Abstract
Background
Timeliness in the administration of recommended vaccines is often evaluated using vaccine delays and provides more information regarding the susceptibility of children to vaccine-preventable diseases compared with vaccine coverage at a given age. The importance of on-time administration of vaccines scheduled at the first visit is well documented, but data are scarce about the impact of vaccine delays at other visits on vaccination status by 24 months of age. Using vaccine delays for the first three doses of DTaP-containing vaccines and for the first dose of measles-containing vaccines as markers of timeliness at the 2, 4, 6 and 12 month visits, we estimated the proportion of incomplete vaccination status by 24 months of age attributable to a vaccine delay at each of these visits.
Methods
We used the data from six cross-sectional coverage surveys conducted in the Province of Quebec from 2006 to 2016 which included 7183 children randomly selected from the universal health insurance database. A vaccine dose was considered delayed if received 30 days or more after the recommended age. The impact of new vaccine delays at each visit on incomplete vaccination status by 24 months of age was estimated with the attributable risk in the population.
Results
The proportion of children with vaccine delay was 5.4% at 2 months, 13.3% at 4 months, 23.1% at 6 months and 23.6% at 12 months. Overall, 72.5% of all 2-year-old children with an incomplete status by 24 months were attributable with a vaccine delay, of which 16.1% were attributable with a first vaccine delay at 2 months, 10.6% at 4 months, 14.0% at 6 months and 31.8% at 12 months.
Conclusions
While great emphasis has been put on vaccine delays at the first vaccination visit, the prevalence of vaccine delays was greater with later visits and most children with an incomplete vaccination status by 24 months had a vaccine delay occurring during these later visits. Interventions to improve timeliness should address vaccine delays at each visit and not only focus on the first visit.
Electronic supplementary material
The online version of this article (10.1186/s12889-018-6235-6) contains supplementary material, which is available to authorized users.
Keywords: Delay, Timeliness, Vaccine coverage, Vaccination, Infant
Background
Vaccine coverage is a common indicator used for the evaluation and monitoring of vaccination programs. It evaluates the proportion of individuals who have received all the recommended vaccines, regardless of the timeliness [1]. Timely vaccination is increasingly used to monitor vaccination programs. By assessing the interval between the recommended age and the age at which a dose was administered, this indicator provides a better estimate of the period during which children are protected [2–4]. Timely vaccination is often evaluated using vaccine delays for which several definitions exist. The definition most commonly used is a delay of 30 days or more after the recommended age for each dose [3–10]. A vaccine delay for a dose may impact on-time administration of subsequent doses and increase the child’s risk of disease targeted by the vaccine [11, 12].
In Quebec (Canada), vaccines recommended to children are free of charge and are mostly administered by public health clinics. In 2005, a survey conducted by the Quebec Ministry of Health reported that vaccine delays to the childhood schedule were occurring in public health clinics of several regions of the province. These delays were attributed to a variety of factors including the introduction of new vaccine programs in the early 2000’s [13, 14], missed opportunities due to providers reluctance to administer several injections at the same visit and barriers like difficulty to get a vaccination appointment. In response, monitoring of vaccine delays has been introduced in 2006 in public health clinics for the 2-month and the 12-month visits. To improve the 2-month visit timeliness, appointment periods with priority given to first visits were added and activities such as reminders and recalls were implemented. For children with vaccine delays, an accelerated vaccination schedule can be applied using the minimum intervals between the vaccine doses instead of the intervals recommended for routine vaccination.
Since 2006, vaccine coverage surveys have been conducted every 2 years in children aged 1 and 2 years. The 2006 survey found that only 17% of 24-month old children had received all recommended vaccine doses with no delays. This survey as well as other studies had shown that vaccine delays of 30 days or more for vaccines scheduled at the first visit at 2 months of age were associated with vaccine delays at later visits or with an incomplete vaccination status by 24 months of age [9, 15–18]. However there are scarce data regarding the frequency of vaccine delays at other visits and their impact on vaccine coverage by 24 months of age. Using Quebec vaccine coverage surveys from 2006 to 2016, we estimated the proportion of children with vaccine delays at 2, 4, 6 and 12 months and the proportion of incomplete vaccination status by 24 months of age attributable to a vaccine delay at each of these visits. To identify more vulnerable populations, factors associated with vaccine delays at each vaccination visit from 2 to 12 months were assessed.
Methods
Study population and survey design
The present study is based on the data from six cross-sectional surveys conducted in Quebec in 2006, 2008, 2010, 2012, 2014 and 2016 [15, 19–23]. With the authorization of the Quebec Access to Information Commission, children in these studies were randomly selected from the Quebec Universal Health Insurance database which includes all children from the province. Each survey included a “1-year cohort” and a “2-year cohort” with children aged 15 to 17 months and 24 to 26 months respectively at the time of the survey. With surveys conducted every other year and cohorts defined by year of birth (e.g. in the survey conducted at the beginning of 2006, the 2-year cohort included children born in 2003 and those in the 1-year cohort were born in 2004), the six surveys assessed the immunization data in 12 different birth cohorts (born between 2003 and 2014). The surveys invited approximately 1000 children in each cohort (only 600 for 2006), a number deemed sufficient to obtain a precision of ±3% in the vaccine coverage for each survey assuming a response rate of about 60% [24]. Were excluded children living in the two northern regions of the province.
Questionnaires were sent by mail and were filled out by parents or legal guardians. A postal reminder was sent to non-respondents 2 weeks and 4 weeks later. In the absence of response, parents were called directly 2 weeks after the last postal reminder and those with unknown phone number or not reached by phone received another questionnaire by mail. Respondents were invited to transcribe on the questionnaire the information available in their child’s vaccination booklet (i.e. vaccine names, date of vaccination and vaccine providers). The questionnaire also collected information on the characteristics of the child, the mother and the parents. For children without vaccination booklets and those with information incomplete or inconsistent with the provincial vaccine schedule, vaccine providers were contacted to collect/validate the information. Only written information on doses from parents or vaccine providers were accepted. Each survey was approved by the Ethic Board Committee of the CHU de Quebec-Université Laval Hospital and written consent was obtained from all the participants.
Vaccination schedule
Since 2004, many new vaccines had been introduced in the Quebec’s vaccination schedule (Table 1).The pneumococcal conjugate vaccine (PCV) was introduced in December 2004 with 3 doses scheduled at 2, 4 and 12 months. A single dose of monovalent varicella vaccine at 12 months of age was introduced in 2006 and replaced in 2008 by the combined measles, mumps, rubella and varicella vaccine (MMRV). Since November 2011, children are receiving two doses of rotavirus vaccine at 2 and 4 months of age. Finally, in June 2013, hepatitis B vaccine program was launched and the combined vaccine against diphtheria, acellular pertussis, tetanus, polio, Haemophilus influenzae type b and Hepatitis B (DTaP-IPV-Hib-HB) replaced the pentavalent vaccine DTaP-IPV-Hib in the schedule at 2, 4 and 18 months of age.
Table 1.
The Quebec vaccine schedule before 24 months of age and definition of vaccine delays used in the analysis
| Recommended age | Vaccine schedule by date of several changes made | Minimum acceptable age | Age of child when vaccine delays were initiated | ||||
|---|---|---|---|---|---|---|---|
| January 2006 | May 2008 | November 2011 | May 2013 | June 2013 | |||
| 2 months | DTaP-IPV-Hib, PCV | DTaP-IPV-Hib, PCV | DTaP-IPV-Hib, PCV Rota |
DTaP-IPV-Hib, PCV Rota |
DTaP-IPV-Hib-HB, PCV, Rota |
42 days | ≥ 91 days (61 days + 30.43 days) |
| 4 months | DTaP-IPV-Hib, PCV | DTaP-IPV-Hib, PCV | DTaP-IPV-Hib, PCV Rota |
DTaP-IPV-Hib, PCV Rota |
DTaP-IPV-Hib-HB, PCV, Rota |
Previous doses + 28 days | ≥ 152 days (122 days + 30.43 days) |
| 6 months | DTaP-IPV-Hib | DTaP-IPV-Hib | DTaP-IPV-Hib | DTaP-IPV-Hib | DTaP-IPV-Hib | Previous dose + 28 days | ≥ 213 days (183 days + 30.43 days) |
| 12 months | MMR + varicella monovalent vaccine, PCV, Men-C-C |
MMRV
PCV, Men-C-C |
MMRV PCV, Men-C-C |
MMR
PCV, Men-C-C |
MMR PCV, Men-C-C |
365 days | ≥ 395 days (365 days + 30.43 days) |
| 18 months | DTaP-IPV-Hib, MMR | DTaP-IPV-Hib, MMR | DTaP-IPV-Hib, MMR | DTaP-IPV-Hib, MMRV | DTaP-IPV-Hib-HB, MMRV | DTaP-IPV-Hib ± HB: Previous dose + 183 days MMR ± V: Previous dose+ 28 days |
≥ 578 days (548 days + 30.43 days) |
Source: Ministère de la Santé et des Services sociaux. Protocole d’immunisation du Québec, 6e edition: http://publications.msss.gouv.qc.ca/msss/document-000105
Changes in the vaccines used appear in boldface
Outcomes
Birth and vaccination dates were used to compute age at vaccination in days. A dose was administered on-time if received within 30 days of the recommended age and was considered delayed after this period (Table 1). Vaccine delays were assessed for each of the first three doses of the DTaP-containing vaccine (DTaP) recommended at 2, 4 and 6 months of age and for the first dose of the measles-containing vaccine (Measles) recommended at 12 months. As most recommended vaccines are administered at the same visit, these vaccines have been used as markers of all vaccines administered at each visit. A vaccine delay at one dose is frequently followed by a vaccine delay at the following dose(s). A new vaccine delay was defined as a delay occurring among children whose previous dose(s) were on-time (e.g. Delay at 4 months among children with no delay at 2 months).
Given the changes in the Quebec vaccination schedule over the study period, the vaccination status was defined for antigens common to all surveys (DTaP-IPV-Hib, Men C-C and measles-mumps-rubella). A complete vaccination status by 24 months of age was defined as having received 4 doses of DTaP-IPV-Hib, 1 dose of Men-C-C vaccine and 2 doses of MMR vaccine before 2 years of age. Otherwise, the vaccination status was incomplete.
Statistical analysis
All analyses were performed with Statistical Analysis System (SAS Institute Inc. Carry, NC, version 9.4). Proportions were compared using Chi-square test or Chi-square test for trend when appropriate. Missed opportunities at 2 and 12-month visits occurred when a vaccine-eligible child did not receive all recommended vaccines at the same vaccination date [25]. Other independent variables included data regarding the child, the family and the vaccine provider. The data on vaccine provider at the 2 month visit were used for the analysis of vaccine delays at 2, 4 and 6 months and the data on vaccine provider for all vaccination visits from 2 to 12 months were used for the analysis of vaccine delays at 12 months.
As vaccine delays occurred before 15 months of age, the analyses on factors associated with new vaccine delays included all participants to maximize the statistical power. In contrast, as children in the “1-year cohort” had not yet reached 2 years of age, the impact of new vaccine delays at 2, 4, 6 or 12 months on vaccination status by 24 months of age was estimated only with children in the “2-year cohort”.
The impact of new vaccine delays at 2, 4, 6 and 12 month on vaccination status by 24 months of age was estimated with a robust Poisson multivariable regression. This alternative to logistic regression for binary outcomes directly estimates relative risk without risking convergence issues associated with log-binomial regressions [26–28]. The SAS GENMOD procedure was used with a log link and a robust estimator of the standard errors. All potential factors associated to incomplete vaccine coverage and to vaccine delay were included in the model without any selection procedure [29]. Risk-ratio modification by the survey year was assessed with an interaction term between vaccine delay and survey year in the model. A model based standardization approach was used to estimate adjusted risk difference (RD) in incomplete vaccination status by 24 months of age between children with and without vaccine delays [30]. Briefly, using predicted probabilities of the model, the standardized risk of outcome considering all children exposed (with vaccine delays) was estimated. The standardized risk considering all children unexposed (without vaccine delays) was also estimated similarly. The standardized RD was obtained as the difference between these two quantities. Adjusted attributable risks in the population (ARp) were estimated for delays at each dose also using a model based standardization approach. That is, ARp were estimated as one minus the ratio of the predicted risk of outcome considering all children unexposed over the observed risk of outcome [31]. Confidence intervals for RD and ARp were obtained through the percentile method by performing non-parametric bootstrap with 1000 samples [32, 33].
Factors associated with new vaccine delays were assessed for each dose with the same SAS procedure used to estimate the effect of vaccine delays on vaccination status by 24 months of age. A backward procedure with a p value < 0.05 was used for the selection criteria. The year of survey was kept in multivariable models to ensure face validity.
Results
Characteristics of participants, vaccine delays and vaccine coverage
Of the 11,200 children invited to participate since 2006, the participation rate was 70% (844/1200) in 2006, 64% (1282/2000) in 2008, 62% (1233/2000) in 2010, 73% (1459/2000) in 2012, 69% (1384/2000) in 2014 and 65% (1295/2000) in 2016. Children born outside Quebec were excluded from the analysis because they were exposed to a different vaccine schedule (n = 140 for the 1-year cohorts and n = 174 for the 2-year cohorts). This left a total of 3675 children in the 1-year cohorts and 3508 children in the 2-year cohorts (95% of participants for both cohorts) for the analysis.
Forty-four percent of participant children were the first child in the family, 76.9% attending daycare and the majority were vaccinated in public health clinics (66.7%). Most parents lived with a partner (91.9%) and most mothers have completed a college or university degree (70.5%). Missed opportunities occurred in 3.1% of children at the 2-month visit for which two injections were to be administered and in 14.8% at the 12-month visit where three injections were recommended (from 31.1% in 2006 to 7.0% in 2016). All characteristics of children participants are presented in Additional file 1.
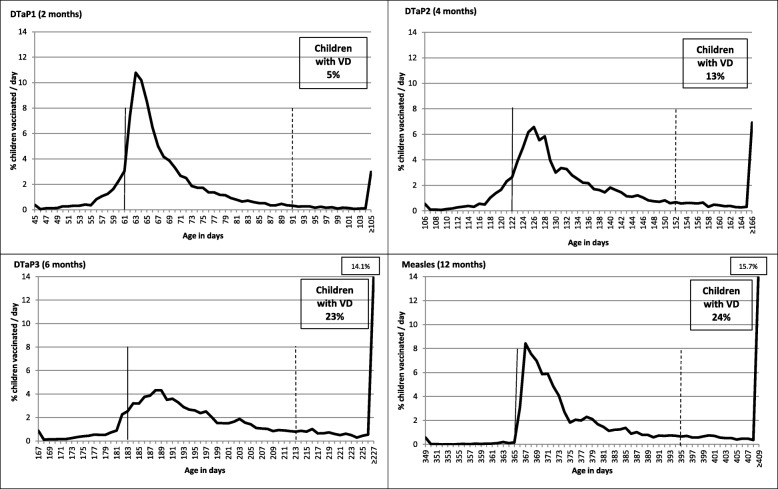
The proportion of children vaccinated by age in days for each dose is presented in Fig. 1. At 2 months, a greater proportion of children received their DTaP1 close to the recommended age compared to subsequent visits. At 6 months, 14.1% of children received DTaP3 after 227 days. The proportion of new vaccine delays increased from 5.4% at 2 months to 14.3% at 12 months (Fig. 2). Among children who experienced vaccine delays at 2 months (n = 386), 77.2% had vaccine delays at other visits (298/386) (Fig. 3) and 33.7% had vaccine delays at each of the next three visits. At 4 months there were 2.4 times more vaccine delays (n = 941) than at 2 months of which 66.6% (627) were new vaccine delays. Among these new vaccine delays, 84.1% experienced vaccine delays at subsequent visits (527/627). At 6 months, there were 4.2 times (1617) more vaccine delays than at the 2 month visit and 49.9% (807) were new vaccine delays. At 12 months, the number of vaccine delays was similar to that at 6 months (1624) and 60.7% (986) were new vaccine delays (Fig. 2 and Table 2). In children with a vaccine delay for DTaP doses, instead of using the minimum interval recommended for accelerated schedule to prevent additional vaccine delays, the majority received their next dose with 2-month intervals as recommended for routine schedule (see Additional file 2).
Fig. 1.
Proportion of children vaccinated according to age in days at 2–4-6 and 12 months, 2006–2016*. *Both cohorts included. The vertical reference lines indicate recommended age at vaccination for each visit and dotted lines indicate age when a dose becomes delayed
Fig. 2.
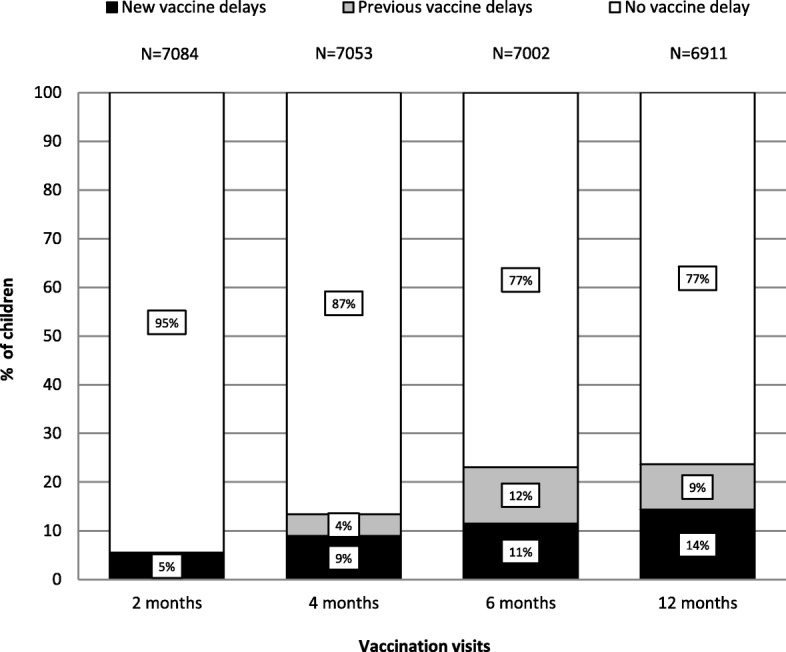
Proportion of children with and without vaccine delays at 2–4-6 and 12 month visits, 2006–2016*. *Both cohorts included. DTaP1 is recommended at 2 months, DTaP2 at 4 months, DTaP3 at 6 months and the first measles-containing vaccine at 12 months
Fig. 3.
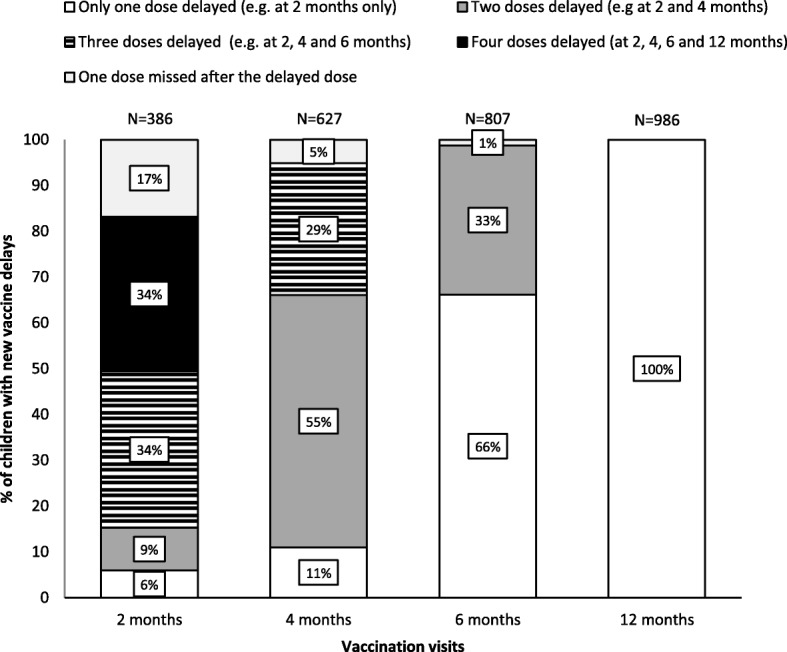
Distribution of total vaccine delays by visit with first (new) vaccine delay, 2006–2016*. *Both cohorts included. DTaP1 was recommended at 2 months, DTaP2 at 4 months, DTaP3 at 6 months and the first measles-containing vaccine at 12 months. Categories of vaccine delays were mutually exclusive. The maximum number of delayed doses was 4 for a first (new) delay at 2 months, 3 at 4 months, 2 at 6 months and 1 at 12 months
Table 2.
Characteristics of the participants according to new vaccine delays (2–4-6 and 12 months), 2006–2016 (N = 7183)a
| Vaccine delays | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DTaP1 (2 month visit) | DTaP2 (4 month visit) | DTaP3 (6 month visit) | Measles (12 month visit) | |||||||||
| Frequencyb | % | P valuec | Frequencyb | % | P valuec | Frequencyb | % | P valuec | Frequencyb | % | P valuec | |
| Dose not administered | 99 | 130 | 181 | 272 | ||||||||
| Total vaccine delays | 386/7084d | 5.4% | 941/7053d | 13.3% | 1617/7002d | 23.1% | 1634/6911d | 23.6% | ||||
| New vaccine delays | 386/386 | 100% | 627/941 | 66.7% | 807/1617 | 49.9% | 986/1634 | 60.3% | ||||
| Denominator used for the analysis | N = 7084 | N = 6682e | N = 6039e | N = 5166e | ||||||||
| Characteristics | ||||||||||||
| Year of survey: | ||||||||||||
| 2006 | 53/791 | 6.7% | 0.003 | 78/738 | 10.6% | 0.04 | 116/659 | 17.6% | 0.001 | 149/535 | 27.9% | < 0.0001 |
| 2008 | 67/1211 | 5.5% | 104/1144 | 9.1% | 138/1039 | 13.3% | 229/891 | 25.7% | ||||
| 2010 | 76/1160 | 6.6% | 117/1081 | 10.8% | 145/962 | 15.1% | 165/804 | 20.5% | ||||
| 2012 | 88/1381 | 6.4% | 133/1286 | 10.3% | 153/1149 | 13.3% | 181/982 | 18.4% | ||||
| 2014 | 58/1321 | 4.4% | 109/1259 | 8.7% | 132/1145 | 11.5% | 140/1003 | 14.0% | ||||
| 2016 | 44/1220 | 3.6% | 86/1174 | 7.3% | 123/1085 | 11.3% | 122/951 | 12.8% | ||||
| Sex: | ||||||||||||
| Female | 188/3445 | 5.5% | 0.98 | 316/3250 | 9.7% | 0.35 | 404/2927 | 13.8% | 0.34 | 449/2486 | 18.1% | 0.07 |
| Male | 198/3639 | 7.5% | 311/3432 | 9.1% | 403/3112 | 12.9% | 537/2680 | 20.0% | ||||
| Maternal age at child’s birth | ||||||||||||
| < 20 years | 3/71 | 4.2% | 0.03 | 6/68 | 8.8% | 0.74 | 8/61 | 13.1% | 0.49 | 7/52 | 13.5% | 0.47 |
| 20–29 years | 168/3107 | 5.4% | 286/2929 | 9.8% | 334/2635 | 12.7% | 426/2266 | 18.8% | ||||
| 30–39 years | 192/3685 | 5.2% | 318/3487 | 9.1% | 434/3163 | 13.7% | 520/2701 | 19.3% | ||||
| ≥ 40 years | 20/197 | 10.2% | 14/177 | 7.9% | 26/162 | 16.0% | 31/135 | 23.0% | ||||
| Unknown / missing | 3/24 | 12.5% | 3 /21 | 14.3% | 5/18 | 27.8% | 2/13 | 15.4% | ||||
| Mother’s languagef: | ||||||||||||
| French | 250/4936 | 5.1% | 0.03 | 400/4673 | 8.6% | 0.004 | 525/4260 | 12.3% | 0.001 | 655/3693 | 17.7% | 0..04 |
| English | 32/389 | 8.2% | 36/355 | 10.1% | 32/317 | 10.1% | 66/279 | 23.7% | ||||
| Other | 47/942 | 5.0% | 107/894 | 12.0% | 131/787 | 16.6% | 113/647 | 17.5% | ||||
| Unknown / missing | 57/817 | 7.0% | 84/760 | 11.1% | 119/675 | 17.6% | 152/547 | 27.8% | ||||
| Mother’s originf: | ||||||||||||
| Canada | 210/4066 | 5.2% | 0.91 | 318/3845 | 8.3% | 0.0004 | 415/3516 | 11.8% | 0.0002 | 501/3063 | 16.4% | 0.64 |
| Other | 52/990 | 5.3% | 122/933 | 13.1% | 134/809 | 16.6% | 104/666 | 15.6% | ||||
| Unknown / missing | 124/2028 | 6.1% | 187/1904 | 9.8% | 258/1714 | 15.1% | 381/1437 | 26.5% | ||||
| Maternal level of education: | ||||||||||||
| < High school | 40/490 | 8.2% | 0.0008 | 55/449 | 12.2% | < 0.0001 | 77/390 | 19.7% | 0.0013 | 63/303 | 20.8% | 0.84 |
| High school | 101/1548 | 6.5% | 175/1445 | 12.1% | 172/1266 | 13.6% | 209/1078 | 19.4% | ||||
| College | 110/2074 | 5.3% | 174/1958 | 8.9% | 220/1780 | 12.4% | 290/1533 | 18.9% | ||||
| University | 129/2925 | 4.4% | 218/2790 | 8.9% | 332/2568 | 12.9% | 417/2224 | 18.8% | ||||
| Unknown / missing | 6/47 | 12.8% | 5/40 | 12.5% | 6/35 | 17.1% | 7/28 | 25.0% | ||||
| Child rank: | ||||||||||||
| First | 118/3118 | 3.8% | < 0.0001 | 206/2995 | 6.9% | < 0.0001 | 300/2784 | 10.8% | < 0.0001 | 437/2465 | 17.7% | 0.01 |
| ≥ 2 | 260/3885 | 6.7% | 405/3615 | 11.2% | 501/3200 | 15.7% | 542/2654 | 20.4% | ||||
| Unknown / missing | 8/81 | 9.9% | 16/72 | 22.2% | 6/55 | 10.9% | 7/47 | 14.3% | ||||
| Daycare attendance: | ||||||||||||
| Yes | 251/5461 | 4.6% | < 0.0001 | 461/5201 | 8.9% | 0.008 | 618/4732 | 13.1% | 0.20 | 760/4066 | 18.7% | 0.21 |
| No | 132/1583 | 8.3% | 161/1444 | 11.1% | 184/1275 | 14.4% | 219/1075 | 20.4% | ||||
| Unknown / missing | 3/40 | 7.5% | 5/37 | 13.5% | 5/32 | 15.6% | 7/25 | 28.0% | ||||
| Child with a chronic disease: | ||||||||||||
| Yes | 23/394 | 7.8% | 0.69 | 46/369 | 12.5% | 0.04 | 53/323 | 16.4% | 0.09 | 65/265 | 24.5% | 0.02 |
| No | 357/6650 | 5.4% | 577/6279 | 9.2% | 747/5686 | 13.1% | 917/4878 | 18.8% | ||||
| Unknown / missing | 6/40 | 15.0% | 4/34 | 11.8% | 7/30 | 23.3% | 4/23 | 17.4% | ||||
| Gestational age at birth: | ||||||||||||
| < 37 weeks | 35/511 | 6.8% | 0.14 | 56/476 | 11.8% | 0.04 | 60/419 | 14.3% | 0.48 | 86/354 | 24.3% | 0.009 |
| ≥ 37 weeks | 336/6338 | 5.3% | 534/5988 | 8.9% | 713/5439 | 13.1% | 870/4667 | 18.6% | ||||
| Unknown / missing | 15/235 | 6.4% | 37/218 | 17.0% | 34/181 | 18.8% | 30/145 | 20.7% | ||||
| Living with a partner | ||||||||||||
| Yes | 339/6504 | 5.2% | 0.004 | 559/6152 | 9.1% | 0.01 | 731/5580 | 13.1% | 0.28 | 901/4795 | 18.8% | 0.11 |
| No | 40/480 | 8.3% | 86/438 | 19.6% | 57/379 | 15.0% | 70/312 | 22.4% | ||||
| Unknown / missing | 7/100 | 7.0% | 12/92 | 13.0% | 19/80 | 23.8% | 15/59 | 25.4% | ||||
| Birth attendantf: | ||||||||||||
| Physician | 221/4791 | 4.6% | < 0.0001 | 418/4557 | 9.2% | 0.55 | 516/4126 | 12.5% | 0.05 | 572/3567 | 16.0% | 0.30 |
| Midwife or other professionnal | 38/252 | 15.1% | 22/212 | 10.4% | 33/189 | 17.5% | 29/151 | 19.2% | ||||
| Unknown / missing | 127/2041 | 6.2% | 187/1914 | 9.8% | 258/1724 | 15.0% | 385/1448 | 26.6% | ||||
| Vaccine provider (at 2 months): | ||||||||||||
| Public health clinics | 277/5033 | 5.5% | 0.68 | 468/4742 | 9.9% | 0.02 | 595/4264 | 14.0% | 0.02 | 610/3629 | 16.8% | < 0.0001 |
| Medical clinic/Hospital | 105/1999 | 5.3% | 153/1892 | 8.1% | 204/1734 | 11.8% | 367/1504 | 24.4% | ||||
| Unknown / missing | 4/52 | 7.7% | 6/48 | 12.5% | 8/41 | 19.5% | 9/33 | 27.3% | ||||
| Main vaccine provider (all vaccines): | ||||||||||||
| Public health clinics only | 574/3473 | 16.5% | < 0.0001 | |||||||||
| Medical clinic/Hospital or both settings | 406/1669 | 24.3% | ||||||||||
| Unknown / missing | 6/24 | 25.0% | ||||||||||
| Missed opportunities at the 2-month visit: | ||||||||||||
| Yes | 48/217 | 22.1% | < 0.0001 | 20/166 | 12.0% | < 0.0001 | 27/146 | 18.5% | 0.07 | 27/114 | 23.7% | 0.21 |
| No | 338/6867 | 4.9% | 607/6516 | 9.3% | 780/5893 | 13.2% | 959/5052 | 19.0% | ||||
| Missed opportunities at the 12-month visit: | ||||||||||||
| Yes | 227/702 | 32.3% | < 0.0001 | |||||||||
| No | 759/4464 | 17.0% | ||||||||||
a Analysis limited to children born in Quebec. Both cohorts included / b Correspond to the proportion of children who experienced new vaccine delays by each variable category / cChi-square test, unknown and missing excluded
dCorrespond to the difference between 7183 and the number of children with a dose not administered
eFor DTaP2 the 6682 represent 6698 children without vaccine delay for DTaP1 minus 16 children who had not received DTaP 2. For DTaP3 the 6039 represent 6055 children without vaccine delay for DTaP1 and DTaP2 minus 16 children who had not received DTaP 3. For MMR1 the 5166 represent 5232 children without vaccine delay for DTaP1, DTaP2 and DTaP3 minus 66 children who had not received MMR1
fThese variables were not collected in 2006 and 2008
The characteristics of participants according to vaccine delays at 2, 4, 6 and 12 months of age are presented in Table 2. The proportion of children with a vaccine delay increased with age, from 5.4% at the 2-month visit to 23.1% at the 6-month visit and 23.6% at the 12-month visit. Among children with a vaccine delay, the interval between 30 days after the recommended age and the time they received their dose was a median of 17 days for DTaP1 at 2 months, 10 days for DTaP2 at 4 months, 13 days for DTaP3 at 6 months and 21 days for measles at 12 months. Overall, 39% of children experienced at least one vaccine delay at one of the four visits, decreasing from 50% in 2006 to 30% in 2016. In univariate analysis, all characteristics except the sex were associated with new vaccine delays for at least one of the visits (Table 2). Between 2006 and 2016, for children immunized in public health clinics, the proportion with new vaccine delays decreased from 9.1 to 3.6% at the first visit, from 13.7 to 6.5% at the 4 month visit, from 20.3 to 11.0% at the 6 month visit and from 31.0 to 11.2% at the 12 month visit. In contrast, no significant differences were observed for children vaccinated in medical clinic/hospital for the 4 and 6 month visits (see Additional file 3).
Impact of new vaccine delays on vaccination status by 24 months of age
Among children with and without a vaccine delay for the DTaP1 at 2 months, 46.4 and 11.1% respectively had an incomplete vaccination status by 24 months for an unadjusted risk difference of 35.3% and a 27.7% adjusted risk difference (RD) (Table 3). The adjusted risk difference for incomplete vaccination status by 24 months was 13.8, 10.5 and 8.8% for new vaccine delays at 4, 6 and 12 months respectively. The relative risk of incomplete vaccination status by 24 months of age between children with and without a vaccine delay varied between 2.3 and 3.6 with overlapping confidence intervals. As computed with predicted probabilities from the multivariable models, 72.5% of 2-year children with an incomplete status by 24 months were attributable to a vaccine delay: 16.1% were attributable to a vaccine delay that first occurred at 2 months, 10.6% at 4 months, 14.0% at 6 months and 31.8% at 12 months. There was no risk-ratio heterogeneity for the survey year.
Table 3.
Proportion of incomplete vaccination status by 24 months of age attributable to new vaccine delays, 2006–2016 (N = 3508)a
| Vaccination visits | Incomplete vaccination status | Risk difference for incomplete vaccination status | Relative risk of incomplete vaccination status | % of cases exposed | Attributable risk in the population | ||
|---|---|---|---|---|---|---|---|
| With a new vaccine delay % (n/N) |
Without a vaccine delay % (n/N) |
Unadjusted | Adjustedb (95% CI) | Adjustedb (95% CI) | Adjusted (95% CI)c | ||
| DTaP at 2 months | 46.4% (96/207) | 11.1% (361/3250) | 35.3% | 27.7% (22.2%; 28.2%) | 3.6 (2.95; 4.37) | 21.0% | 16.1% (8.4%; 23.7%) |
| DTaP at 4 months | 24.3% (64/263) | 9.8% (292/2982) | 14.5% | 13.8% (13.4%; 14.0%) | 2.5 (1.91; 3.18) | 18.0% | 10.6% (1.4%; 19.3%) |
| DTaP at 6 months | 19.6% (71/362) | 8.3% (217/2616) | 11.3% | 10.5% (10.3%; 10.8%) | 2.3 (1.81; 3.02) | 24.7% | 14% (3.9%; 23.5%) |
| Measles at 12 months | 17.5% (99/567) | 4.6% (94/2025) | 12.8% | 8.8% (8.5%; 9.0%) | 2.9 (2.12; 3.85) | 51.3% | 31.8% (20.5%; 40.1%) |
CI Confidence interval estimated using bootstrap with 1000 replications
aAnalysis restricted to the 2-year cohort and to children born in Quebec. Unvaccinated children: 51 at 2 months, 5 at 4 months, 4 at 6 months and 24 at 12 months
bRisk difference estimated with standardization using predicted probabilities calculated from Robust Poisson multivariable regression. Incomplete vaccination for common antigens only (ie. DTaP-VPI-Hib. MMR and Men-C-C vaccines). Risk difference and relative risk adjusted for survey year, mother’s age at child birth, sex, mother’s education, child’s rank, daycare attendance, child’s health condition, gestational age, family type, main vaccine provider, missed opportunities. Analysis restricted to children with no vaccine delays at previous dose. Mother’s language, mother’s country of origin and the birth attendant were excluded from this analysis as they were not collected in 2006 and 2008 (more than 10% of missing)
cSimilar results were observed using the following formula: PCE = proportion of cases exposed (i.e. proportion of children with incomplete vaccination status with new vaccine delays)
Factors associated with new vaccine delays
Compared to the firstborn, other children were more likely to have a new vaccine delay at 2, 4, 6 and 12 months (Table 4). Missed opportunity at the 2-month visit was the most important factor associated with vaccine delays for DTaP1 (RR 4.28 (IC 3.18; 5.77)). At 2 months, children who were not attending daycare (versus attending daycare) and children whose parent who completed the survey was living without a partner (versus with a partner) were more likely to experience delays. Vaccination in medical clinic/hospital (versus in public health clinics) at the 2-month visit was associated with lower risk of vaccine delay for DTaP2 and DTaP3. The risk of vaccine delays for DTaP1 and DTaP2 decreased with higher maternal education. Missed opportunity at the 12-month visit was the most important factor associated with vaccine delays for measles at 12 months. Gestational age at birth lower than 37 weeks (vs ≥ 37 weeks) was also associated with vaccine delays at 12 months. Finally, in contrast to results observed for DTaP2 and DTaP3, children vaccinated in medical clinic/hospital or in both settings for the 12-month visit were more likely to experience measles vaccine delays at 12 months compared with children vaccinated in public health clinics.
Table 4.
Factors significantly associated with new vaccine delays at 2–4-6 and 12 months of age, 2006–2016 (N = 7183)a
| Factors associated with vaccine delaysb | DTaP1 at 2 months | DTaP2 at 4 monthsc | DTaP3 at 6 monthsd | Measles at 12 monthse | ||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted RR (95% CI) | Adjusted RR (95% CI) | Unadjusted RR (95% CI) | Adjusted RR (95% CI) | Unadjusted RR (95% CI) | Adjusted RR (95% CI) | Unadjusted RR (95% CI) | Adjusted RR (95% CI) | |
| Year of survey: | ||||||||
| 2006 | 1.86 (1.26; 2.74) | 1.38 (0.91; 2.09) | 1.44 (1.08;1.93) | 1.56 (1.15; 2.12) | 1.55 (1.23; 1.96) | 1.71 (1.35; 2.17) | 2.17 (1.75; 2.69) | 1.84 (1.45; 2.32) |
| 2008 | 1.53 (1.06; 2.23) | 1.20 (0.82; 1.75) | 1.24 (0.94; 1.63) | 1.29 (0.97; 1.71) | 1.17 (0.93; 1.47) | 1.24 (0.98; 1.56) | 2.00 (1.64; 2.45) | 1.74 (1.41; 2.15) |
| 2010 | 1.82 (1.26; 2.61) | 1.55 (1.08; 2.22) | 1.48 (1.13; 1.93) | 1.53 (1.17; 2.02) | 1.33 (1.06; 1.66) | 1.40 (1.12; 1.76) | 1.60 (1.29; 1.98) | 1.53 (1.23; 1.90) |
| 2012 | 1.77 (1.24; 2.52) | 1.57 (1.11; 2.24) | 1.41 (1.09; 1.83) | 1.38 (1.06; 1.81) | 1.18 (0.94; 1.47) | 1.20 (0.96; 1.51) | 1.44 (1.16; 1.78) | 1.39 (1.12; 1.73) |
| 2014 | 1.22 (0.83;1.79) | 1.13 (0.78; 1.66) | 1.18 (0.90; 1.55) | 1.20 (0.91; 1.58) | 1.02 (0.81; 1.28) | 1.02 (0.81; 1.29) | 1.09 (0.87; 1.36) | 1.09 (0.87; 1.37) |
| 2016 (reference) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Maternal level of education for each increase of one level | 0.82 (0.74; 0.90) | 0.90 (0.81; 0.99) | 0.83 (0.77; 0.90) | 0.86 (0.79; 0.92) | ||||
| Child’s rank: | ||||||||
| ≥ 2 | 1.72 (1.39; 2.12) | 1.68 (1.36; 2.09) | 1.63 (1.39; 1.91) | 1.63 (1.39; 1.92) | 1.45 (1.27; 1.66) | 1.47 (1.28; 1.68) | 1.15 (1.03; 1.30) | 1.16 (1.03; 1.30) |
| First (reference) | 1 | 1 | 1 | 1 | 1 | |||
| Gestational age at birth | ||||||||
| < 37 weeks | 1.30 (1.58; 1.07) | 1.26 (1.04; 1.53) | ||||||
| ≥ 37 weeks (reference) | 1 | 1 | ||||||
| Daycare attendance: | ||||||||
| No | 1.81 (1.48; 2.22) | 1.48 (1.20; 1.84) | ||||||
| Yes (reference) | 1 | 1 | ||||||
| Living with a partner: | ||||||||
| No | 1.60 (1.17; 2.19) | 1.48 (1.07; 2.05) | ||||||
| Yes (reference) | 1 | 1 | ||||||
| Vaccine provider (2-months): | ||||||||
| Medical clinic or Hospital | 0.82 (0.69; 0.98) | 0.80 (0.67; 0.96) | 0.84 (0.73; 0.98) | 0.79 (0.68; 0.92) | ||||
| Public health clinics (reference) | 1 | 1 | 1 | 1 | ||||
| Main vaccine provider (all vaccines): | ||||||||
| Medical clinic/ Hospital or both settings | 1.47 (1.32; 1.65) | 1.22 (1.08; 1.39) | ||||||
| Public health clinics only (reference) | 1 | 1 | ||||||
| Missed opportunities at the 2-month visit: | ||||||||
| Yes | 4.49 (3.43; 5.89) | 4.28 (3.18; 5.77) | ||||||
| No (reference) | 1 | 1 | ||||||
| Missed opportunities at the 12-month visit: | ||||||||
| Yes | 1.90 (1.68; 2.16) | 1.47 (1.26; 1.71) | ||||||
| No (reference) | 1 | 1 | ||||||
RR relative risk, CI confidence interval
a Both cohorts were included in this analysis. Analysis limited to children born in Quebec. Multivariable analysis exclude children unvaccinated since birth (n = 43 for 1-year cohort and n = 42 for 2-year cohort)
bFactors selected with robust Poisson multivariable regression with backward selection. Mother’s language, mother’s country of origin and the birth attendant were excluded from this analysis as they were not collected in 2006 and 2008 (more than 10% of missing). For each visit, results are presented only for covariates significant in the models
cRestricted to children without vaccine delay at 2 month vaccines /d Restricted to children without vaccine delay at 2 and 4 month vaccines / eRestricted to children without vaccine delay at 2, 4 and 6 month vaccines
Discussion
While the literature on vaccine coverage put an emphasis on vaccine delays at 2 months, this study shows that the proportion of vaccine delays was greater at later visits increasing from 5.4% at 2 months, to 13.3% at 4 months, to 23.1% at 6 months and to 23.6% at 12 months. Vaccine delay at one visit had an impact on the timing and administration of subsequent doses as most children with vaccine delay for a DTaP dose received their subsequent dose with a 2-month interval rather than the shortened interval recommended in the accelerated schedule. A vaccine delay decreased the probability of having received all recommended vaccines by 24 months of age and 72.5% of incomplete status by 24 months of age were attributed to a new vaccine delay at 2, 4, 6 or 12 months (16.1% at 2 months, 10.6% at 4 months, 14.0% at 6 months and 31.8% at 12 months).
This study found that delays at the first visit did contribute to incomplete vaccination status by 24 months of age and that more than 75% of children with a vaccine delay at 2-months also had vaccine delays at later visits including a third with vaccine delays at all next three visits. Association between vaccine delays at the first vaccination visit and later vaccine delays or incomplete vaccination status by 24 months of age has been widely reported [9, 16–18]. While other studies also identified that vaccine delays increased at subsequent vaccine visits [9, 11, 34, 35], they did not assess their impact on incomplete vaccination status by 24 months, the additional step this study provides.
The interventions deployed in public health clinics in Quebec since 2006 to improve timeliness at the 2 and 12 month visits seem to have successfully reduced vaccine delays at all visits. While only 17% of 2-year-old children had received all recommended doses with no delay in 2006, this proportion had increased to 50% in 2016 [15, 23]. In a cohort of 361,901 children born from 2004 through 2012 in United States, 55% of children had been fully vaccinated with no delay by 24 months of age [36]. Reminders or recall interventions sent by vaccine providers are effective to increase the likelihood of being vaccinated and to reduce the number of underimmunized days [37, 38]. In the current study, for children vaccinated in public health clinics, the proportion with new vaccine delays decreased from 2006 to 2016 for each vaccination visits: a decrease of 5.5% at 2 months, 7.2% at 4 months, 9.3% at 6 months and 19.8% at 12 months. The risk of vaccine delays for the 12 month visit was 1.22 times higher for children vaccinated in medical clinic/hospital versus those vaccinated in public health clinics. In contrast, in medical clinic/hospital where no intervention was done, no major changes have been observed in the proportion of new vaccine delays from 2006 to 2016. In public health clinics, efforts to reduced vaccine delays were more important for the 2 and 12 month visits and the proportions of children with vaccine delays at 4 and 6 months remained higher in this setting compared with medical clinic/hospital for each survey year except in 2016, resulting in a higher risk of vaccine delays.
In the present study, children who are not the firstborn and those with missed opportunities at the 2-month and the 12-month visits were more likely to had vaccine delays. These two factors have been frequently associated with vaccine delays in other similar studies [6, 10, 34, 39]. A high number of children in the household may impact the accessibility to healthcare settings, including vaccination services. In addition, children with older siblings are possibly more exposed to minor illness, resulting in missed opportunities and vaccine delays [40–42]. We observed that children of single parent (versus living with a partner) had a greater risk for vaccine delays at 2-months and these results might be associated with constraints to access vaccination services [6, 34]. In the current study, the risk of vaccine delays for DTaP1 and DTaP2 decreases with higher maternal education. The literature is inconsistent on this issue; some authors found an association similar to ours while others observed a higher risk of delays with an increasing education level [6, 10]. A literature review found that higher maternal level of education was associated with vaccine hesitancy which is itself associated with vaccine delays [43]. This subject may require future studies.
This study has some limitations. The response rate to vaccine coverage surveys varied from 61 to 73% depending upon the year and cohort. Participants may have had more positive behaviours regarding vaccination than non-participants, resulting in less vaccine delays and higher vaccine coverage. Validation of the information on immunization from questionnaires was restricted to children whose information was not consistent with the provincial vaccine schedule. We cannot rule out that this practice has led to an overestimation of vaccine coverage, but the impact on our results might be minimal as vaccination card compared to medical chart usually has a good positive predictive value [44, 45]. As only written data were accepted in this study, underestimation of vaccine coverage cannot be ruled out but is unlikely as many sources were consulted to obtain vaccine information. As markers of timeliness, vaccine delays were estimated only for DTaP and measles containing vaccines despite other vaccines being administered at 2, 4, 6 and 12 months. While most recommended vaccines are administered at the same visit, the burden associated with vaccine delays presented in this analysis has probably been underestimated.
The current study estimated the risk of incomplete vaccination status by 24 months attributable to vaccine delays at different ages an information that can be useful to decide or prioritize public health interventions. However, the interpretation of ARp as the fraction of the outcome that could be eliminated if exposure could be totally removed from the population is valid only under certain conditions [46]. First, exposure has to be causal rather than merely associated with the disease. By estimating counterfactual risk with model-based standardization, we attempted to estimate the average causal effect of new vaccine delays at 2, 4, 6 or 12 months on vaccination status by 24 months. However, root causes of vaccine delays remained unknown and the elimination of vaccine delays may not result in an improvement of complete vaccination status by 24 months as large as estimated by ARp. Second, estimation of ARp has to be unbiased. While multivariable models included most of the determinants identified in the literature, as for any observational study, residual confounding cannot be ruled out. Finally, the elimination of exposure has to be without any effect on distribution of other risk factors. As other factors included in this analysis are mainly unmodifiable risk factors, it is unlikely that the elimination of vaccine delays has any effect on their distribution. As the attributable risk depends upon the prevalence of vaccine delays at various visits, which may vary between populations, this limits the generalizability of this result to other jurisdictions [46].
Conclusion
While great emphasis has been put on vaccine delays at the first vaccination visit, the prevalence of vaccine delays in this study was greater with later visits and most of incomplete vaccination status by 24 months of age was associated with vaccine delays occurring after the 2 month visit. Interventions to improve timeliness deployed in public health clinics in Quebec seem to have reduced vaccine delays at all visits from 2006 to 2016. These interventions should also focus on other vaccine visits which contributed to the burden associated with vaccine delays and on more vulnerable groups identified in this study. Accelerated vaccination schedules recommended for children with vaccine delays may prevent further delays and reduce their impact.
Additional files
Characteristics of children participants, 1-year and 2-year cohorts, 2006–2016 (n = 7183). (DOCX 20 kb)
Intervals following the next vaccine dose for children with new vaccine delays at the DTaP1 and DTaP2. (DOCX 26 kb)
New vaccine delays at 2, 4, 6 and 12 months by vaccine provider and survey year, 1-year and 2-year cohorts, 2006–2016. (DOCX 17 kb)
Acknowledgements
Not applicable.
Funding
MK has received a scholarship from the Fonds de recherche du Quebec-Santé (FRQS).
DT is a FRQS junior 1 Chercheur-Boursier.
Vaccine coverage surveys from 2006 were funded by the Ministère de la Santé et des Services sociaux du Québec. Funding sources had no involvement on the conduct of this research and on the redaction of this manuscript.
Availability of data and materials
The datasets used and analysed during this current study are available from the corresponding author on reasonable request.
Abbreviations
- ARp
Attributable risk in the population
- CI
Confidence interval
- DTaP
Diphtheria, tetanus, acellular pertussis and polio containing vaccine
- DTaP-IPV-Hib
Diphtheria, tetanus, acellular pertussis, polio virus and Haemophilus Influenzae type b vaccine
- DTaP-IPV-Hib-HB
Diphtheria, tetanus, acellular pertussis, polio virus, Haemophilus Influenzae type b vaccine and Hepatitis B vaccine
- Measles
Measles-containing vaccine
- Men-C-C
Meningococcal C conjugate vaccine
- MMR
Measles, mumps and rubella vaccine
- MMRV
Measles, mumps, rubella and varicella vaccine
- PCV
Pneumococcal conjugate vaccine
- Rota
Rotavirus vaccine
- RR
Risk ratio
- VPD
Vaccine-preventable diseases
Authors’ contributions
NB, GDS and MK (since 2016) conceptualized the research and collected the data. MK performed analyses, interpreted the results and prepared the initial draft of the manuscript. GDS, DT and NB were supervisors of MK's PhD candidature and assisted in performing analyses and provided overall guidance to MK. MK, NB, GDS, MO, MG, ML and CS participated in the study design and methodology. All authors contributed significantly to towards drafting and editing the manuscript. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Each survey from 2006 to 2016 was approved by the Ethic Board Committee of the CHU de Quebec-University Laval Hospital and written consent was obtained from all the participants.
Consent for publication
Not applicable.
Competing interests
Gaston De Serres received research grand for investigator-initiated studies from Pfizer and GlaxoSmithKline (GSK) and was reimbursed expenses to travel to an ad hoc advisory board meeting by GSK but received no honoraria. He has equally provided paid expert testimony in a grievance against a vaccine-or-mask healthcare worker influenza vaccination policy for the Ontario Nurse Association. Other authors have no conflicts of interest to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marilou Kiely, Email: marilou.kiely@inspq.qc.ca.
Nicole Boulianne, Email: nicole.boulianne@inspq.qc.ca.
Denis Talbot, Email: denis.talbot@fmed.ulaval.ca.
Manale Ouakki, Email: manale.ouakki@inspq.qc.ca.
Maryse Guay, Email: maryse.guay.agence16@ssss.gouv.qc.ca.
Monique Landry, Email: monique.landry@msss.gouv.qc.ca.
Chantal Sauvageau, Email: chantal.sauvageau@inspq.qc.ca.
Gaston De Serres, Email: gaston.deserres@inspq.qc.ca.
References
- 1.Goldstein ND, Newbern EC, Evans AA, Drezner K, Welles SL. Choice of measures of vaccination and estimates of risk of pediatric pertussis. Vaccine. 2015;33(32):3970–3975. doi: 10.1016/j.vaccine.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Dannetun E, Tegnell A, Hermansson G, Törner A, Giesecke J. Timeliness of MMR vaccination--influence on vaccination coverage. Vaccine. 2004;22(31–32):4228–4232. doi: 10.1016/j.vaccine.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Akmatov MK, Kretzschmar M, Kramer A, Mikolajczyk RT. Timeliness of vaccination and its effects on fraction of vaccinated population. Vaccine. 2008;26:3805–3811. doi: 10.1016/j.vaccine.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Luman ET, Barker LE, Shaw KM, McCauley MM, Buehler JW, Pickering LK. Timeliness of childhood vaccinations in the United States days Undervaccinated and number of vaccines delayed. JAMA. 2005;293(10):1204–1211. doi: 10.1001/jama.293.10.1204. [DOI] [PubMed] [Google Scholar]
- 5.Bolton P, Hussain A, Hadpawat A, Holt E, Hughart N, Guyer B. Deficiencies in current childhood immunization indicators. Public Health Rep Wash DC 1974. 1998;113(6):527–532. [PMC free article] [PubMed] [Google Scholar]
- 6.Dombkowski KJ, Lantz PM, Freed GL. Risk factors for delay in age-appropriate vaccination. Public Health Rep Wash DC 1974. 2004;119(2):144–155. doi: 10.1177/003335490411900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietz V, Zell E, Eddins D, Bernier R, Orenstein W. Vaccination coverage in the USA. Lancet Lond Engl. 1994;344(8934):1439–1440. doi: 10.1016/s0140-6736(94)90610-6. [DOI] [PubMed] [Google Scholar]
- 8.Hull BP, McIntyre PB. Timeliness of childhood immunisation in Australia. Vaccine. 2006;24(20):4403–4408. doi: 10.1016/j.vaccine.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 9.Strine TW, Luman ET, Okoro CA, McCauley MM, Barker LE. Predictors of age-appropriate receipt of DTaP dose 4. Am J Prev Med. 2003;25(1):45–49. doi: 10.1016/s0749-3797(03)00093-x. [DOI] [PubMed] [Google Scholar]
- 10.Dayan GH, Shaw KM, Baughman AL, Orellana LC, Forlenza R, Ellis A, et al. Assessment of delay in age-appropriate vaccination using survival analysis. Am J Epidemiol. 2006;163(6):561–570. doi: 10.1093/aje/kwj074. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Z, Smith PJ, Hill HA. Missed opportunities for simultaneous administration of the fourth dose of DTaP among children in the United States. Vaccine. 2017;35:3191–3195. doi: 10.1016/j.vaccine.2017.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W-T, Lin H-C, Yang C-H. Undervaccination with diphtheria, tetanus, and pertussis vaccine: national trends and association with pertussis risk in young children. Hum Vaccines Immunother. 2017;13(4):757–761. doi: 10.1080/21645515.2016.1249552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Public Health Agency of Canada. Canadian immunization guide. Available from: https://www.canada.ca/en/public-health/services/canadian-immunization-guide.html. [cited 6 Mar 2017].
- 14.Ministère de la Santé et des Services sociaux. Protocole d’immunisation du Québec, 6e édition. Available from: http://publications.msss.gouv.qc.ca/msss/document-000105. [cited 20 Mar 2017]
- 15.Boulianne N, Audet D, Ouakki M, Guay M, Duval B, De Serres G. Enquête sur la couverture vaccinale des enfants québécois en 2006. Québec: Institut national de santé publique du Québec; 2007. [Google Scholar]
- 16.Fiks AG, Alessandrini EA, Luberti AA, Ostapenko S, Zhang X, Silber JH. Identifying factors predicting immunization delay for children followed in an urban primary care network using an electronic health record. Pediatrics. 2006;118(6):e1680–e1686. doi: 10.1542/peds.2005-2349. [DOI] [PubMed] [Google Scholar]
- 17.Hanna JN, Wakefield JE, Doolan CJ, Messner JL. Childhood immunisation: factors associated with failure to complete the recommended schedule by two years of age. Aust J Public Health. 1994;18(1):15–21. doi: 10.1111/j.1753-6405.1994.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 18.Guerra FA. Delays in immunization have potentially serious health consequences. Paediatr Drugs. 2007;9(3):143–148. doi: 10.2165/00148581-200709030-00002. [DOI] [PubMed] [Google Scholar]
- 19.Boulianne N, Bradet R, Audet D, Deceuninck G. Enquête sur la couverture vaccinale des enfants de 1 an et 2 ans au Québec en 2008. Québec: Institut national de santé publique du Québec; 2009. [Google Scholar]
- 20.Boulianne N, Bradet R, Audet D, Ouakki M, Guay M, De Serres G. Enquête sur la couverture vaccinale des enfants de 1 an et 2 ans au Québec en 2010. Québec: Institut national de santé publique du Québec; 2011. [Google Scholar]
- 21.Boulianne N, Bradet R, Audet D, Ouakki M, De Serres G, Guay M. Enquête sur la couverture vaccinale des enfants de 1 an et 2 ans au Québec en 2012. Québec: Institut national de santé publique du Québec; 2013. [Google Scholar]
- 22.Boulianne N, Audet D, Ouakki M, Dubé E, De Serres G, Guay M. Enquête sur la couverture vaccinale des enfants de 1 an et 2 ans au Québec en 2014. Québec: Institut national de santé publique du Québec; 2015. [Google Scholar]
- 23.Kiely M, Boulianne N, Ouakki M, Audet D, Gariépy MC, Guay M, De Serres G, Dubé E. Enquête sur la couverture vaccinale des enfants de 1 an et 2 ans au Québec en 2016. Québec: Institut national de santé publique du Québec; 2017.
- 24.Hanley JA, Moodie E. Sample size, precision and power calculations: a unified approach. J Biomet Biost. 2011;2(5):1000124. [Google Scholar]
- 25.Sabnis SS, Pomeranz AJ, Lye PS, Amateau MM. Do missed opportunities stay missed? A 6-month follow-up of missed vaccine opportunities in inner city Milwaukee children. Pediatrics. 1998;101(5):E5. doi: 10.1542/peds.101.5.e5. [DOI] [PubMed] [Google Scholar]
- 26.Skov T, Deddens J, Petersen MR, Endahl L. Prevalence proportion ratios: estimation and hypothesis testing. Int J Epidemiol. 1998;27(1):91–95. doi: 10.1093/ije/27.1.91. [DOI] [PubMed] [Google Scholar]
- 27.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 28.Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanderWele TJ, Shpitser I. A new criterion for confounder selection. Biometrics. 2011;67(4):1406–1413. doi: 10.1111/j.1541-0420.2011.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernan MA, Robins JM. Causal inference. Boca Raton: Chapman & Hall/ CRC, forthcoming; 2017. [Google Scholar]
- 31.Gust DA, Strine TW, Maurice E, Smith P, Yusuf H, Wilkinson M, et al. Underimmunization among children: effects of vaccine safety concerns on immunization status. Pediatrics. 2004;114(1):e16–e22. doi: 10.1542/peds.114.1.e16. [DOI] [PubMed] [Google Scholar]
- 32.Greenland S. Interval estimation by simulation as an alternative to and extension of confidence intervals. Int J Epidemiol. 2004;33(6):1389–1397. doi: 10.1093/ije/dyh276. [DOI] [PubMed] [Google Scholar]
- 33.Kovar JG, JNK R, CFJ W. Bootstrap and other methods to measure errors in survey estimates. Can J Stat. 1988;16:25–45.
- 34.Lernout T, Theeten H, Hens N, Braeckman T, Roelants M, Hoppenbrouwers K, et al. Timeliness of infant vaccination and factors related with delay in Flanders, Belgium. Vaccine. 2014;32(2):284–289. doi: 10.1016/j.vaccine.2013.10.084. [DOI] [PubMed] [Google Scholar]
- 35.Walton S, Cortina-Borja M, Dezateux C, Griffiths LJ, Tingay K, Akbari A, et al. Measuring the timeliness of childhood vaccinations: using cohort data and routine health records to evaluate quality of immunisation services. Vaccine. 2017;35(51):7166–7173. doi: 10.1016/j.vaccine.2017.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daley MF, Glanz JM, Newcomer SR, Jackson ML, Groom HC, Lugg MM, et al. Assessing misclassification of vaccination status: implications for studies of the safety of the childhood immunization schedule. Vaccine. 2017;35(15):1873–1878. doi: 10.1016/j.vaccine.2017.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hambidge SJ, Phibbs SL, Chandramouli V, Fairclough D, Steiner JF. A stepped intervention increases well-child care and immunization rates in a disadvantaged population. Pediatrics. 2009;124(2):455–464. doi: 10.1542/peds.2008-0446. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson Vann JC, Jacobson RM, Coyne-Beasley T, Asafu-Adjei JK, Szilagyi PG. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst Rev. 2018;(1). Art. No.: CD003941. [DOI] [PMC free article] [PubMed]
- 39.Tozzi AE, Piga S, Corchia C, Di Lallo D, Carnielli V, Chiandotto V, et al. Timeliness of routine immunization in a population-based Italian cohort of very preterm infants: results of the ACTION follow-up project. Vaccine. 2014;32(7):793–799. doi: 10.1016/j.vaccine.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 40.Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics. 2008;122(4):718–725. doi: 10.1542/peds.2007-0538. [DOI] [PubMed] [Google Scholar]
- 41.Luman ET, McCauley MM, Shefer A, Chu SY. Maternal characteristics associated with vaccination of young children. Pediatrics. 2003;111(5 Pt 2):1215–1218. [PubMed] [Google Scholar]
- 42.Reading R, Surridge H, Adamson R. Infant immunization and family size. J Public Health Oxf Engl. 2004;26(4):369–371. doi: 10.1093/pubmed/fdh173. [DOI] [PubMed] [Google Scholar]
- 43.Larson HJ, Jarrett C, Eckersberger E, Smith DMD, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine. 2014;32(19):2150–2159. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein KP, Kviz FJ, Daum RS. Accuracy of immunization histories provided by adults accompanying preschool children to a pediatric emergency department. JAMA. 1993;270(18):2190–2194. [PubMed] [Google Scholar]
- 45.Miles M, Ryman TK, Dietz V, Zell E, Luman ET. Validity of vaccination cards and parental recall to estimate vaccination coverage: a systematic review of the literature. Vaccine. 2013;31(12):1560–1568. doi: 10.1016/j.vaccine.2012.10.089. [DOI] [PubMed] [Google Scholar]
- 46.Benichou J, Palma M. Handbook of epidemiology. Second. Germany: Wolfgang Ahrens & Iris Pigeot; 2014. Measures of impact; p. 2489. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of children participants, 1-year and 2-year cohorts, 2006–2016 (n = 7183). (DOCX 20 kb)
Intervals following the next vaccine dose for children with new vaccine delays at the DTaP1 and DTaP2. (DOCX 26 kb)
New vaccine delays at 2, 4, 6 and 12 months by vaccine provider and survey year, 1-year and 2-year cohorts, 2006–2016. (DOCX 17 kb)
Data Availability Statement
The datasets used and analysed during this current study are available from the corresponding author on reasonable request.