Abstract
Research on the inter-relationship between drug abuse and social stress has primarily focused on the role of stress exposure during adulthood and more recently, adolescence. Adolescence is a time of heightened reward sensitivity, but it is also a time when earlier life experiences are expressed. Exposure to stress early in postnatal life is associated with an accelerated age of onset for drug use. Lifelong addiction is significantly greater if drug use is initiated during early adolescence. Understanding how developmental changes following stress exposure interact with sensitive periods to unfold over the course of maturation is integral to reducing their later impact on substance use. Arousal levels, gender/sex, inflammation, and the timing of stress exposure play a role in the vulnerability of these circuits. The current review focuses on how early postnatal stress impacts brain development during a sensitive period to increase externalizing and internalizing behaviors in adolescence that include social interactions (aggression; sexual activity), working memory impairment, and depression. How stress effects the developmental trajectories of brain circuits that are associated with addiction are discussed for both clinical and preclinical studies.
Keywords: Adolescence, Arousal, Inflammation, Maltreatment, Sensitive period
Highlights
-
•
Exposure to early stress increases addiction.
-
•
Stress exposure during sensitive periods shapes brain development.
-
•
Sex differences exist in early life stress effects
-
•
Social buffering, reward, arousal and inflammation are important for outcome.
1. Introduction
Early life stress exposure has a significant impact on later behavior and has been associated with a number of clinical disorders. Notwithstanding, early life stress exposure has been associated with anxiety, depression, drug abuse, and schizophrenia. The effects of early stress exposure emerge later in adolescence. The following review examines the inter-relationships between early life stress exposure, addiction, and the mediating factors that have received less attention. Namely, arousal, inflammation, and the timing of stress exposure are discussed within a sensitive period framework. During a sensitive period, low levels of GABA and BDNF maintain a high state of plasticity, implying a state of immaturity. Preventative interventions given during a sensitive period may reduce later appearing abnormalities.
Developmental exposure to toxic stress has been found in 54% of the addiction population. Toxic stress includes physical/sexual abuse, neglect, loss of a caregiver, and exposure to a dysfunctional household (e.g. domestic violence, exposure to drug use, or exposure to a natural disaster). Epidemiology studies show that exposure to early life adversity increases vulnerability to a number of disorders that include affective and reward dysfunction (e.g., addiction, depression, schizophrenia) in a sex-dependent manner (Clark et al., 1997). Stress exposure can be quantified with the Adverse Childhood Experiences scale (ACEs) (Anda et al., 2006), which has helped to standardize the field. Increases in the number of ACEs are associated the risk of addiction in general (Dube et al., 2003). For example, more than four ACES increase the risk of addiction 7–10 fold (Dube et al., 2003). The impact of these statistics lies in the relationship between the ages of initiation of drug use. Specifically, more ACES are associated with increased risk of initiating drug use early (<14 years of age) by 2–4 fold (Dube et al., 2003). Significant abuse (molestation and abuse) resulted in odds ratios of 3.5 for alcohol/cigarette experimentation and 12.2 for marijuana use/regular drinking by age 10 (Bensley et al., 1999). While a number of risk factors for addiction are associated with stress, early initiation of drug use (possibly the consequence of these risk factors (Hyucksun Shin, 2012)) increases the chance of lifelong addiction by 4–10 fold across different drug classes (McGue et al., 2001; Wagner and Anthony, 2007).
Developmental maltreatment in animals is experimentally produced in a number of ways. Early life stress paradigms include maternal separation, deprivation, or early weaning. How these different animal stress paradigms compare to the human condition is dicussed in (Andersen, 2015). The naturalistic model of early stress exposure categorizes the dam as having low or high licking and grooming behavior (low/high-LG; (Caldji et al., 1998; Champagne et al., 2003). High-LG is beneficial to the rat pup, while low LG is stressful. The experimental approach involves isolating pups from the dam and/or littermates for as little as 24 h (Levine, 1967) to an average of 2–6 h a day and between the ages of P2-20 depending on individual investigators (Lehmann and Feldon, 2000; Plotsky and Meaney, 1993; Reus et al., 2011). Pups and dam are reunited after the separation. This separation can occur over the stress-hypo responsive period (SHRP), when stress responsiveness is low due to increased GABA effects on the hypothalamic-pituitary-adrenal axis (Dent et al., 2007). The SHRP programs reduced corticosterone levels (Sanchez et al., 2015) and may provide a period of increased plasticity that is found in other systems (i.e., visual processing (Fagiolini and Hensch, 2000)). Maternal separation or neonatal separation has revealed important and persistent changes in neuroanatomy, neurochemistry, and behavior (Andersen and Teicher, 2004; 2008b; Matthews et al., 2001; Meaney et al., 1996). Together, the maternal separation paradigm provides a species-relevant, early life stressor that yields similar changes in brain morphometry and behavior to those observed in child abuse (Andersen, 2015; Teicher et al., 2006).
2. Stress and the role of externalizing and internalizing behaviors in addiction
Broadly conceptualized as externalizing and internalizing disorders (Tandon et al., 2009), this nosology has been used to identify latent correlations with drug use (Kessler et al., 2011). There is tremendous overlap between externalizing and internalizing disorders that are linked to dysfunctional reward and affect processing. The timing of stress exposure may differentially shape individuals to be vulnerable to substance use (see Section 3). A number of outcomes are possible following exposure to early life stress (reviewed by (Cicchetti and Rogosch, 2002; Vachon et al., 2015)).
2.1. Clinical
While a number of studies suggest that early life stress increases risk for substance use (Chauhan and Widom, 2012; Widom et al., 2006), this relationship is complex. Inconsistencies in the persistence of drug use suggest that mediating or moderating variables play a significant role in drug abuse vulnerability in a maltreated population (Fergusson et al., 2008; Wilson et al., 2015). A recent longitudinal study conducted between the ages of 14–17 years in a maltreated population found that externalizing symptoms and substance use persist across this timeframe, whereas the relationship between internalizing symptoms and drug use does not (Wilson et al., 2015). Together, these studies (and those below) raise the question regarding what are the other mediating/moderating factors that underlie the relationship between abuse and drug abuse.
Externalizing disorders include attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder, and conduct disorder. These disorders are characterized by oppositional, aggressive, impulsive, disruptive, and rule-breaking behavior and are further associated with poor social interactions. Overall, individual externalizing behaviors are often part of a trajectory of more serious illness (Woolfenden et al., 2001). For example, exposure to early childhood adversity is associated with a greater likelihood of a substance use disorder, incarceration, and early pregnancy later in life (Andersen and Teicher, 2008a; Biederman et al., 2001; Copeland et al., 2010; Disney et al., 1999; Elkins et al., 2007; Stewart et al., 1980). Physically aggressive behavior is more predominant in males than in females with conduct disorder (Cleverley et al., 2012). Early pregnancy is common in girls with conduct disorder (Copeland et al., 2010; Stewart et al., 1980). Specifically, childhood maltreatment in girls is associated with increased sexual activity and a 66% higher chance of teen pregnancy (Elkins et al., 2007; Garwood et al., 2015; Keenan et al., 1999). Over 50% of individuals diagnosed with conduct disorder meet criteria for a substance use disorder (Reebye et al., 1995). In fact, a diagnosis of conduct disorder between 11 and 14 years of age predicts substance use disorders by 18 years of age (all odds ratios, > 4.27) (Elkins et al., 2007; Sung et al., 2004). Externalizing disorders are more strongly associated with alcohol use disorder than internalizing disorders (Farmer et al., 2016).
Internalizing disorders include anxiety and depression (Tandon et al., 2009). Both anxiety and depression have their own individual developmental trajectories of impairment, although anxiety often precedes depression (Beesdo et al., 2007). Anxiety disorders are more prevalent in individuals with higher ACES. The prevalence of depression is ∼67% of an early life stress population (Andersen and Teicher, 2008b; Teicher et al., 2009; Widom et al., 2007) compared to a lifetime prevalence of ∼20% in the general population (Kessler et al., 1994). Like externalizing disorders, poor social interactions often lead to isolation (Woolfenden et al., 2001). The presence of an internalizing disorder meditates the effects of early child neglect and later substance use (Douglas et al., 2010; Duprey et al., 2017). Specifically, early sexual abuse in females is associated with greater risk for alcohol abuse and/or dependence in adulthood as determined in a cross-sectional study (Meng and D'Arcy, 2016) as well as a longitudinal study (Skinner et al., 2016). Early life stress affects both genders at the level of internalizing behaviors, whereas externalizing disorders are more prevalent in males.
2.2. Preclinical
Exposure to early life stress increases both externalizing and internalizing behaviors (Marquez et al., 2013) that are risk factors for drug use. The presence of behavioral disinhibition as discussed above characterizes symptoms of externalizing behaviors in animals (Flagel et al., 2010). Early stressed animals demonstrate more impulsivity than controls (Gondre-Lewis et al., 2016). Maternally separated rats have increased levels of aggression (Haller and Kruk, 2006; Veenema and Neumann, 2009) that is mediated by impaired social recognition (Lukas et al., 2011). Males of low-LG dams (Parent and Meaney, 2008) or following maternal separation (Haller and Kruk, 2006) have increased aggressive play behavior relative to controls. Females show greater aggressive play strategies when low-LG rats are compared to high-LG counterparts (and not control rats (Parent et al., 2013)). In females, but not males, low-LG programs reproductive success (Cameron et al., 2008a). Low-LG females also demonstrate precocial puberty and are more sexually receptive relative to high-LG females (Cameron et al., 2008b; Uriarte et al., 2007). Elevated estrogen increases aggression in females probably due to its aromatization to testosterone (Zeidan et al., 2011). Testosterone is able to moderate the effects of high- and low-LG on sexual receptivity of females with a maternal separation history (Borrow et al., 2013).
Early stress increases responses to stimulants. Male mice show hypersensitive stress responses to the locomotor effects of low doses of cocaine (Kikusui et al., 2005). However, discrepancies exist as to whether male rats exposed to maternal separation show increased stimulant intake (Kosten et al., 2000l; van der Veen et al., 2008) or show no change (Mathews and McCormick, 2007). Female rats separated for 60 min between P2-9 acquire cocaine self-administration more rapidly than controls (Kosten et al., 2004) that are independent of estrogen-mediated increases in self-administration (Kosten et al., 2006). Separated females also show increased sensitivity to cocaine relative to separated males and controls (Matthews et al., 1999), but show lower baseline responding on a progressive ratio schedule for intracranial self-stimulation relative to controls (Michaels et al., 2007). Curiously, locomotor responses to amphetamine are evident in maternally separated animals when they are in a novel, but not habituated, environment (Hensleigh et al., 2011). These data raise the possibility that increased susceptibility to drug use (e.g., stimulants) occurs under stressful conditions in early stressed animals.
Internalizing behaviors of depressive-like effects have been observed in animals. Exposure to early life stress in animals is associated with elevated anxiety-like behaviors (Levine, 1967 #86} and depressive-like behaviors (Andersen, 2015). Both of these behaviors are common to addiction and are sex-dependent. For example, elevated alcohol intake is observed in males exposed to early life stress (separation between P1-20 for 6 h/day), with little to no effect in stressed females in adulthood (Roman et al., 2004). Alcohol consumption reduces the anhedonic effects of alcohol withdrawal in adolescent rats (Boutros et al., 2014). However, maternal separation (P2-20 for 4 h/day) increases anhedonia during adolescence in females, but not males (Leussis et al., 2012; Lukkes et al., 2017). These sex differences could result from different durations in stress exposures or the age of assessment. Greater alcohol consumption in adolescence is observed in longer separations (360 min) from the dam compared to shorter separations (15 min) (Daoura et al., 2011; Huot et al., 2001). Besides anhedonia, chronic pain mediates a relationship between a history of child abuse and depression in humans (Goldberg, 1994). It should be no surprise that maternally separated animals, who have a high rate of depression, show a 78% progressive decrease in morphine aversion (Vazquez et al., 2005) and place preferences for morphine and increased intake (Vazquez et al., 2005).
Mechanistic changes that underlie behavioral changes are reviewed elsewhere. A brief review of predisposing factors that are relevant to drug-taking can be found in (Moffett et al., 2007). Relatedly, dopamine (Flagel et al.),2010) and opiate changes (Ploj et al., 2003) following maternal separation are reviewed. Finally, the genetic basis underlying vulnerability to the adverse effects of stress can be found in (Somaini et al., 2011).
2.3. Integration of clinical and preclinical findings
Drug choice amongst humans or animals reflects whether they exhibit externalizing or internalizing behaviors, although there is overlap. In both humans and animals, deficits in executive function including the regulation of affect and reward are involved in risk behaviors. Behaviors such as aggression, impulsivity, and poor social function are risk factors for increased substance use and are found in both humans and animals following exposure to early adversity. Other behaviors observed following maltreatment include anhedonia, pain exposure, and anxiety. The inter-relationship between risk behaviors and substance use has been characterized more in association studies in humans than in animals. Animal studies have examined the developmental expression of risk behaviors in early stress models; the relationship between stress, early expression of risk factors and subsequent drug use is sorely missing. Understanding how different forms of abuse (physical, sexual, or neglect) or its duration influence risk behavior and subsequent drug-taking may offer insight for preventative treatment.
3. The timing of stress exposures and their relationship to addiction
The timing of stress exposure is important for regional brain changes that are associated with addiction. Exposure to early life stress accelerates the appearance of behaviors and brain development relative to controls (Gee et al., 2013; Hyucksun Shin, 2012). Brain regions that share commonalities between stress exposure and addiction will be presented. Early maturity can lead to earlier initiation of drug use, which in turn, is associated with greater prevalence of lifelong addiction (McGue et al., 2001; Wagner and Anthony, 2007). The emergence of addiction requires activity in the prefrontal cortex (and other regions) that regulates, or fails to regulate, behavior.
The timing of stress exposure interacts with a sensitive period to uniquely effect behavior (Andersen and Teicher, 2008b). Experiences that occur during a sensitive period will have maximal effect on regional brain development to fine-tune neuronal circuits (Andersen, 2003; Greenough et al., 1987). Sensitive periods differ from critical periods, which require specific information to guide development (Greenough et al., 1987). The limited time course of development of ocular dominance columns is a classic example of a critical period (Lensjo et al., 2017). Alternatively, sensitive periods may represent a critical period that occurs during the second phase of synaptic overproduction and elimination. Stress exposure that occurs during active development will permanently alter the course of maturation. As a result, stress during a sensitive period will impact addiction-related behaviors as a function of age. The manifestation of stress-related changes is often delayed until adolescence (Andersen and Teicher, 2008b).
3.1. Clinical
The caveat for all human studies, unless otherwise noted, is the directionality of the effect. Whether there is existing brain dysfunction prior to the onset of abuse or is the abuse the outcome of the dysfunction can be answered by animal studies. Unfortunately, very few human studies in early maltreated individuals have examined neuroanatomical changes that are relevant to drug use (Delavari et al., 2016), and even fewer have characterized the age of abuse onset. Through structural equation modeling, the associations between the age of maltreatment and morphometric MRI changes were determined and believed to reflect times when the region is most sensitive to exposure to adversity (Andersen et al., 2008; Pechtel et al., 2014).
3.1.2 While estimated ages, reductions in the volume of the hippocampus are associated with abuse occurring between 3 and 5 years of age. Reduced hippocampal function results in less regulation of the hypothalamic-pituitary-adrenal axis that modulates glucocorticoid release and more drug craving (Kilts et al., 2001; Volkow et al., 2002). Maltreated individuals have reduced hippocampal size (De Bellis et al., 2000; Stein, 1997). Smaller hippocampal volume in alcoholics (De Bellis et al., 2000) predicts the severity of drug abuse relapse (Van Dam et al., 2014). In a non-abused population, hippocampal volume correlates positively with the early age of onset of alcohol use (De Bellis et al., 2000) and negatively with the duration of an alcohol use disorder. Alcohol use disorder has an accelerated age of onset in teens with a history of physical and sexual abuse (Clark et al., 2003).
3.1.3 Exposure to maltreatment between 10 and 11 years of age has maximal effects on the size of the right amygdala (Pechtel et al., 2014). It is within this timeframe that exposure to child maltreatment accelerates negative amygdala-prefrontal cortex connectivity causing it to attain maturity earlier than age-matched controls (Gee et al., 2013). Reduced amygdala volume is associated with increased cocaine use in adults (Mei et al., 2015) probably due to its role in forming the associations between drug and formally neutral cues involved in relapse (See et al., 2003; Volkow et al., 2002).
3.1.4 The sensitive period for the effects of sexual abuse in the prefrontal cortex occurs between 14 and 16 years of age. The prefrontal cortex plays a significant role in reward valuation and regulating risky behaviors. For example, the prefrontal cortex modulates impulsivity, novelty preferences, and responses to drug-associated cues in humans (Casey and Jones, 2010). Together, the timing of maltreatment is associated with selective vulnerability of brain regions. The timing of maltreatment results in different behavioral outcomes that include addiction. Personalized scripts that are read during an fMRI assessment determine activation (or deactivation) responses to drug-associated cues. Cocaine-dependent men with a history of childhood maltreatment have increased left dorsolateral prefrontal cortex activation in response to drug cues and decreased regulatory processes compared with cocaine-dependent men without abuse (Elton et al., 2015).
3.2. Preclinical
Animal studies enable the study of timing of stress exposure as it relates to the onset of drug addiction. Of equal importance, the maturation of the brain after the stress experience interacts with the original experience to delay or accelerate the emergence of drug addiction. A number of brain regions that are affected by early stress are associated with delayed outcomes. The majority of subjects in early stress models are tested in adulthood and not immediately after the end of their stress exposure. As a result we know the endpoint of stress exposure, but little about the process across age. Finally, the direct relationship between the brain area and addiction-related behavior has not been assessed as directly as those found in human MRI studies.
3.2.1 The expression of synaptophysin, a measure of synaptic density, in the hippocampus in rats exposed to maternal separation (4 h/day P2-20) fails to undergo normal overexpression of synapses during adolescence relative to control rats (Andersen and Teicher, 2004). Chronic cold stress (4° between P7-27) in mice decreased hippocampal expression of glutamate-related proteins of the excitatory amino-acid transporter-3 (EAAT-3), the glial glutamate transporter-1 (GLT-1) and increased glutamate aspartate transporter (GLAST) levels (Odeon et al., 2015). These stressed mice also had greater alcohol consumption. Overall, the effect of early stress on the hippocampus as it relates directly to addictive behaviors is minimally characterized.
3.2.2 The amygdala is involved in anxiety responses that increase reward value via negative reinforcement (Aston-Jones and Harris, 2004). Rats exposed to maternal separation are anxious. They spend more time in the closed arm of the elevated plus maze and show greater preferences for alcohol (Huot et al., 2001; Kalinichev et al., 2002). Alcohol consumption is negatively associated with Pro-opiomelanocortin (POMC) expression in the amygdala in maternally separated (360 min/day) rats (Granholm et al., 2017).
3.2.3 Synaptic density in the prefrontal cortex is not significantly affected by maternal separation until adulthood in the rat (Andersen and Teicher, 2004). No age differences were observed in synaptophysin between maternally separated males and age-matched controls between the ages of P20-80. A 16% increase in synpatophysin was observed at P100 in the maternally separated rats. The prefrontal cortex is involved in the risk behavior of impulsivity (Dalley et al., 2008; Sonntag et al., 2014; St Onge and Floresco, 2010; Winstanley et al., 2005). Glutamatergic projections from the dorsal prefrontal cortex to the accumbens core are necessary for the expression of behavioral sensitization to cocaine (Pierce et al., 1998). The dopamine D1 receptor in the prefrontal cortex is important for motivational effects of drug-related cues (Weiss et al., 2001). The D1 receptor is overproduced on glutamatergic projects from the prefrontal cortex to the accumbens in normal animals (Brenhouse et al., 2008), but reduced in animals that were maternally separated (Brenhouse et al., 2013). The decrease in D1 receptors on glutamatergic afferents is associated with anhedonia (Freund et al., 2016), a risk factor for addictive behaviors. Maternally separated animals tested in a dark-cold living conditions as adults exhibit reduced motivation to obtain sucrose in a progressive ratio schedule (Ruedi-Bettschen et al., 2005).
While the majority of studies fail to find changes in motivational properties of cocaine cues following early life stress (e.g. (Matthews et al., 2001),), one possibility is that an adolescent stressor is needed to produce prefrontal cortex mediated effects. Decreased cortical activity or volume is observed in both abused humans (Andersen and Teicher, 2008b) and in adolescent rats that underwent social isolation (Hall, 1998; Leussis et al., 2008). Investigations show that later stress increases addictive behaviors that are associated with cortical function. Adolescent stress models include social isolation and social defeat stress. As an example, rats that were isolation reared as adolescents, but not maternally deprived, show locomotor sensitization to intermittent 1.5 mg/kg of amphetamine (Miczek et al., 2008). Notably, early stress rats will initiate drug use earlier than controls due to the influence of accelerated development of the other brain regions influencing the development of the prefrontal cortex.
3.3. Integration of clinical and preclinical findings
Sensitive periods and their relationship to early stress exposure and drug use open the window to a relatively new approach to treatment. By knowing the age of the stress exposure, the promise of sensitive periods lies in the optimal time for intervention and possibly, the type of drug to target addiction. Clinical investigations have not paid enough attention to the age of abuse; preclinical studies have missed the opportunity to determine relationships between age, duration, and type of stressor and how they influence risk behaviors and subsequent drug use. Both clinical and preclinical studies have similar decreases in hippocampal volume and adolescent effects of stress in the prefrontal cortex. Stress-induced increases in drug-taking will emerge once the cortex reaches a threshold level of maturity. Complementary clinical and preclinical studies can determine whether stress-induced brain changes are a cause or consequence of stress and how perturbation of one region can effect the development of another.
4. Mechanisms of sensitive periods of stress exposure
Critical/sensitive periods are defined by changes in growth factors, such as brain-derived growth factor (BDNF; or its receptor, tropomyosin receptor kinase B [TrkB]), and the GABAergic fast-spiking interneuron that expresses the calcium-binding protein of parvalbumin (PV) (Huang et al., 1999; Morishita et al., 2015a). A decrease in these factors signals the initiation of a sensitive period. Decreased inhibition allows excitatory activity to shape the development of synapses (Toyoizumi and Miller, 2009) and in essence, tips the balance towards more excitatory activity. Elevated glutamatergic activity is associated with more dendritic branching, increased signaling, and general plasticity. This glutamatergic overproduction is followed by pruning and a rise of PV and BDNF/TrkB levels. Together low excitatory and higher inhibition that closes the sensitive period resulting in less plasticity. The final stage occurs when perineuronal nets (PNNs) surround the PV neurons, thus protecting the synapse and consolidating the information (Ye and Miao, 2013).
In addition, increases in the GABA-Aα1 receptor subtype close sensitive periods in concert with rising PV levels, as exemplified by changes in the visual system (Fagiolini et al., 2004; Fagiolini and Hensch, 2000). GABA-Aα1 receptors are fast-acting ionotropic receptors. Similarly, both PV and GABA-Aa1 rise in concert in the non-human primate PFC (Hoftman et al., 2015). GABA-A levels are reduced in the amygdala of adult MS subjects (Caldji et al., 2000), although the age that this first occurs is not known. Injections of the GABA-Aa1 agonist muscimol into the amygdala are associated with more anxiety-like behavior (Raineki et al., 2012).
4.1. The role of GABA and BDNF in externalizing and internalizing behaviors
4.1.1. Clinical
Both GABA and BDNF are involved in developmental plasticity that shapes the trajectory of brain development (discussed in more detail below). The results from studies that examine GABA and behavior are dependent on which region of the brain was examined. For an excellent review of GABA changes and impulsivity, see (Hayes et al., 2014). Generally, low GABA levels (as measured with magnetic resonance spectroscopy) characterize externalizing behaviors. Low cortical GABA is found in individuals characterized as having a more cognitive impulsive phenotype (versus motor impulsivity (Silveri et al., 2013)), aggressive behavior (Ende et al., 2015), and reduced fear-associated behavior (Nagamitsu et al., 2015). However, increased GABA levels have been reported in conduct disorder (Dalsgaard et al., 2014). Elevated impulsivity in borderline personality disorder and ADHD were associated with elevated cortical GABA (Ende et al., 2015). Changes in BDNF levels are found in individuals with addiction (Chen et al., 2014).
4.1.2. Preclinical
Adult rats characterized as highly impulsive with the 5-choice serial reaction task (a task of motor impulsivity) have lower GABA-Aα1 receptor binding in the anterior cingulate cortex, thus disinhibiting outgoing activity (Jupp et al., 2013). Similarly, infusions of muscimol, a GABA-Aα1 agonist into the infralimbic prefrontal cortex (Murphy et al., 2012) and the prelimbic region (Feja and Koch, 2014) increased impulsivity. Synthesis inhibition of GABA in the medial prefrontal cortex increases impulsivity (Paine et al., 2015). In contrast, increased GAD-67 expression in the nucleus accumbens is lower in impulsive rats (Caprioli et al., 2014; Sawiak et al., 2016). Aggressive behavior in rodents is characterized by low GABA levels measured with 1H-MRS GABA (a 40% decrease) in the ventral anterior cingulate cortex (Jager et al., 2017) and measured directly in the lateral hypothalamus (Haller and Kruk, 2006). Other drug abuse risk behaviors (novelty preferences, social deficits) are enhanced by developmental stress exposure and are associated with increased glutamate activity and lower GAD-67 expression (Tzanoulinou et al., 2014); these results suggest a protracted sensitive period (see below). Changes in GABA that are related to depressive behavior also exist. Both sexes show reduced GABA in teenagers (Gabbay et al., 2012) and in PV in animals (Brenhouse and Andersen, 2011; Leussis et al., 2012; Lukkes et al., 2017) with anhedonia. Furthermore, TrkB levels correlate with the degree of depressive-like behavior (Lukkes et al., 2018).
In terms of drug abuse, non-contingent cocaine exposure in adolescent, but not adult, rodents produced an enduring attenuation of medial prefrontal GABAergic activity and PV cell expression, which is postulated to increase the risk of SUD long-term (Cass et al., 2013). This hypothesized increase vulnerability would be further exacerbated by stress-induced losses of PV.
4.2. The role of early life stress in sensitive periods
4.2.1. Parvalbumin changes
Different types of stress (probably those that are more relevant to the maturational stage) will produce unique effects on behavior and brain development. Exposure to chronic variable stress increases cortical GABA levels (McKlveen et al., 2016) while social isolation stress has no effect on PV (Lukkes et al., 2017). Parvalbumin undergoes significant changes during the adolescent period (Caballero and Tseng 2016a, 2016b). Exposure to juvenile stress reduces PV into adulthood, but also alters activity at GABAergic gene promoters including dysregulated DNA methylation, histone modification, and change promoter-enhancer interactions (Morishita et al., 2015b).
Recent work compared two different stress periods: early life with the maternal separation paradigm and a social isolation paradigm in females only. Previous studies have established that these two stress paradigms produce similar corticosterone levels (Vargas et al., 2016). We found that early life stress, but not social isolation, increased anhedonia (Lukkes et al., 2018). PV and TrkB levels were significantly reduced in the prelimbic cortex following early exposure, whereas adolescent exposure had no effect on these measures. However, social isolation decreased the percentage of PV neurons that were encapsulated by Wisteria floribunda agglutinin (WFA; e.g., perineuronal nets) (Ueno et al., 2017). Decreased PV/WFA suggests a vulnerable sensitive period to adolescent stress. Part of this vulnerability may be related to increased reduction-oxidation reactions that might occur following stress exposure during development. To test this hypothesis, mice with glutathoine synthesis gene Gclm knock out that produces oxidative stress were examined for changes in PV. Under these re-dox conditions, the dopamine transport blocker GBR-12909 suppressed co-expression of PV and WFA in the cortex (Cabungcal et al., 2013). These data suggest that under conditions of increased oxidative stress, exposure to a stressful challenge (i.e., elevated dopamine in the prefrontal cortex) reduces the perineuronal nets that surround PV neurons, thereby increasing plasticity.
Decreases in PV levels have been associated with reduced working memory in rats exposed to early life stress (Brenhouse and Andersen, 2011) and depressive effects (Leussis et al., 2012; Lukkes et al., 2018). Specifically, levels of anhedonia correlated with PV levels in the prefrontal cortex (r = 0.93; n = 9) in early stress animals, but not controls (Lukkes et al., 2018). Together, these data are consistent with a prolonged sensitive period where the environment affects cortical plasticity longer than in control animals. Other studies have found that PV loss occurred earlier in female MS rats (Holland et al., 2014), while one study of MS (Seidel et al., 2008) failed to find stress-induced changes in PV.
4.2.2. Gonadal hormones
The effects on PV are regionally dependent and hormone-specific (Sotonyi et al., 2010). By adulthood, females have more PV neurons in the amygdala and medial prefrontal cortex than males (Rowniak et al., 2015; Wischhof et al., 2015). ERβ receptors co-localize exclusively on PV neurons in layer V in PFC (Blurton-Jones and Tuszynski, 2002; Kritzer, 2002), with less expression of ERα on BDNF containing neurons (Blurton-Jones and Tuszynski, 2006). Estrogen increases general neuronal loss between puberty (P35) and adulthood (P90) in the prefrontal cortex of rats (Juraska and Markham, 2004; Markham et al., 2007). Pruning of synapses and PV are prevented by ovariectomy before puberty (Wu et al., 2014). Intracellular androgen receptors are neither detected on PV neurons nor are PV neurons affected by gonadectomy in males; instead androgen receptors are predominantly localized to pyramidal cells in the PFC (Kritzer, 2004). However, androgens predispose males to GABA-A mediated excitoxicity (Nunez and McCarthy, 2008).
4.3. Brain-derived growth factor (BDNF)
Changes in the methylation status of BDNF alter susceptibility to depression that is associated with early life stress (Roth et al., 2009). DNA methylation of BDNF from the saliva BDNF changes in the human brain (Smith et al., 2015). BDNF polymorphisms have been associated with increased risk for depression in individuals with a history of abuse (Aguilera et al., 2009) and even more so if the serotonin transporter polymorphism is present. In animals, investigation into the ValMet polymorphism that decreases the effectiveness of BDNF interacts with sex and age and the type of stressor (reviewed in (Bath et al., 2013)). With a direct examination of rats that underwent maternal separation, changes in BDNF or its receptor, TrkB, correlate with depressive-like behavior (Lukkes et al., 2018). Overall, exposure to maternal separation reduced levels of TrkB in the amygdala and the prelimbic prefrontal cortex were found in animals that had depressive-like behavior, but was elevated in behaviorally ‘resilient’ stressed rats. The elevation of TrkB levels discussed above could reflect a different group of rats that are resilient or an externalizing group. When maternal care was low during the first few days of life, amygdala BDNF levels showed early developmental decreases relative to controls (Macri et al., 2010). We have recently found that mature BDNF analyzed from saliva reflects changes in prefrontal cortex BDNF levels of young adolescent, but not adult, animals (Jordan and Andersen, 2018). Cortical BDNF in young adolescents correlates with increased reinstatement to cocaine, whereby low BDNF is associated with more cocaine intake (Jordan and Andersen, 2018). Future studies may be able to track early BDNF changes by non-invasively collecting saliva.
4.4. Sensitive periods and their relationship to addiction
The role that the neurochemistry of sensitive periods has relative to vulnerability to addiction is not well understood. The above discussion of the primary players (PV, BDNF/TrkB, and glutamate) have on opening and closing sensitive periods should be taken into account for future studies.
5. Reward reactivity, arousal, and inflammation as mediating factors in stress outcomes
5.1. Reward sensitivity and addiction
5.1.1. Clinical
Reactivity to reward is a moderating variable for depressive behavior in a maltreated population (Dennison et al., 2016). Exposure to early life stress markedly increases the risk for addiction to drugs of abuse (Andersen and Teicher, 2008a) in part by increasing reward insensitivity (Pechtel and Pizzagalli, 2013). Reward insensitivity is found in both externalizing and internalizing disorders. Further, reward deficits are associated with increased aggression in humans (Dodge et al., 1990; Fergusson et al., 1996).
Reduced salience in events that are normally rewarding could produce reward insensitivity. Reward salience is partially mediated by the dopamine D1 receptor in the prefrontal cortex. The DRD1 gene is associated with behaviors found in conduct disorder: males show increased aggression (Pardini et al., 2014) and girls initiate sexual activity early and have more of it (Miller et al., 1999). Childhood maltreatment is also common to both boys and girls with conduct disorder (Elkins et al., 2007; Garwood et al., 2015; Keenan et al., 1999) suggesting that early life experiences interact with the DRD1 to elevate the risk of addiction in this population. The DRD1 has been linked to aggression and sexual behavior (Miller et al., 1999; Pardini et al., 2014).
5.1.2. Preclinical
A key characteristic of early life stress exposure in animal models is altered reward processing. Maladaptive social relationships and increased drug use following exposure to early adversity can be partially explained by a dysfunctional reward system. Social behavior is normally rewarding through its actions on the accumbens (Gordon et al., 2002; Trezza et al., 2014; van Kerkhof et al., 2013). Under normal conditions, social interactions peak during adolescence before declining in adulthood (Panksepp and Beatty, 1980; Pellis and Pellis, 1990; Vanderschuren et al., 1995). Elevated offensive play is observed by juvenility in male separated rats, whereby these subjects show increases in nape attacks, offensive pulling and biting, with a decrease in submissive play (Veenema and Neumann, 2009). By adulthood, exposure to maternal separation increases aggression compared with controls (Haller and Kruk, 2006).
We, and others (Hostetler et al., 2011), suggest that transient over-expression of D1R in the cortex programs behavioral change to yield persistent changes into adulthood. Our data support the hypothesis that reduced reward function can be explained by a loss of D1 receptors on the prelimbic prefrontal cortex projections to the nucleus accumbens pathway in early life stress males (Brenhouse et al., 2013). Play studies using cortical microinjections (Trezza et al., 2014) or systemic D1 agonists/antagonists (Gariepy et al., 1998) show D1 involvement. Therefore, less salience attributed to social relationships may explain reward insensitivity in individuals with an early life stress history. In contrast to this D1R reduction in separated males, the majority of glutamatergic projection neurons have elevated D1 receptors (Brenhouse et al., 2008). Increased prefrontal expression of the D1R increases sexual activity (Freund et al., 2016), cocaine seeking, sensitivity, and motivation to take more cocaine (Sonntag et al., 2014). Increased D1R expression is likely on prelimbic connections to the insula, amygdala and lateral hypothalamus amongst other regions (Sesack et al., 1989).
5.2. Arousal and addiction
5.2.1. Clinical cortisol
Child maltreatment has further been associated with long-term changes in hypothalamic—pituitary—adrenal (HPA) axis function (Tarullo and Gunnar, 2006). Initial reports of elevated cortisol levels following maltreatment in adult women (Heim et al., 1997) may be more reflective of the control group than maltreatment. The majority of studies in the last decade report decreased levels of cortisol in response to a stressor in individuals with a history of early life stress and externalizing symptoms and elevated cortisol in those with internalizing symptoms (Cicchetti and Rogosch, 2001; Hinkelmann et al., 2013). Early life stress was associated with an earlier onset of puberty than controls and a blunted cortisol awakening response compared to lower early life stress (King et al., 2017). The later onset of puberty predicted the opposite for cortisol responses.
While this suppression in responsiveness is an adaptive response to chronic stress exposure, low cortisol levels have been associated with increased addiction rates (reviewed by (Sinha, 2008)) and aggressive behavior (Fishbein et al., 1992; Hawes et al., 2009). Further, humans with antisocial behavior have low levels of cortisol and a greater risk for drug use (Hawes et al., 2009). Studies have shown that high maternal aggression in humans changes the testosterone-cortisol relationship from inverse (more testosterone, less cortisol) to positive in boys (more of both), and reduces the relationship in girls (Simmons et al., 2015). The former is a characteristic found in boys with conduct disorder.
5.2.2. Peripheral measures of stress and arousal
5.2.2.1. Clinical
A well-established literature of arousal and motivation exists, whereby individuals are motivated to seek an optimal level of arousal (Yerkes-Dodson) and emotional arousal enhances perception of salient information; arousal also heightens the ability to learn (Berlyne, 1969). While most research is focused on the effects of cortisol/corticosterone, measures of sympathetic arousal include galvanic skin response (GSR) and heart rate variability (HRV) that can be acquired non-invasively, which increases the likelihood of consent. More importantly, GSR and HRV are lower in a subgroup of children with high aggression (Lorber, 2004; Maliphant et al., 1990; Oosterlaan et al., 2005; Raine et al., 1997) and in a subgroup of teens that are high-risk for substance use (van Bokhoven et al., 2005). In other words, these children do not become aroused in the same way as normal children because their HRV is inversely related to aggression. Not surprisingly, children with low levels of early life stress in challenging situations may be insensitive to social cues or negative social consequences due to low arousal levels (Gatzke-Kopp et al., 2002). Low GSR reflects insensitivity to perceived negative consequences, which may underlie proactive aggression and antisocial behaviors (Erath et al., 2011; Scarpa and Raine, 1997).
5.2.3. Preclinical
Animals with an early stress history have reduced glucocorticoid (Yusko et al., 2012; Yusko et al., 2012) in (some), but not all, cases (Dettling et al., 2002; Haller and Kruk, 2006). However, elevated plasma levels of corticosterone have been reported (Veenema and Neumann, 2009). Corticosterone administration at high physiological range for young rats (10 mg/kg i.p. between P2-20 (Alonso, 2000; Zahorodna et al., 2000) reduces PV levels in the hippocampus. The loss of synapses, in general, is similar to our observations with our maternal separation paradigm (Brenhouse and Andersen, 2011; Leussis et al., 2012; Lukkes et al., 2017). Reduced corticosterone levels produced by metyropone treatment (50 μg/g, ip 30 min) decreases over eating in maternally separated rats suggestive of metabolic effects as well (Murphy et al., 2017).
The effects of early life stress further interact with temperament. In studies examining rats bred for high or low novelty behavior, low novelty rats developed an exaggerated corticosterone response to challenge (Clinton et al., 2014). In contrast, high novelty rats show a blunted corticosterone response; these animals are also more aggressive. However, at this stage we do not know what influences arousal levels other than modulation by the dam (Zhang et al., 2006).
5.2.4. Gonadal hormones
An inverse relationship between corticosterone and gonadal hormones (Elias et al., 1981) emerges during adolescence (Dismukes et al., 2015) that may further explain increased aggression. As corticosterone falls, testosterone is predicted to rise. High maternal aggression in humans changes this testosterone-cortisol relationship to positive in boys, and reduces the relationship in girls (Simmons et al., 2015). For example, an inverse relationship between corticosterone and gonadal hormones emerges during adolescence normally (Dismukes et al., 2015; Elias et al., 1981).
5.3. Inflammation and addiction
5.3.1. Immune effects
Elevated neuroimmune function has been found in individuals with addiction (Crews et al., 2017; Frank et al., 2011), depression (Lindqvist et al., 2009), schizophrenia (Behrens and Sejnowski, 2009), PTSD (Gill et al., 2008), exposure to adversity (Carpenter et al., 2010; Ganguly and Brenhouse, 2015), and a host of other conditions. The elevated inflammation described in drug users is usually interpreted as secondary to the harmful effects of drug and alcohol use and have been expertly reviewed (Crews et al., 2006; Harricharan et al., 2017; Lacagnina et al., 2017). Here, we posit that a pre-existing condition of inflammation due to early life stress will increase the vulnerability to develop as substance use disorder (Frank et al., 2011).
The immune system in the brain is comprised of astrocytes and microglia, both of which release proinflammatory cytokines. Initial studies showed that conditioned media derived from either of these cells reduced morphine and oxycodone place preferences (Narita et al., 2006). Microglia and astrocytes express Toll-like receptors (TLRs). The TLR3, TLR4, TLR5, and TLR9 (Farina et al., 2007). The TLRs are increased following exposure to alcohol (Crews et al., 2017). Adolescent morphine exposure increases TLR4 on microglia within the nucleus accumbens (Schwarz and Bilbo, 2013). Alcohol increased expression of the astrocytic marker, glial fibrillary acidic protein, has been described three weeks post exposure (Bowers and Kalivas, 2003). Increases in the proinflammatory molecule cyclooxygenase 2 (COX-2) or IL-6 are observed in maternally separated rats; these effects can be reduced with the anti-inflammatory agent IL-10 (Brenhouse and Schwarz, 2016). Exposure to early life stress will further enhance vulnerability to drugs of abuse by keeping the window of enhanced plasticity open.
Within a sensitive period framework, changes in neuroimmune function can influence developmental processes in three ways. First, normal synaptic pruning processes during maturation that are mediated by microglia (Schafer et al., 2012; Schwarz and Bilbo, 2012) would be further increased following early life stress. During increased inflammation, microglial and other phagocytic markers engulf ∼30% of the cells (Moir et al., 1992), which may further promote pruning. Microglia activation works in part by increasing intracellular calcium levels that lead to elevated secretion of IL-6, TNFα, and nitric oxide (Farber et al., 2009). IL-6 has also been shown to elevate BDNF in hippocampal neurons (Kairisalo et al., 2009). In other words, these data suggest IL-6 could play a role in closing the sensitive period early, leaving PV in an immature, low state. The other two mechanisms involve glutamate and GABA, or the excitatory/inhibitory balance. Pro-inflammatory cytokines increase glutamate activity, decrease GABA receptors, and up-regulate AMPA/NMDA expression during opioid reward processes (Hutchinson and Watkins, 2014). Elevated IL-6 levels are found in patients with depression (Carpenter et al., 2010).
Second, cytokine-induced increases in glutamate activity would further alter plasticity by shifting the excitatory/inhibitory balance. Cytokine release increases glutamate levels by reducing the glutamate transporter 1 (GLT-1)/excitatory amino acid transporter-2 (EAAT2) and GLAST (glutamate and aspartate transporter), excitatory amino acid transporter-1 (EAAT1) (reviewed in (Harricharan et al., 2017)). We have found increased NR2a receptor expression in the prefrontal cortex of adolescent male rats exposed to maternal separation relative to controls (Wieck et al., 2013). Elevated NR2a has been observed following exposure to nicotine (rats (Adriani et al., 2004) and humans (Wang et al., 2007)), cocaine abstinence (Ben-Shahar et al., 2009), and prenatal alcohol (Nixon et al., 2002). Local microinjections of the anti-inflammatory cytokine IL-10 decrease the NR2a and have no effect in controls, showing neuroimmune involvement (Wieck et al., 2013).
Third, decreased inhibition by a loss of GABA cells would further elevate glutamate activity (Brenhouse and Andersen, 2011; Leussis et al., 2012; Lukkes et al., 2017). Parvalbumin neurons are decreased following exposure to maternal separation due to elevated levels of interleukin-6 (IL-6 (Wieck et al., 2013);). The source of IL-6 could be derived from the periphery, microglia (Schwarz and Bilbo, 2012), or neurons themselves (Lemke et al., 1998). IL-6 has been shown to mark PV cells as “sick” for later demise (Behrens and Sejnowski, 2009; Dugan et al., 2009). IL-6 is elevated in the plasma and prefrontal cortex of MS rats relative to control rats (Wieck et al., 2013), suggesting that these effects are locally mediated. Plasma levels of IL-1β have been reported to be elevated in MS males at P40 (Wieck et al., 2013), however, this effect was neither replicated in males nor found in females (Grassi-Oliveira et al., 2016). The brain cytokines, IL-1β and IL-2, can modulate rage either by acting through the 5-HT2 receptors or GABAA, respectively (Siegel et al., 2007).
5.3.2. Gonadal hormones
Microglia are affected by testosterone (Garcia-Ovejero et al., 2002), which is reduced in MS rats (Grassi-Oliveira et al., 2016). Decreased testosterone levels are associated with less anti-inflammatory IL-10, providing even less anti-inflammation protection in separated males. If GABA levels were to decrease and leave the neurons exposed to greater glutamate activity, both of which has been observed in MS rats (Wieck et al., 2013), then female microglia are likely to shift to the pro-inflammatory (M1) state more so than males (Schwarz and Bilbo, 2012).
6. Prevention approaches
6.1. Timing of intervention
During human adolescence the trajectories between individuals exposed and not exposed to early life stress diverge. Abused and non-abused children begin to separate in developmental trajectories at <11 years of age (Widom et al., 2007), before the onset of puberty. Evidence for a delay in the manifestation of early life stress exists in both humans (Teicher et al., 2009) and in animals (Andersen and Teicher, 2004; Brenhouse et al., 2013). Adolescence is also the stage when elevated amygdala activity is observed in children with an early life stress history (Malter Cohen et al., 2013; Marusak et al., 2015).
While early stress exposure increases vulnerability, it also important to recognize that this same interaction may also underlie the ideal timing for a preventative intervention (Andersen and Teicher, 2008b; McCrory et al., 2017). In both humans and animals, reduced hippocampal volume is associated with early life stress, whereas prefrontal changes are associated with adolescent stress (Andersen and Teicher, 2004; 2008b; Andersen et al., 2008); adolescence is also the stage when changes associated with early stress exposure become evident (Brenhouse and Andersen, 2011). If stress exposure can accelerate the emergence of depression (Teicher et al., 2009; Widom et al., 2007), it is likely that stress will accelerate the initiation of drug use (Hyucksun Shin, 2012). Earlier initiation is associated with greater duration of addiction (McGue et al., 2001; Wagner and Anthony, 2007). One likely reason for this elevated vulnerability is due to the specificity of stress exposure on unique regions during a sensitive period (Andersen et al., 2008).
6.2. Social buffering
Reinforced, positive social behavior influences later behavior by shaping the development of the prefrontal cortex to respond to social cues (Bell et al. 2009, 2010), increase behavioral flexibility (Himmler et al. 2013, 2014), and modulate physiologic responsiveness (Hofer and Shair, 1982). Other brain regions also play a role in this process, but are likely to receive activity from the prelimbic cortex to influence the behavioral outcome. Evidence of the impact of social interaction on vulnerability to mental illness, addiction, or even neuroimmune challenge is observed at the clinical and preclinical level.
Differences in parenting styles support this position. The Romanian orphan study is a superb example of how children with a significant history of early life stress (Johnson et al., 1992) show low cortisol levels, ADHD, and peer difficulties and have fewer problems when placed into foster home (Pitula et al., 2017). Rodent studies have shown that different levels of social buffering are evident in differences in maternal care. Differences in high/low-LG dams (reviewed above) or under experimental conditions of low maternal care found in the maternal separation paradigm or communal nesting (Macri et al., 2010) reduces social interaction later in life (Veenema and Neumann, 2009). Modulation of passive behavior demonstrated by guinea pigs in the absence of the mother can be remediated by the presence of the dam and mimicked by lipopolysaccharide (a challenge that engages an immune response) (Hennessy et al., 2013). The dam further buffers maternal separation effects on sexual behavior (Popoola and Cameron, 2018).
Peer support is also important, especially during adolescence. Social isolation during this time is associated with greater depression, drug use, internet use, and other disruptive behaviors in humans (Ellickson and Hays, 1992; NIDA, 2007) and in animals (Eitan et al., 2017; Lesscher et al., 2015; Morgan et al., 2002). The loss of peer support in rats is associated with more anhedonic behavior and corticosterone, although these effects are reduced in rats with a maternal separation history (Biggio et al., 2014; Lukkes et al., 2017).
6.3. Existing pharmacotherapies
Prevention is already possible with medications that are currently available when their use is optimally timed. Enduring reductions in substance use are found in adolescents who were treated in childhood in a subpopulation of individuals with ADHD (Mannuzza et al. 2003, 2008; McCabe et al., 2016; Wilens et al., 2003). However, not all studies, including the Multimodal Treatment of ADHD study, observe an overall reduction in drug use after treatment (Hechtman et al., 2016; Hinshaw and Arnold, 2015). Animal studies support the findings that preventative effects depend on timing. Juvenile exposure to methylphenidate in rats (P20-35) produces an aversion to cocaine-associated environments. In contrast, post-pubertal exposure (P50-65) produces a place preference for cocaine (Andersen et al., 2002). Cocaine self-administration is reduced following methylphenidate treatment from P30-240 (Thanos et al., 2007) and is increased in animals exposed later to methylphenidate (P35-60 (Brandon et al., 2001);) or earlier (Crawford et al., 2011).
6.4. Novel pharmacotherapies
Preventative interventions to reduce the effects of early life stress on brain development should capitalize on a sensitive period of development for its timing and its biological basis. In human (Teicher et al. 2006, 2009) and in animal studies, early life stress exposure does not manifest until adolescence (reviewed (Andersen and Teicher, 2008b)). Treatment prior to the emergence of symptoms therefore would be aimed at reducing inflammation, decreasing glutamate activity, or increasing GABA/PV and/or BDNF levels. An intervention with COX-2 inhibitor (an anti-inflammatory) reduces elevated COX-2 levels and IL-6 in maternally separated animals (Brenhouse and Andersen, 2011). Exposure to a COX-2 inhibition reduced stress-induced working memory impairment (Brenhouse and Andersen, 2011) and depressive-like behavior (Lukkes et al., 2017). Anti-inflammatory treatment can reduce depressive-like behaviors (Brenhouse and Andersen, 2011; Hennessy et al., 2011; Lukkes et al.; Sukoff Rizzo et al., 2012). Exposure to a probiotic diet would also decrease inflammation (de Timary et al., 2017). Probiotics would also aid in sleep and social interaction because of feeling better.
Also within a sensitive period manipulation, increasing BDNF levels by antidepressants (Duman and Monteggia, 2006; Shirayama et al., 2002) can provide some degree of improvement. Finally, reducing glutamatergic activity with treatment with adinazolam and MK-801, but not by augmenting serotonin activity with tianeptine (Leussis et al., 2008) was shown to reduce the effects of a social stressor in rats (see Fig. 1, Fig. 2).
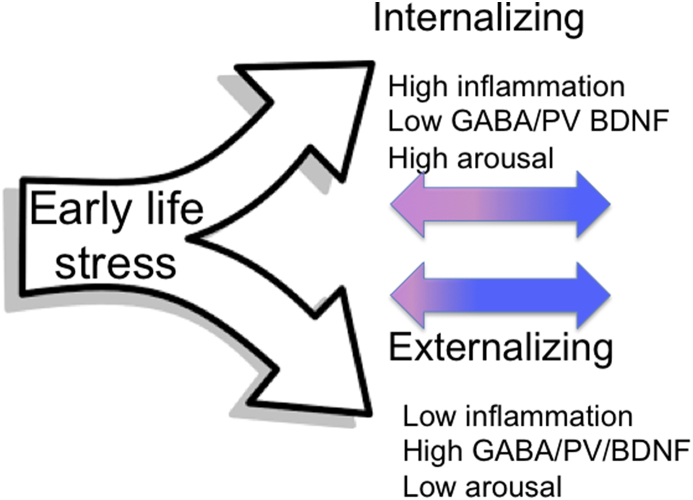
Fig. 1.
The outcomes of early life stress exposure on behavior. The arrows represent the prevalence of gender effects (pink for females, blue for males) for internalizing and externalizing disorders. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
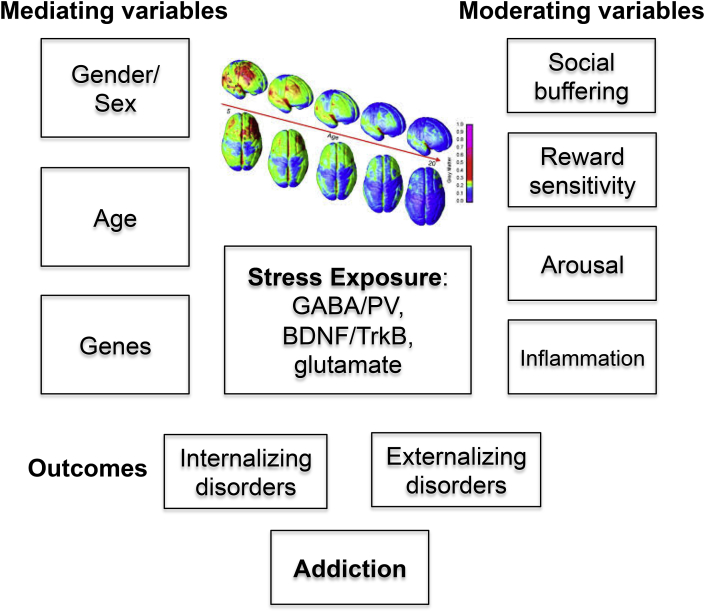
Fig. 2.
The mediating and moderating variables of the outcomes of early life stress exposure. These factors interact with sensitive periods of brain development (color picture shows the progressive pruning processes in the brain (Gogtay et al., 2004)) by altering levels of parvalbumin (PV), BDNF and its receptor TrkB, and glutamate levels to shape the adult brain. The behavioral outcomes of internalizing and externalizing disorders increase the vulnerability to develop addiction. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
7. Conclusions
Early life stress exposure interacts with sensitive periods of development to permanently alter the trajectory of behavior and biochemistry. Exposure to stress early in postnatal life is associated with an accelerated age of onset for drug use, but also greater vulnerability to addiction. A number of moderating and mediating factors are discussed. The factors of arousal levels, inflammation, and the timing of stress exposure influence the outcome of early life adversity. By carefully timed interventions we may be able to prevent the cascade of adverse consequences of child maltreatment.
Declarations of interest
None.
Acknowledgments
This work was supported by the National Institutes of Health (DA-015403 and DA-026485) and the Simches Family.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2018.100140.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adriani W., Granstrem O., Macri S., Izykenova G., Dambinova S., Laviola G. Behavioral and neurochemical vulnerability during adolescence in mice: studies with nicotine. Neuropsychopharmacology. 2004;29:869–878. doi: 10.1038/sj.npp.1300366. [DOI] [PubMed] [Google Scholar]
- Aguilera M., Arias B., Wichers M., Barrantes-Vidal N., Moya J., Villa H., van Os J., Ibanez M.I., Ruiperez M.A., Ortet G., Fananas L. Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychol. Med. 2009;39:1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Anda R.F., Felitti V.J., Bremner J.D., Walker J.D., Whitfield C., Perry B.D., Dube S.R., Giles W.H. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatr. Clin. Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen S.L. Exposure to early adversity: points of cross-species translation that can lead to improved understanding of depression. Dev. Psychopathol. 2015;27:477–491. doi: 10.1017/S0954579415000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L., Arvanitogiannis A., Pliakas A.M., LeBlanc C., Carlezon W.A., Jr. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat. Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Teicher M.H. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Teicher M.H. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci. Biobehav. Rev. 2008;33(4):516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L., Teicher M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Tomada A., Vincow E.S., Valente E., Polcari A., Teicher M.H. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J. Neuropsychiatry. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G., Harris G.C. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Suppl. 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Bath K.G., Schilit A., Lee F.S. Stress effects on BDNF expression: effects of age, sex, and form of stress. Neuroscience. 2013;239:149–156. doi: 10.1016/j.neuroscience.2013.01.074. [DOI] [PubMed] [Google Scholar]
- Beesdo K., Bittner A., Pine D.S., Stein M.B., Hofler M., Lieb R., Wittchen H.U. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch. Gen. Psychiatr. 2007;64:903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Behrens M.M., Sejnowski T.J. Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology. 2009;57:193–200. doi: 10.1016/j.neuropharm.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell H.C., McCaffrey D.R., Forgie M.L., Kolb B., Pellis S.M. The role of the medial prefrontal cortex in the play fighting of rats. Behav. Neurosci. 2009;123:1158–1168. doi: 10.1037/a0017617. [DOI] [PubMed] [Google Scholar]
- Bell H.C., Pellis S.M., Kolb B. Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortices. Behav. Brain Res. 2010;207:7–13. doi: 10.1016/j.bbr.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O., Obara I., Ary A.W., Ma N., Mangiardi M.A., Medina R.L., Szumlinski K.K. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensley L.S., Spieker S.J., Van Eenwyk J., Schoder J. Self-reported abuse history and adolescent problem behaviors. II. Alcohol and Drug Use. J. Adolesc. Health. 1999;24:173–180. doi: 10.1016/s1054-139x(98)00112-8. [DOI] [PubMed] [Google Scholar]
- Berlyne D.E. Arousal, reward and learning. Ann. N. Y. Acad. Sci. 1969;159:1059–1070. doi: 10.1111/j.1749-6632.1969.tb12997.x. [DOI] [PubMed] [Google Scholar]
- Biederman J., Mick E., Faraone S.V., Burback M. Patterns of remission and symptom decline in conduct disorder: a four- year prospective study of an ADHD sample. J. Am. Acad. Child Adolesc. Psychiatr. 2001;40:290–298. doi: 10.1097/00004583-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Biggio F., Pisu M.G., Garau A., Boero G., Locci V., Mostallino M.C., Olla P., Utzeri C., Serra M. Maternal separation attenuates the effect of adolescent social isolation on HPA axis responsiveness in adult rats. Eur. Neuropsychopharmacol. 2014;24:1152–1161. doi: 10.1016/j.euroneuro.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M., Tuszynski M.H. Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. J. Comp. Neurol. 2002;452:276–287. doi: 10.1002/cne.10393. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M., Tuszynski M.H. Estradiol-induced modulation of estrogen receptor-beta and GABA within the adult neocortex: a potential transsynaptic mechanism for estrogen modulation of BDNF. J. Comp. Neurol. 2006;499:603–612. doi: 10.1002/cne.21122. [DOI] [PubMed] [Google Scholar]
- Borrow A.P., Levy M.J., Soehngen E.P., Cameron N.M. Perinatal testosterone exposure and maternal care effects on the female rat's development and sexual behaviour. J. Neuroendocrinol. 2013;25:528–536. doi: 10.1111/jne.12035. [DOI] [PubMed] [Google Scholar]
- Boutros N., Semenova S., Markou A. Adolescent intermittent ethanol exposure diminishes anhedonia during ethanol withdrawal in adulthood. Eur. Neuropsychopharmacol. 2014;24:856–864. doi: 10.1016/j.euroneuro.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers M.S., Kalivas P.W. Forebrain astroglial plasticity is induced following withdrawal from repeated cocaine administration. Eur. J. Neurosci. 2003;17:1273–1278. doi: 10.1046/j.1460-9568.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- Brandon C.L., Marinelli M., Baker L.K., White F.J. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Brenhouse H.C., Andersen S.L. Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol. Psychiatry. 2011;70:434–440. doi: 10.1016/j.biopsych.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H.C., Lukkes J.L., Andersen S.L. Early life adversity alters the developmental profiles of addiction-related prefrontal cortex circuitry. Brain Sci. 2013;3:143–158. doi: 10.3390/brainsci3010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H.C., Schwarz J.M. Immunoadolescence: neuroimmune development and adolescent behavior. Neurosci. Biobehav. Rev. 2016;70:288–299. doi: 10.1016/j.neubiorev.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H.C., Sonntag K.C., Andersen S.L. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J. Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A., Tseng K. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci. Biobehav. Rev. 2016;70:4–12. doi: 10.1016/j.neubiorev.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A., Tseng K.Y. GABAergic function as a limiting factor for prefrontal maturation during adolescence. Trends Neurosci. 2016;39:441–448. doi: 10.1016/j.tins.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal J.H., Steullet P., Kraftsik R., Cuenod M., Do K.Q. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol. Psychiatry. 2013;73:574–582. doi: 10.1016/j.biopsych.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Caldji C., Francis D., Sharma S., Plotsky P.M., Meaney M.J. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Caldji C., Tannenbaum B., Sharma S., Francis D., Plotsky P.M., Meaney M.J. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N., Del Corpo A., Diorio J., McAllister K., Sharma S., Meaney M.J. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PloS One. 2008;3 doi: 10.1371/journal.pone.0002210. e2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N.M., Fish E.W., Meaney M.J. Maternal influences on the sexual behavior and reproductive success of the female rat. Horm. Behav. 2008;54:178–184. doi: 10.1016/j.yhbeh.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Caprioli D., Sawiak S.J., Merlo E., Theobald D.E., Spoelder M., Jupp B., Voon V., Carpenter T.A., Everitt B.J., Robbins T.W., Dalley J.W. Gamma aminobutyric acidergic and neuronal structural markers in the nucleus accumbens core underlie trait-like impulsive behavior. Biol. Psychiatry. 2014;75:115–123. doi: 10.1016/j.biopsych.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter L.L., Gawuga C.E., Tyrka A.R., Lee J.K., Anderson G.M., Price L.H. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J. Am. Acad. Child Adolesc. Psychiatr. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. quiz 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass D.K., Thomases D.R., Caballero A., Tseng K.Y. Developmental disruption of gamma-aminobutyric acid function in the medial prefrontal cortex by noncontingent cocaine exposure during early adolescence. Biol. Psychiatry. 2013;74:490–501. doi: 10.1016/j.biopsych.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F.A., Francis D.D., Mar A., Meaney M.J. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Chauhan P., Widom C.S. Childhood maltreatment and illicit drug use in middle adulthood: the role of neighborhood characteristics. Dev. Psychopathol. 2012;24:723–738. doi: 10.1017/S0954579412000338. [DOI] [PubMed] [Google Scholar]
- Chen P.H., Huang M.C., Lai Y.C., Chen P.Y., Liu H.C. Serum brain-derived neurotrophic factor levels were reduced during methamphetamine early withdrawal. Addict. Biol. 2014;19:482–485. doi: 10.1111/j.1369-1600.2012.00444.x. [DOI] [PubMed] [Google Scholar]
- Cicchetti D., Rogosch F.A. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Dev. Psychopathol. 2001;13:783–804. [PubMed] [Google Scholar]
- Cicchetti D., Rogosch F.A. A developmental psychopathology perspective on adolescence. J. Consult. Clin. Psychol. 2002;70:6–20. doi: 10.1037//0022-006x.70.1.6. [DOI] [PubMed] [Google Scholar]
- Clark D.B., De Bellis M.D., Lynch K.G., Cornelius J.R., Martin C.S. Physical and sexual abuse, depression and alcohol use disorders in adolescents: onsets and outcomes. Drug Alcohol Depend. 2003;69:51–60. doi: 10.1016/s0376-8716(02)00254-5. [DOI] [PubMed] [Google Scholar]
- Clark D.B., Lesnick L., Hegedus A.M. Traumas and other adverse life events in adolescents with alcohol abuse and dependence. J. Am. Acad. Child Adolesc. Psychiatr. 1997;36:1744–1751. doi: 10.1097/00004583-199712000-00023. [DOI] [PubMed] [Google Scholar]
- Cleverley K., Szatmari P., Vaillancourt T., Boyle M., Lipman E. Developmental trajectories of physical and indirect aggression from late childhood to adolescence: sex differences and outcomes in emerging adulthood. J. Am. Acad. Child Adolesc. Psychiatr. 2012;51:1037–1051. doi: 10.1016/j.jaac.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Clinton S.M., Watson S.J., Akil H. High novelty-seeking rats are resilient to negative physiological effects of the early life stress. Stress. 2014;17:97–107. doi: 10.3109/10253890.2013.850670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W., Shanahan L., Miller S., Costello E.J., Angold A., Maughan B. Outcomes of early pubertal timing in young women: a prospective population-based study. Am. J. Psychiatry. 2010;167:1218–1225. doi: 10.1176/appi.ajp.2010.09081190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford C.A., Baella S.A., Farley C.M., Herbert M.S., Horn L.R., Campbell R.H., Zavala A.R. Early methylphenidate exposure enhances cocaine self-administration but not cocaine-induced conditioned place preference in young adult rats. Psychopharmacology. 2011;213:43–52. doi: 10.1007/s00213-010-2011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F.T., Bechara R., Brown L.A., Guidot D.M., Mandrekar P., Oak S., Qin L., Szabo G., Wheeler M., Zou J. Cytokines and alcohol. Alcohol Clin. Exp. Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Crews F.T., Walter T.J., Coleman L.G., Jr., Vetreno R.P. Toll-like receptor signaling and stages of addiction. Psychopharmacology. 2017;234:1483–1498. doi: 10.1007/s00213-017-4560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley J.W., Mar A.C., Economidou D., Robbins T.W. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol. Biochem. Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Dalsgaard S., Mortensen P.B., Frydenberg M., Thomsen P.H. ADHD, stimulant treatment in childhood and subsequent substance abuse in adulthood - a naturalistic long-term follow-up study. Addict. Behav. 2014;39:325–328. doi: 10.1016/j.addbeh.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Daoura L., Haaker J., Nylander I. Early environmental factors differentially affect voluntary ethanol consumption in adolescent and adult male rats. Alcohol Clin. Exp. Res. 2011;35:506–515. doi: 10.1111/j.1530-0277.2010.01367.x. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Clark D.B., Beers S.R., Soloff P.H., Boring A.M., Hall J., Kersh A., Keshavan M.S. Hippocampal volume in adolescent-onset alcohol use disorders. Am. J. Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- de Timary P., Starkel P., Delzenne N.M., Leclercq S. A role for the peripheral immune system in the development of alcohol use disorders? Neuropharmacology. 2017;22:148–160. doi: 10.1016/j.neuropharm.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Delavari F., Sheibani V., Esmaeili-Mahani S., Nakhaee N. Maternal separation and the risk of drug abuse in later life. Addiction & Health. 2016;8:107–114. [PMC free article] [PubMed] [Google Scholar]
- Dennison M.J., Sheridan M.A., Busso D.S., Jenness J.L., Peverill M., Rosen M.L., McLaughlin K.A. Neurobehavioral markers of resilience to depression amongst adolescents exposed to child abuse. J. Abnorm. Psychol. 2016;125:1201–1212. doi: 10.1037/abn0000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent G., Choi D.C., Herman J.P., Levine S. GABAergic circuits and the stress hyporesponsive period in the rat: ontogeny of glutamic acid decarboxylase (GAD) 67 mRNA expression in limbic-hypothalamic stress pathways. Brain Res. 2007;1138:1–9. doi: 10.1016/j.brainres.2006.04.082. [DOI] [PubMed] [Google Scholar]
- Dettling A.C., Feldon J., Pryce C.R. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacol. Biochem. Behav. 2002;73:259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Dismukes A.R., Johnson M.M., Vitacco M.J., Iturri F., Shirtcliff E.A. Coupling of the HPA and HPG axes in the context of early life adversity in incarcerated male adolescents. Dev. Psychobiol. 2015;57:705–718. doi: 10.1002/dev.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney E.R., Elkins I.J., McGue M., Iacono W.G. Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. Am. J. Psychiatry. 1999;156:1515–1521. doi: 10.1176/ajp.156.10.1515. [DOI] [PubMed] [Google Scholar]
- Dodge K.A., Bates J.E., Pettit G.S. Mechanisms in the cycle of violence. Science. 1990;250:1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- Douglas K.R., Chan G., Gelernter J., Arias A.J., Anton R.F., Weiss R.D., Brady K., Poling J., Farrer L., Kranzler H.R. Adverse childhood events as risk factors for substance dependence: partial mediation by mood and anxiety disorders. Addict. Behav. 2010;35:7–13. doi: 10.1016/j.addbeh.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube S.R., Felitti V.J., Dong M., Chapman D.P., Giles W.H., Anda R.F. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Dugan L.L., Ali S.S., Shekhtman G., Roberts A.J., Lucero J., Quick K.L., Behrens M.M. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PloS One. 2009;4 doi: 10.1371/journal.pone.0005518. e5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Monteggia L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duprey E.B., Oshri A., Caughy M.O. Childhood neglect, internalizing symptoms and adolescent substance use: does the neighborhood context matter? J. Youth Adolesc. 2017;46:1582–1597. doi: 10.1007/s10964-017-0672-x. [DOI] [PubMed] [Google Scholar]
- Eitan S., Emery M.A., Bates M.L.S., Horrax C. Opioid addiction: who are your real friends? Neurosci. Biobehav. Rev. 2017;83:697–712. doi: 10.1016/j.neubiorev.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Elias A.N., Gwinup G., Valenta L.J. Effects of valproic acid, naloxone and hydrocortisone in Nelson's syndrome and cushing's disease. Clin. Endocrinol. 1981;15:151–154. doi: 10.1111/j.1365-2265.1981.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Elkins I.J., McGue M., Iacono W.G. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch. Gen. Psychiatr. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Ellickson P.L., Hays R.D. On becoming involved with drugs: modeling adolescent drug use over time. Health Psychol. : official journal of the Division of Health Psychology, American Psychological Association. 1992;11:377–385. doi: 10.1037//0278-6133.11.6.377. [DOI] [PubMed] [Google Scholar]
- Elton A., Smitherman S., Young J., Kilts C.D. Effects of childhood maltreatment on the neural correlates of stress- and drug cue-induced cocaine craving. Addict. Biol. 2015;20:820–831. doi: 10.1111/adb.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G., Cackowski S., Van Eijk J., Sack M., Demirakca T., Kleindienst N., Bohus M., Sobanski E., Krause-Utz A., Schmahl C. Impulsivity and aggression in female BPD and ADHD patients: association with ACC glutamate and GABA concentrations. Neuropsychopharmacology. 2015;41(2):410–418. doi: 10.1038/npp.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erath S.A., El-Sheikh M., Hinnant J.B., Cummings E.M. Skin conductance level reactivity moderates the association between harsh parenting and growth in child externalizing behavior. Dev. Psychol. 2011;47:693–706. doi: 10.1037/a0021909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M., Fritschy J.M., Low K., Mohler H., Rudolph U., Hensch T.K. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Fagiolini M., Hensch T.K. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Farber K., Cheung G., Mitchell D., Wallis R., Weihe E., Schwaeble W., Kettenmann H. C1q, the recognition subcomponent of the classical pathway of complement, drives microglial activation. J. Neurosci. Res. 2009;87:644–652. doi: 10.1002/jnr.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C., Aloisi F., Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Farmer R.F., Gau J.M., Seeley J.R., Kosty D.B., Sher K.J., Lewinsohn P.M. Internalizing and externalizing disorders as predictors of alcohol use disorder onset during three developmental periods. Drug Alcohol Depend. 2016;164:38–46. doi: 10.1016/j.drugalcdep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feja M., Koch M. Ventral medial prefrontal cortex inactivation impairs impulse control but does not affect delay-discounting in rats. Behav. Brain Res. 2014;264:230–239. doi: 10.1016/j.bbr.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Fergusson D.M., Boden J.M., Horwood L.J. The developmental antecedents of illicit drug use: evidence from a 25-year longitudinal study. Drug Alcohol Depend. 2008;96:165–177. doi: 10.1016/j.drugalcdep.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Fergusson D.M., Horwood L.J., Lynskey M.T. Childhood sexual abuse and psychiatric disorder in young adulthood: II. Psychiatric outcomes of childhood sexual abuse. J. Am. Acad. Child Adolesc. Psychiatr. 1996;35:1365–1374. doi: 10.1097/00004583-199610000-00024. [DOI] [PubMed] [Google Scholar]
- Fishbein D.H., Dax E., Lozovsky D.B., Jaffe J.H. Neuroendocrine responses to a glucose challenge in substance users with high and low levels of aggression, impulsivity, and antisocial personality. Neuropsychobiology. 1992;25:106–114. doi: 10.1159/000118818. [DOI] [PubMed] [Google Scholar]
- Flagel S.B., Robinson T.E., Clark J.J., Clinton S.M., Watson S.J., Seeman P., Phillips P.E., Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.G., Watkins L.R., Maier S.F. Stress- and glucocorticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav. Immun. 2011;25(Suppl. 1):S21–S28. doi: 10.1016/j.bbi.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund N., Thompson B.S., Sonntag K., Meda S., Andersen S.L. When the party is over: depressive-like states in rats following termination of cortical D1 receptor overexpression. Psychopharmacology. 2016;233:1191–1201. doi: 10.1007/s00213-015-4200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V., Mao X., Klein R.G., Ely B.A., Babb J.S., Panzer A.M., Alonso C.M., Shungu D.C. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch. Gen. Psychiatr. 2012;69:139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly P., Brenhouse H.C. Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Developmental Cognitive Neuroscience. 2015;11:18–30. doi: 10.1016/j.dcn.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ovejero D., Veiga S., Garcia-Segura L.M., Doncarlos L.L. Glial expression of estrogen and androgen receptors after rat brain injury. J. Comp. Neurol. 2002;450:256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- Gariepy J.L., Gendreau P.L., Cairns R.B., Lewis M.H. D1 dopamine receptors and the reversal of isolation-induced behaviors in mice. Behav. Brain Res. 1998;95:103–111. doi: 10.1016/s0166-4328(97)00215-5. [DOI] [PubMed] [Google Scholar]
- Garwood S.K., Gerassi L., Jonson-Reid M., Plax K., Drake B. More than poverty: the effect of child abuse and neglect on teen pregnancy risk. J. Adolesc. Health. 2015;57:164–168. doi: 10.1016/j.jadohealth.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzke-Kopp L.M., Raine A., Loeber R., Stouthamer-Loeber M., Steinhauer S.R. Serious delinquent behavior, sensation seeking, and electrodermal arousal. J. Abnorm. Child Psychol. 2002;30:477–486. doi: 10.1023/a:1019816930615. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Hare T.A., Bookheimer S.Y., Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J., Vythilingam M., Page G.G. Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J. Trauma Stress. 2008;21:530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., 3rd, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R.T. Childhood abuse, depression, and chronic pain. Clin. J. Pain. 1994;10:277–281. doi: 10.1097/00002508-199412000-00006. [DOI] [PubMed] [Google Scholar]
- Gondre-Lewis M.C., Warnock K.T., Wang H., June H.L., Jr., Bell K.A., Rabe H., Tiruveedhula V.V., Cook J., Luddens H., Aurelian L., June H.L., Sr Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABA(A) mechanism. Stress. 2016;19:235–247. doi: 10.3109/10253890.2016.1160280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon N.S., Kollack-Walker S., Akil H., Panksepp J. Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Res. Bull. 2002;57:651–659. doi: 10.1016/s0361-9230(01)00762-6. [DOI] [PubMed] [Google Scholar]
- Granholm L., Todkar A., Bergman S., Nilsson K., Comasco E., Nylander I. The expression of opioid genes in non-classical reward areas depends on early life conditions and ethanol intake. Brain Res. 2017;1668:36–45. doi: 10.1016/j.brainres.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Grassi-Oliveira R., Honeycutt J.A., Holland F.H., Ganguly P., Brenhouse H.C. Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: impacts of sex, experience, and cytokines. Psychoneuroendocrinology. 2016;71:19–30. doi: 10.1016/j.psyneuen.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough W.T., Black J.E., Wallace C.S. Experience and brain development. Child Dev. 1987;58:539–559. [PubMed] [Google Scholar]
- Hall F.S. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit. Rev. Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Haller J., Kruk M.R. Normal and abnormal aggression: human disorders and novel laboratory models. Neurosci. Biobehav. Rev. 2006;30:292–303. doi: 10.1016/j.neubiorev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Harricharan R., Abboussi O., Daniels W.M.U. Addiction: a dysregulation of satiety and inflammatory processes. Prog. Brain Res. 2017;235:65–91. doi: 10.1016/bs.pbr.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Hawes D.J., Brennan J., Dadds M.R. Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Curr. Opin. Psychiatr. 2009;22:357–362. doi: 10.1097/YCO.0b013e32832bfa6d. [DOI] [PubMed] [Google Scholar]
- Hayes D.J., Jupp B., Sawiak S.J., Merlo E., Caprioli D., Dalley J.W. Brain gamma-aminobutyric acid: a neglected role in impulsivity. Eur. J. Neurosci. 2014;39:1921–1932. doi: 10.1111/ejn.12485. [DOI] [PubMed] [Google Scholar]
- Hechtman L., Swanson J.M., Sibley M.H., Stehli A., Owens E.B., Mitchell J.T., Arnold L.E., Molina B.S., Hinshaw S.P., Jensen P.S., Abikoff H.B., Perez Algorta G., Howard A.L., Hoza B., Etcovitch J., Houssais S., Lakes K.D., Nichols J.Q. Functional adult outcomes 16 Years after childhood diagnosis of attention-deficit/hyperactivity disorder: MTA results. J. Am. Acad. Child Adolesc. Psychiatr. 2016;55:945–952 e2. doi: 10.1016/j.jaac.2016.07.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Owens M.J., Plotsky P.M., Nemeroff C.B. Persistent changes in corticotropin-releasing factor systems due to early life stress: relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacol. Bull. 1997;33:185–192. [PubMed] [Google Scholar]
- Hennessy M.B., Jacobs S., Schiml P.A., Hawk K., Stafford N., Deak T. Maternal inhibition of infant behavioral response following isolation in novel surroundings and inflammatory challenge. Dev. Psychobiol. 2013;55:395–403. doi: 10.1002/dev.21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy M.B., Paik K.D., Caraway J.D., Schiml P.A., Deak T. Proinflammatory activity and the sensitization of depressive-like behavior during maternal separation. Behav. Neurosci. 2011;125:426–433. doi: 10.1037/a0023559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensleigh E., Smedley L., Pritchard L.M. Sex, but not repeated maternal separation during the first postnatal week, influences novel object exploration and amphetamine sensitivity. Dev. Psychobiol. 2011;53:132–140. doi: 10.1002/dev.20499. [DOI] [PubMed] [Google Scholar]
- Himmler B.T., Nakahashi A., Snow E., McMickle A., Muhammad A., Biondolillo K.D., Pellis S.M., Kolb B. Juvenile play experience does not affect nicotine sensitization and voluntary consumption of nicotine in adult rats. Dev. Psychobiol. 2014;56:1052–1060. doi: 10.1002/dev.21189. [DOI] [PubMed] [Google Scholar]
- Himmler B.T., Pellis S.M., Kolb B. Juvenile play experience primes neurons in the medial prefrontal cortex to be more responsive to later experiences. Neurosci. Lett. 2013;556:42–45. doi: 10.1016/j.neulet.2013.09.061. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K., Muhtz C., Dettenborn L., Agorastos A., Wingenfeld K., Spitzer C., Gao W., Kirschbaum C., Wiedemann K., Otte C. Association between childhood trauma and low hair cortisol in depressed patients and healthy control subjects. Biol. Psychiatry. 2013;74:e15–e17. doi: 10.1016/j.biopsych.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Hinshaw S.P., Arnold L.E. Attention-deficit hyperactivity disorder, multimodal treatment, and longitudinal outcome: evidence, paradox, and challenge. Wiley interdisciplinary Reviews Cognitive Science. 2015;6:39–52. doi: 10.1002/wcs.1324. [DOI] [PubMed] [Google Scholar]
- Hofer M.A., Shair H. Control of sleep-wake states in the infant rat by features of the mother-infant relationship. Dev. Psychobiol. 1982;15:229–243. doi: 10.1002/dev.420150307. [DOI] [PubMed] [Google Scholar]
- Hoftman G.D., Volk D.W., Bazmi H.H., Li S., Sampson A.R., Lewis D.A. Altered cortical expression of GABA-related genes in schizophrenia: illness progression vs developmental disturbance. Schizophr. Bull. 2015;41:180–191. doi: 10.1093/schbul/sbt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland F.H., Ganguly P., Potter D.N., Chartoff E.H., Brenhouse H.C. Early life stress disrupts social behavior and prefrontal cortex parvalbumin interneurons at an earlier time-point in females than in males. Neurosci. Lett. 2014;566:131–136. doi: 10.1016/j.neulet.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler C.M., Harkey S.L., Krzywosinski T.B., Aragona B.J., Bales K.L. Neonatal exposure to the D1 agonist SKF38393 inhibits pair bonding in the adult prairie vole. Behav. Pharmacol. 2011;22:703–710. doi: 10.1097/FBP.0b013e32834affd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.J., Kirkwood A., Pizzorusso T., Porciatti V., Morales B., Bear M.F., Maffei L., Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Huot R.L., Thrivikraman K.V., Meaney M.J., Plotsky P.M. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology. 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Hutchinson M.R., Watkins L.R. Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology. 2014;76 Pt B:218–227. doi: 10.1016/j.neuropharm.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyucksun Shin S. A longitudinal examination of the relationships between childhood maltreatment and patterns of adolescent substance use among high-risk adolescents. Am. J. Addict./American Academy of Psychiatrists in Alcoholism and Addictions. 2012;21:453–461. doi: 10.1111/j.1521-0391.2012.00255.x. [DOI] [PubMed] [Google Scholar]
- Jager A., Amiri H., Bielczyk N., van Heukelum S., Heerschap A., Aschrafi A., Poelmans G., Buitelaar J.K., Kozicz T., Glennon J.C. Cortical control of aggression: GABA signalling in the anterior cingulate cortex. Eur. Neuropsychopharmacol. 2017 doi: 10.1016/j.euroneuro.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Johnson D.E., Miller L.C., Iverson S., Thomas W., Franchino B., Dole K., Kiernan M.T., Georgieff M.K., Hostetter M.K. The health of children adopted from Romania. J. Am. Med. Assoc. 1992;268:3446–3451. [PubMed] [Google Scholar]
- Jordan C.J., Andersen S.L. Working memory and salivary brain-derived neurotrophic factor as developmental predictors of cocaine seeking in males and females. Addict. Biol. 2018;23:868–879. doi: 10.1111/adb.12535. [DOI] [PubMed] [Google Scholar]
- Jupp B., Caprioli D., Saigal N., Reverte I., Shrestha S., Cumming P., Everitt B.J., Robbins T.W., Dalley J.W. Dopaminergic and GABA-ergic markers of impulsivity in rats: evidence for anatomical localisation in ventral striatum and prefrontal cortex. Eur. J. Neurosci. 2013;37:1519–1528. doi: 10.1111/ejn.12146. [DOI] [PubMed] [Google Scholar]
- Juraska J.M., Markham J.A. The cellular basis for volume changes in the rat cortex during puberty: white and gray matter. Ann. N. Y. Acad. Sci. 2004;1021:431–435. doi: 10.1196/annals.1308.058. [DOI] [PubMed] [Google Scholar]
- Kairisalo M., Korhonen L., Sepp M., Pruunsild P., Kukkonen J.P., Kivinen J., Timmusk T., Blomgren K., Lindholm D. NF-kappaB-dependent regulation of brain-derived neurotrophic factor in hippocampal neurons by X-linked inhibitor of apoptosis protein. Eur. J. Neurosci. 2009;30:958–966. doi: 10.1111/j.1460-9568.2009.06898.x. [DOI] [PubMed] [Google Scholar]
- Kalinichev M., Easterling K.W., Plotsky P.M., Holtzman S.G. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol. Biochem. Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Keenan K., Loeber R., Green S. Conduct disorder in girls: a review of the literature. Clin. Child Fam. Psychol. Rev. 1999;2:3–19. doi: 10.1023/a:1021811307364. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., McGonagle K.A., Zhao S., Nelson C.B., Hughes M., Eshleman S., Wittchen H.U., Kendler K.S. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the national comorbidity survey. Arch. Gen. Psychiatr. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Ormel J., Petukhova M., McLaughlin K.A., Green J.G., Russo L.J., Stein D.J., Zaslavsky A.M., Aguilar-Gaxiola S., Alonso J., Andrade L., Benjet C., de Girolamo G., de Graaf R., Demyttenaere K., Fayyad J., Haro J.M., Hu C., Karam A., Lee S., Lepine J.P., Matchsinger H., Mihaescu-Pintia C., Posada-Villa J., Sagar R., Ustun T.B. Development of lifetime comorbidity in the World Health Organization world mental health surveys. Arch. Gen. Psychiatr. 2011;68:90–100. doi: 10.1001/archgenpsychiatry.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T., Faccidomo S., Miczek K.A. Repeated maternal separation: differences in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology. 2005;178:202–210. doi: 10.1007/s00213-004-1989-1. [DOI] [PubMed] [Google Scholar]
- Kilts C.D., Schweitzer J.B., Quinn C.K., Gross R.E., Faber T.L., Muhammad F., Ely T.D., Hoffman J.M., Drexler K.P. Neural activity related to drug craving in cocaine addiction. Arch. Gen. Psychiatr. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- King L.S., Colich N.L., LeMoult J., Humphreys K.L., Ordaz S.J., Price A.N., Gotlib I.H. The impact of the severity of early life stress on diurnal cortisol: the role of puberty. Psychoneuroendocrinology. 2017;77:68–74. doi: 10.1016/j.psyneuen.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten K.S., Miserendino M.J.D., Kehoe P. Ehanced acquision of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:45–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Kosten T.A., Sanchez H., Zhang X.Y., Kehoe P. Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behav. Brain Res. 2004;151:137–149. doi: 10.1016/j.bbr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Kosten T.A., Zhang X.Y., Kehoe P. Heightened cocaine and food self-administration in female rats with neonatal isolation experience. Neuropsychopharmacology. 2006;31:70–76. doi: 10.1038/sj.npp.1300779. [DOI] [PubMed] [Google Scholar]
- Kritzer M. The distribution of immunoreactivity for intracellular androgen receptors in the cerebral cortex of hormonally intact adult male and female rats: localization in pyramidal neurons making corticocortical connections. Cerebr. Cortex. 2004;14:268–280. doi: 10.1093/cercor/bhg127. [DOI] [PubMed] [Google Scholar]
- Kritzer M.F. Regional, laminar, and cellular distribution of immunoreactivity for ER alpha and ER beta in the cerebral cortex of hormonally intact, adult male and female rats. Cerebr. Cortex. 2002;12:116–128. doi: 10.1093/cercor/12.2.116. [DOI] [PubMed] [Google Scholar]
- Lacagnina M.J., Rivera P.D., Bilbo S.D. Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharmacology. 2017;42:156–177. doi: 10.1038/npp.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J., Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev. Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Lemke R., Hartig W., Rossner S., Bigl V., Schliebs R. Interleukin-6 is not expressed in activated microglia and in reactive astrocytes in response to lesion of rat basal forebrain cholinergic system as demonstrated by combined in situ hybridization and immunocytochemistry. J. Neurosci. Res. 1998;51:223–236. doi: 10.1002/(SICI)1097-4547(19980115)51:2<223::AID-JNR11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lensjo K.K., Lepperod M.E., Dick G., Hafting T., Fyhn M. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J. Neurosci. 2017;37:1269–1283. doi: 10.1523/JNEUROSCI.2504-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher H.M., Spoelder M., Rotte M.D., Janssen M.J., Hesseling P., Lozeman-van't Klooster J.G., Baars A.M., Vanderschuren L.J. Early social isolation augments alcohol consumption in rats. Behav. Pharmacol. 2015;26:673–680. doi: 10.1097/FBP.0000000000000165. [DOI] [PubMed] [Google Scholar]
- Leussis M.P., Freund N., Brenhouse H.C., Thompson B.S., Andersen S.L. Depressive-like behavior in adolescents after maternal separation: sex differences, controllability, and GABA. Dev. Neurosci. 2012;34:210–217. doi: 10.1159/000339162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis M.P., Lawson K., Stone K., Andersen S.L. The enduring effects of an adolescent social stressor on synaptic density, part II: poststress reversal of synaptic loss in the cortex by adinazolam and MK-801. Synapse. 2008;62:185–192. doi: 10.1002/syn.20483. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- Lindqvist D., Janelidze S., Hagell P., Erhardt S., Samuelsson M., Minthon L., Hansson O., Bjorkqvist M., Traskman-Bendz L., Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol. Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Lorber M.F. Psychophysiology of aggression, psychopathy, and conduct problems: a meta-analysis. Psychol. Bull. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Lukas M., Bredewold R., Landgraf R., Neumann I.D., Veenema A.H. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology. 2011;36:843–853. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Lukkes J.L., Meda S., Norman K.J., Andersen S.L. Anhedonic behavior and gamma-amino butyric acid during a sensitive period in female rats exposed to early adversity. J. Psychiatr. Res. 2018;100:8–15. doi: 10.1016/j.jpsychires.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes J.L., Meda S., Thompson B.S., Freund N., Andersen S.L. Early life stress and later peer distress on depressive behavior in adolescent female rats: effects of a novel intervention on GABA and D2 receptors. Behav. Brain Res. 2017;330:37–45. doi: 10.1016/j.bbr.2017.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S., Laviola G., Leussis M.P., Andersen S.L. Abnormal behavioral and neurotrophic development in the younger sibling receiving less maternal care in a communal nursing paradigm in rats. Psychoneuroendocrinology. 2010;35:392–402. doi: 10.1016/j.psyneuen.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Maliphant R., Hume F., Furnham A. Autonomic nervous system (ANS) activity, personality characteristics and disruptive behaviour in girls. J. Child Psychol. Psychiatry Allied Discip. 1990;31:619–628. doi: 10.1111/j.1469-7610.1990.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Malter Cohen M., Jing D., Yang R.R., Tottenham N., Lee F.S., Casey B.J. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc. Natl. Acad. Sci. U. S. A. 2013;110:18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzza S., Klein R.G., Moulton J.L., 3rd Does stimulant treatment place children at risk for adult substance abuse? A controlled, prospective follow-up study. J. Child Adolesc. Psychopharmacol. 2003;13:273–282. doi: 10.1089/104454603322572606. [DOI] [PubMed] [Google Scholar]
- Mannuzza S., Klein R.G., Truong N.L., Moulton J.L., 3rd, Roizen E.R., Howell K.H., Castellanos F.X. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am. J. Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J.A., Morris J.R., Juraska J.M. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Marquez C., Poirier G.L., Cordero M.I., Larsen M.H., Groner A., Marquis J., Magistretti P.J., Trono D., Sandi C. Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl. Psychiatry. 2013;3:e216. doi: 10.1038/tp.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak H.A., Martin K.R., Etkin A., Thomason M.E. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology. 2015;40:1250–1258. doi: 10.1038/npp.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews I.Z., McCormick C.M. Female and male rats in late adolescence differ from adults in amphetamine-induced locomotor activity, but not in conditioned place preference for amphetamine. Behav. Pharmacol. 2007;18:641–650. doi: 10.1097/FBP.0b013e3282effbf5. [DOI] [PubMed] [Google Scholar]
- Matthews K., Dalley J.W., Matthews C., Tsai T.H., Robbins T.W. Periodic maternal separation of neonatal rats produces region- and gender-specific effects on biogenic amine content in postmortem adult brain. Synapse. 2001;40:1–10. doi: 10.1002/1098-2396(200104)40:1<1::AID-SYN1020>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Matthews K., Robbins T.W., Everitt B.J., Caine S.B. Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology. 1999;141:123–134. doi: 10.1007/s002130050816. [DOI] [PubMed] [Google Scholar]
- McCabe S.E., Dickinson K., West B.T., Wilens T.E. Age of onset, duration, and type of medication therapy for attention-deficit/hyperactivity disorder and substance use during adolescence: a multi-cohort national study. J. Am. Acad. Child Adolesc. Psychiatr. 2016;55:479–486. doi: 10.1016/j.jaac.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E.J., Gerin M.I., Viding E. Annual Research Review: childhood maltreatment, latent vulnerability and the shift to preventative psychiatry - the contribution of functional brain imaging. J. Child Psychol. Psychiatry Allied Discip. 2017;58:338–357. doi: 10.1111/jcpp.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M., Iacono W.G., Legrand L.N., Malone S., Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin. Exp. Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- McKlveen J.M., Morano R.L., Fitzgerald M., Zoubovsky S., Cassella S.N., Scheimann J.R., Ghosal S., Mahbod P., Packard B.A., Myers B., Baccei M.L., Herman J.P. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol. Psychiatry. 2016;80:754–764. doi: 10.1016/j.biopsych.2016.03.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M.J., Diorio J., Francis D., Widdowson J., LaPlante P., Caldji C., Sharma S., Seckl J.R., Plotsky P.M. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev. Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Mei S., Xu J., Carroll K.M., Potenza M.N. Self-reported impulsivity is negatively correlated with amygdalar volumes in cocaine dependence. Psychiatr. Res. 2015;233:212–217. doi: 10.1016/j.pscychresns.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., D'Arcy C. Gender moderates the relationship between childhood abuse and internalizing and substance use disorders later in life: a cross-sectional analysis. BMC Psychiatr. 2016;16:401. doi: 10.1186/s12888-016-1071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels C.C., Easterling K.W., Holtzman S.G. Maternal separation alters ICSS responding in adult male and female rats, but morphine and naltrexone have little affect on that behavior. Brain Res. Bull. 2007;73:310–318. doi: 10.1016/j.brainresbull.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Miczek K.A., Yap J.J., Covington H.E., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol. Therapeut. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.B., Pasta D.J., MacMurray J., Chiu C., Wu H., Comings D.E. Dopamine receptor genes are associated with age at first sexual intercourse. J. Biosoc. Sci. 1999;31:43–54. doi: 10.1017/s0021932099000437. [DOI] [PubMed] [Google Scholar]
- Moffett M.C., Vicentic A., Kozel M., Plotsky P., Francis D.D., Kuhar M.J. Maternal separation alters drug intake patterns in adulthood in rats. Biochem. Pharmacol. 2007;73:321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir R.D., Martins R.N., Bush A.I., Small D.H., Milward E.A., Rumble B.A., Multhaup G., Beyreuther K., Masters C.L. Human brain beta A4 amyloid protein precursor of Alzheimer's disease: purification and partial characterization. J. Neurochem. 1992;59:1490–1498. doi: 10.1111/j.1471-4159.1992.tb08465.x. [DOI] [PubMed] [Google Scholar]
- Morgan D., Grant K.A., Gage H.D., Mach R.H., Kaplan J.R., Prioleau O., Nader S.H., Buchheimer N., Ehrenkaufer R.L., Nader M.A. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat. Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morishita H., Cabungcal J.H., Chen Y., Do K.Q., Hensch T.K. Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biol. Psychiatry. 2015;78:396–402. doi: 10.1016/j.biopsych.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H., Kundakovic M., Bicks L., Mitchell A., Akbarian S. Interneuron epigenomes during the critical period of cortical plasticity: implications for schizophrenia. Neurobiol. Learn. Mem. 2015;124:104–110. doi: 10.1016/j.nlm.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E.R., Fernando A.B., Urcelay G.P., Robinson E.S., Mar A.C., Theobald D.E., Dalley J.W., Robbins T.W. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology. 2012;219:401–410. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.O., Herald J.B., Wills C.T., Unfried S.G., Cohn D.M., Loria A.S. Postnatal treatment with metyrapone attenuates the effects of diet-induced obesity in female rats exposed to early-life stress. Am. J. Physiol. Endocrinol. Metabol. 2017;312:E98–E108. doi: 10.1152/ajpendo.00308.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamitsu S., Yamashita Y., Tanigawa H., Chiba H., Kaida H., Ishibashi M., Kakuma T., Croarkin P.E., Matsuishi T. Upregulated GABA inhibitory function in ADHD children with child behavior checklist-dysregulation profile: 123I-iomazenil SPECT study. Front. Psychiatr. 2015;6:84. doi: 10.3389/fpsyt.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M., Miyatake M., Narita M., Shibasaki M., Shindo K., Nakamura A., Kuzumaki N., Nagumo Y., Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- NIDA . 2007. Community Monitoring Systems: Tracking and Improving the Well-being of America's Children and Adolescents. NIH 07-5852. [Google Scholar]
- Nixon K., Hughes P.D., Amsel A., Leslie S.W. NMDA receptor subunit expression following early postnatal exposure to ethanol. Brain Research Developmental Brain Research. 2002;139:295–299. doi: 10.1016/s0165-3806(02)00515-1. [DOI] [PubMed] [Google Scholar]
- Nunez J.L., McCarthy M.M. Androgens predispose males to GABAA-mediated excitotoxicity in the developing hippocampus. Exp. Neurol. 2008;210:699–708. doi: 10.1016/j.expneurol.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeon M.M., Andreu M., Yamauchi L., Grosman M., Acosta G.B. Chronic postnatal stress induces voluntary alcohol intake and modifies glutamate transporters in adolescent rats. Stress. 2015;18:427–434. doi: 10.3109/10253890.2015.1041909. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J., Geurts H.M., Knol D.L., Sergeant J.A. Low basal salivary cortisol is associated with teacher-reported symptoms of conduct disorder. Psychiatr. Res. 2005;134:1–10. doi: 10.1016/j.psychres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Paine T.A., Cooke E.K., Lowes D.C. Effects of chronic inhibition of GABA synthesis on attention and impulse control. Pharmacol. Biochem. Behav. 2015;135:97–104. doi: 10.1016/j.pbb.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J., Beatty W.W. Social deprivation and play in rats. Behav. Neural. Biol. 1980;30:197–206. doi: 10.1016/s0163-1047(80)91077-8. [DOI] [PubMed] [Google Scholar]
- Pardini M., Krueger F., Hodgkinson C.A., Raymont V., Strenziok M., Amore M., Wassermann E.M., Goldman D., Grafman J.H. Aggression, DRD1 polymorphism, and lesion location in penetrating traumatic brain injury. CNS Spectr. 2014;19:382–390. doi: 10.1017/S1092852914000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent C.I., Del Corpo A., Cameron N.M., Meaney M.J. Maternal care associates with play dominance rank among adult female rats. Dev. Psychobiol. 2013;55:745–756. doi: 10.1002/dev.21070. [DOI] [PubMed] [Google Scholar]
- Parent C.I., Meaney M.J. The influence of natural variations in maternal care on play fighting in the rat. Dev. Psychobiol. 2008;50:767–776. doi: 10.1002/dev.20342. [DOI] [PubMed] [Google Scholar]
- Pechtel P., Lyons-Ruth K., Anderson C.M., Teicher M.H. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. NeuroImage. 2014;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D.A. Disrupted reinforcement learning and maladaptive behavior in women with a history of childhood sexual abuse: a high-density event-related potential study. JAMA psychiatry. 2013;70:499–507. doi: 10.1001/jamapsychiatry.2013.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis S.M., Pellis V.C. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev. Psychobiol. 1990;23:215–231. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- Pierce R.C., Reeder D.C., Hicks J., Morgan Z.R., Kalivas P.W. Ibotenic acid lesions of the dorsal prefrontal cortex disrupt the expression of behavioral sensitization to cocaine. Neuroscience. 1998;82:1103–1114. doi: 10.1016/s0306-4522(97)00366-7. [DOI] [PubMed] [Google Scholar]
- Pitula C.E., DePasquale C.E., Mliner S.B., Gunnar M.R. Child development; 2017. Peer Problems Among Postinstitutionalized, Internationally Adopted Children: Relations to Hypocortisolism, Parenting Quality, and ADHD Symptoms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploj K., Roman E., Nylander I. Long-term effects of short and long periods of maternal separation on brain opioid peptide levels in male Wistar rats. Neuropeptides. 2003;37:149–156. doi: 10.1016/s0143-4179(03)00043-x. [DOI] [PubMed] [Google Scholar]
- Plotsky P.M., Meaney M.J. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain research Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Popoola D.O., Cameron N.M. Maternal care-related differences in males and females rats' sensitivity to ethanol and the associations between the GABAergic system and steroids in males. Dev. Psychobiol. 2018;60:380–394. doi: 10.1002/dev.21607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A., Venables P.H., Mednick S.A. Low resting heart rate at age 3 years predisposes to aggression at age 11 years: evidence from the Mauritius Child Health Project. J. Am. Acad. Child Adolesc. Psychiatr. 1997;36:1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- Raineki C., Cortes M.R., Belnoue L., Sullivan R.M. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J. Neurosci. 2012;32:7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reebye P., Moretti M.M., Lessard J.C. Conduct disorder and substance use disorder: comorbidity in a clinical sample of preadolescents and adolescents. Canadian Journal of Psychiatry Revue canadienne de psychiatrie. 1995;40:313–319. doi: 10.1177/070674379504000606. [DOI] [PubMed] [Google Scholar]
- Reus G.Z., Stringari R.B., Ribeiro K.F., Cipriano A.L., Panizzutti B.S., Stertz L., Lersch C., Kapczinski F., Quevedo J. Maternal deprivation induces depressive-like behaviour and alters neurotrophin levels in the rat brain. Neurochem. Res. 2011;36:460–466. doi: 10.1007/s11064-010-0364-3. [DOI] [PubMed] [Google Scholar]
- Roman E., Ploj K., Nylander I. Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol. 2004;33:31–39. doi: 10.1016/j.alcohol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Roth T.L., Lubin F.D., Funk A.J., Sweatt J.D. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowniak M., Bogus-Nowakowska K., Robak A. The densities of calbindin and parvalbumin, but not calretinin neurons, are sexually dimorphic in the amygdala of the Guinea pig. Brain Res. 2015;1604:84–97. doi: 10.1016/j.brainres.2015.01.048. [DOI] [PubMed] [Google Scholar]
- Ruedi-Bettschen D., Pedersen E.M., Feldon J., Pryce C.R. Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav. Brain Res. 2005;156:297–310. doi: 10.1016/j.bbr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Sanchez M.M., McCormack K.M., Howell B.R. Social buffering of stress responses in nonhuman primates: maternal regulation of the development of emotional regulatory brain circuits. Soc. Neurosci. 2015;10:512–526. doi: 10.1080/17470919.2015.1087426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawiak S.J., Jupp B., Taylor T., Caprioli D., Carpenter T.A., Dalley J.W. In vivo gamma-aminobutyric acid measurement in rats with spectral editing at 4.7T. J. Magn. Reson. Imag. : JMRI. 2016;43:1308–1312. doi: 10.1002/jmri.25093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A., Raine A. Psychophysiology of anger and violent behavior. Psychiatr. Clin. 1997;20:375–394. doi: 10.1016/s0193-953x(05)70318-x. [DOI] [PubMed] [Google Scholar]
- Schafer D.P., Lehrman E.K., Kautzman A.G., Koyama R., Mardinly A.R., Yamasaki R., Ransohoff R.M., Greenberg M.E., Barres B.A., Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J.M., Bilbo S.D. Sex, glia, and development: interactions in health and disease. Horm. Behav. 2012;62:243–253. doi: 10.1016/j.yhbeh.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J.M., Bilbo S.D. Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction. J. Neurosci. 2013;33:961–971. doi: 10.1523/JNEUROSCI.2516-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See R.E., Fuchs R.A., Ledford C.C., McLaughlin J. Drug addiction, relapse, and the amygdala. Ann. N. Y. Acad. Sci. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- Seidel K., Helmeke C., Poeggel G., Braun K. Repeated neonatal separation stress alters the composition of neurochemically characterized interneuron subpopulations in the rodent dentate gyrus and basolateral amygdala. Developmental Neurobiology. 2008;68:1137–1152. doi: 10.1002/dneu.20651. [DOI] [PubMed] [Google Scholar]
- Sesack S.R., Deutch A.Y., Roth R.H., Bunney B.S. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shirayama Y., Chen A.C., Nakagawa S., Russell D.S., Duman R.S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A., Bhatt S., Bhatt R., Zalcman S.S. The neurobiological bases for development of pharmacological treatments of aggressive disorders. Curr. Neuropharmacol. 2007;5:135–147. doi: 10.2174/157015907780866929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri M.M., Sneider J.T., Crowley D.J., Covell M.J., Acharya D., Rosso I.M., Jensen J.E. Frontal lobe gamma-aminobutyric acid levels during adolescence: associations with impulsivity and response inhibition. Biol. Psychiatry. 2013;74:296–304. doi: 10.1016/j.biopsych.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J.G., Byrne M.L., Schwartz O.S., Whittle S.L., Sheeber L., Kaess M., Youssef G.J., Allen N.B. Dual-axis hormonal covariation in adolescence and the moderating influence of prior trauma and aversive maternal parenting. Dev. Psychobiol. 2015;57:670–687. doi: 10.1002/dev.21275. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M.L., Hong S., Herrenkohl T.I., Brown E.C., Lee J.O., Jung H. Longitudinal effects of early childhood maltreatment on Co-occurring substance misuse and mental health problems in adulthood: the role of adolescent alcohol use and depression. J. Stud. Alcohol Drugs. 2016;77:464–472. doi: 10.15288/jsad.2016.77.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.K., Kilaru V., Klengel T., Mercer K.B., Bradley B., Conneely K.N., Ressler K.J., Binder E.B. DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to brain. Am. J. Med. Genet. Part B, Neuropsychiatric genetics. 2015;168B:36–44. doi: 10.1002/ajmg.b.32278. the official publication of the International Society of Psychiatric Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somaini L., Donnini C., Manfredini M., Raggi M.A., Saracino M.A., Gerra M.L., Amore M., Leonardi C., Serpelloni G., Gerra G. Adverse childhood experiences (ACEs), genetic polymorphisms and neurochemical correlates in experimentation with psychotropic drugs among adolescents. Neurosci. Biobehav. Rev. 2011;35:1771–1778. doi: 10.1016/j.neubiorev.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Sonntag K.C., Brenhouse H.C., Freund N., Thompson B.S., Puhl M., Andersen S.L. Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: comparison with adolescents. Psychopharmacology. 2014;231:1615–1626. doi: 10.1007/s00213-013-3399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotonyi P., Gao Q., Bechmann I., Horvath T.L. Estrogen promotes parvalbumin expression in arcuate nucleus POMC neurons. Reprod. Sci. 2010;17:1077–1080. doi: 10.1177/1933719110379651. [DOI] [PubMed] [Google Scholar]
- St Onge J.R., Floresco S.B. Prefrontal cortical contribution to risk-based decision making. Cerebr. Cortex. 2010;20:1816–1828. doi: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- Stein M.B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol. Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Stewart M.A., deBlois C.S., Meardon J., Cummings C. Aggressive conduct disorder of children. The clinical picture. J. Nerv. Ment. Dis. 1980;168:604–610. doi: 10.1097/00005053-198010000-00003. [DOI] [PubMed] [Google Scholar]
- Sukoff Rizzo S.J., Neal S.J., Hughes Z.A., Beyna M., Rosenzweig-Lipson S., Moss S.J., Brandon N.J. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl. Psychiatry. 2012;2:e199. doi: 10.1038/tp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung M., Erkanli A., Angold A., Costello E.J. Effects of age at first substance use and psychiatric comorbidity on the development of substance use disorders. Drug Alcohol Depend. 2004;75:287–299. doi: 10.1016/j.drugalcdep.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Tandon M., Cardeli E., Luby J. Internalizing disorders in early childhood: a review of depressive and anxiety disorders. Child and adolescent psychiatric clinics of North America. 2009;18:593–610. doi: 10.1016/j.chc.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo A.R., Gunnar M.R. Child maltreatment and the developing HPA axis. Horm. Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Polcari A., Andersen S.L. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. J. Clin. Psychiatr. 2009;70:684–691. doi: 10.4088/jcp.08m04235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Tomoda A., Andersen S.L. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann. N. Y. Acad. Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- Thanos P.K., Michaelides M., Benveniste H., Wang G.J., Volkow N.D. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol. Biochem. Behav. 2007;87:426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Toyoizumi T., Miller K.D. Equalization of ocular dominance columns induced by an activity-dependent learning rule and the maturation of inhibition. J. Neurosci. 2009;29:6514–6525. doi: 10.1523/JNEUROSCI.0492-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V., Baarendse P.J., Vanderschuren L.J. On the interaction between drugs of abuse and adolescent social behavior. Psychopharmacology. 2014;231:1715–1729. doi: 10.1007/s00213-014-3471-z. [DOI] [PubMed] [Google Scholar]
- Tzanoulinou S., Riccio O., de Boer M.W., Sandi C. Peripubertal stress-induced behavioral changes are associated with altered expression of genes involved in excitation and inhibition in the amygdala. Transl. Psychiatry. 2014;4 doi: 10.1038/tp.2014.54. e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H., Suemitsu S., Murakami S., Kitamura N., Wani K., Okamoto M., Matsumoto Y., Ishihara T. Region-specific impairments in parvalbumin interneurons in social isolation-reared mice. Neuroscience. 2017;359:196–208. doi: 10.1016/j.neuroscience.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Uriarte N., Breigeiron M.K., Benetti F., Rosa X.F., Lucion A.B. Effects of maternal care on the development, emotionality, and reproductive functions in male and female rats. Dev. Psychobiol. 2007;49:451–462. doi: 10.1002/dev.20241. [DOI] [PubMed] [Google Scholar]
- Vachon D.D., Krueger R.F., Rogosch F.A., Cicchetti D. Assessment of the harmful psychiatric and behavioral effects of different forms of child maltreatment. JAMA psychiatry. 2015;72:1135–1142. doi: 10.1001/jamapsychiatry.2015.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bokhoven I., Matthys W., van Goozen S.H., van Engeland H. Prediction of adolescent outcome in children with disruptive behaviour disorders--a study of neurobiological, psychological and family factors. Eur. Child Adolesc. Psychiatr. 2005;14:153–163. doi: 10.1007/s00787-005-0455-x. [DOI] [PubMed] [Google Scholar]
- Van Dam N.T., Rando K., Potenza M.N., Tuit K., Sinha R. Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA psychiatry. 2014;71:917–925. doi: 10.1001/jamapsychiatry.2014.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen R., Koehl M., Abrous D.N., de Kloet E.R., Piazza P.V., Deroche-Gamonet V. Maternal environment influences cocaine intake in adulthood in a genotype-dependent manner. PloS One. 2008;3:e2245. doi: 10.1371/journal.pone.0002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof L.W., Damsteegt R., Trezza V., Voorn P., Vanderschuren L.J. Social play behavior in adolescent rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacology. 2013;38:1899–1909. doi: 10.1038/npp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren L.J., Niesink R.J., Spruijt B.M., Van Ree J.M. Influence of environmental factors on social play behavior of juvenile rats. Physiol. Behav. 1995;58:119–123. doi: 10.1016/0031-9384(94)00385-i. [DOI] [PubMed] [Google Scholar]
- Vargas J., Junco M., Gomez C., Lajud N. Early life stress increases metabolic risk, HPA Axis reactivity, and depressive-like behavior when combined with postweaning social isolation in rats. PloS One. 2016;11 doi: 10.1371/journal.pone.0162665. e0162665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez V., Penit-Soria J., Durand C., Besson M.J., Giros B., Dauge V. Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood. J. Neurosci. 2005;25:4453–4462. doi: 10.1523/JNEUROSCI.4807-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema A.H., Neumann I.D. Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology. 2009;34:463–467. doi: 10.1016/j.psyneuen.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Fowler J.S., Wang G.J., Goldstein R.Z. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol. Learn. Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Wagner F.A., Anthony J.C. Male-female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug Alcohol Depend. 2007;86:191–198. doi: 10.1016/j.drugalcdep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wang F., Chen H., Steketee J.D., Sharp B.M. Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharmacology. 2007;32:103–109. doi: 10.1038/sj.npp.1301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F., Ciccocioppo R., Parsons L.H., Katner S., Liu X., Zorrilla E.P., Valdez G.R., Ben-Shahar O., Angeletti S., Richter R.R. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann. N. Y. Acad. Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Widom C.S., DuMont K., Czaja S.J. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch. Gen. Psychiatr. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- Widom C.S., Marmorstein N.R., White H.R. Childhood victimization and illicit drug use in middle adulthood. Psychol. Addict. Behav. 2006;20:394–403. doi: 10.1037/0893-164X.20.4.394. [DOI] [PubMed] [Google Scholar]
- Wieck A., Andersen S.L., Brenhouse H.C. Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: relationship to cortical NMDA receptor expression. Brain Behav. Immun. 2013;28:218–226. doi: 10.1016/j.bbi.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Wilens T.E., Faraone S.V., Biederman J., Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Wilson H.W., Samuelson S.L., Staudenmeyer A.H., Widom C.S. Trajectories of psychopathology and risky behaviors associated with childhood abuse and neglect in low-income urban African American girls. Child Abuse Neglect. 2015;45:108–121. doi: 10.1016/j.chiabu.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Winstanley C.A., Theobald D.E., Dalley J.W., Cardinal R.N., Robbins T.W. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cerebr. Cortex. 2005;4:669–682. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Wischhof L., Irrsack E., Osorio C., Koch M. Prenatal LPS-exposure--a neurodevelopmental rat model of schizophrenia--differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2015;57:17–30. doi: 10.1016/j.pnpbp.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Woolfenden S.R., Williams K., Peat J. Family and parenting interventions in children and adolescents with conduct disorder and delinquency aged 10-17. Cochrane Database Syst. Rev. 2001:CD003015. doi: 10.1002/14651858.CD003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.C., Du X., van den Buuse M., Hill R.A. Sex differences in the adolescent developmental trajectory of parvalbumin interneurons in the hippocampus: a role for estradiol. Psychoneuroendocrinology. 2014;45:167–178. doi: 10.1016/j.psyneuen.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Ye Q., Miao Q.L. Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex. Matrix Biol. 2013;32:352–363. doi: 10.1016/j.matbio.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Yusko B., Hawk K., Schiml P.A., Deak T., Hennessy M.B. Sensitization of depressive-like behavior during repeated maternal separation is associated with more-rapid increase in core body temperature and reduced plasma cortisol levels. Physiol. Behav. 2012;105:861–867. doi: 10.1016/j.physbeh.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahorodna A., Tokarski K., Bijak M. Repeated corticosterone administration increases excitatory effect of 5-HT4 receptor agonist in the rat hippocampus. Pol. J. Pharmacol. 2000;52:107–109. [PubMed] [Google Scholar]
- Zeidan M.A., Igoe S.A., Linnman C., Vitalo A., Levine J.B., Klibanski A., Goldstein J.M., Milad M.R. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol. Psychiatry. 2011;70:920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.Y., Bagot R., Parent C., Nesbitt C., Bredy T.W., Caldji C., Fish E., Anisman H., Szyf M., Meaney M.J. Maternal programming of defensive responses through sustained effects on gene expression. Biol. Psychiatry. 2006;73:72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.