Abstract
Background
Although the cause of acute eosinophilic pneumonia (AEP) has not yet been fully clarified, cigarette smoking is reported to be a risk factor for developing AEP. The heat-not-burn cigarette (HNBC) was developed to reduce the adverse effects of smoke on the user's surroundings. However, the health risks associated with HNBCs have not yet been clarified. We report a successfully treated case of fatal AEP presumably induced by HNBC use.
Presentation of case
A 16-year-old man commenced HNBC smoking two weeks before admission and subsequently suffered from shortness of breath that gradually worsened. The patient was transferred to emergency department and immediately intubated because of respiratory failure. Computed tomography showed mosaic ground-glass shadows on the distal side of both lungs with a PaO2/FIO2 ratio of 76. The patient required veno-venous extracorporeal membrane oxygenation (ECMO) for severe respiratory failure. He was diagnosed with AEP by clinical course and detection of eosinophils in sputum; thus, methylprednisolone was administrated. The patient was weaned off ECMO four days after initiation and extubated the day after. He fully recovered without sequelae.
Conclusion
As far as we know, our patient is the first case of AEP induced by HNBC use successfully treated with ECMO. Emergency physicians must be aware that HNBCs can induce fatal AEP.
Keywords: Tobacco, Cigarettes, Heat-not-burn cigarettes, Acute eosinophilic pneumonia, Extracorporeal membrane oxygenation, ECMO
Abbreviations: AEP, acute eosinophilic pneumonia; BAL, bronchoalveolar lavage; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; FIO2, fraction of inspiratory oxygen; HNBC, heat-not-burn cigarette; IV, intravenous administration; mPSL, methylprednisolone; PaO2, partial pressure of arterial oxygen; PEEP, positive end-expiratory pressure; Pplat, plateau pressure; PSL, prednisolone; SpO2, oxygen saturation of pulse oximetry; VV, veno-venous
Highlights
-
•
The use of heat-not-burn cigarettes has been increasing in recent years.
-
•
The effects of heat-not-burn cigarettes on health have not yet been clarified.
-
•
Heat-not-burn cigarettes possibly induce acute eosinophilic pneumonia.
-
•
Extracorporeal membrane oxygenation was effective in a patient with a severe case of acute eosinophilic pneumonia.
1. Background
Acute eosinophilic pneumonia (AEP), an uncommon but important cause of severe respiratory failure, is usually triggered by inhalation of allergic antigens such as chemical substances and fungi [1]. Cigarette smoking in particular is a well-known cause of AEP, as the condition normally happens within a few weeks after starting smoking [2].
The heat-not-burn cigarette (HNBC) was developed to reduce the negative effects of smoke on the user's surroundings and has been on the market since 2014 in Japan. HNBC use has spread worldwide; 4.7% of all cigarette smokers in Japan used HNBCs in 2017 according to an internet survey [3]. HNBCs are widely believed to expose smokers and their surroundings to lesser amounts of toxic substances such as nicotine compared to conventional cigarettes [4]; however, the latest research shows the amount of nicotine released by HNBCs is almost equal to that released by conventional cigarettes [5]. Furthermore, the health risks associated with HNBCs have not yet been clarified.
Herein, we report a case of fulminant HNBC-induced AEP successfully treated with extracorporeal membrane oxygenation (ECMO). Only one report of HNBC-induced AEP has been published previously as far as we know [6]. Since HNBC use has been recently increasing, emergency physicians should consider HNBC smoking as one of the significant causes of AEP. Our experience may help emergency physicians determine the cause of AEP.
2. Case presentation
A 16-year-old man with a history of crustacea allergy and bronchial asthma in childhood was transferred to emergency department (original hospital) with complaints of severe cough, fatigue, and shortness of breath. These symptoms started immediately after smoking HNBCs and worsened in the two weeks before hospitalization.
His Glasgow Coma Scale score at presentation indicated health deterioration (E1V3M5). His vital signs were as follows: blood pressure 109/50 mmHg, heart rate 136 bpm, respiratory rate 30 breaths per minute, body temperature 37.3 °C, and oxygen saturation of pulse oximetry (SpO2), 81%. Blood test results revealed leukocytosis (white blood cell count of 28000/μL; neutrophils 98%, lymphocytes 1.5%, eosinophils 0%, monocytes 0.5%) and an increase of C-reactive protein, 32.3 mg/dL. Renal function and liver enzymes were unremarkable: blood urea nitrogen 17.1 mg/dL, serum creatinine 0.67 mg/dL, aspartate aminotransferase 11 U/L, and alanine aminotransferase 8U/L, with increased lactic acid levels of 2.0 mmol/L. Toxicological screening was negative.
Both lungs showed a ground-glass appearance in the chest X-ray and computed tomography (CT) showed consolidation spreading in a mosaic pattern from the middle to distal side of both lungs (Fig. 1). Based on the patient's clinical course and CT findings, he was strongly suspected of having AEP, and 500mg of methylprednisolone (mPSL) was administered.
Fig. 1.
CT findings at emergency department. Non-contrast CT scan was performed using slice thickness 5 mm. The picture shows ground-grass findings spreading in both lungs. Such infiltrative shadows spread diffusely from the apex of each lung to above the diaphragm.
Since oxygen therapy was ineffective, the patient was intubated and mechanical ventilation started. On the day after, his respiratory status was deteriorated and he was transferred to our hospital for further treatment. The ventilation settings at our hospital admission were a fraction of inspiratory oxygen (FIO2) of 1.0, respiratory frequency of 15 breaths per minute, positive end-expiratory pressure (PEEP) of 15 cmH2O, and plateau pressure (Pplat) of 30 cmH2O. Blood gas analysis under this setting showed a pH of 7.188, pO2 of 76 mmHg, pCO2 of 79.3 mmHg, base excess −1.8 mmHg. A bloody foamy secretion oozed out of the tracheal tube. At that time, the PaO2/FIO2 ratio (PFR) was 76, that satisfied a criterion for severe ARDS in Berlin definition [7]. We continued the conventional ventilation; however, no improvement was found.
We subsequently initiated veno-venous (VV) ECMO by 23 french 38cm drainage cannula inserted at the right jugular vein and 20 french 15cm infusion cannula inserted at the right femoral vein with 4.0 L per minute of blood flow. We adjusted the ventilator settings to 0.4 of FIO2, 10 cmH2O of PEEP, and 20 cmH2O of Pplat for resting lung. The patient's lung totally collapsed and he had no tidal volume at that time (Supplementary Fig. a and b).
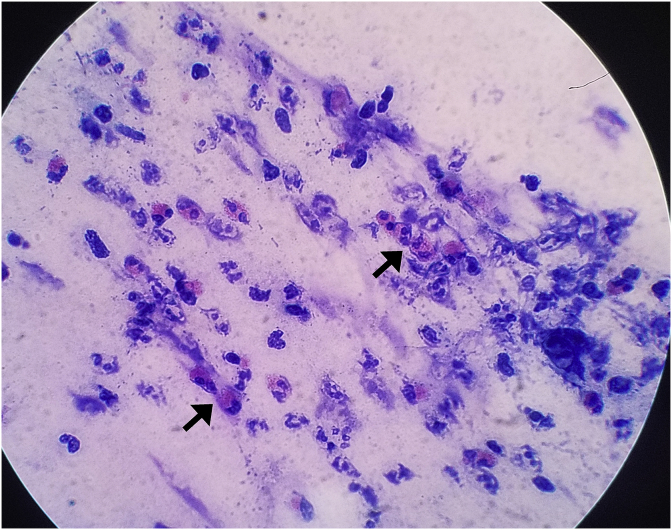
To make an accurate diagnosis, we examined sputum cytology of tracheal secretions instead of bronchoalveolar lavage (BAL) fluid after ECMO initiation. The cytological findings showed that aggregated eosinophils were accumulating and the percentage of eosinophils, neutrophils, and lymphocytes were 14.7%, 51.7%, and 33.6%, respectively (Fig. 2). 1000mg of mPSL was administrated intravenously for three days, followed by 60mg of oral prednisolone. Tidal volume started to increase on the fourth hospital day and oxygenation and carbon dioxide emission were gradually recovered (Fig. 3). ECMO was removed on the fifth hospital day. The patient was extubated on the sixth hospital day and transferred back to the original hospital on the 10th day. Blood tests showed the number of eosinophils had increased from the fifth hospital day up to 2980/μL on the seventh day (Fig. 3). Chest x-ray and CT findings on the ninth hospital day showed that the alveolar consolidation had disappeared and showed a healthy alveolar structure in both lungs (Supplementary Fig. c and d).
Fig. 2.
Sputum cytology on the first hospital day. The picture shows aggregation of eosinophils (arrows). The percentage of eosinophils increased slightly (14.7%). Many neutrophils were present (51.7%).
Fig. 3.
Clinical course. On the first day, the patient had little tidal volume. ECMO was needed for four days and the tidal volume (blue bar) increased gradually with administration of methylprednisolone. Interestingly, although blood tests showed no eosinophils on the first day, the elevation of eosinophils (orange line) reached 2980/μL on the seventh day.
After transferring back to the original hospital, the patient rehabilitated in the general ward. Oral prednisolone was gradually decreased and stopped. The patient was discharged without sequelae 10 days after transferring. Currently, the patient is well enough to lead a normal life. The patient and his family were told to never smoke again, not even HNBCs.
3. Discussion
To draw health-conscious smokers and revamp smoking's public image, “safer” products such as HNBCs have been developed. HNBC use has been increasing in recent years due to the absence of smoke and its adverse effects on the user's surroundings. It is widely believed that HNBC users inhale less tar and accordingly, incur less harmful effects on their health than with smoking conventional cigarettes. However, previous studies have demonstrated that HNBCs' benefits are controversial [5,6]. Conflicts of interests may have influenced the endorsement of these devices in the past, since tobacco companies promote cigarette smoking while globally undermining tobacco control. Health professionals and scholarly journals should consider both the ethics and conflicts of interest associated with advocating tobacco product substitution.
AEP can develop soon after initiation of smoking as well as rechallenge with smoking after a long abstinence [2]. The mechanism of how smoking causes AEP has not been clarified; however, some chemical substances contained in the cigarette could contribute to activating an allergic reaction and eosinophils. In the mechanism of allergic reaction, the theory that “if the amount of toxic substance is small, the risk proportionally decreases” is not necessarily true. Auer reported that HNBCs release nicotine in the almost same amount, 4–82% of volatile organic compounds, and 0.1–9% polycyclic aromatic hydrocarbons as conventional cigarettes [5]. Notably, HNBCs release more acenaphthene, one of the polycyclic aromatic hydrocarbons, compared to conventional cigarettes [5].
AEP is marked by acute febrile respiratory failure associated with pulmonary eosinophilia and diffuse radiographic infiltrates. The diagnostic criteria for AEP include: acute onset of symptoms seven days or less before presentation; fever ≥37.2 °C; bilateral infiltrates on chest film; severe hypoxemia: partial pressure of arterial oxygen (PaO2) on room air ≤60, SpO2 on room air <90%, or alveolar-arterial gradient >40; lung eosinophilia: BAL differential with ≥25% eosinophils or predominance of eosinophils on open lung biopsy; and no history of drug hypersensitivity, no historical or laboratory evidence of infection, and no other known origin of acute eosinophilic lung disease [8,9].
While eosinophils are found in very few numbers, usually less than 2% in BAL in a normal lung, the percentage of eosinophils was 14.6% in sputum cytology with aggregations in our patient, which did not meet the criteria for AEP (Fig. 2). We made the final diagnosis of AEP based on other criteria, even though our patient did not meet the BAL criteria. It is also important to exclude other differential diagnoses, such as bacterial pneumonia, viral pneumonia, vasculitis, autoimmune disease, leukemia and lymphoma. We performed possible pathogen examinations (culture, serological diagnosis etc.), examinations of vasculitis, autoimmune diseases, leukemia and lymphoma; however, these tests were all negative. Furthermore, our patient rapidly and dramatically improved after administration of mPSL, which is typical with AEP [10]. It is reported that 75% of AEP in the acute phase does not show elevation in the number of eosinophils in the blood [1]; however, it may increase in the recovery phase of the second to eighth day after onset [10]. In our case, there was no elevation of eosinophils in the acute phase, and it increased to 2980/μL on the seventh hospital day. This is considered a key finding for AEP diagnosis.
As far as we know, only one report of HNBC-induced AEP has previously been published [6]. In that case, the symptoms developed six months after starting HNBC use. The patient required oxygen therapy but not invasive ventilation. We report a successfully treated case of fulminant HNBC-induced AEP where lung function deteriorated rapidly and heavily, eventually requiring invasive ventilation.
Our case showed more rapid and massive progression compared to the previous report [6], and VV ECMO was required. In this case, the PFR of less than 80 persisted for more than 6 hours, that fulfills the criteria for ECMO initiation in EOLIA study [11]. We performed VV ECMO with the right jugular vein drainage and femoral vein infusion, that is the normal method in our department. Previous studies indicate that drainage from IVC realizes less recirculation and therefore the route from the IVC to the right atrium is the standard in most centers regarding VV ECMO with two cannulas. However, recent study has shown that recirculation can be minimized by using multistage drainage cannula through the right internal jugular vein with infusion to the femoral vein [12].
Currently, two cases of AEP have been reported (including our report), despite the short time period HNBCs have been on the market and limited HNBC use. It is suggested that the incidence of AEP caused by HNBC is equal to or greater than that caused by conventional cigarettes. This implies that HNBCs can cause a rapid and fatal respiratory failure requiring ECMO. Since AEP is reversible and usually improves in a short time with administration of corticosteroid, VV ECMO should be employed if the patient's life cannot be maintained by conventional mechanical ventilation [13].
4. Conclusion
Our experience may suggest that HNBCs possibly induce fatal AEP. Since this is only a case report, adverse effects and health risks cannot be compared between HNBC and conventional cigarette use. A large-scale epidemiological survey is advocated.
Declarations of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2018.12.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Fig. Transition of chest x-ray and CT findings.
X-ray/CT after ECMO initiation (a, b) shows a totally collapsed lung. This may have been because the alveoli were occupied by inflammatory cells, including eosinophils and neutrophils. After aggressive methylprednisolone treatment, all the inflammatory cells disappeared and x-ray/CT on the 9th hospital day (c, d) showed an almost healthy alveolar structure. Both CT scans (b, d) were performed using slice thickness 5 mm without contrast medium.
References
- 1.Allen J.N., Pacht E.R., Gadek J.E., Davis W.B. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N. Engl. J. Med. 1989;32:569–574. doi: 10.1056/NEJM198908313210903. [DOI] [PubMed] [Google Scholar]
- 2.Uchiyama H., Suda T., Nakamura Y., Shirai M., Gemma H., Shirai T., Toyoshima M., Imokawa S., Yasuda K., Ida M., Nakano Y., Inui N., Sato J., Hayakawa H., Chida K. Alterations in smoking habits are associated with acute eosinophilic pneumonia. Chest. 2008;133:1174–1180. doi: 10.1378/chest.07-2669. [DOI] [PubMed] [Google Scholar]
- 3.Tabuchi T., Gallus S., Shinozaki T., Nakaya T., Kunugita N., Colwell B. Heat-not-burn tobacco product use in Japan: its prevalence, predictors and perceived symptoms from exposure to secondhand heat-not-burn tobacco aerosol. Tob. Contr. 2018;27:e25–e33. doi: 10.1136/tobaccocontrol-2017-053947. pii: tobaccocontrol-2017-053947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werley M.S., Freelin S.A., Wrenn S.E., Gerstenberg B., Roemer E., Schramke H., Van Miert E., Vanscheeuwijck P., Weber S., Coggins C.R. Smoke chemistry, in vitro and in vivo toxicology evaluations of the electrically heated cigarette smoking system series K. Regul. Toxicol. Pharmacol. 2008;52:122–139. doi: 10.1016/j.yrtph.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Auer R., Concha-Lozano N., Jacot-Sadowski I., Cornuz J., Berthet A. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Int. Med. 2017;177:1050–1052. doi: 10.1001/jamainternmed.2017.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamada T., Yamashita Y., Tomioka H. Acute eosinophilic pneumonia following heat-not-burn cigarette smoking. Respirol. Case Rep. 2016;4 doi: 10.1002/rcr2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: the berlin definition. J. Am. Med. Assoc. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.Cottin V., Cordier J.F. Eosinophilic pneumonias. Allergy. 2005;60:841–857. doi: 10.1111/j.1398-9995.2005.00812.x. [DOI] [PubMed] [Google Scholar]
- 9.Allen J. Acute eosinophilic pneumonia. Semin. Respir. Crit. Care Med. 2006;27:142–147. doi: 10.1055/s-2006-939517. [DOI] [PubMed] [Google Scholar]
- 10.Philit F., Etienne-Mastroïanni B., Parrot A., Guérin C., Robert D., Cordier J.F. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am. J. Respir. Crit. Care Med. 2002;166:1235–1239. doi: 10.1164/rccm.2112056. [DOI] [PubMed] [Google Scholar]
- 11.Combes A., Hajage D., Capellier G., Demoule A., Lavoué S., Guervilly C., Da Silva D., Zafrani L., Tirot P., Veber B., Maury E., Levy B., Cohen Y., Richard C., Kalfon P., Bouadma L., Mehdaoui H., Beduneau G., Lebreton G., Brochard L., Ferguson N.D., Fan E., Slutsky A.S., Brodie D., Mercat A., EOLIA Trial Group, REVA. ECMONet Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N. Engl. J. Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 12.Frenckner B., Broman M., Broomé M. Position of draining venous cannula in extracorporeal membrane oxygenation for respiratory and respiratory/circulatory support in adult patients. Crit. Care. 2018;22:163. doi: 10.1186/s13054-018-2083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauvaget E., Dellamonica J., Arlaud K., Sanfiorenzo C., Bernardin G., Padovani B., Viard L., Dubus J.C. Idiopathic acute eosinophilic pneumonia requiring ECMO in a teenager smoking tobacco and cannabis. Pediatr. Pulmonol. 2010;45:1246–1249. doi: 10.1002/ppul.21314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.