Abstract
Background
Injection drug use (IDU) is a major risk factor for infective endocarditis (IE). Few data exist on repeat IE (rIE) in persons who inject drugs (PWID).
Methods
Patients ≥18 years old seen at Wake Forest Baptist Medical Center from 2004 to 2017 who met Duke criteria for IE and who self-reported IDU in the 3 months before admission were identified. The subset of PWID who developed rIE, defined as another episode of IE at least 10 weeks after diagnosis of the first episode, was then reviewed.
Results
Of the 87 PWID who survived their first episode of IE, 22 (25.3%) experienced rIE and 77.3% had rIE within a year of the first episode. All patients who experienced rIE resumed IDU between episodes of IE. Of the patients with rIE, 54.5% had an infection caused by S. aureus and 22.7% required surgical intervention. Mortality at 1 year was 36.3%. Compared with their first IE episode, patients with rIE had fewer S. aureus infections (P = .01). Compared with PWID who experienced single-episode IE, intravenous prescription opioid use (P = .01), surgery (P < .01), tricuspid valve involvement (P = .02), and polymicrobial infection (P = .03) occurred more often during first episodes of IE in individuals who then developed rIE.
Conclusions
rIE is common among IDU-related IE and confers a high 1-year mortality rate. The microbiology of rIE is varied, with S. aureus being less frequently isolated. More studies on modification of social and clinical risk factors are needed to prevent rIE.
Keywords: infective endocarditis, recurrence, injection drug use, persons who inject drugs, opioid abuse
Injection drug use (IDU) is a well-known risk factor for infective endocarditis (IE), and people who inject drugs (PWID) now account for an increasing proportion of cases of community-onset IE [1]. As a consequence, approximately 16% of cases of IE in North America are now attributable to IDU [2]. In North Carolina, there was a 12-fold increase in hospitalization related to injection drug use–associated IE (IDU-IE) from 2010 to 2015, with the hospital costs for managing those infections exceeding $22 million by 2015 [3]. Not surprisingly, IE has been identified as 1 of the infections of concern in relation to the opioid crisis [4]. Among all persons who experience an initial episode of IE, repeat IE (rIE) is estimated to occur in 2%–31% [5–7]. A recent review from the International Collaboration on Endocarditis (ICE) identified IDU as the strongest independent risk factor for rIE [5]. Although prior reviews on rIE include PWID as 1 of the risk groups for rIE [5, 6, 8], a dedicated review of rIE in PWID has not previously been published. In particular, the timing of onset after the first episode, causative pathogens, illness severity, and outcomes have not been well characterized in PWID. In this retrospective study, we describe the demographics, clinical characteristics, and outcomes of rIE in PWID and attempt to determine which clinical factors within a group of PWID with prior IE might predispose to recurrence.
METHODS
Study Subjects and Inclusion Criteria
Medical records of patients ≥18 years of age admitted to Wake Forest Baptist Medical Center (WFBMC) from January 2004 to January 2017 were retrospectively queried for admissions associated with International Classification of Diseases (ICD)–9 and ICD-10 diagnosis codes for endocarditis in any encounter setting. Patients with an ICD-9 of 996.0 were removed in an attempt to exclude cases related to cardiac devices. The remaining 1141 individual records were manually reviewed to evaluate inclusion criteria and extract all data. Cases were restricted to patients with a diagnosis of possible or definite endocarditis by the modified Duke criteria [9] who acknowledged IDU in the 3 months before admission. The subset of PWID who experienced rIE, defined as another episode of possible or definite endocarditis occurring at least 10 weeks after the date of diagnosis of the first documented episode [5], were then selected for further detailed review. The study was approved by the institutional review board, with the need for informed consent waived given the retrospective nature of the investigation.
Study Data and Definitions
Basic demographic data including age, gender, race, and county of residence were extracted. Area of residence was defined as metro or nonmetro using the US Department of Agriculture guidelines [10]. Route, type, and timing of any reported drug use were recorded. The results of admission laboratory studies, including HIV and hepatitis C virus testing, and all culture and susceptibility results were extracted from the record. Microbial growth from 2 or more blood cultures or from a single valve tissue culture was accepted as proof of etiology for the episode of IE. To characterize illness severity, laboratory evaluations at the time of admission, days of fever ≥100.4°F, Pitt bacteremia score [11], and APACHE II [12] score were assessed. Duration of bacteremia, type and results of echocardiography, and complications of infection including need for intensive care admission were assessed. Invasive management interventions including surgical procedures (valve repair, replacement, or annuloplasty) and removal of vegetations using a percutaneous vegectomy device [13] were documented. For repeat IE admissions, an episode caused by a microorganism of the same species (same organism and no more than 1 discrepancy on susceptibility testing) within 6 months of the initial episode was classified as relapse, whereas all other episodes were classified as reinfections [14]. Outcomes in 87 patients with IDU-IE were followed for at least 12 months from the initial IE episode using both the electronic medical record and the North Carolina Vital Statistics database. Costs presented are total costs extracted directly from the records of the bill created for the patient encounter. Patient data were managed using Excel 2016 (Microsoft Corp., Redmond, WA) and Research Electronic Data Capture (REDCap) [15].
Statistical Analysis
Categorical variables were analyzed with the chi-square, Fisher exact, and McNemar tests, and continuous variables were evaluated using the t test or Mann-Whitney U test; 2-tailed P values <.05 were considered statistically significant. Those who survived but experienced a single episode of IE (sIE) were compared using stepwise logistic regression with those with rIE to look for variables that were associated with rIE; all variables meeting P < .2 were included in the model and retained at a threshold of P < .05. All statistical tests were performed using SPSS, v.18.0 (IBM Corp., Armonk, NY). Kaplan-Meier survival curves were used to examine 1-year survival between those with sIE and those with rIE.
RESULTS
Clinical and Demographic Characteristics of PWID With rIE
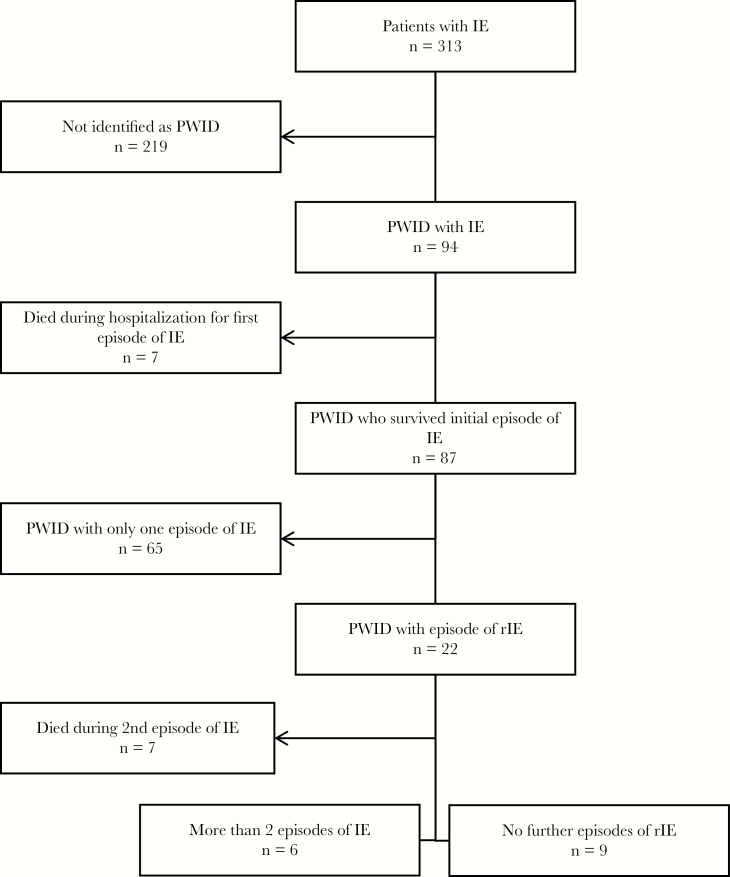
Ninety-four patients with IE were classified as PWID (Figure 1). Of these, 7 (7.5%) died during their initial episode of IE. Among the 87 survivors, 22 (25.3%) experienced rIE (Figure 1). Seventeen episodes of rIE (77.3%) occurred within 1 year of the initial episode. Those with recurrences were Caucasian (100%), resided predominately in nonmetro areas (68.2%), and injected primarily prescription opioids between admissions (68.2%). Thirteen (59.1%) patients received referrals to outpatient substance use clinics during their first admission (Table 1). Staphylococcus aureus was the causative pathogen in the rIE episodes in 12 patients (54.5%), with methicillin-susceptible S. aureus (MSSA) more frequently cultured (36.4% of all infections, 66.7% of S. aureus infections) than methicillin-resistant S. aureus (MRSA; 22.7% of all infections, 41.7% of S. aureus infections). One patient had concurrent MSSA and MRSA infection. The majority of rIE episodes were classified as reinfections (86.4%) rather than relapses. The median length of stay for rIE was 16 days, and the cost of hospitalization was $90 675.
Figure 1.
Selection of persons who inject drugs with repeat infective endocarditis. Abbreviations: IE, infective endocarditis; PWID, persons who inject drugs; rIE, repeat infective endocarditis.
Table 1.
Repeat Infective Endocarditis in Persons Who Inject Drugs: Demographic and Clinical Characteristics (n = 22)
| Characteristic | Result |
|---|---|
| Male, No. (%) | 11 (50) |
| Caucasian, No. (%) | 22 (100) |
| Age, median (IQR), y | 28.5 (22–38) |
| Nonmetro residence, No. (%) | 15 (68.2) |
| Days since completion of prior antimicrobial therapy for previous episode of IE, median (IQR) | 109 (72–151) |
| Referred to substance counseling during first admission, No. (%) | 13 (59.1) |
| Time since last use of intravenous drugs before admission, No. (%) | |
| <1 wk | 13 (59.1) |
| 1–4 wk | 5 (22.3) |
| Unclear timing of last use | 4 (18.2) |
| Preferred drug for injection, No. (%) | |
| Prescription opioids | 15 (68.2) |
| Heroin | 7 (31.8) |
| Cocaine | 2 (9.1) |
| Methamphetamines | 5 (22.3) |
| Comorbidities, No. (%) | |
| Hepatitis C virus infection | 18 (81.2) |
| Old/resolved (antibody-positive, RNA-negative) | 4 (18.2) |
| Active (antibody- and RNA-positive) | 8 (36.4) |
| Unknown (viral RNA not checked) | 6 (27.3) |
| Negative | 4 (18.2) |
| HIV infection | 0 |
| Charlson Comorbidity Index, median (IQR) | 0 (0–0.8) |
| Duration of symptoms before presentation, No. (%) | |
| <1 wk | 12 (54.5) |
| 1–2 wk | 5 (22.7) |
| Duration unknown | 5 (22.7) |
| Symptoms before presentation, No. (%) | |
| Subjective fevers | 8 (36.4) |
| Chills | 6 (27.3) |
| Chest pain | 8 (36.4) |
| Shortness of breath | 4 (18.2) |
| Musculoskeletal pain | 8 (36.4) |
| Neurologic symptomsa | 3 (13.4) |
| Severity of illness at time of admission for repeat episode, median (IQR) | |
| Pitt bacteremia score | 3.0 (0–2) |
| Apache II score | 12.0 (8–23) |
| Admission WBC, 10*3/uL | 14.4 (6.8–23) |
| Admission serum creatinine, mg/dL | 1.70 (1.1–2.0) |
| Time to defervescence of fever,b d | 3 (1–7) |
| Time to clearance of bacteremia,b d | 3 (1–5) |
| Echocardiographic findings, No. (%) | |
| Patients undergoing TTE/No. with demonstrated vegetations | 22 (100)/19 (86.4) |
| Patients undergoing TEE/No. with demonstrated vegetations | 14 (63.6)/13 (59.1) |
| Site of vegetations, No. (%) | |
| Tricuspid valve | 17 (77.3) |
| Mitral valve | 5 (22.7) |
| Aortic valve | 3 (13.4) |
| Pulmonic valve | 1 (4.5) |
| Multiple valves | 3 (13.4) |
| Vegetation on same valve as prior episode | 19 (86.4) |
| Type of valve involved, No. (%) | |
| Native | 15 (68.2) |
| Prosthetic | 7 (31.8) |
| Microbiology of rIE, No. (%) | |
| Staphylococcus aureus | 12 (54.5) |
| Streptococci | 5 (22.3) |
| Enterococcus | 3 (13.6) |
| Fungal | 3 (13.6) |
| Polymicrobial | 4 (18.2) |
| Classification of rIEb, No. (%) | |
| Relapse | 3 (13.6) |
| Re-infection | 19 (86.4) |
| Complications associated with rIE, No. (%) | 13 (59.1) |
| Septic arthritis/osteomyelitis | 2 (9.1) |
| Septic pulmonary emboli +/- pneumonia +/- empyema | 10 (45.5) |
| CNS emboli | 4 (18.2) |
| Splenic emboli +/- abscess | 0 |
| Requirement for hemodialysis, No. (%) | 2 (9.1) |
| Management in ICU, No. (%) | 18 (81.2) |
| Length of ICU stay, median (IQR), d | 4 (2–9) |
| Invasive cardiac interventions, No. (%) | 5 (22.7) |
| Valve repair | 1 (4.5) |
| Valve replacement | 1 (4.5) |
| Annuloplasty | 3 (13.4) |
| Percutaneous vegectomy | 0 |
| Length of hospitalization for rIE, median (IQR), d | 16 (8–46) |
| Duration of antimicrobial therapy for rIE, median (IQR), d | 16 (7–43) |
| Left AMA before completing antimicrobial course, No. (%) | 2 (9.1) |
| Costs of hospitalization for management of rIE, median (IQR), $ | 90 675 (39 167–134 624) |
| Outcomes, No. (%) | |
| Death during hospitalization for rIE | 7 (31.8) |
| 1-y mortality | 8 (36.4) |
| Another episode of rIE in subsequent 12 mo | 4 (18.2) |
Abbreviations: AMA, against medical advice; CNS, central nervous system; ICU, intensive care unit; IE, infective endocarditis; IQR, interquartile range; rIE, repeat infective endocarditis; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram; WBC, white blood cell count.
aNeurological symptoms included stroke, transient ischemic attack, numbness, tingling, visual changes, speech impairment.
bRefer to the “Methods” for a definition.
Comparison of First and Repeat Episodes of IE in PWID With rIE
Comparative features of the first and second episodes of IE in those who experienced rIE are presented in Table 2. Rates of tricuspid valve (TV) vegetations (72.3% vs 77.3%, P = 1), left-sided involvement (18.2% vs 31.8%, P = .25), and multivalve involvement (9.1% vs 18.2%, P = .62) were similar between episodes. S. aureus was less commonly identified as the microbial etiology with rIE (95.5% vs 54.5%, P = .01). Repeat IE was associated with shorter median duration of bacteremia (1 vs 3 days, P = .04) and trended toward fewer surgical interventions (22.7% vs 59.1%, P = .06), but the frequency of detection of vegetations by transthoracic echocardiogram (TTE; 86.4% vs 45.5%, P = .04) and the median Pitt bacteremia score (3.0 vs 1.0, P = .06) were both higher.
Table 2.
Comparison of the First and Second Episodes of Infective Endocarditis in Persons Who Inject Drugs Who Experienced Repeat Infective Endocarditis
| Characteristic | First Episode (n = 22) |
Second Episode (n = 22) |
P Value |
|---|---|---|---|
| Prescription opioid use, No. (%) | 21 (95.5) | 15 (68.2) | .289 |
| Severity of illness at time of admission, median (IQR) | |||
| Pitt bacteremia score | 1.0 (0–2) | 3.0 (0–6) | .06 |
| APACHE II score | 11 (7–16) | 12 (8–23) | .09 |
| Echocardiographic findings, No. (%) | |||
| TTE-positive for vegetation | 10 (45.5) | 19 (86.4) | .04 |
| TEE done | 19 (86.4) | 13 (59.1) | .11 |
| Site of vegetation, No. (%) | |||
| Multiple valves involved | 1 (4.5) | 3 (13.4) | .5 |
| Tricuspid valve | 16 (72.7) | 17 (77.3) | 1 |
| Pulmonic valve | 0 | 1 (4.5) | 1 |
| Mitral valve | 2 (9.1) | 5 (22.7) | .25 |
| Aortic valve | 2 (9.1) | 3 (13.6) | 1 |
| Left-sided involvement | 4 (18.2) | 7 (31.8) | .25 |
| Echocardiogram-negative, No. (%) | 3 (13.4) | 1 (4.5) | .48 |
| Microbiology, No. (%) | |||
| Staphylococcus aureus | 21 (95.4) | 12 (54.5) | .04 |
| MSSA | 10 (45.5) | 8 (36.4) | .77 |
| MRSA | 12 (54.5) | 5 (22.7) | .07 |
| Streptococci | 1 (4.5) | 5 (22.7) | .22 |
| Enterococcus | 0 | 3 (13.6) | .25 |
| Fungal | 0 | 3 (13.6) | .25 |
| Culture-negative | 0 | 2 (9.1) | .48 |
| Polymicrobial | 4 (18.2) | 4 (18.2) | 1 |
| Definite Duke criteria, No. (%) | 19 (86.4) | 19 (86.4) | 1 |
| Complications, No. (%) | |||
| Hemodialysis required | 2 (9.1) | 2 (9.1) | 1 |
| ICU management needed | 17 (77.3) | 18 (81.2) | 1 |
| Length of ICU stay, median (IQR), d | 2 (0–9) | 4 (2–9) | .77 |
| Embolic event/metastatic focus of infection, No. (%) | |||
| None | 2 (9.1) | 3 (13.6) | 1 |
| Pulmonary | 16 (72.7) | 10 (45.5) | .03 |
| Central nervous system | 1 (4.5) | 4 (18.2) | .36 |
| Musculoskeletal | 4 (18.2) | 2 (9.1) | .5 |
| Renal | 1 (4.5) | 1 (4.5) | 1 |
| Splenic | 1 (4.5) | 0 | 1 |
| Duration of bacteremiaa, median (IQR), d | 3 (1–5) | 2 (1–3) | .03 |
| Invasive cardiac interventions, No. (%) | 13 (59.1) | 5 (22.7) | .06 |
| Valve repair | 3 (13.4) | 0 | .25 |
| Annuloplasty | 2 (9.1) | 3 (13.6) | 1 |
| Valve replacement | 7 (31.2) | 1 (4.5) | .07 |
| Percutaneous vegectomy | 1 (4.5) | 0 | 1 |
| Duration of antimicrobial therapya, median (IQR), d | 43 (24–48) | 16 (7–43) | .10 |
| Length of hospitalization, median (IQR), d | 23 (17–41) | 16 (8–46) | .82 |
| Costs of hospitalization, median (IQR), $ | 144 880b (48 583–231 216) | 90 675c (39 167–134 624) | .71 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram.
aRefer to the “Methods” for a definition.
bn = 17.
cn = 21.
Comparison of First Episodes of IE in PWID Who Did and Did Not Experience rIE
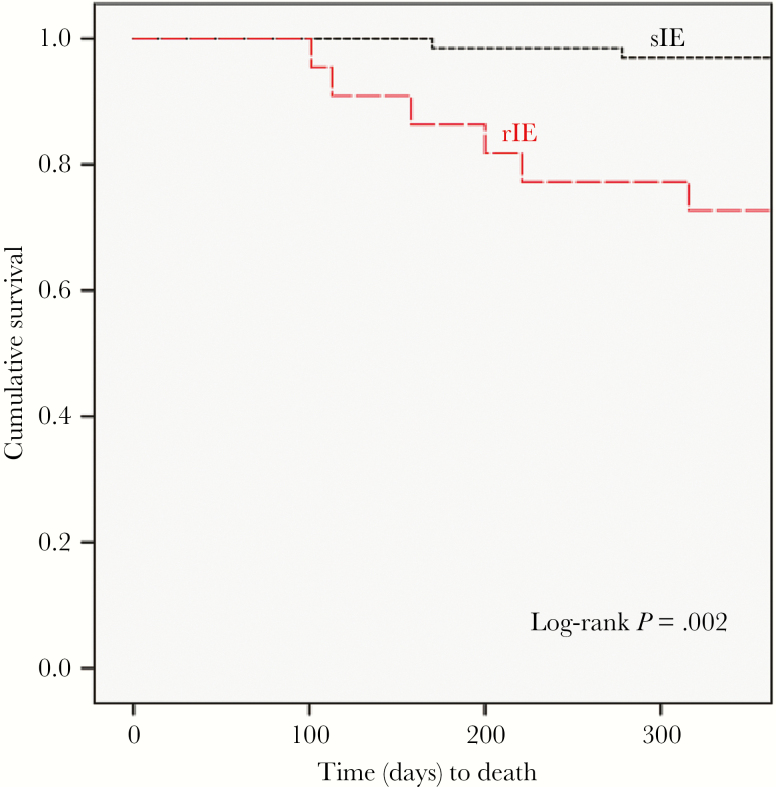
Clinical features of the initial episode of IE in PWID who experienced and survived only a single episode of endocarditis were then compared with the clinical features of first episodes of IE in those patients who developed rIE (Table 3). During their initial episode of IE, PWID who later experienced rIE had a greater incidence of prescription opioid injection (95.5% vs 67.7%, P = .01), more frequent visualization of vegetations on the TV (72.7% vs 35.3%, P = .02), lower rates of echocardiogram-negative IE (13.6% vs 40%), and higher rates of surgical intervention (59.1% vs 24.6%, P < .01) as compared with PWID with sIE, whereas the median number of days of bacteremia (1 vs 3 days, P = .04) was lower. Multivariate analysis identified the presence of echocardiographically demonstrated TV vegetations (odds ratio [OR], 3.8; 95% confidence interval [CI], 1.2–11.9) and surgical intervention (OR, 5.1; 95% CI, 1.6–16.3) as being associated with rIE. One-year survival of patients from their initial episode of IE was 63.6% in the rIE group (14 patients) compared with 95.4% (62 patients) in the sIE group (P < .01) (Figure 2). Of the 3 sIE patients who died after surviving their initial hospitalization for IE, 1 died from overdose and 2 died from unknown causes. Of the 8 individuals with rIE who died, 7 expired while hospitalized for management of their recurrent episode of IE, and 1 died postdischarge from unknown causes.
Table 3.
Predictors of Repeat Infective Endocarditis: Comparison of the Features of the First Episode of Infective Endocarditis in Persons Who Inject Drugs With Single Infective Endocarditis vs Those With Repeat Endocarditis
| Characteristic | Single Episode (n = 65) |
Repeat Episode (n = 22) |
P Value | Multivariate Analysis |
|---|---|---|---|---|
| Age, median (IQR), y | 29 (24–38.5) | 28.5 (23–37.3) | .63 | |
| Caucasian, No. (%) | 62 (95.4) | 22 (100) | .57 | |
| Male, No. (%) | 32 (49.2) | 11 (50) | .95 | |
| Nonmetro residency,a No. (%) | 46 (70.8) | 15 (68.2) | .82 | |
| Prescription opioid use, No. (%) | 44 (67.7) | 21 (95.4) | .01 | |
| Hepatitis C virus infection, No. (%) | 40 (61.5) | 18 (71.8) | .08 | |
| Old/resolved (antibody-positive, RNA-negative) | 9 (13.8) | 4 (18.2) | .73 | |
| Active (antibody- and RNA-positive) | 22 (33.8) | 8 (36.4) | 1 | |
| Unknown (viral RNA not checked) | 10 (15.4) | 6 (27.3) | .22 | |
| Pitt bacteremia score, median (IQR) | 1 (0–2) | 1 (0–2) | .54 | |
| APACHE II score, median (IQR) | 9 (4–13) | 9.5 (5.8–12.3) | .78 | |
| Duration of bacteremia,a median (IQR), d | 1 (1–3) | 3 (1–5.3) | .04 | |
| Echocardiographic findings, No. (%) | ||||
| TTE-positive for vegetation | 36 (55.4) | 10 (45.4) | .42 | |
| TEE done | 40 (61.5) | 19 (86.4) | .03 | |
| Site of vegetation, No. (%) | ||||
| Tricuspid | 23 (35.3) | 16 (72.7) | .02 | OR, 3.8; 95% CI, 1.2–11.9; P = .02 |
| Mitral | 13 (20) | 2 (9.1) | .34 | |
| Aortic | 8 (12.3) | 2 (9.1) | 1 | |
| Pulmonic | 2 (3.1) | 0 | 1 | |
| Echocardiogram-negative, No. (%) | 26 (40.0) | 3 (13.6) | .03 | |
| Microbiology, No. (%) | ||||
| Staphylococcus aureus | 49 (70.8) | 21 (95.5) | .06 | |
| MSSA | 16 (24.6) | 10 (45.4) | .07 | |
| MRSA | 34 (52.3) | 12 (54.5) | .86 | |
| Streptococci | 9 (13.8) | 2 (9.1) | .72 | |
| Polymicrobial | 2 (3.1) | 4 (18.2) | .03 | |
| Definite Duke criteria, No. (%) | 51 (78.5) | 19 (86.4) | .54 | |
| ICU admission needed, No. (%) | 39 (60.0) | 17 (77.2) | .2 | |
| ICU length of stay, median (IQR), d | 1 days (0–6) | 2 days (0.8–6.8) | .24 | |
| Vasopressor agent used, No. (%) | 8 (12.3) | 3 (13.6) | 1 | |
| Intubation required, No. (%) | 26 (40) | 13 (59.1) | .12 | |
| Embolic events/metastatic focus of infection, No. (%) | ||||
| None | 15 (23.1) | 2 (9.1) | .22 | |
| Lung | 33 (50.7) | 16 (72.3) | .07 | |
| Central nervous system | 4 (6.2) | 1 (4.5) | 1 | |
| Musculoskeletal | 16 (24.6) | 4 (18.2) | .78 | |
| Invasive cardiac interventions done, No. (%) | 16 (24.6) | 13 (59.1) | < .01 | OR, 5.1; 95% CI, 1.6–16.3; P = .005 |
| Valve repair | 2 (3.1) | 4 (18.1) | .03 | |
| Valve replacement | 10 (15.4) | 7 (31.8) | .09 | |
| Annuloplasty | 4 (6.2) | 2 (9.1) | .64 | |
| Percutaneous vegectomy | 0 | 1 (4.5) | .25 | |
| Length of hospitalization, median (IQR), d | 18 (10–30) | 21 (12.8–41.5) | .22 | |
Abbreviations: CI, confidence interval; ICU, intensive care unit; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; OR, odds ratio; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram.
aSee the “Methods” for a definition.
Figure 2.
Kaplan-Meier curves comparing survival between repeat and single infective endocarditis (no repeat infective endocarditis). Abbreviations: sIE, single infective endocarditis; rIE, repeat infective endocarditis.
DISCUSSION
The opioid epidemic is a growing public health crisis in the United States, with drug overdose deaths, viral hepatitis, and infective endocarditis all increasing significantly over the past decade [16–18]. Compared with previous decades, a significant proportion of PWID now utilize prescription opioids intended for oral use [19, 20]. Further differences compared with the past are a higher number of rural dwelling and female PWID [16, 21]. Rural patients have different patterns of abuse than their urban peers, including an increased propensity to inject prescription opioids; the preferred prescription opioid in a region and its associated injection frequency and behaviors vary by geography [19, 22, 23]. Residence in rural areas has also proven to be a significant barrier to appropriate substance use disorder treatment as these areas are often medically underserved [24].
Consistent with the published literature, 25% of PWID in our study population who experienced an episode of IE developed rIE, with most episodes representing re-infections rather than relapses [5, 8, 25]. All of our patients with rIE acknowledged IDU relapse after their first episode of IE. Most cases occurred within 1 year of the prior episode, a phenomenon noted in other studies as well [5, 8]. These facts highlight the urgent need to provide effective substance use disorder treatment after hospital discharge [8, 26].
There were a number of clinical features of rIE in our population, which suggests that a second episode of IDU-IE has specific characteristics as compared with initial episodes. First, the microbiology of rIE differed from that of first infections. Although S. aureus was still the most common cause of infection, isolation of that organism occurred less frequently with rIE than initial episodes; the proportion of MRSA also declined, though this decrease did not reach statistical significance. The decline in occurrence of MRSA during rIE is somewhat surprising in light of a recent study showing that PWID are 16-fold more likely to develop invasive MRSA infections than other populations [27]. Streptococci, Candida infections, and enterococci were more commonly encountered in episodes of rIE, but those increases were not statistically significant. The reasons for the more varied microbiology of repeat episodes are unknown but may relate to lingering effects of prior antistaphylococcal therapy on cutaneous colonization with S. aureus or a change in injection practices after the first episode of IE. Second, surgery was less commonly performed with rIE even though vegetations were more frequently visualized. This observation likely reflects the ongoing concerns over repeat surgical intervention in a patient population at high risk for repeat infection [1, 28–31]. Third, mortality at 1 year after an episode of rIE was high at ~36%, compared with a 1-year mortality of ~5% in PWID with sIE. We attribute this observation to the occurrence of a second severe infection, more varied microbiology, including more frequent Candida infections, and the fact that rIE may be a marker of more severe addiction. Given this high mortality, those with an admission for rIE should be prioritized for intensive addiction treatment.
Multivariate analysis comparing first episodes of IE in those PWID with sIE with those who went on to develop rIE (Table 3) found that surgical intervention and a visualized tricuspid valve vegetation during initial episodes were associated with subsequent development of rIE. Regarding the role of surgery, the presence of prosthetic material could have further predisposed a patient to repeat infection, especially in the setting of ongoing IDU, as both IDU and prosthetic valves are associated with rIE [5, 25, 31, 32]. Visualization of TV vegetations was the other factor associated with rIE in multivariate analysis. Tricuspid valve vegetations are considered a hallmark of IDU-IE [28], but the presence of demonstrable vegetations on that valve by TTE has not previously been identified as a risk factor for recurrence of IE in PWID or in other populations of patients [5, 8, 25]. The reasons for that association are unclear. However, others have noted that echocardiogram-positive IE is associated with more complications and higher risk of death than echocardiogram-negative IE [33] and that mortality is associated with the size of the vegetations in right-sided infective endocarditis (RSIE) in PWID [34]. Thus, in PWID, another consequence of demonstrable TV vegetations via TTE may be an increased risk for rIE.
A complex aspect of these cases is cost [35, 36]. As was apparent in our study, prolonged hospitalizations for rIE are common as these infections are severe and parenteral therapy is standard of care for IE [29, 30]. Not only are many physicians unwilling to discharge PWID home with intravenous access due to safety concerns, but practical barriers such as lack of home health services for this patient population also exist [37]. However, algorithms to identify patients who may be safely discharged home on outpatient parenteral antimicrobial therapy with central vascular catheters are now being developed [38]. The average total hospital costs for managing an episode of IE at our institution were ~$145 000, which is similar to previous studies [35]. Somewhat surprisingly, the costs of the repeat episode were no more than for the first episode despite higher clinical severity, as reflected by the Pitt bacteremia and APACHE II scores; this discrepancy may reflect the lower rates of surgical intervention during rIE. The summative costs of all episodes of endocarditis for this population of 22 individuals (1 patient had 5 endocarditis admissions), which occurred over a span of 5 years, exceeded $8 million. This cost represents an enormous economic burden for these patients and their families, as well as for the institutions that provide care to this frequently underinsured group of patients [37].
Conceptually, the most effective intervention to reduce morbidity and mortality as well as the economic costs of rIE would be to address the root cause of these infections, which is substance use disorder [16, 26, 36]. In response to this crisis, our institution is working to expand its addiction treatment capabilities. However, this initiative remains a work in progress in our region and elsewhere as states are facing challenges from similar inadequate infrastructure [37, 39].
There are several limitations to our study. First, this study was conducted at a single center located in the western Piedmont region of North Carolina, which is 1 of the epicenters of the injection opioid epidemic [40]. Thus, the population of PWID encompassed by our study may not reflect community characteristics in other locales. Second, the study was retrospective in design, which limits the precision of the data and the ability to access information not initially obtained by care providers. Third, identification of cases was dependent upon discharge diagnosis coding and collection of accurate information about IDU, both of which may have resulted in underestimating the true population of PWID with IE. Fourth, information about drug use was self-reported by the patients with no mechanism to ascertain the accuracy of that information. Last, given the social circumstances of many of the patients, follow-up data were sometimes challenging to obtain, and patients may have been admitted to other hospitals with rIE. We believe that this number would be small, however, as we are the major referral center in our catchment area. In addition, with the use of the NC death registry, we are confident that we captured most of the mortality data that pertained to our patients even though we recognize that patients who expired out of state would not be included in our mortality calculations.
In conclusion, PWID who experience IE are at high risk for recurrences, the majority of which are reinfections rather than relapses and most of which occur within 1 year. Repeat episodes of IE in PWID are clinically severe, manifest a more varied microbiology than first episodes, and are most often managed medically with fewer surgical interventions than first episodes. TV involvement and cardiac surgical intervention during the first episode of IE were associated with rIE by multivariate analysis. The mortality of rIE in PWID is high and dictates a need for more effective management strategies and preventive interventions in this population, which is at high risk for infective endocarditis.
Supplementary Material
Footnotes
From “Piece of My Heart,” written by Jerry Ragovoy and Bert Berns, originally recorded by Erma Franklin in 1967 and immortalized by Janis Joplin and Big Brother and the Holding Company in 1968.
References
- 1. Wang A, Gaca JG, Chu VH. Management considerations in infective endocarditis: a review. JAMA 2018; 320:72–83. [DOI] [PubMed] [Google Scholar]
- 2. Murdoch DR, Corey GR, Hoen B, et al. ; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009; 169:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fleischauer AT, Ruhl L, Rhea S, Barnes E. Hospitalizations for endocarditis and associated health care costs among persons with diagnosed drug dependence - North Carolina, 2010-2015. MMWR Morb Mortal Wkly Rep 2017; 66:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolitski R, Dan C. Three medical societies identify specific infections of concern in relation to the opioid crisis https://www.hiv.gov/blog/three-medical-societies-identify-specific-infections-concern-relation-opioid-crisis. Accessed 10 October 2018.
- 5. Alagna L, Park LP, Nicholson BP, et al. Repeat endocarditis: analysis of risk factors based on the International Collaboration on Endocarditis - Prospective Cohort Study. Clin Microbiol Infect 2014; 20:566–75. [DOI] [PubMed] [Google Scholar]
- 6. Welton DE, Young JB, Gentry WO, et al. Recurrent infective endocarditis: analysis of predisposing factors and clinical features. Am J Med 1979; 66:932–8. [DOI] [PubMed] [Google Scholar]
- 7. Levison ME, Kaye D, Mandell GL, Hook EW. Characteristics of patients with multiple episodes of bacterial endocarditis. JAMA 1970; 211:1355–7. [PubMed] [Google Scholar]
- 8. Baddour LM. Twelve-year review of recurrent native-valve infective endocarditis: a disease of the modern antibiotic era. Rev Infect Dis 1988; 10:1163–70. [DOI] [PubMed] [Google Scholar]
- 9. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–8. [DOI] [PubMed] [Google Scholar]
- 10. Cromartie J, Parker T. Data for rural analysis https://www.ers.usda.gov/topics/rural-economy-population/rural-classifications/data-for-rural-analysis/. Accessed 22 February 2018.
- 11. Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial infections. Ann Intern Med 2004; 140:26–32. [DOI] [PubMed] [Google Scholar]
- 12. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–29. [PubMed] [Google Scholar]
- 13. Thiagaraj AK, Malviya M, Htun WW, et al. A novel approach in the management of right-sided endocarditis: percutaneous vegectomy using the AngioVac cannula. Future Cardiol 2017; 13:211–7. [DOI] [PubMed] [Google Scholar]
- 14. Chu VH, Sexton DJ, Cabell CH, et al. Repeat infective endocarditis: differentiating relapse from reinfection. Clin Infect Dis 2005; 41:406–9. [DOI] [PubMed] [Google Scholar]
- 15. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users - United States, 2002-2013. MMWR Morb Mortal Wkly Rep 2015; 64:719–25. [PMC free article] [PubMed] [Google Scholar]
- 18. Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health 2018; 108:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Young AM, Havens JR, Leukefeld CG. Route of administration for illicit prescription opioids: a comparison of rural and urban drug users. Harm Reduct J 2010; 7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Havens JR, Walker R, Leukefeld CG. Prevalence of opioid analgesic injection among rural nonmedical opioid analgesic users. Drug Alcohol Depend 2007; 87:98–102. [DOI] [PubMed] [Google Scholar]
- 21. Tung MK, Light M, Giri R, et al. Evolving epidemiology of injecting drug use-associated infective endocarditis: a regional centre experience. Drug Alcohol Rev 2015; 34:412–7. [DOI] [PubMed] [Google Scholar]
- 22. Kirsh K, Peppin J, Coleman J. Characterization of prescription opioid abuse in the United States: focus on route of administration. J Pain Palliat Care Pharmacother 2012; 26:348–61. [DOI] [PubMed] [Google Scholar]
- 23. Young AM, Havens JR. Transition from first illicit drug use to first injection drug use among rural Appalachian drug users: a cross-sectional comparison and retrospective survival analysis. Addiction 2012; 107:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis MM, Spurlock M, Dulacki K, et al. Disparities in alcohol, drug use, and mental health condition prevalence and access to care in rural, isolated, and reservation areas: findings from the South Dakota Health Survey. J Rural Health 2016; 32:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mansur AJ, Dal Bó CM, Fukushima JT, et al. Relapses, recurrences, valve replacements, and mortality during the long-term follow-up after infective endocarditis. Am Heart J 2001; 141:78–86. [DOI] [PubMed] [Google Scholar]
- 26. Rosenthal ES, Karchmer AW, Theisen-Toupal J, et al. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med 2016; 129:481–5. [DOI] [PubMed] [Google Scholar]
- 27. Jackson KA, Bohm MK, Brooks JT, et al. Invasive methicillin-resistant Staphylococcus aureus infections among persons who inject drugs - six sites, 2005-2016. MMWR Morb Mortal Wkly Rep 2018; 67:625–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hussain ST, Witten J, Shrestha NK, et al. Tricuspid valve endocarditis. Ann Cardiothorac Surg 2017; 6:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baddour LM, Wilson WR, Bayer AS, et al. ; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 30. Habib G, Lancellotti P, Antunes MJ, et al. ; ESC Scientific Document Group 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36:3075–128. [DOI] [PubMed] [Google Scholar]
- 31. Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100:875–82. [DOI] [PubMed] [Google Scholar]
- 32. Renzulli A, Carozza A, Romano G, et al. Recurrent infective endocarditis: a multivariate analysis of 21 years of experience. Ann Thorac Surg 2001; 72:39–43. [DOI] [PubMed] [Google Scholar]
- 33. Vicent L, Saldivar HG, Bouza E, et al. ; GAMES investigators (Appendix 1) Prognostic implications of a negative echocardiography in patients with infective endocarditis. Eur J Intern Med 2018; 52:40–8. [DOI] [PubMed] [Google Scholar]
- 34. Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117:560–6. [DOI] [PubMed] [Google Scholar]
- 35. Tookes H, Diaz C, Li H, et al. A cost analysis of hospitalizations for infections related to injection drug use at a county safety-net hospital in Miami, Florida. PLoS One 2015; 10:e0129360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oliver SE, Cope AB, Rinsky JL, et al. ; Ocular Syphilis Disease Investigation Specialists Workgroup Increases in ocular syphilis-North Carolina, 2014-2015. Clin Infect Dis 2017; 65:1676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rapoport AB, Fischer LS, Santibanez S, et al. Infectious diseases physicians’ perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infect Dis 2018; 5(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eaton EF, Mathews RE, Lane PS, et al. A 9-point risk assessment for patients who inject drugs and require intravenous antibiotics: focusing inpatient resources on patients at greatest risk of ongoing drug use. Clin Infect Dis. In press. [DOI] [PubMed] [Google Scholar]
- 39. Fanucchi LC, Lofwall MR, Nuzzo PA, Walsh SL. In-hospital illicit drug use, substance use disorders, and acceptance of residential treatment in a prospective pilot needs assessment of hospitalized adults with severe infections from injecting drugs. J Subst Abuse Treat 2018; 92:64–9. [DOI] [PubMed] [Google Scholar]
- 40. Wasserman W. New data visualization tool enables in-depth, county-by-county look at impact of opioid epidemic in appalachian region https://www.arc.gov/news/article.asp?ARTICLE_ID=622. Accessed 10 July 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.