, Cameron DD, Gray JE (2017) Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiol 174: 776–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangasjärvi S, Nurmi M, Tikkanen M, Aro EM (2009) Cell-specific mechanisms and systemic signalling as emerging themes in light acclimation of C3 plants. Plant Cell Environ 32: 1230–1240 [DOI] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284: 654–657 [DOI] [PubMed] [Google Scholar]
- Karpiński S, Szechyńska-Hebda M, Wituszyńska W, Burdiak P (2013) Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant Cell Environ 36: 736–744 [DOI] [PubMed] [Google Scholar]
- Kerchev P, Waszczak C, Lewandowska A, Willems P, Shapiguzov A, Li Z, Alseekh S, Mühlenbock P, Hoeberichts FA, Huang J, et al. (2016) Lack of GLYCOLATE OXIDASE1, but not GLYCOLATE OXIDASE2, attenuates the photorespiratory phenotype of CATALASE2-deficient Arabidopsis. Plant Physiol 171: 1704–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Zandalinas SI, Sengupta S, Nuhkat M, Kangasjärvi J, Mittler R (2018) Rapid responses to abiotic stress: priming the landscape for the signal transduction network. Trends Plant Sci (in press) [DOI] [PubMed] [Google Scholar]
- Kulasek M, Bernacki MJ, Ciszak K, Witoń D, Karpiński S (2016) Contribution of PsbS function and stomatal conductance to foliar temperature in higher plants. Plant Cell Physiol 57: 1495–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo M, Oelmüller R (2015) REDOX RESPONSIVE TRANSCRIPTION FACTOR1 is involved in age-dependent and systemic stress signaling. Plant Signal Behav 10: e1051279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhamdi A, Mauve C, Gouia H, Saindrenan P, Hodges M, Noctor G (2010) Cytosolic NADP-dependent isocitrate dehydrogenase contributes to redox homeostasis and the regulation of pathogen responses in Arabidopsis leaves. Plant Cell Environ 33: 1112–1123 [DOI] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2: ra45. [DOI] [PubMed] [Google Scholar]
- Miller MAE, O’Cualain R, Selley J, Knight D, Karim MF, Hubbard SJ, Johnson GN (2017) Dynamic acclimation to high light in Arabidopsis thaliana involves widespread reengineering of the leaf proteome. Front Plant Sci 8: 1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Müller SM, Wang S, Telman W, Liebthal M, Schnitzer H, Viehhauser A, Sticht C, Delatorre C, Wirtz M, Hell R, et al. (2017) The redox-sensitive module of cyclophilin 20-3, 2-cysteine peroxiredoxin and cysteine synthase integrates sulfur metabolism and oxylipin signaling in the high light acclimation response. Plant J 91: 995–1014 [DOI] [PubMed] [Google Scholar]
- Mullineaux P, Ball L, Escobar C, Karpinska B, Creissen G, Karpinski S (2000) Are diverse signalling pathways integrated in the regulation of Arabidopsis antioxidant defence gene expression in response to excess excitation energy? Philos Trans R Soc Lond B Biol Sci 355: 1531–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munekage YN, Inoue S, Yoneda Y, Yokota A (2015) Distinct palisade tissue development processes promoted by leaf autonomous signalling and long-distance signalling in Arabidopsis thaliana. Plant Cell Environ 38: 1116–1126 [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH (2016) Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ 39: 1140–1160 [DOI] [PubMed] [Google Scholar]
- Oelze ML, Vogel MO, Alsharafa K, Kahmann U, Viehhauser A, Maurino VG, Dietz KJ (2012) Efficient acclimation of the chloroplast antioxidant defence of Arabidopsis thaliana leaves in response to a 10- or 100-fold light increment and the possible involvement of retrograde signals. J Exp Bot 63: 1297–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F, Birtic S, Cuiné S, Triantaphylidès C, Ravanat JL, Havaux M (2012) Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol 158: 1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena JR, Chao CC, Levine RL, Stadtman ER (2001) Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc Natl Acad Sci USA 98: 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide: the response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134: 1683–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossel JB, Wilson PB, Hussain D, Woo NS, Gordon MJ, Mewett OP, Howell KA, Whelan J, Kazan K, Pogson BJ (2007) Systemic and intracellular responses to photooxidative stress in Arabidopsis. Plant Cell 19: 4091–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL. (2017) Plants in Action, Ed 2 Australian Society of Plant Scientists, Macmillan Education Australia Pty Ltd [Google Scholar]
- Shah J, Chaturvedi R, Chowdhury Z, Venables B, Petros RA (2014) Signaling by small metabolites in systemic acquired resistance. Plant J 79: 645–658 [DOI] [PubMed] [Google Scholar]
- Shuman JL, Cortes DF, Armenta JM, Pokrzywa RM, Mendes P, Shulaev V (2011) Plant metabolomics by GC-MS and differential analysis. Methods Mol Biol 678: 229–246 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K, et al. (2013) Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25: 3553–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Devireddy AR, Inupakutika MA, Baxter A, Miller G, Song L, Shulaev E, Azad RK, Shulaev V, Mittler R (2015) Ultra-fast alterations in mRNA levels uncover multiple players in light stress acclimation in plants. Plant J 84: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechyńska-Hebda M, Karpiński S (2013) Light intensity-dependent retrograde signalling in higher plants. J Plant Physiol 170: 1501–1516 [DOI] [PubMed] [Google Scholar]
- Szechyńska-Hebda M, Kruk J, Górecka M, Karpińska B, Karpiński S (2010) Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 22: 2201–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechyńska-Hebda M, Lewandowska M, Karpiński S (2017) Electrical signaling, photosynthesis and systemic acquired acclimation. Front Physiol 8: 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta A, Rahikainen M, Konert G, Finazzi G, Kangasjärvi S (2014) Signalling crosstalk in light stress and immune reactions in plants. Philos Trans R Soc Lond B Biol Sci 369: 20130235. [DOI] [PMC free article] [PubMed] [Google Scholar]