Abstract
The mammalian target of rapamycin (mTOR) has a fundamental role in the metabolism, growth, and regulation of the immune system. The interferon gamma (IFN-γ)derived from T helper 1 (Th1) cells is a prominent pro-inflammatory cytokine in multiple sclerosis (MS) and its animal model, the experimental autoimmune encephalomyelitis (EAE). Due to the exclusive role of rapamycin (RAPA) in mTOR complex 1 (mTORC1) inhibition, essentially Th1 differentiation and IFN-γ production, we evaluated the potential therapeutic effects of hemp seed/evening primrose oils (HSO/EPO) in comparison with RAPA administration in EAE. To evaluate the therapeutic effects of EPO/HSO supplement in comparison with RAPA, EAE was induced using myelin oligodendrocyte glycoprotein (MOG) peptide and complete Freund's adjuvant in C57BL/6 mice. The weight, clinical score, and histological findings were evaluated. Total mRNA was extracted from local lymph nodes and qRT-PCR was used for the purpose of the genes expression level of regulatory associated protein of TORC1 (RAPTOR) and IFN-γ. Our results indicated that the relative expression of RAPTOR and IFN-γ genes were significantly reduced in HSO/EPO, RAPA, and RAPA + HSO/EPO treated groups in comparison with the untreated group. Interestingly, histological findings have shown that the HSO/EPO treated group remarkably regenerated the myelin sheath, but this did not occur in the case of RAPA or combined RAPA and HSO/EPO treated groups. Our findings suggeste that HSO/HPO can be used as a potent immunomodulator and as a good candidate for re-myelination and downregulation of immune response for treatment of MS.
Keywords: Experimental autoimmune encephalomyelitis, Inflammation, Multiple sclerosis, Myelin sheath, Sirolimus
INTRODUCTION
The mammalian target of rapamycin (mTOR) signaling is an important intracellular pathway in the regulation of the cell cycle and many fundamental metabolic and physiological processes. mTOR pathway forms two structurally and functionally distinct complexes identified as mTORC1 in which mTOR is bound to regulatory associated protein of TORC1 (RAPTOR), sensitive to rapamycin (RAPA) and mTORC2 in which is mTOR bound to RAPA-insensitive companion of mTORC2 (RICTOR). mTORC2 is activated by growth factors, but, in contrast to mTORC1, seems to be nutrient-insensitive(1). The critical role for two complexes, in the activation, differentiation, and function of T helper (Th) cells has been strongly established, and in particular the expression of forkhead box P3 (FOXP3+), the 'master' transcription factor for T regulatory cells (Treg)(2).
Evidence has suggested that mTORC1 tends to promote Th1 cells differentiation(3), while mTORC2 may bias the response to Th2(4), whereas the inhibition of both mTOR complexes by RAPA is necessary for optimal Treg cell responses. Th17 cell development seems to be unrelated to TORC2 but is inhibited by RAPA in favor of Treg cells(5). Th1 cells, with the hallmark of the cytokine interferon gamma (IFN-γ) and Th17 cells interleukin-17 (IL-17) play critical roles in EAE pathogenesis, whereas Treg cells transforming growth factor-β (TGF-β) mediate immunological tolerance and reduce inflammation and prevent autoimmune diseases(6). Clinical observations suggest that defects of omega-6 and omega-3-polyunsaturated fatty acid (ω6-PUFAs and ω3-PUFAs) synthesis may be complicated in multiple sclerosis (MS)(7), and the risk of developing MS is related to increased dietary intake of saturated fatty acids (SFAs)(8). It has reported taht, PUFAs and antioxidant deficiencies along with reduced cellular antioxidant defense mechanisms have been detected in MS patients. Also PUFAs and antioxidant treatment in EAE have reduced the clinical signs of disease(8). ω3-PUFAs can suppress IFN-γ production in MS patients(9), and the effectiveness of ω6/ω3 essential fatty acids in the body reaches the optimal level when used at the same time in the ratio of 2.5/1(10). Accordingly, studies on the effect of HSO/EPO as a natural food/medicine with therapeutic and antioxidant properties have been performed in relapsing-remitting MS patients in clinical trials(11,12). The EPO (8-12% gamma-linolenic acid (GLA)) and HSO (6-8% GLA) are used for treating inflammatory condition. GLA is a functionally EFA that studies have confirmed its anti-inflammatory properties and it can correct the symptoms of EFA deficiency(13,14). The HSO/EPO could repair the phospholipids of cell membrane lipids by ω6/ω3 PUFAs in 2.3/1 ratio and modulate immunological responses by establishing a balance between Th1 and Th2 due to antioxidant compounds(11,12). RAPA or sirolimus is the natural product that was used as an immunosuppressive therapy in organ transplantation and cancer. Unlike chemotherapy in cancer that has not been paid to immune tendency(15), RAPA forms an immunosuppressive complex with intracellular protein, FK binding proteins (FKBP12) and blocks the activation of the mTOR kinase(16). Therefore, the current study was conducted to investigate the effects of RAPA, HSO/EPO, and the co-administration of the two agents on the weight, clinical score, histological findings, and expression of RAPTOR and IFN-γ genes in mononuclear lymph nodes cells in EAE mice.
MATERIALS AND METHODS
Animal
Female C57BL/6 mice (age, 6-8 weeks; weight, 18-22 g) were purchased from the Pasteur Institute of Iran, the Production and Research Complex. Female mice are superior to males for the study of neuropathic studies associated with MOG35-55-induced EAE. MOG35-55 produced behavioral signs of neuropathic hypersensitivity in females, but not males(17). All mice were housed in a specific pathogen-free environment. All experiments were performed in accordance with the animal care and the protocol of Urmia university of medical science, Urmia, I.R. Iran (Ethics committee approval No. IR.umsu.rec.1396.73).
Experimental autoimmune encephalomyelitis induction
For induction of chronic-EAE (C-EAE) in mice, the total volume of the emulsion containing a 1:1 ratio of MOG35-55(300 μg/mouse, Sigma-Aldrich, USA)/ complete Freund's adjuvant (Sigma-Aldrich, USA) was dissolved in phosphate buffer saline (PBS; Sigma-Aldrich, USA) and supplemented with Mycobacterium tuberculosis (500 μg/mouse, Sigma-Aldrich, USA) and administered in two different flanking sites to each mouse. Mice received pertussis toxin (500 ng/mouse, Sigma-Aldrich, USA) i.p. at the time of immunization and 48 h later(18).
Clinical evaluation
Mice were weighed daily and assessed for EAE clinical signs. Signs of ascending paralysis of EAE were scored according to the following criteria: (0 = no clinical signs; 0.5 = partly limp tail; 1 = paralyzed tail; 2 = hind limb paresis; 2.5 = one hind limb paralyzed; 3 = both hind limbs paralyzed; 3.5 = hind legs paralyzed and weakness in forelimbs; 4 = forelimbs paralyzed and 5 = moribund or dead)(19).
Experimental animal groups
After induction of EAE in mice, on the day 15 after immunization when the clinical signs of EAE appeared, based on routine clinical scoring for EAE(19), the animals were checked and scored. EAE mice were randomly assigned to 4 groups in comparison with naive control as follows:
Group A, EAE + RAPA + HSO/EPO (n = 6): mice subjected to EAE were treated with HSO/EPO (50 λ/mouse)(11)and RAPA (1 mg/kg/50 λ)(20); group B, EAE + RAPA (n = 6): mice subjected to EAE treated with RAPA (1 mg/kg/50 λ)(20); group C, EAE + HSO/EPO (n = 6): mice subjected to EAE treated with HSO/EPO (50 λ/mouse)(11); group D, EAE control (n = 6): mice subjected to EAE treated with ethyl alcohol diluted with distilled water (1:9)(20); group E, naive control (n = 5): mice subjected to EAE treated with ethyl alcohol diluted with distilled water (1:9)(20). Mice were treated until sacrificed on day 28 after immunization. RAPA was injected daily (i.p.) into groups A and B immediately after the onset of disease symptoms and HSO/EPO was administered orally to groups A and C.
Treatment of rapamycin and hemp seed/evening primrose oils
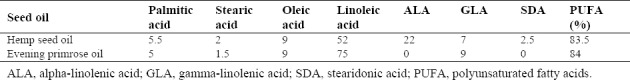
Pure HSO and EPO were isolated from the tops of fresh varieties of industrial seeds by the cold pressing standardized method, a mechanical extraction process, in Giah Essence Agro-Industry & Phytopharm Company, Gorgan, Golestan Province, I.R. Iran. The analysis of the fatty acids of the extracted oils was determined by gas chromatography (Table 1). RAPA in powder form (Santa Cruz Biotechnology, United States) was dissolved in 1 mL of ethyl alcohol (Merck, Germany) 1 mL of ethyl alcohol (Merck, Germany) which was diluted with distilled water. The RAPA solution was stored at 4 °C in the dark according to the manufacturer's instruction.
Table 1.
Fatty acid profiles (%) of hemp seed and evening primrose oils by gas chromatography.
Histological assessment
At the end of the experiment, animals were sacrificed after administration of (i.p.) ketamine/xylazine (80/10 mg/kg) and the brain tissue was isolated to assess clinical pathology. Histologically, EAE is characterized by a predominant cerebellar or brainstem involvement. The brain of the mice in each group was enucleated and fixed in a mixture of 10% formaldehyde and deionized water for 24 h, then dehydrated in graded ethanol solutions and embedded in paraffin wax. The thickness of each section was 6-7 μm approximately. The sections for histological examination were subjected to routine hematoxylin and eosin (H&E) and luxol fast blue (LFB) staining, to confirm infiltration of inflammatory cells, demyelination and re-myelination of axons after demyelination. We performed LFB staining that marks lipoproteins in myelin sheath and gives them a blue appearance under the light microscope (X400). LFB staining, of brain sections showed severe loss of LFB staining presenting myelin sheath loss. Demyelination and inflammation were determined by the presence of inflammatory cells and spongy tissue of the brain and the presence of strings of the non-myelin axon(21). The resulting slides each area of brain were stained with H&E and LFB was graded on a 4-point scale: 0 = not any pathology; 1 = not any tissue damage but slight inflammation; 2 = moderate inflammation, primary tissue damage and demyelination; 3 = moderate tissue destruction (demyelination, neuronal loss, tissue damage, cell death, neuronal vacuolation, neuronophagia); and 4 = necrosis (loss of all tissue elements completely with associated cellular remains). The zone with maximum tissue damage was used to evaluate each brain area(21).
Quantitative real time-polymerase chain reaction
In order to investigate the expression of IFN-γ and RAPTOR, real time-polymerase chain reaction (qRT-PCR) was performed. The total RNA was extracted from the lymph node cells using kit (Gene All, South Korea) after separation from the mice and the isolated RNA was reverse transcribed using random hexamer primers and reverse transcriptase kit (Gene All, South Korea).
The extracted RNA purity was measured by measuring the ratio of optical density at 260 nm to 280 nm.
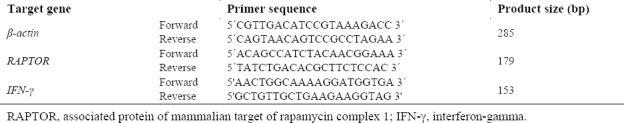
The cDNA was amplified using the SYBR-green PCR master mix kit (Ampliqon, Denmark) according to the advice of the manufacturer's directions. RT-PCR with gene-specific primers for RAPTOR, an essential component of mTORC1), IFN-γ and β-actin was done. RT-PCR with cDNAs of specific RAPTOR, IFN-γ, and β-actin were performed in the following condition: initial denaturation; 95 °C for 51 s, annealing; 60.5 °C for 60 s and β-actin were used as an internal control. Sequences of primers are presented in Table 2.
Table 2.
Primers sequences to evaluate the expression of RAPTOR, IFN-γ, and β-actin genes in lymph nodes cells.
A melting curve analysis was used to verify the specificity of the amplification reactions. For relative quantitation, the 2-ΔΔCT formula was used(22,23,24).
Statistical analysis
For statistical analysis and differences between groups, one-way analysis of variance (ANOVA) was performed, and all experiments were repeated in duplicate. P ≤ 0.05 was considered as statistically significant. Data presented as mean ± standard error of mean (SEM).
RESULTS
Clinical assessments
Clinical assessments include weight and clinical score (CS) as described in material and methods. The body weight of HSO/EPO treated group was significantly increased in comparison with EAE, RAPA + HSO/EPO, and RAPA groups (Fig. 1). The highest scores of mice were found in the RAPA + HSO/EPO and EAE groups, respectively. The lowest score was found primarily in the HSO/EPO group. ANOVA analysis showed that differences in clinical scores were statistically significant among the five groups. Furthermore, the comparisons among the different groups showed significantly higher and lower clinical EAE scores in RAPA + HSO/EPO and HSO/EPO respectively, 28 days after induction. We observed that HSO/EPO treatment significantly reduced the development of EAE (Fig. 2).
Fig. 1.
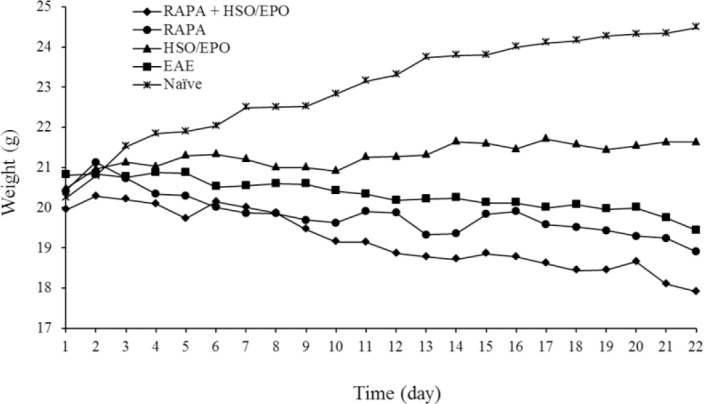
Weighing of EAE animals in comparison with naïve mice. Data presented as mean ± SEM. EAE, experimental autoimmune encephalomyelitis; RAPA, rapamycin; HSO/EPO, hemp seed oil/evening primrose oil.
Fig. 2.
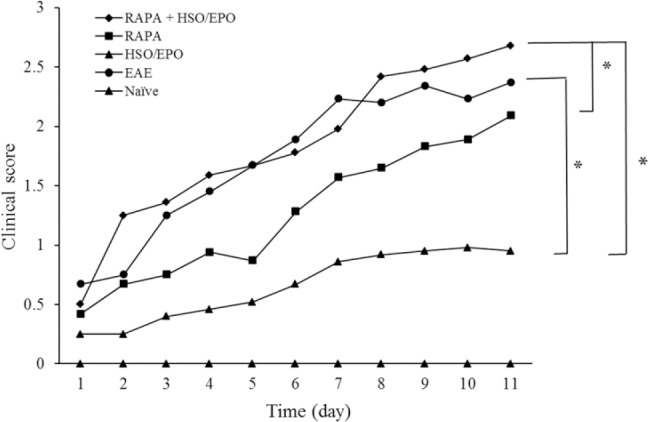
Clinical course of EAE in animals. Data presented as mean ± SEM. *P value ≤ 0.05 is significant. EAE, experimental autoimmune encephalomyelitis; RAPA, rapamycin; HSO/EPO, hemp seed oil/evening primrose oil.
Histological analysis
Sections were stained with H&E and LFB to evaluate the infiltration of inflammatory cells, spongiotic zones, and loss in the brain tissue of EAE. To assess demyelination and re-myelination after demyelination, we used the previous described method(21,25)and showed a presence of spongiform degenerations in the brain tissue, which confirmed the demyelinated zones(21).
Histological analysis (Fig. 3) showed that treatment with HSO/EPO significantly reduced the infiltration of inflammatory cells and promoted re-myelination in comparison with RAPA, RAPA + HSO/EPO, and EAE control groups. As shown in (Fig. 3 A2,3 and B2,3), co-administration of both RAPA and HSO/EPO was associated with more demyelination compared to RAPA and the HSO/EPO treated groups (P = 0.000).
Fig. 3.
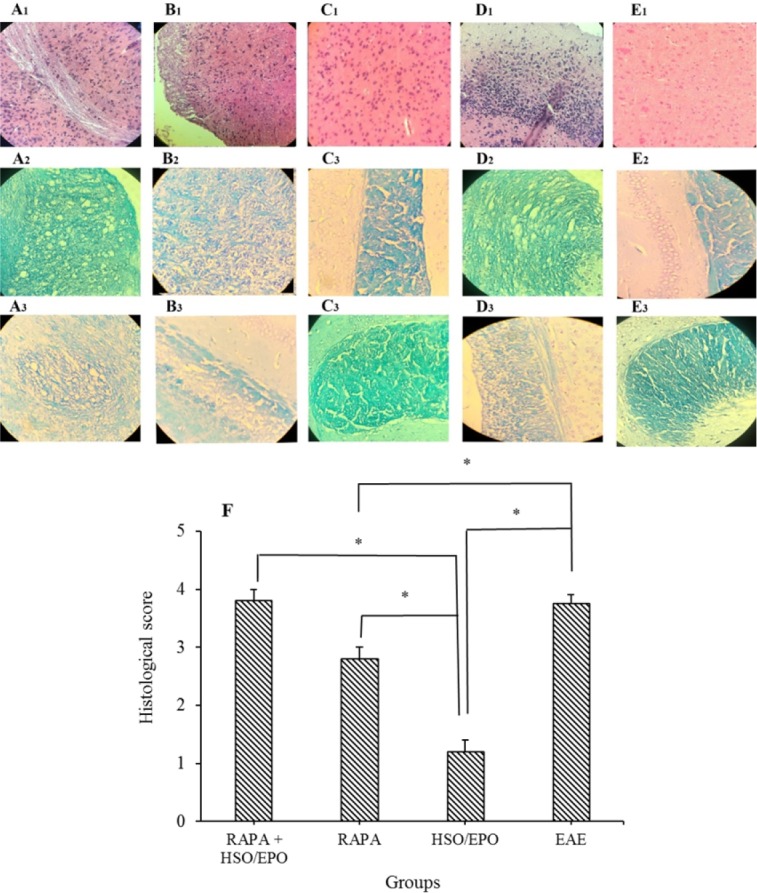
Pathological analysis of brain sections. Group A, RAPA + HSO/EPO-treated mice showed (A1) a large number of inflammatory cells, strings of the non-myelin axon, neuronophagia, (A2) vacuolation, spongy lesions, and (A3) extensive demyelination; group B, rapamycin-treated mice showed (B1) infiltration of inflammatory cells, (B2) spongiotic zones, and (B3) demyelination; group C, HSO/EPO-treated mice showed (C1), a few inflammatory cells and (C2,3) without spongy lesions and demyelination; group D, EAE mice showed (D1) extensive focal inflammatory cells, (D2) extensive vacuolation, zones of spongy degeneration, and (D3) demyelination; group E1-3, the section of the brain of naive mice exhibited no clinical signs. The first row was stained with H&E, the second and third rows were stained with LFB; F, histological score: 0 = no pathology, 1 = no tissue damage but minor inflammation, 2 = modest inflammation, prime tissue damage and demyelination, 3 = moderate tissue damage (demyelination, neuronal loss, tissue damage, cell death, neuronal vacuolation, and neuronophagia), 4 = necrosis (loss of all tissue elements entirely with associated cellular remains). Data presented as mean ± SEM. *P value ≤ 0.05 is significant. EAE, experimental autoimmune encephalomyelitis; HSO/EPO, hemp seed oil/evening primrose oil; RAPA, rapamycin; H&E, hematoxylin and eosin; LFB, luxol fast blue.
We found that the anti-inflammatory effects of HSO/EPO are uniquely effective than RAPA, and this effect is related to the quality of the combination of fatty acids and antioxidant properties, which prevents demyelination process and promotes regeneration of myelin sheath. In most cases of HSO/EPO group, the white matter was judged free of inflammatory cells. These results indicate that administration of HSO/EPO after EAE induction can induce specific tolerance and eliminate acute tissue damage associated with clinical EAE.
The relative expression of mTORC1 and IFN-γ genes in the extracted mononuclear cells
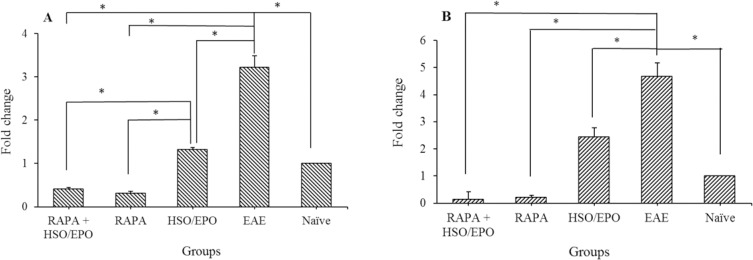
To evaluate the anti-inflammatory effects of HSO/EPO in comparison with rapamycin, we analyzed the expression patterns of RAPTOR and IFN-γ genes in all groups of the lymph nodes. As illustrated in (Fig. 4), it has shown that the expression of IFN-γ mRNA, as well as RAPTOR, is significantly reduced in mice treated with HSO/EPO in comparison with the EAE group (IFN-γ, P = 0.003; RAPTOR, P = 0.000) but the expression of IFN-γ and mTORC1 mRNAs are not completely suppressed as we can see in rapamycin treated groups. Although the expression of mTORC1 in HSO/EPO, rapamycin + HSO/EPO and rapamycin treated groups is significantly down-regulated, but due to the fundamental roles of mTORC1 in cell metabolism regulation, the complete down-regulation of mTORC1 is not interested. Thus, from the point of this view, only the HSO/EPO treated group is not statistically different from normal mice. Interestingly, similar to rapamycin, HSO/EPO has a great effect on IFN-γ down-regulation which suggests the immunomodulatory properties of HSO/EPO (P = 0.000).
Fig. 4.
The fold changes in the mRNA expression of mTORC1 and IFN-γ genes in the lymphocytes. The expression of (A)RAPTOR and (B)IFN-γ in the HSO/EPO had a significant reduction compared with the EAE group. Data presented as mean ± SEM. *P value ≤ 0.05 is significant. mTORC1, mammalian target of rapamycin complex 1; IFN-γ, interferon gamma; RAPTOR, regulatory associated protein of TORC1; HSO/EPO, hemp seed oil/evening primrose oil; EAE, experimental autoimmune encephalomyelitis.
DISCUSSION
MS is a chronic inflammatory and neurodegenerative disease of the brain and spinal cord, which leads to disability and functional loss due to demyelination and neuronal injury(26). SFAs, animal fat without fish fat is positively correlated with MS mortality. While PUFAs may not only exert immunosuppressive actions through their incorporation in immune responses but also may affect cell function within the central nervous system (CNS). Antioxidants can support cellular defenses in various ways, including radical scavenging, interfering with gene transcription, mRNA expression, enzyme activity, and chelation. Both dietary antioxidants and PUFAs have the potential to reduce disease symptoms by targeting specific mechanisms and supporting recovery in MS(8). HSO has been used to treat various disorders for many years in traditional medicine. Recent clinical trials have known HSO as a functional food(27). HSO has over 80% PUFAs and is a remarkably rich source of the two essential fatty acids (LA and alpha-LA). The ω6/ω3-PUFA ratio in HSO is routinely between 2:1 and 3:1, which is considered optimal for human health(10). From a nutritional point of view, up to 7% GLA and 2.5% stearidonic acid (SDA or STA) is very remarkable(28). The corporation of both GLA and SDA in HSO, naturally at a favorable ω6/ω3 ratio of 2:1, allows this enzymatic phase with delta-6-desaturase to be efficiently bypassed(29). The delta-6-desaturase, which is the slowest and rate-limiting step in the metabolic pathway to GLA, catalyzes this reaction. GLA is rapidly elongated to dihomo-gamma-LA (DGLA) by the elongase enzyme, and DGLA is acetylated and incorporated into the cell membrane phospholipids. DGLA competes with the arachidonic acid for cyclooxygenase (COX) enzymes, and the metabolites of DGLA and arachidonic acid that are produced by the prostaglandins E3 and E2, respectively have actions that oppose each other. Following an inflammatory stimulus in MS-like diseases, proinflammatory eicosanoids derived from arachidonic acid are prostaglandin E2, whereas GLA and DGLA produce anti-inflammatory eicosanoids including prostaglandin E1 and prostaglandin E3 that inhibit IFN-γ, tumor necrosis factor-α, IL-1β and IL-6(30). Analysis revealed that HSO contains over 3.6-6.7 g antioxidant/kg oil including terpenes, phytosterols, and tocopherols and polyphenols(28). The antioxidant properties of HSO are scavenger free radicals and regulate signaling pathways to modulate inflammatory reactions(31,32). EPO is rich in GLA that precursors of anti-inflammatory eicosanoids, which are elements of cell membranes. The metabolism of dietary GLA ultimately leads to PGE1, which has an effective anti-inflammatory activity and is regularly recommended for the treatment of inflammatory and autoimmune disorders(33). The earliest results in the use of EPO and colchicine combined therapy in MS patients suggest that it may be of considerable value(34). Rapamycin is a natural product produced by Streptomyces hygroscopic strain, which was used as an immunosuppressive therapy in organ transplantation and certain types of cancer(16). The adverse side effects of RAPA include anemia, thrombocytopenia, nausea, headache, fever, urinary tract infection, and interstitial pneumonitis(35), limited the usefulness of this drug. RAPA predominantly inhibits differentiation of effector T cells by inhibiting mTORC1 and mitigating Th1 and Th17 differentiation but not Th2 cells(36). As shown in our experiment, the expression of mTORC1 mRNA levels was elevated in EAE mice while, an immunomodulatory agent (HSO/HPO) could reduce the inflammatory condition and improve re-myelination in comparison with RAPA in EAE mice alone, but not along with RAPA. In our previous study, HSO/EPO was shown to reduce the secretion of inflammatory cytokines. The mechanism by HSO/EPO exerts its beneficial effects is not limited to attenuating the inflammation, but it is also related to its effects on phospholipids synthesis in erythrocytes membrane cells in MS patients(37).
Analyzes have shown that the expression of mTORC1 and IFN-γ mRNA was significantly reduced both in RAPA and HSO/EPO treated groups compared to EAE group. According to previous findings(38), our data indicated that completely down-regulation of RAPTOR is not appropriate and it is largely because of the contribution of RAPTOR in Th2 cell differentiation. Also, histological findings showed that HSO/EPO group had less activated inflammatory cells and spongy degeneration in the brain tissue. Likely, HSO/EPO affects the activation of inflammatory cells and promoting re-myelination in the CNS tissue. Since re-myelination occurs during the initial phases of the disease, this is rare in more developed stages(39). Additionally, we found that co-administration of RAPA and HSO/EPO was not effective as HSO/EPO, mainly due to the deleterious effects of RAPA on the suppression of the mTORC1 pathway and consequently as a result of the accumulation of fatty acids in the blood stream and causes a condition similar to the metabolic syndrome. Evidence suggests that chronic inhibition of mTOR with RAPA leads to an exacerbation of hyperglycemia and insulin resistance(40). Since the cells cannot absorb fatty acids from the bloodstream, the accumulation of fatty acids causes metabolic disturbances to exacerbate inflammation and thus lead to myelin degradation. Therefore, that treatment with RAPA+ HSO/EPO led to greater vacuolation, spongy lesions, and demyelination in the brain. The addition of RAPA to the HSO/EPO re-myelination model appeared to prevent re-myelination from occurring during the treatment period. The findings with the identified adverse effects of RAPA on the uptake of fatty acids in the RAPA+ HSO/EPO group may well explain the reduction in weight.
Inhibitory effects of RAPA on mTOR not only enhance demyelination in the HSO/EPO model but also provide insight into signaling mechanisms through mTOR that regulate re-myelination. Therefore, our finding according to reports of others(41)indicating the efficacy of RAPA-mediated inhibition of mTOR, possibly reduced signaling through RAPTOR, as well as spongy degeneration and demyelination, was observed in mice treated with RAPA alone and RAPA+ HSO/EPO. These data support the role of mTOR, and more specifically mTORC1, as a positive mediator in the context of re-myelination. In the RAPA treated group, despite the IFN-γ suppression, there is a significant demyelination and infiltration of inflammatory cells. The RAPA inhibitory effects on mTORC1 expression cause disturbance in the USFAs in cellular structures and USFAs cannot be corporate in cell membranes, especially myelin(42). Also RAPA or mTORC1 inhibitor promotes lipolysis by enhancing protein kinase A-mediated phosphorylation of the hormone-sensitive lipase(43). It was reported that ω3-LC-PUFAs mediated function modulates mTORC1 activity and may increase Treg cell count and function(44). Therefore, the quality of fatty acids, including reduced proportions of ω6/ω3 fatty acids or intake increased ω3-PUFAs, could alleviate autoimmune diseases by modulating Th1/Th2/Th17 cells and upregulating Treg cells through control of mTOR signaling such as RAPA(37,45,46). Finally, HSO/EPO treatment improved the histological EAE score by attenuating infiltration of inflammatory cells and promoting re-myelination, suggesting a protective effect in CNS tissues. The route of administration, oral, is another beneficial effect of our experiment. It is suggested that oral administration of polyphenolic compounds in HSO/EPO, which contains a mixture of various chemicals with potential bio-reactivity(37), could be a reasonable way of using this supplement in MS patients.
CONCLUSION
Taken together, our research has shown that HSO/EPO may be an effective treatment for cognitive impairment of MS patients to promote re-myelination, whereas RAPA treatment has not been reported to be effective in EAE or MS treatment by promoting myelin sheath regeneration.
ACKNOWLEDGMENTS
The content of this paper is extracted from the Ph.D thesis (No. 139501002835) which was financially supported by the Research Deputy of Cellular and Molecular Research Center, Cellular and Molecular Medicine Institute Urmia University of Medical Sciences, Urmia, I.R. Iran.
REFERENCES
- 1.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205(3):565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32(6):743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7(13):1819–1824. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korn T, Anderson AC, Bettelli E, Oukka M. The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis. J Neuroimmunol. 2007;191(1-2):51–60. doi: 10.1016/j.jneuroim.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghadirian P, Jain M, Ducic S, Shatenstein B, Morisset R. Nutritional factors in the aetiology of multiple sclerosis: a case-control study in Montreal, Canada. Int J Epidemiol. 1998;27(5):845–852. doi: 10.1093/ije/27.5.845. [DOI] [PubMed] [Google Scholar]
- 8.van Meeteren ME, Teunissen CE, Dijkstra CD, van Tol EA. Antioxidants and polyunsaturated fatty acids in multiple sclerosis. Eur J Clin Nutr. 2005;59(12):1347–1361. doi: 10.1038/sj.ejcn.1602255. [DOI] [PubMed] [Google Scholar]
- 9.Gallai V, Sarchielli P, Trequattrini A, Franceschini M, Floridi A, Firenze C, et al. Cytokine secretion and eicosanoid production in the peripheral blood mononuclear cells of MS patients undergoing dietary supplementation with n-3 polyunsaturated fatty acids. J Neuroimmunol. 1995;56(2):143–153. doi: 10.1016/0165-5728(94)00140-j. [DOI] [PubMed] [Google Scholar]
- 10.Simopoulos AP, Leaf A, Salem N., Jr Workshop statement on the essentiality of and recommended dietary intakes for Omega-6 and Omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2000;63(3):119–121. doi: 10.1054/plef.2000.0176. [DOI] [PubMed] [Google Scholar]
- 11.Rezapour-Firouzi S, Arefhosseini SR, Mehdi F, Mehrangiz EM, Baradaran B, Sadeghihokmabad E, et al. Immunomodulatory and therapeutic effects of Hot-nature diet and co-supplemented hemp seed, evening primrose oils intervention in multiple sclerosis patients. Complement Ther Med. 2013;21(5):473–480. doi: 10.1016/j.ctim.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Rezapour-Firouzi S, Arefhosseini SR, Ebrahimi-Mamaghani M, Farhoudi M, Baradaran B, Ali TM, et al. Erythrocyte membrane fatty acids in multiple sclerosis patients and hot-nature dietary intervention with co-supplemented hemp-seed and evening-primrose oils. Afr J Tradit Complement Altern Med. 2013;10(6):519–527. doi: 10.4314/ajtcam.v10i6.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassam AG, Rivers JP, Crawford MA. Metabolism of gamma-linolenic acid in essential fatty acid-deficient rats. J Nutr. 1977;107(4):519–524. doi: 10.1093/jn/107.4.519. [DOI] [PubMed] [Google Scholar]
- 14.Hassam AG. The influence of alpha-linolenic acid (18: 3omega3) on the metabolism of gamma-linolenic acid (18: 3omega6) in the rat. Br J Nutr. 1977;38(1):137–140. doi: 10.1079/bjn19770069. [DOI] [PubMed] [Google Scholar]
- 15.Mohammadian M, Zeynali S, Azarbaijani AF, Khadem Ansari MH, Kheradmand F. Cytotoxic effects of the newly-developed chemotherapeutic agents 17-AAG in combination with oxaliplatin and capecitabine in colorectal cancer cell lines. Res Pharm Sci. 2017;12(6):517–525. doi: 10.4103/1735-5362.217432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35(3 Suppl):7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 17.Rahn EJ, Iannitti T, Donahue RR, Taylor BK. Sex differences in a mouse model of multiple sclerosis: neuropathic pain behavior in females but not males and protection from neurological deficits during proestrus. Biol Sex Differ. 2014;5(1):4–21. doi: 10.1186/2042-6410-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammadzadeh A, Pourfathollah AA, Shahrokhi S, Fallah A, Tahoori MT, Amari A, et al. Evaluation of AD-MSC (adipose-derived mesenchymal stem cells) as a vehicle for IFN-β delivery in experimental autoimmune encephalomyelitis. Clin Immunol. 2016;169:98–106. doi: 10.1016/j.clim.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Benson JM, Campbell KA, Guan Z, Gienapp IE, Stuckman SS, Forsthuber T, et al. T-cell activation and receptor downmodulation precede deletion induced by mucosally administered antigen. J Clin Invest. 2000;106(8):1031–1038. doi: 10.1172/JCI10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lisi L, Aceto P, Navarra P, Dello Russo C. mTOR kinase: a possible pharmacological target in the management of chronic pain. Biomed Res Int. 2015. 2015 doi: 10.1155/2015/394257. Article ID: 394257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangalam AK, Luo N, Luckey D, Papke L, Hubbard A, Wussow A, et al. Absence of IFN-gamma increases brain pathology in experimental autoimmune encephalomyelitis-susceptible DRB1*0301. DQ8 HLA transgenic mice through secretion of proinflammatory cytokine IL-17 and induction of pathogenic monocytes/microglia into the central nervous system. J Immunol. 2014;193(10):4859–5870. doi: 10.4049/jimmunol.1302008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machado AM, de Souza WM, de Padua M, da Silva Rodrigues Machado AR, Figueiredo LT. Development of a one-step SYBR Green I real-time RT-PCR assay for the detection and quantitation of Araraquara and Rio Mamore hantavirus. Viruses. 2013;5(9):2272–2281. doi: 10.3390/v5092272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11(1):107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Nun A, Mendel I, Bakimer R, Fridkis-Hareli M, Teitelbaum D, Arnon R, et al. The autoimmune reactivity to myelin oligodendrocyte glycoprotein (MOG) in multiple sclerosis is potentially pathogenic: effect of copolymer 1 on MOG-induced disease. J Neurol. 1996;243(4 Suppl 1):S14–S22. doi: 10.1007/BF00873697. [DOI] [PubMed] [Google Scholar]
- 27.Callaway JC. Hempseed as a nutritional resource: An overview. Euphytica. 2004;140(1-2):65–72. [Google Scholar]
- 28.Matthaus B, Brühl L. Virgin hemp seed oil: An interesting niche product. Eur J Lipid Sci Technol. 2008;110(7):655–661. [Google Scholar]
- 29.Okuyama H, Kobayashi T, Watanabe S. Dietary fatty acids the N-6/N-3 balance and chronic elderly diseases. Excess linoleic acid and relative N-3 deficiency syndrome seen in Japan. Prog Lipid Res. 1996;35(4):409–457. doi: 10.1016/s0163-7827(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 30.Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. Curr Opin Clin Nutr Metab Care. 2001;4(2):115–121. doi: 10.1097/00075197-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Oomah BD, Busson M, Godfrey DV, Drover JCG. Characteristic of hemp (Cannabis sativa L.) seed oil. Food Chemistry. 2002;76(1):33–43. [Google Scholar]
- 32.Christie WW. The analysis of evening primrose oil. Ind Crops Prod. 1999;10(2):73–83. [Google Scholar]
- 33.Taylor M. Alternative medicine and the perimenopause an evidence-based review. Obstet Gynecol Clin North Am. 2002;29(3):555–573. doi: 10.1016/s0889-8545(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 34.Horrobin DF. Multiple sclerosis: the rational basis for treatment with colchicine and evening primrose oil. Med Hypotheses. 1979;5(3):365–378. doi: 10.1016/0306-9877(79)90018-5. [DOI] [PubMed] [Google Scholar]
- 35.Merkel S, Mogilevskaja N, Mengel M, Haller H, Schwarz A. Side effects of sirolimus. Transplant Proc. 2006;38(3):714–715. doi: 10.1016/j.transproceed.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 36.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rezapour-Firouzi S. Herbal Oil Supplement With Hot-Nature Diet for Multiple Sclerosis. In: Watson RR, Killgore WDS, editors. Nutrition and Lifestyle in Neurological Autoimmune Diseases. 1st ed. Academic Press; 2017. pp. 229–245. [Google Scholar]
- 38.Yang K, Shrestha S, Zeng H, Karmaus PW, Neale G, Vogel P, et al. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity. 2013;39(6):1043–1056. doi: 10.1016/j.immuni.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lassmann H, Bruck W, Lucchinetti C, Rodriguez M. Remyelination in multiple sclerosis. Mult Scler. 1997;3(2):133–136. doi: 10.1177/135245859700300213. [DOI] [PubMed] [Google Scholar]
- 40.Pavlakis M, Goldfarb-Rumyantzev AS. Diabetes after transplantation and sirolimus: what's the connection. J Am Soc Nephrol? 2008;19(7):1255–1256. doi: 10.1681/ASN.2008050474. [DOI] [PubMed] [Google Scholar]
- 41.Sachs HH, Bercury KK, Popescu DC, Narayanan SP, Macklin WB. A new model of cuprizone-mediated demyelination/remyelination. ASN Neuro. 2014;6(5):pii:1759091414551955. doi: 10.1177/1759091414551955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids and the brain: from infancy to aging. Neurobiol Aging. 2005;26(Suppl 1):98–102. doi: 10.1016/j.neurobiolaging.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Brown NF, Stefanovic-Racic M, Sipula IJ, Perdomo G. The mammalian target of rapamycin regulates lipid metabolism in primary cultures of rat hepatocytes. Metabolism. 2007;56(11):1500–1507. doi: 10.1016/j.metabol.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4653–4658. doi: 10.1073/pnas.1000083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 46.Yuan LF, Li GD, Ren XJ, Nian H, Li XR, Zhang XM. Rapamycin ameliorates experimental autoimmune uveoretinitis by inhibiting Th1/Th2/Th17 cells and upregulating CD4+CD25+ Foxp3 regulatory T cells. Int J Ophthalmol. 2015;8(4):659–664. doi: 10.3980/j.issn.2222-3959.2015.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]