Abstract
Lung aeration is the critical first step that triggers the transition from fetal to postnatal cardiopulmonary physiology after birth. When an infant is apneic or does not breathe sufficiently, intervention is needed to support this transition. Effective ventilation is therefore the cornerstone of neonatal resuscitation. In this article, we review the physiology of cardiopulmonary transition at birth, with particular attention to factors the caregiver should consider when providing ventilation. We then summarize the available clinical evidence for strategies to monitor and perform positive pressure ventilation in the delivery room setting.
Keywords: ventilation, lung aeration, newborn, resuscitation
1. Introduction
Lung liquid clearance and aeration is the critical first step that initiates a series of cardiopulmonary events needed for successful newborn transition at birth.[1] When an infant is insufficiently breathing or apneic this transition fails and prompt intervention is needed in order to avoid (further) hypoxia and ischemic insults. Thus, effective ventilation is the cornerstone of resuscitation after birth.[2] While resuscitation is defined as the process of reviving someone from unconsciousness or apparent death, most infants only need to be stabilized with respiratory support to restore breathing. It is rare for newborns to require extensive resuscitation measures after birth, and in most cases this is because effective ventilation has not been established.[3]
While effective ventilation is the most critical step in newborn resuscitation, current recommendations are largely based on historical practice, evidence from animal models and dogma. Very little clinical evidence exists to support the current approach to ventilation, in terms of inflation time and pressures used to clear lung liquid, aerate the lung, and establish gas exchange. Our understanding of the mechanisms regulating lung aeration and maintaining the functional residual capacity is largely informed by experimental studies of animal models using phase-contrast X-ray imaging.[4] This knowledge provides a physiologic rationale to approach respiratory support in newborn infants after birth.
In the first part of this review, we discuss the physiological mechanisms of cardiopulmonary transition that the caregiver should consider when providing ventilation at birth. We then review the existing clinical evidence for providing positive pressure ventilation (PPV) in the delivery room setting.
2. The physiology of newborn transition and how this alters during neonatal resuscitation
In the last decade, there has been a renewed interest in performing experimental and observational human studies in neonatal transition and resuscitation at birth. Several recent reviews provide a comprehensive summary of the current knowledge.[4–6] For the purpose of this review we will describe the most important features that caregivers need to take into account when an infant fails transition and requires intervention.
2.1. The respiratory transition at birth passes through three distinct phases
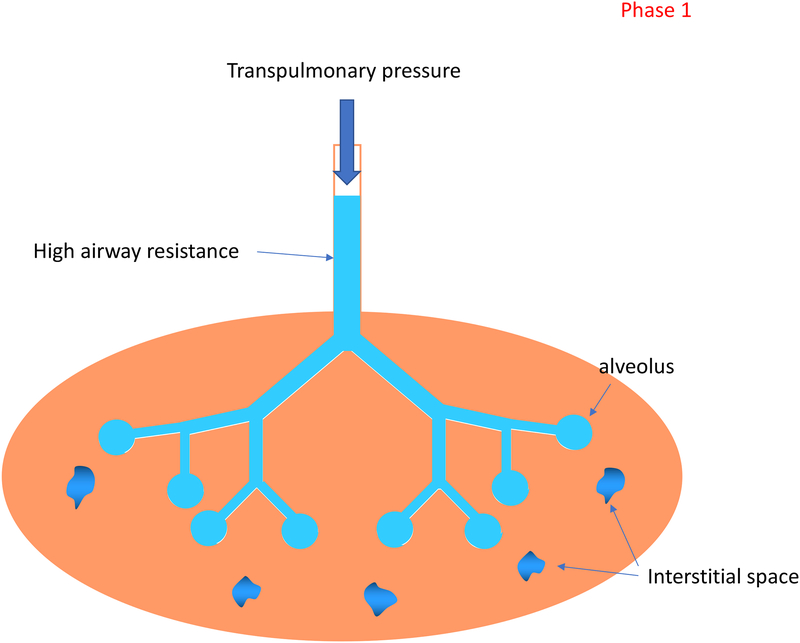
When an infant is born, the lungs are filled with liquid, preventing pulmonary gas exchange. In this first phase the support should be entirely focused on clearing the lung liquid (Figure 1). A trans-pulmonary pressure gradient plays a key role in driving the lung liquid towards the end-distal airways where it is cleared across the distal airway wall and enters the surrounding interstitial tissue space.[7] Lung liquid has a much higher resistance than air and therefore it is likely that the pressure needs to be higher or given for a longer period. There is very little gas exchange possible during this phase and it is a logical proposition that when an inflation is applied, a period of expiration in between is unnecessary.
Figure 1: Phases of lung aeration during newborn transition.
Phase 1: The airways are still fluid-filled, and the emphasis is to clear the fetal lung liquid and aerate the lung.
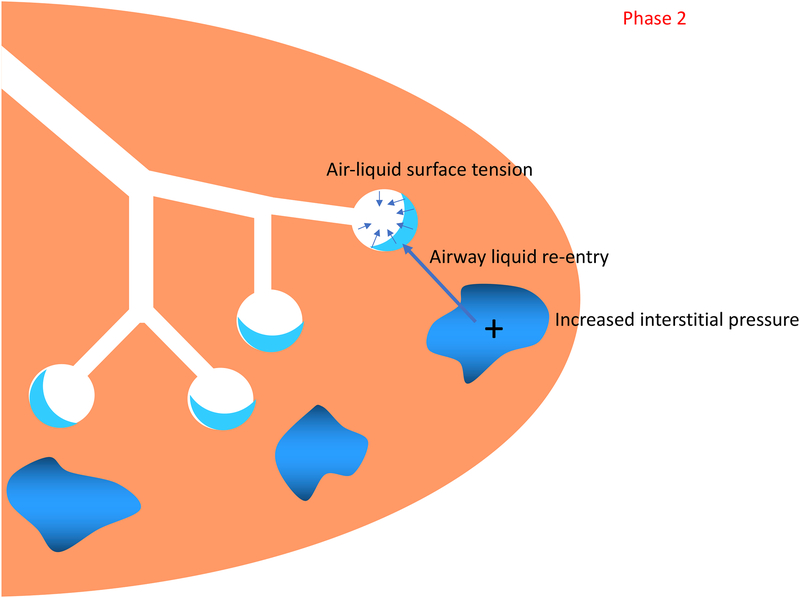
Phase 2: Lung aeration has been achieved and gas exchange is now possible. The air-liquid surface tension is present, and fetal lung liquid (now in the interstitial tissue) increases perialveolar interstitial tissue pressures. The focus of respiratory support is minimizing alveolar collapse and/or reflooding during expiration.
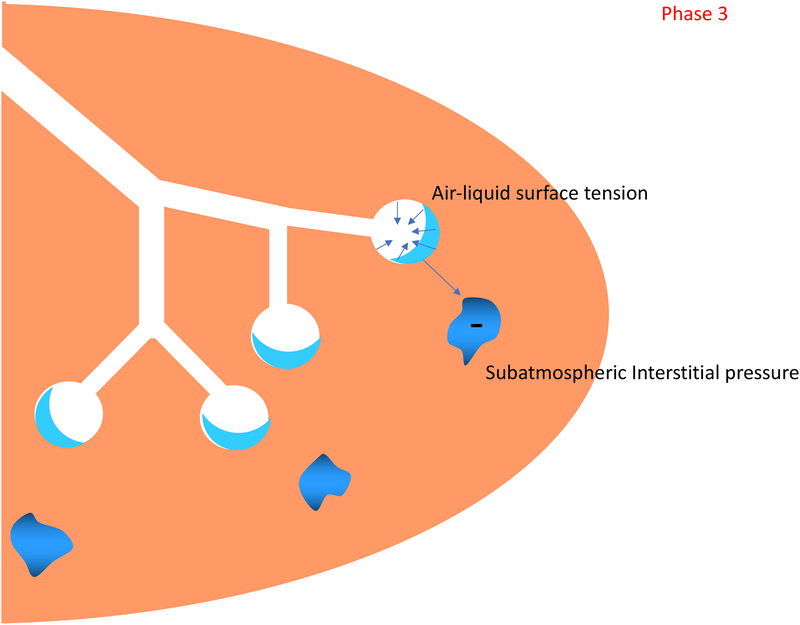
Phase 3: The lungs is aerated and liquid is cleared from the tissue. Ventilation is now focused on gas exchange and metabolic homeostasis.
After most of the lung liquid is cleared, the second phase commences and pulmonary gas exchange is possible. Between the air and the thin lining of liquid in the alveoli a tension force arises, the air-liquid surface tension, and depending on how much surfactant is present, the alveoli now have the tendency to collapse. In addition, the liquid cleared from the airways during the first phase accumulates and initially resides within the interstitial tissue, increasing perialveolar interstitial tissue pressures and posing the potential for liquid re-entry back into the airways.[8,9] In this second phase the focus of respiratory support should be on minimizing alveolar collapse and/or reflooding during expiration. Thus, adequate levels of positive end expiratory pressure or continuous positive airway pressure should be applied.[9]
In the third phase the infant has passed the immediate transition, the lungs are aerated and the liquid is cleared from the tissue. Ventilation is now more focused on gas exchange and metabolic homeostasis, which depend on the functional and structural maturity of the lung, respiratory muscles, and chest wall.
2.2. The pulmonary and hemodynamic transition are intimately linked
Lung aeration plays a key role in the sudden increase pulmonary blood flow (PBF) that is needed after birth. It has been shown in preterm rabbit pups that aeration of the lung, even with 100% nitrogen, increases both PBF and heart rate.[1] The mechanism has not been elucidated yet, but the global increase in PBF is likely a vagal response to lung aeration.[1,10] Aerating the lungs indicates a rapid accumulation of liquid in the interstitium and could activate juxta capillary receptors (J-receptors).[11] It has been described that in lung edema J-receptors are triggered by fluid accumulation and via vagal C-fibers cause global pulmonary vasodilatation.[12] The increase in heart rate might be due to triggering of the J-receptors, but it could be also that the rapid increase in PBF, which increases pulmonary venous return and left atrial filling, may increase the heart rate by increasing left ventricular preload.[1]
In asphyxiated near-term lambs, heart rate and PBF did not increase until oxygen was added to the gas mixture. However, only a small fraction was needed (5%) while increasing the oxygen to 100% did not have any additional effect.[13] This likely indicates that, once aerated, only a small amount of oxygen is needed in the inhaled gas to increase PBF and myocardial energetics. This is likely to result from a direct effect of increasing oxygen levels on both the myocardium and the pulmonary vasculature as well as the effect of lung aeration (irrespective of inspired oxygen level) on PBF.[13]
Thus, it is clear that an increase in heart rate is an important indicator of effective ventilation, as it indicates that lung aeration has been successful. However, when the lungs are not uniformly aerated, the neural reflex will cause a prompt increase PBF in both the aerated and non-aerated part, which leads to a ventilation-perfusion mismatch.[1] This is clinically often compensated by increasing the oxygen concentration and thus increasing the alveolar-capillary partial pressure difference.
This also indicates that when lung aeration and thus increased PBF has not been established, the left ventricle output is still largely dependent on the venous return from umbilical venous flow, which flows via the foramen ovale directly into the left atrium.[14] When the umbilical cord is clamped before lung aeration, the loss of umbilical venous return leads to a sudden decrease in preload for the left ventricle. The lung must be aerated with resultant increased PBF in order to restore cardiac output.[14]
2.3. The transition of the larynx
A patent larynx is required for effective breathing and ventilation at birth. At birth laryngeal activity needs to change from a fetal to neonatal pattern. Before birth, the glottis is adducted during apnea. This provides a high resistance to liquid efflux from the trachea, promotes fetal lung expansion, and thereby regulates lung growth and development.[15] The glottis dilates phasically during fetal breathing movements, and the resistance to liquid efflux is temporarily reduced and liquid leaves the lung.[16] It was recently demonstrated that apneic preterm rabbit pups retain their fetal pattern of glottic activity at birth and the glottis is closed unless a breath is taken.[17] The glottis will also relax when an infant becomes severely hypoxic and asphyxiated.[17]
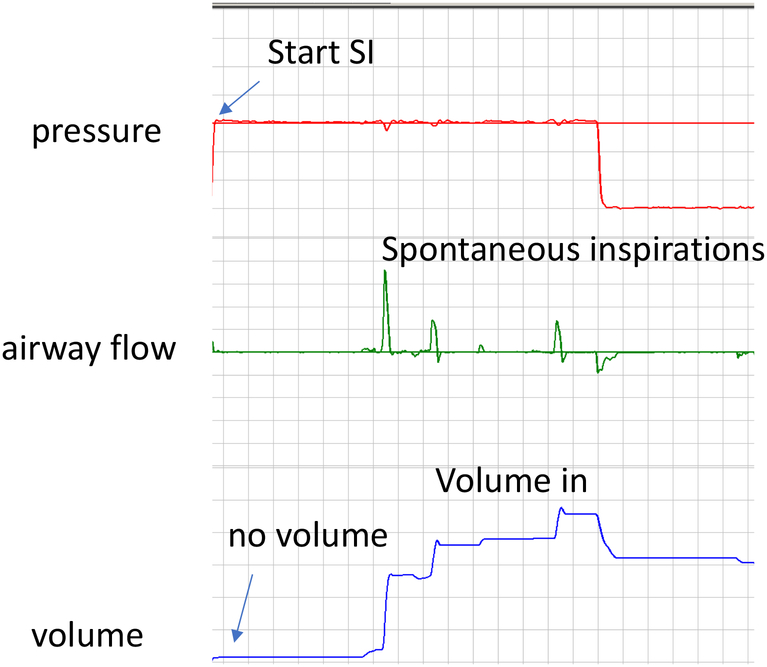
Caregivers should be aware that in all infants a closed glottis will prevent face mask ventilation from inflating the lung (Figure 2). Logically, the best way to open the larynx is to stimulate and support spontaneous breathing, which is also likely to be the most gentle and effective way of providing respiratory care without causing injury.
Figure 2: Impact of closed glottis on tidal volume delivery during sustained inflation (SI).
In this respiratory tracing, when the sustained inflation begins, the pressure rises but there is almost no positive flow and no volume enters the lung. When the infant starts spontaneously breathing (and opens the glottis), there is positive flow to the infant with improved tidal volume delivery.
2.4. Breathing at birth and oxygenation
For a successful pulmonary transition, the infant’s respiratory drive has to change from fetal breathing movements (FBM), which are discontinuous and generate low transpulmonary pressures and small tidal volumes,[18] to breathing after birth, which is regular and with large inspiratory efforts and tidal volumes. The factors that trigger the onset of large inspiratory efforts at birth are not clear but are thought to include activation of chemoreceptors, increased PaCO2 levels, loss of inhibitory factors on respiratory center activity (prostaglandins, progesterone, adenosine) and physical stimuli (light, temperature and handling).
A low oxygen level has a direct inhibitory neural input to the respiratory center in the upper lateral pons and suppresses the breathing effort.[19] Increasing the fetal oxygen level removes the inhibition and the fetus will breathe regularly and with large effort.[20] In contrast, hypoxia potentates the inhibitory neural input and the fetus becomes apneic.[21] During parturition, the infant may become mildly hypoxic. While hypoxia stimulates breathing effort later in life, immediately after birth hypoxia may inhibit respiratory drive, particularly in preterm infants where the maturation of the increase in O2 sensitivity is delayed.[22] Then during the first few weeks after birth, hypoxia increasingly stimulates respiratory drive due a temporal change in O2 sensitivity.[22] However, hyperoxia could also inhibit the chemoreceptors as a delay in onset of breathing was observed in asphyxiated infants and animals resuscitated with 100% oxygen.[23]
Most preterm infants breathe at birth, but the effort is often insufficient to clear the lung liquid and to aerate the lung in an adequate manner.[24] The caregiver should be aware that breathing can be subtle and is often missed while positive pressure is applied. Because we now use a lower oxygen concentration in order to avoid hyperoxia, hypoxia may occur in the first minutes after birth,[25,26] and this should also be avoided as it negatively influences the breathing effort.
3. Clinical evidence for the most effective methods to provide ventilation in the delivery room
Initial recommendations for PPV were grounded in historical practice, not clinical evidence. In recent years, many observational studies and clinical trials have focused on identifying the most effective methods to provide PPV in the delivery room setting. Here we discuss residual questions related to delivery room PPV and review the available clinical evidence for each.
3.1. What are the targets for positive pressure ventilation in the delivery room?
The critical target values of key respiratory parameters (such as pressure, volume and duration) during PPV remain poorly defined. In addition, the most appropriate targets for these parameters will depend on the infant’s gestational age and what phase of transition the caregiver is supporting. If the lung is still liquid-filled, the first task is to clear the lung liquid and aerate the lung. Once this process is complete, the caregiver can focus on ventilating the lung with phasic ventilation.[5] Therefore, appropriate respiratory targets for PPV are dynamic, depending on the phase of transition.
3.1.1. Peak Pressure
Pressure is currently one of the only respiratory parameters monitored during resuscitation, as manometers are readily available in the delivery room. If the glottis is open, the critical opening pressure needed to achieve initial lung aeration is likely influenced by individual differences in physiology, such as the amount of lung liquid present and the size of the airways and cross-sectional surface area of the epithelium of alveoli.[27] These factors vary by gestational age, the mode of delivery, and whether the infant is apneic or has spontaneously breathed. As discussed below (Duration) the amount of pressure required to aerate the lung is also influenced by how long the pressure is held.
When term asphyxiated infants (n=12) were intubated during delivery room resuscitation in the 1970s, peak pressures of 13–20cm H2O were necessary for functional residual capacity formation.[28] The critical peak pressures to aerate the lung in preterm infants are unknown but are likely higher as the more narrow airways and less surface area of the epithelium leads to a higher resistance to move liquid distally and across the epithelium. In addition, surfactant deficiency and a distensible chest wall in the preterm infant lower lung compliance.
In the absence of high-quality evidence to guide starting pressures during PPV, it remains logical to initiate PPV with low pressures and subsequently titrate both peak inspiratory pressures and positive end expiratory pressure according to the infant’s clinical response. It is also worth noting that after lung aeration is achieved, there are large and sudden changes in lung characteristics. Thus, the same pressures required to first aerate the lung will likely result in excessive tidal volumes during phasic ventilation.
3.1.2. Duration
As described above, the newly born infant must first clear the lung of residual lung fluid in order to recruit and aerate the lung in the first phase of newborn transition. One method to support lung liquid clearance is to increase peak inflation pressures, but this causes heterogeneous lung recruitment and can induce acute lung injury within regions of the lung that are already aerated. An alternative strategy is to apply pressure for a longer duration, ie: a “sustained inflation”. The available preclinical and clinical evidence for sustained inflation have been extensively reviewed. [29] In brief, some of the available randomized controlled trial (RCT) evidence from preterm infants suggests that sustained inflation improves short-term outcomes, such as need for mechanical ventilation in the first 72 hours after birth.[30] However, most trials of sustained inflation were not designed to assess the impact of sustained inflation on clinically relevant outcomes, such as bronchopulmonary dysplasia. Until safety and long-term outcome data are available from sufficiently powered trials, such as the ongoing Sustained Aeration of Infant Lungs trial [31], sustained inflation is not currently recommended for routine clinical practice.[2]
3.1.3. Tidal Volume (TV)
In a historic study of spontaneously breathing full-term infants (n=16), the inspiratory TV of initial inflations was the parameter most closely associated with establishment of the functional residual capacity.[32] The mean inspiratory TV required to form the functional residual capacity was 37.7mL, well over 12mL/kg for a typical term infant with a birth weight of 3kg. Many studies of preterm infants have defined an “appropriate” exhaled TV range of 4–8mL/kg during phasic PPV,[33,34] which is largely extrapolated from physiologic data in infants with an aerated lung.[35] However, the tidal volume needed to displace the fetal lung fluid and initially aerate the preterm lung is unknown.
3.2. What are the obstacles to performing effective PPV?
PPV is the most important intervention performed during neonatal resuscitation, but it is technically challenging to perform. In preterm infants, there is a narrow therapeutic TV range needed to sufficiently aerate the lung without inducing acute lung injury from over-distention and subsequent brain injury from a systemic inflammatory cascade.[36]
To minimize injury, empiric intubation and mechanical ventilation are now avoided, and the focus of respiratory care is non-invasive PPV. While mask ventilation is the first choice of respiratory support after birth, mask technique is more difficult than assumed, even for experienced caregivers. As detailed in other reviews, major obstacles to performing effective ventilation include mask leak and airway obstruction.[37,38]
Mask leak is typically caused by a poor fitting mask or poor mask position or hold. Airway obstruction can be iatrogenic from incorrect head position, insufficient chin lift, or applying the mask to tightly to the face. In addition, a closed larynx can also cause airway obstruction, especially in preterm infants.[17] Finally, if the lung is not yet aerated, there may be a residual liquid column in the airways that needs to be displaced distally; this causes a functional airway obstruction.
Recognizing these common impediments, newborn resuscitation algorithms recommend ventilation corrective steps if the heart rate does not rise in response to initial PPV. Corrective steps include actions to address mask leak (mask adjustment) and physical or functional airway obstruction (repositioning the airway, opening and suctioning the mouth, and increasing peak pressure).[39,40] These ventilation corrective steps are necessary skills for providers to learn as part of neonatal resuscitation education and training.
Lastly, appropriate recognition of spontaneous breathing is difficult during resuscitation of preterm infants, especially when the infant is wrapped in plastic for thermoregulation. While PPV is recommended for apneic or gasping infants, continuous positive airway pressure and stimulation are appropriate alternative strategies when breathing is present. Observational studies have demonstrated that preterm infants often breathe between and during the inflations given during PPV, which could be counterproductive and potentially lead to injury.[41,42]
3.3. How should we monitor PPV in the delivery room?
Limited methods exist to monitor the safety and efficacy of PPV in the delivery room setting. Rise in heart rate is considered the best indication of effective ventilation. However, in the absence of rise in heart rate, clinicians have few tools to objectively identify technical impediments or measure the quality of delivered ventilation. In addition, rise in heart rate alone will not identify excessive ventilation, which predispose the preterm infant to lung and systemic inflammation.[43][36] Potential methods to monitor delivery room PPV include visual assessment of chest rise, exhaled CO2 detection (both qualitative and quantitative), and respiratory function monitoring.
3.3.1. Chest Rise
Visual assessment of chest rise is currently recommended to guide ventilation and indicate ventilation corrective steps.[39,40] However, clinicians’ subjective assessment of chest rise during PPV is poorly associated with measured TV delivery.[44] Further, although mask leak and airway obstruction are common during delivery room PPV,[45] providers’ subjective assessment of mask leak is inaccurate.[46] Importantly, providers are more likely to underestimate than overestimate the delivered TV,[44,46] suggesting that reliance on chest rise alone may contribute to excessive TV delivery during PPV.
3.3.2. Exhaled CO2
Continuous CO2 measurements are commonly used to monitor ventilation in the neonatal intensive care unit setting. In contrast, absolute exhaled CO2 levels do not reflect arterial CO2 levels immediately after birth, due to partially fluid-filled lungs, reduced pulmonary blood flow, dead space in the respiratory circuit, and rebreathing CO2. In addition, exhaled CO2 values are typically lower in apneic infants, compared with infants who spontaneously breathe.[47,48]
Qualitative exhaled CO2 detection and rising trends in CO2 preceed rise in heart rate during PPV [49,50] and likely reflect lung aeration.[48,51,52][53] While many observational studies have demonstrated that exhaled CO2 monitoring is feasible during delivery room PPV,[54] there are limited data suggesting that CO2 monitoring in the delivery room improves clinical outcomes.
Kong et al. randomly assigned preterm infants who required PPV after birth to have resuscitation guided by exhaled CO2 monitoring vs. clinical assessment alone. In that trial, there were no significant differences in median PCO2 levels at the end of the resuscitation or upon NICU admission between groups. In addition, the proportion of normocarbic infants at both time points was not significantly different.[55] Hawkes et al. randomized preterm infants to undergo resuscitation with qualitative CO2 detector vs. colorimetric CO2 detector. There was no significant difference between groups with regrds to the primary outcome, normocarbia on first blood gas, or other clinically relevant clinical outcomes.[56]
3.3.3. Respiratory Function Monitoring
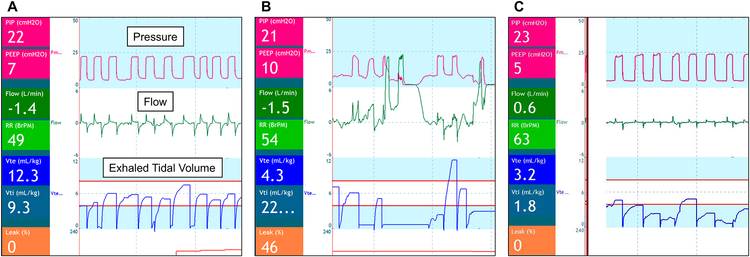
A respiratory function monitor (RFM) uses an in-line flow transducer between the gas flow and facemask to instantaneously calculate and display spirometric data.[57] Displayed inflation parameters include peak pressures, PEEP, expiratory TV, flow patterns, and mask leak (Figure 3). The RFM provides the potential for volume-targeted delivery room PPV, as opposed to reliance on subjective assessment of chest rise to titrate peak pressures. In addition, the numeric data and respiratory waveforms can be used to identify common impediments to PPV, such as mask leak or airway obstruction (Figure 3), which would allow a more targeted approach to ventilation corrective steps.
Figure 3: Respiratory function monitor display during positive pressure ventilation.
In each display, respiratory data are presented numerically on left side and with waveforms displayed centrally, as labelled on panel A
A: Successful PPV: Unobstructed air flow, exhaled tidal volume between 4–8 ml/kg, and there is no leak.
B: PPV with consistent mask leak. Air flow is consistently positive, exhaled tidal volumes are inconsistent and often low, and calculated leak is displayed numerically in the bottom left corner.
C: PPV with anatomic or functional airway obstruction. Peak pressures are consistent, minimal positive and negative air flow, exhaled tidal volumes are low, and there is no leak.
In preliminary manikin studies, use of an RFM improved PPV performance.[58–60] Few studies have investigated the impact of RFM on provider performance and patient outcomes in the clinical environment. In Schmolzer’s single site feasibility RCT, providers performed PPV for preterm infants with either a visible vs. masked RFM display; RFM data were recorded in all cases. The median face mask leak was significantly lower in the visible RFM group. In addition, the proportion of infants intubated during PPV- a secondary outcome- was significantly lower in the RFM-visible group.[61] The ongoing MONITOR trial is a multi-site RCT of infants <28 weeks gestation receiving PPV after birth designed to determine whether a masked versus visible RFM display results in a significantly increased proportion of inflations delivered within a targeted range of exhaled TV.[33]
3.4. How should we deliver PPV?
Historically, PPV has been applied via facemask and bag in the delivery room setting. Recognition of the technical impediments to safe and effective facemask ventilation have prompted interest in alternative devices and interfaces.
3.4.1. Respiratory Devices
Recommended respiratory devices to deliver PPV include a self-inflating bag, flow-inflating bag, and the T-piece resuscitator. These devices all have benefits and pitfalls, and there are insufficient data to identify any one of these as the optimal device for delivery room PPV. In manikin studies, providers deliver PPV with lower and more consistent pressures using the T-piece resuscitator,[62][63] prompting an interest in the clinical effectiveness of this device. Guinsburg at al. published a large (n=1962) cohort study of preterm infants <33 weeks who underwent resuscitation with either T-piece resuscitator or self-inflating bag. After adjusting for important maternal and infant demographic characteristics, infants who received PPV with the T-piece were significantly more likely to survive to discharge without bronchopulmonary dysplasia or severe intraventricular hemorrhage, or periventricular leukomalacia.[64]
In by far the largest randomized trial of resuscitation devices, Szyld et al. performed a cluster-randomized crossover trial comparing the T-piece resuscitator with the self-inflating bag among 1,027 infants ≥ 26 weeks gestation who required PPV after birth.[65] There was no significant difference in the primary outcome of heart rate ≥ 100 beats per minute at 2 minutes of life. However, peak pressures delivered with the T-piece were significantly lower and less variable, fewer infants in the T-piece group were intubated in the delivery room, and in a post-hoc analysis of very low birth weight infants, significantly fewer infants in the T-piece group developed bronchopulmonary dysplasia.
Similarly, Thakur et al. reported that infants resuscitated with the T-piece received PPV for a significantly shorter duration and were less likely to be intubated in the delivery room compared with infants resuscitated with the self-inflating bag in their quasi-randomized trial of 90 infants >26 weeks gestation.[66] In contrast, Dawson et al. compared oxygenation parameters among 80 infants <29 weeks gestation resuscitated with the T-piece resuscitator versus the self-inflating bag.[67] In that RCT, there was no significant difference in median SpO2 levels at 5 minutes of life between groups (primary outcome) or delivery room intubation (secondary outcome).
Thus, while the T-piece resuscitator is not necessarily more a more effective ventilation device than the self-inflating bag, the more consistent pressures delivered with the T-piece resuscitator may make it a safer device for populations at risk for acute lung injury after birth, such as preterm infants.
3.4.2. Interface
Although PPV is most commonly delivered via a facemask, alternative interfaces include nasal prongs or a nasal tube, and the laryngeal mask airway (LMA).
Two RCTs compared nasal tube with facemask for PPV in preterm infants. Kamlin et al. randomized 363 infants <30 weeks gestation to undergo PPV with either single nasal tube or facemask.[68] There was no significant difference in the primary outcome of intubation in the first 24 hours of life or any of the short-term secondary clinical outcomes. RFM recordings analyzed from 43 infants enrolled in the Kamlin trial demonstrated that airway obstruction and leak were significantly more common in inflations delivered via nasal tube than facemask. [69] Similarly, McCarthy et al. randomized 144 infants <31 weeks gestation to receive PPV delivered via nasal tube or facemask. The primary outcome, delivery room intubation, was not significantly different between groups.[70]
A laryngeal mask airway (LMA) is inserted orally with the laryngeal mask lumen sitting directly over the supraglottic airway. A LMA is easily placed without laryngoscopy,[71] making it ideal for inexperienced providers. Current guidelines recommend the use of LMA as an alternative to endotracheal intubation when facemask ventilation is unsuccessful.[39,40] However, the LMA is also a potential alternative device to the facemask for initiation of PPV. A recent systematic review included 4 clinical trials comparing LMA to facemask ventilation for primary delivery room PPV.[72] These trials varied in design but all demonstrated that a LMA is a safe and feasible method to deliver PPV in infants >34 weeks gestation. In one RCT in that review, an LMA significantly reduced the time to spontaneous breathing during PPV, compared with facemask.[73] In two of the additional trials, infants who received LMA PPV were significantly less likely to be intubated for ineffective ventilation compared with infants who received facemask PPV.[74,75]
Thus, there are convincing preliminary evidence that PPV delivered via LMA may be more effective than facemask PPV. However, all infants in these trials were >34 weeks gestation. There is insufficient evidence to assess the safety and efficacy of LMA in preterm infants <34 weeks gestation, who are at highest need for PPV in the delivery room.
4. Conclusion
Establishing lung aeration and ventilation is the most critical phase of newborn transition after birth. Respiratory interventions to support this transition should be individualized based on several factors, such as the infant’s underlying physiology, the phase of lung aeration, and the infant’s response to resuscitation. As there are limited data to identify one best method of providing PPV, providers should be aware of the strengths and limitations of the available techniques, monitoring, and equipment for delivery room PPV.
Clinical Practice Points
Lung aeration triggers the cardiorespiratory adaptation to the post-uterine environment after birth
Effective ventilation is the most critical intervention for newborns who need support during neonatal transition
Lung aeration occurs in distinct phases, and the focus of respiratory support varies based on the phase of transition
Research Directions
To identify the impact of respiratory function monitoring and continuous CO2 monitoring on providers’ PPV performance and clinically relevant outcomes in the newborn
To characterize the safety and clinical effectiveness of sustained inflation for lung aeration after birth
To determine the safest and most effective devices and interfaces to provide delivery room positive pressure ventilation
Acknowledgments
Funding Support: Dr. Foglia is supported by a NICHD Career Development Award (K23HD084727). Dr. te Pas is recipient of a NWO innovational research incentives scheme (VIDI 91716428)
Abbreviations:
- FBM
fetal breathing movements
- LMA
laryngeal mask airway
- PBF
pulmonary blood flow
- PPV
positive pressure ventilation
- RCT
randomized controlled trial
- RFM
respiratory function monitor
- TV
tidal volume
Footnotes
Disclosures: The authors have no financial conflicts of interest to disclose. The authors are both investigators on the ongoing SAIL trial (Sustained Aeration of Infant Lungs), Clinicaltrials.gov Identifier NCT02139800 and MONITOR trial (Monitoring Neonatal Resuscitation) trial, Clinicaltrials.gov Identifier NCT NCT03256578.
Contributor Information
Elizabeth E. Foglia, Division of Neonatology, The Children’s Hospital of Philadelphia and The University of Pennsylvania Perelman School of Medicine, Philadelphia PA, USA, foglia@email.chop.edu.
Arjan B. te Pas, Division of Neonatology, Department of Pediatrics, Leiden University Medical Center, Leiden, Netherlands, A.B.te_Pas@lumc.nl.
References
- [1].Lang JAR, Pearson JT, Binder-Heschl C, Wallace MJ, Siew ML, Kitchen MJ, et al. Increase in Pulmonary blood flow at birth; role of oxygen and lung aeration. J Physiol 2016; 594:1389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2015;132:S204–41. [DOI] [PubMed] [Google Scholar]
- [3].Perlman JM, Risser R. Cardiopulmonary resuscitation in the delivery room. Associated clinical events. Arch Pediatr Adolesc Med 1995;149:20–5. [DOI] [PubMed] [Google Scholar]
- [4].Hooper SB, Siew ML, Kitchen MJ, te Pas AB. Establishing functional residual capacity in the non-breathing infant. Semin Fetal Neonatal Med 2013;18:336–43. [DOI] [PubMed] [Google Scholar]
- [5].Hooper SB, te Pas AB, Kitchen MJ. Respiratory transition in the newborn: a three-phase process. Arch Dis Child Fetal Neonatal Ed 2016:101:F266–71. [DOI] [PubMed] [Google Scholar]
- [6].Hooper SB, te Pas AB, Lang J, van Vonderen JJ, Roehr CC, Kluckow M, et al. Cardiovascular transition at birth: a physiological sequence. Pediatr Res 2015;77. [DOI] [PubMed] [Google Scholar]
- [7].Siew ML, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, te Pas AB, et al. Inspiration regulates the rate and temporal pattern of lung liquid clearance and lung aeration at birth. J Appl Physiol 2009;106:1888–95. [DOI] [PubMed] [Google Scholar]
- [8].Bland RD, McMillan DD, Bressack MA, Dong L. Clearance of liquid from lungs of newborn rabbits. J Appl Physiol 1980;49:171–7. [DOI] [PubMed] [Google Scholar]
- [9].McGillick EV, Lee K, Yamaoka S, te Pas AB, Crossley KJ, Wallace MJ, et al. Elevated airway liquid volumes at birth: a potential cause of transient tachypnea of the newborn. J Appl Physiol 2017;123:1204–13. [DOI] [PubMed] [Google Scholar]
- [10].Lang JAR, Pearson JT, Binder-Heschl C, Wallace MJ, Siew ML, Kitchen MJ, et al. Vagal denervation inhibits the increase in pulmonary blood flow during partial lung aeration at birth. J Physiol 2017;595:1593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Siew ML, Wallace MJ, Allison BJ, Kitchen MJ, te Pas AB, Islam MS, et al. The role of lung inflation and sodium transport in airway liquid clearance during lung aeration in newborn rabbits. Pediatr Res 2013;73:443–9. [DOI] [PubMed] [Google Scholar]
- [12].Paintal AS. Mechanism of stimulation of type J pulmonary receptors. J Physiol 1969;203:511–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sobotka KS, Ong T, Polglase GR, Crossley KJ, Moss TJM, Hooper SB. The effect of oxygen content during an initial sustained inflation on heart rate in asphyxiated near-term lambs. Arch Dis Child Fetal Neonatal Ed 2015;100:F337–43. [DOI] [PubMed] [Google Scholar]
- [14].Hooper SB, Binder-Heschl C, Polglase GR, Gill AW, Kluckow M, Wallace EM, et al. The timing of umbilical cord clamping at birth: physiological considerations. Matern Health Neonatol Perinatol 2016;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hooper SB, Harding R. Fetal lung liquid: A major determinant of the growth and functional development of the fetal lung. Clin Exp Pharmacol Physiol 1995;22:235–41. [DOI] [PubMed] [Google Scholar]
- [16].Harding R, Bocking AD, Sigger JN. Influence of upper respiratory tract on liquid flow to and from fetal lungs. J Appl Physiol 1985 1986;61:68–74. [DOI] [PubMed] [Google Scholar]
- [17].Crawshaw JR, Kitchen MJ, Binder-Heschl C, Thio M, Wallace MJ, Kerr LT, et al. Laryngeal closure impedes non-invasive ventilation at birth. Arch Dis Child Fetal Neonatal Ed Published online first: 20 October 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Harding R Fetal breathing movements In: C R, W E, B P, editors. The Lung: Scientific Foundations. New York: Lippincott-Raven; 1997. p 2093. [Google Scholar]
- [19].Gluckman PD, Johnston BM. Lesions in the upper lateral pons abolish the hypoxic depression of breathing in unanaesthetized fetal lambs in utero. J Physiol 1987;382:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Baier RJ, Hasan SU, Cates DB, Hooper D, Nowaczyk BJ, Rigatto H. Hyperoxemia profoundly alters breathing pattern and arouses the fetal sheep. J Dev Physiol 1992; 18:143–150. [PubMed] [Google Scholar]
- [21].Alvarez JE, Baier RJ, Fajardo CA, Nowaczyk BJ, Cates DB, Rigatto H. The effect of 10% O2 on the continuous breathing induced by O2 or O2 plus cord occlusion in the fetal sheep. J Dev Physiol 1992;17:227–32. [PubMed] [Google Scholar]
- [22].Davey MG, Moss TJ, McCrabb GJ, Harding R. Prematurity alters hypoxic and hypercapnic ventilatory responses in developing lambs. Respir Physiol 1996;105:57–67. [DOI] [PubMed] [Google Scholar]
- [23].Bookatz GB, Mayer CA, Wilson CG, Vento M, Gelfand SL, Haxhiu MA, et al. Effect of Supplemental Oxygen on Reinitiation of Breathing after Neonatal Resuscitation in Rat Pups. Pediatr Res 2007;61:698–702. [DOI] [PubMed] [Google Scholar]
- [24].Huberts TJP, Foglia EE, Narayen IC, van Vonderen JJ, Hooper SB, te Pas AB. The Breathing Effort of Very Preterm Infants at Birth. J Pediatr Published Online First 12 January 2018. [DOI] [PubMed] [Google Scholar]
- [25].White LN, Thio M, Owen LS, Kamlin CO, Sloss S, Hooper SB, et al. Achievement of saturation targets in preterm infants <32 weeks’ gestational age in the delivery room. Arch Dis Child Fetal Neonatal Ed 2017;102:F423–7. [DOI] [PubMed] [Google Scholar]
- [26].Goos TG, Rook D, van der Eijk AC, Kroon AA, Pichler G, Urlesberger B, et al. Observing the resuscitation of very preterm infants: Are we able to follow the oxygen saturation targets? Resuscitation 2013;84:1108–13. [DOI] [PubMed] [Google Scholar]
- [27].te Pas AB, Kitchen MJ, Lee K, Wallace MJ, Fouras A, Lewis RA, et al. Optimizing lung aeration at birth using a sustained inflation and positive pressure ventilation in preterm rabbits. Pediatr Res 2016;80:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boon AW, Milner AD, Hopkin IE. Lung expansion, tidal exchange, and formation of the functional residual capacity during resuscitation of asphyxiated neonates. J Pediatr 1979;95:1031–6. [DOI] [PubMed] [Google Scholar]
- [29].Foglia EE, te Pas AB. Sustained Lung Inflation: Physiology and Practice. Clin Perinatol 2016;43:633–46. [DOI] [PubMed] [Google Scholar]
- [30].Schmölzer GM, Kumar M, Aziz K, Pichler G, O’Reilly M, Lista G, et al. Sustained inflation versus positive pressure ventilation at birth: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2015; 100:F361–8. [DOI] [PubMed] [Google Scholar]
- [31].Foglia EE, Owen LS, Thio M, Ratcliffe SJ, Lista G, te Pas A, et al. Sustained Aeration of Infant Lungs (SAIL) trial: study protocol for a randomized controlled trial. Trials 2015;16:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vyas H, Field D, Milner AD, Hopkin IE. Determinants of the first inspiratory volume and functional residual capacity at birth. Pediatr Pulmonol 1986;2:189–93. [DOI] [PubMed] [Google Scholar]
- [33].Monitoring Neonatal Resuscitation Trial. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03256578 (accessed December 22, 2017).
- [34].Murthy V, Dattani N, Peacock JL, Fox GF, Campbell ME, Milner AD, et al. The first five inflations during resuscitation of prematurely born infants. Arch Dis Child Fetal Neonatal Ed 2012;97:F249–253. [DOI] [PubMed] [Google Scholar]
- [35].Keszler M, Abubakar K. Volume guarantee: stability of tidal volume and incidence of hypocarbia. Pediatr Pulmonol 2004;38:240–5. [DOI] [PubMed] [Google Scholar]
- [36].Polglase GR, Miller SL, Barton SK, Baburamani AA, Wong FY, Aridas JDS, et al. Initiation of resuscitation with high tidal volumes causes cerebral hemodynamic disturbance, brain inflammation and injury in preterm lambs. PLoS One 2012;7:e39535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fuchs H, Schilleman K, Hummler HD, te Pas AB. Techniques and Devices to Improve Noninvasive Ventilation in the Delivery Room. NeoReviews 2012;13:e353–63. [Google Scholar]
- [38].van Vonderen JJ, van Zanten HA, Schilleman K, Hooper SB, Kitchen MJ, Witlcox RS, et al. Cardiorespiratory Monitoring during Neonatal Resuscitation for Direct Feedback and Audit. Front Pediatr 2016;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wyllie J, Bruinenberg J, Roehr CC, Rüdiger M, Trevisanuto D, Urlesberger B. European Resuscitation Council Guidelines for Resuscitation 2015: Section 7. Resuscitation and support of transition of babies at birth. Resuscitation 2015;95:249–63. [DOI] [PubMed] [Google Scholar]
- [40].Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Perlman JM, et al. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S543–60. [DOI] [PubMed] [Google Scholar]
- [41].Schilleman K, van der Pot CJM, Hooper SB, Lopriore E, Walther FJ, te Pas AB. Evaluating manual inflations and breathing during mask ventilation in preterm infants at birth. J Pediatr 2013;162:457–63. [DOI] [PubMed] [Google Scholar]
- [42].van Vonderen JJ, Hooper SB, Hummler HD, Lopriore E, te Pas AB. Effects of a sustained inflation in preterm infants at birth. J Pediatr 2014;165:903–908. e1. [DOI] [PubMed] [Google Scholar]
- [43].Hillman NH, Moss TJM, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, et al. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med 2007;176:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Poulton DA, Schmolzer GM, Morley CJ, Davis PG. Assessment of chest rise during mask ventilation of preterm infants in the delivery room. Resuscitation 2011;82:175–9. [DOI] [PubMed] [Google Scholar]
- [45].Schmölzer GM, Dawson JA, Kamlin COF, O’Donnell CP, Morley CJ, Davis PG. Airway obstruction and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed 2011;96:F254–7. [DOI] [PubMed] [Google Scholar]
- [46].Schmölzer GM, Kamlin OCOF, O’Donnell CPF, Dawson JA, Morley CJ, Davis PG. Assessment of tidal volume and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed 2010;95:F393–7. [DOI] [PubMed] [Google Scholar]
- [47].van Vonderen JJ, Lista G, Cavigioli F, Hooper SB, te Pas AB. Effectivity of ventilation by measuring expired CO2 and RIP during stabilisation of preterm infants at birth. Arch Dis Child Fetal Neonatal Ed 2015;100:F514–518. [DOI] [PubMed] [Google Scholar]
- [48].Murthy V, O’Rourke-Potocki A, Dattani N, Fox GF, Campbell ME, Milner AD, et al. End tidal carbon dioxide levels during the resuscitation of prematurely born infants. Early Hum Dev 2012;88:783–7. [DOI] [PubMed] [Google Scholar]
- [49].Blank D, Rich W, Leone T, Garey D, Finer N. Pedi-cap color change precedes a significant increase in heart rate during neonatal resuscitation. Resuscitation 2014;85:1568–72. [DOI] [PubMed] [Google Scholar]
- [50].Hooper SB, Fouras A, Siew ML, Wallace MJ, Kitchen MJ, te Pas AB, et al. Expired CO2 levels indicate degree of lung aeration at birth. PLoS One 2013;8:e70895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Palme-Kilander C, Tunell R. Pulmonary gas exchange during facemask ventilation immediately after birth. Arch Dis Child 1993;68:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kang LJ, Cheung P-YY, Pichler G, O’Reilly M, Aziz K, Schmölzer GM. Monitoring Lung Aeration during Respiratory Support in Preterm Infants at Birth. PLoS One 2014;9:e102729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hawkes GA, Kenosi M, Finn D, O’Toole JM, O’Halloran KD, Boylan GB, et al. Delivery room end tidal CO2 monitoring in preterm infants <32 weeks. Arch Dis Child Fetal Neonatal Ed 2016;101:F62–65. [DOI] [PubMed] [Google Scholar]
- [54].Hawkes GA, Kelleher J, Ryan CA, Dempsey EM. A review of carbon dioxide monitoring in preterm newborns in the delivery room. Resuscitation 2014;85:1315–9. [DOI] [PubMed] [Google Scholar]
- [55].Kong JY, Rich W, Finer NN, Leone TA. Quantitative end-tidal carbon dioxide monitoring in the delivery room: a randomized controlled trial. J Pediatr 2013;163:104–8.e1. [DOI] [PubMed] [Google Scholar]
- [56].Hawkes GA, Finn D, Kenosi M, Livingstone V, O’Toole JM, Boylan GB, et al. A Randomized Controlled Trial of End-Tidal Carbon Dioxide Detection of Preterm Infants in the Delivery Room. J Pediatr 2017;182:74–78. e2. [DOI] [PubMed] [Google Scholar]
- [57].Schmölzer GM, Kamlin OCOF, Dawson JA, te Pas AB, Morley CJ, Davis PG. Respiratory monitoring of neonatal resuscitation. Arch Dis Child Fetal Neonatal Ed 2010;95:F295–303. [DOI] [PubMed] [Google Scholar]
- [58].Wood FE, Morley CJ, Dawson JA, Davis PG. A respiratory function monitor improves mask ventilation. Arch Dis Child Fetal Neonatal Ed 2008;93:F380–1. [DOI] [PubMed] [Google Scholar]
- [59].Kattwinkel J, Stewart C, Walsh B, Gurka M, Paget-Brown A. Responding to compliance changes in a lung model during manual ventilation: perhaps volume, rather than pressure, should be displayed. Pediatrics 2009;123:e465–70. [DOI] [PubMed] [Google Scholar]
- [60].Bowman TA, Paget-Brown A, Carroll J, Gurka MJ, Kattwinkel J. Sensing and responding to compliance changes during manual ventilation using a lung model: can we teach healthcare providers to improve? J Pediatr 2012;160:372–376. e1. [DOI] [PubMed] [Google Scholar]
- [61].Schmölzer GM, Morley CJ, Wong C, Dawson JA, Kamlin COF, Donath SM, et al. Respiratory function monitor guidance of mask ventilation in the delivery room: a feasibility study. J Pediatr 2012;160:377–381. e2. [DOI] [PubMed] [Google Scholar]
- [62].Dawson JA, Gerber A, Kamlin COF, Davis PG, Morley CJ. Providing PEEP during neonatal resuscitation: which device is best? J Paediatr Child Health 2011;47:698–703. [DOI] [PubMed] [Google Scholar]
- [63].Bennett S, Finer NN, Rich W, Vaucher Y. A comparison of three neonatal resuscitation devices. Resuscitation 2005;67:113–8. [DOI] [PubMed] [Google Scholar]
- [64].Guinsburg R, de Almeida MFB, de Castro JS, Gonçalves-Ferri WA, Marques PF, Caldas JPS, et al. T-piece versus self-inflating bag ventilation in preterm neonates at birth. Arch Dis Child Fetal Neonatal Ed 2018;103:F49–55. [DOI] [PubMed] [Google Scholar]
- [65].Szyld E, Aguilar A, Musante GA, Vain N, Prudent L, Fabres J, et al. Comparison of Devices for Newborn Ventilation in the Delivery Room. J Pediatr 2014;165:234–239. e3. [DOI] [PubMed] [Google Scholar]
- [66].Thakur A, Saluja A, Modi M, Kler N, Garg P, Soni A, et al. T-piece or self inflating bag for positive pressure ventilation during delivery room resuscitation: An RCT. Resuscitation 2015;90:21–4. [DOI] [PubMed] [Google Scholar]
- [67].Dawson JA, Schmölzer GM, Kamlin COF, te Pas AB, O’Donnell CPF, Donath SM, et al. Oxygenation with T-piece versus self-inflating bag for ventilation of extremely preterm infants at birth: a randomized controlled trial. J Pediatr 2011;158:912–918. e1–2. [DOI] [PubMed] [Google Scholar]
- [68].Kamlin COF, Schilleman K, Dawson JA, Lopriore E, Donath SM, Schmölzer GM, et al. Mask versus nasal tube for stabilization of preterm infants at birth: a randomized controlled trial. Pediatrics 2013;132:e381–388. [DOI] [PubMed] [Google Scholar]
- [69].van Vonderen JJ, Kamlin CO, Dawson JA, Walther FJ, Davis PG, te Pas AB. Mask versus Nasal Tube for Stabilization of Preterm Infants at Birth: Respiratory Function Measurements. J Pediatr 2015;167:81–85. e1. [DOI] [PubMed] [Google Scholar]
- [70].McCarthy LK, Twomey AR, Molloy EJ, Murphy JFA, O’Donnell CPF. A randomized trial of nasal prong or face mask for respiratory support for preterm newborns. Pediatrics 2013;132:e389–395. [DOI] [PubMed] [Google Scholar]
- [71].Gandini D, Brimacombe J. Manikin training for neonatal resuscitation with the laryngeal mask airway. Pediatr Anesth 2004;14:493–4. [DOI] [PubMed] [Google Scholar]
- [72].Bansal SC, Caoci S, Dempsey E, Trevisanuto D, Roehr CC. The Laryngeal Mask Airway and Its Use in Neonatal Resuscitation: A Critical Review of Where We Are in 2017/2018. Neonatology 2018;113:152–61. [DOI] [PubMed] [Google Scholar]
- [73].Pejovic NJ, Trevisanuto D, Lubulwa C, Myrnerts Höök S, Cavallin F, Byamugisha J, et al. Neonatal resuscitation using a laryngeal mask airway: a randomised trial in Uganda. Arch Dis Child Fetal Neonatal Ed Published online 14 September 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Trevisanuto D, Cavallin F, Nguyen LN, Nguyen TV, Tran LD, Tran CD, et al. Supreme Laryngeal Mask Airway versus Face Mask during Neonatal Resuscitation: A Randomized Controlled Trial. J Pediatr 2015;167:286–291.e1. [DOI] [PubMed] [Google Scholar]
- [75].Zhu XY, Lin B, Zhang QS, Ye HM, Yu RJ. A prospective evaluation of the efficacy of the laryngeal mask airway during neonatal resuscitation. Resuscitation 2011;82:1405–9. [DOI] [PubMed] [Google Scholar]