Abstract
Helicteres isora L. (H. isora) has been used in traditional medicine in Asia. This study was aimed to determine biological activities of H. isora fruit extracts. Chemopreventive effect was examined by cell proliferation assay and differentiation-inducing effect. Anti-inflammatory activity of extracts was studied on the levels of nitric oxide (NO), tumor necrosis factor alpha (TNF-α), production of prostaglandin E2 (PGE-2), and cyclooxygenas-2 (COX-2). Cell proliferation assay revealed that H. isora extracts and its major compound, rosmarinic acid, showed no cytotoxicity in THP-1 and RCM-1 cells. Methylthio acetic acid from Cucumis melo var.conomon used as a positive control and 80% ethanol extracts demonstrated significant cell differentiation induction. Hexane extract of H. isora could lower the levels of TNF-α, PGE-2, and NO in THP-1 cells with 51.61 ± 0.79%, 69.68 ± 0.017%, and 69.93 ± 9.41% inhibition, respectively. The highest inhibitory effect on COX-2 was obtained from dichloromethane extract. Dexamethasone inhibited the secretion of TNF-α with 95.82 ± 0.50% while celecoxib showed the inhibitory effect on COX-2 and PGE-2 with 100% and 99.86%, respectively. The ethanol extract showed the best antioxidant activity by DPPH and FRAP assays at IC50 of 5.43 ± 1.01 μg/mL and 22.83 ± 0.13 mmol FeSO4/g sample, respectively, while the positive control, trolox, showed the antioxidant activity with IC50 and FRAP values at 4.08 ± 0.85 μg/mL and 10.84 ± 0.04 mmol FeSO4/g sample, respectively. Taken together, H. isora possess chemopreventive and antioxidant activity. Further studies on in vivo activities of this plant are suggested.
Keywords: anti-inflammation, anti-oxidation, chemoprevention, Helicteres isora L.
INTRODUCTION
Helicteres isora L. (H. isora) or East Indian screw tree is belonging to Sterculiaceae family usually found in Asia and has been widely used in traditional medicine(1). Fruit, root, and bark of this plant are mild astringents used for flatulence and skin irritation(2,3). Acetone extracts of dried H. isora fruits showed strong antioxidant activity compared to hexane and iso-propyl alcohol extracts and also exhibited cytotoxicity against human lung cancer cells (NCI-H460)(3). Antimicrobial activity of methanolic extract, isolated alkaloids, flavonoids and phenolic compounds from dried fruits were tested against Escherichia coli, Pseudomonas aeruginosa, Salmonella abony, and Staphylococcus aureus by cup-plate diffusion method. The methanolic extracts of H. isora bark have been reported on anthelmintic activity against Indian adult earthworms (Pheretima posthuma) and showed the best activity at 50 mg/mL with paralysis time of 12.54 min and death time of 16.55 min when compared to the standard albendazole(4). Anti-inflammatory activity from the methanolic extract of stem bark was demonstrated in carrageenan-induced inflammatory in albino rats which showed stronger activity as compared to the petroleum ether extract(5).
The hexane, ethanol, and water extracts could inhibit cyclooxygenase-2 (COX-2) more than COX-1 as determined by colorimetric COX inhibitor screening assay kit(6).
Recently, three major compounds of H. isora have been identified which were 4'-O-β-D-glucopyranosyl rosmarinic acid, 4,4'-O-di-β-D-glucopyranosyl rosmarinic acid, and 2R-O-(4'-O-β-D-glucopyranosyl caffeoyl)-3-(4-hydroxyphenyl) lactic acid. Leaves were isolated and characterized for new flavones, 7,41-di-o-methyl isoscutellarein i.e.(5,8-dihydroxy-7,41 flavones) along with kaempferol-3-o-galactoside (trifolin) and herbacetin-8-o-glucoronide (hibifolin). Stem barks contain chloroplasts, pigments, phytosterols, hydroxyl carboxylic acid, orange-yellow colouring matter, saponins, phlobotannins, sugars, and lignins. Seeds possess phytosterols, fixed oils and fats, phenolic compounds, tannins, amino acid, and carbohydrates. Cucurbitacin B and isocucurbitacin B are presented in roots(1).
According to the American Cancer Society, colorectal cancer was the second most leading cause of cancer death in both men and women in the United States in 2016(7). It has been ranked only behind breast, cervix, liver, and bile duct cancers in women(8). If colorectal cancer invades the wall of colon or rectum through the blood or lymph vessels, cancer can metastasize to other parts of body such as ovary or breast(9). The diagnosis of colorectal cancer in the early stage is important for therapeutic efficacy but unfortunately, most cancers are detected in the late stage(10). Although several therapeutics including surgery, chemotherapy, and radiotherapy are available, some patients still suffer from serious side effects after therapy. The concept of chemoprevention is mainly to reverse, suppress cell proliferation or prevent carcinogenesis by using natural compounds such as phytochemicals or biological agents(11,12,13). Phytochemicals derived from many other fruits and vegetables such as diallyl sulfide, capsaicin, curcumin, eugenol and polyphenols have been reported on chemopreventive activity(14,15,16). Nevertheless, there have been no data in the literature on bioactivities of the extracts from the fruits of H. isora, which encourages the investigation of its biological potential. Therefore, in this study, H. isora fruit extracts were evaluated for chemopreventive effect and other biological activities.
MATERIALS AND METHODS
Preparation of Helicteres isora fruit extracts
The fruit samples of H. isora used in this study were collected from Kanchanaburi, Thailand. Verification of the plant materials was done by using the taxonomic key in the Flora of Thailand (volume six, part two)(17)and a voucher specimen (PBM 05249) was deposited at the Herbarium of Department of Pharmaceutical Botany (PBM), Faculty of Pharmacy, Mahidol University, Bangkok, Thailand. Dried H. isora. fruits were sliced into small pieces and grounded to powder. The extractions were performed by maceration using hexane, dichloromethane, 80% ethanol, and water. The plant powders were separately extracted three times by maceration with three different solvents of hexane, dichloromethane, and 80% ethanol for 72 h at room temperature. The water extract was boiled twice at 80 °C for 30 min. Each extract was filtered and the filtrate was evaporated by a rotary evaporator at 40 °C and then freeze-dried to yield each crude extracts. All extracts were stored in refrigerator at 4 °C until used.
Cell cultivation
Human rectum adenocarcinoma cells (RCM-1) were maintained in 45% RPMI1640 medium with 45% Ham's F12 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% streptomycin and penicillin G. Human monocytic leukemia cell line (THP-1 cells) was cultured in RPMI1640 medium supplemented with 10% FBS, 3.5 μL/L 2-mercaptoethanol, 0.99 g/L glucose, 1% L-glutamine and prevented microbial contamination by streptomycin and penicillin G. The cells were incubated at 37 °C under a humidified 5% CO2 incubator(18,19,20).
Cell proliferative assay
To determine the effect of fruit extract on H. isora cell proliferation, RCM-1 and THP-1 cells were examined by 3-(4 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Rosmarinic acid (Sigma, USA) which was previously reported to be one of the major compounds of H. isora fruits was also tested. The 105 cultured cells were seeded per well on 96-well plates. THP-1 cells were added with 40 ng/mL phorbol-12-myristate-13-acetate (PMA). Both cells were incubated for 48 h at 37 °C in 5% CO2 incubator. After the medium was removed, various concentrations of the plants extracts (0-1000 μg/mL) were added into triplicate wells to assure the reliable results. After 48 h, the samples were replaced with MTT reagent then incubation for 4 h. Isopropanol was added to dissolve formazan crystals. The values were measured by spectrophotometer at 570 nm(21).
Assay for differentiation-inducing effect
The RCM-1 colon cancer cell line is well-differentiated rectum adenocarcinoma derived from a 73-year-old female human and was kindly provided by Kyoto Prefectural University, Japan. The RCM-1 cell line is characterized as a partially-differentiated and spontaneously differentiated as determined by the formation of ducts resembling villiform structures. RCM-1 cells were seeded into 96-well flat bottom culture plates at 105 cells/well before incubating overnight or 90% exhibited contact-insensitive growth at 37 °C in 5% CO2. The cells were then treated in triplicate with various concentrations of the plants extracts from 0-200 μg/mL. Methylthio acetic acid (MTA) from Japanese pickling melon Cucumis melo var. conomon was used as a positive control. The duct formations were measured after treated with samples for 72 h(19).
Differentiation and stimulation of human monocytic leukemia cell line
Various concentrations of the extracts were added in each well. Dexamethasone at 10 and 100 μg/mL and 0.05 μM celecoxib were used as the positive controls. Dimethyl sulfoxide (DMSO) was used as a negative control. Lipo-polysaccharide (LPS) was added to stimulate inflammation. The supernatants were kept from each well and centrifuged before stored at -40 °C until analyzed. The cells were then scraped and kept in tris (hydroxymethyl)aminomethane hydrochloride (Tris-HCl) pH 7.8 containing 1 mM ethylenediaminetetraacetic acid (EDTA) and (4 -(2 -hydroxyethyl)- 1 -piperazine-ethanesulfonic acid) (HEPES) buffer pH 7.2 for further analysis(20).
Determination of tumor necrosis factor-alpha and nitric oxide levels
The treated cell supernatants were analyzed according the manual of cytokine specific sandwich quantitative enzyme-linked immunosorbent assays (ELISAs) specifically designed for tumor necrosis factor-alpha (TNF-α) detection. The plate was specifically determined at 450 nm within 30 min. For nitric oxide (NO), the supernatant in LPS-stimulated THP-1 cells was mixed with Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% naphthylethylenediamine dihydrochloride in water) in 96-well plates and incubated for 5 min at 18-25 °C before measured the absorbance at 540 nm(22).
Prostaglandin E2 and cyclooxygenase-2 inhibitory activity
The production of prostaglandin E2 (PGE-2) was measured by enzyme immunoassay kit (Cayman Chemical, USA). The supernatants in LPS-stimulated THP-1 cells were collected according to the instructions of the manufacturer. The plate was read at a wavelength between 405 and 420 nm(23).
The LPS-stimulated THP-1 cells were tested for COX-2 by enzyme immunoassay kit. The COX activity assay kit (Cayman Chemical, USA) were detected for colorimetric oxidized N,N,N',N'-tetramethyl-p-phenylenediamine (TMPD) that produced by peroxidase activity of COX. To determine COX-2 activity, the COX-1 inhibitor (SC-560) was added into each well and the results of absorbance were used to subtract from those of total COX activity. The plate was read at 590 nm(24).
Antioxidation activity
Radical scavenging activity of the plant extracts were examined by the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) radical scavenging assay. Reaction mixtures contained various concentrations of plants extracts with 0.1 mM DPPH (Sigma, USA) methanolic solution. Trolox (Sigma, USA) was used as a positive control and DPPH methanolic solution was set as a blank. The plate was kept in the dark for 30 min and the results were measured at 517 nm(25). Percent DPPH radical scavenging activity was calculated using equation 1 in which Ab is the absorbance at 517 nm.
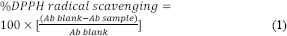
For ferric reducing ability of plasma (FRAP) assay, FRAP reagent was prepared freshly by adding acetic buffer (300 mM), 2,4,6-tripyridyl-s-triazine (TPTZ) (Sigma, USA) solution (10 mM), FeCl3·6H2O (20 mM) and distilled water. The FRAP reagent was kept in water bath at 37°C during use. Various concentrations of crude extracts with FRAP reagent was added in cuvette and incubated for 3 min. The absorbance was read at 593 nm and distilled water with FRAP reagent set as blank zero(26).
RESULTS
Differentiation-inducing effect of Helicteres isora fruit extracts
H. isora fruit extracts and one of its major compounds, rosmarinic acid, were studied on differentiation-inducing effect in human colon cancer cells (RCM-1). The RCM-1 cell line was human primary rectum adenocarcinoma which was characterized as spontaneously differentiated cells by the formation of ducts resembling villiform structures. MTA and DMSO were used as positive and negative controls in this study, respectively. The results demonstrated that MTA and the ethanol extract of H. isora effectively induced RCM-1 cell differentiation while other solvent extracts and rosmarinic acid showed no significant effect (Table 1). When treated with 80% ethanol extract of H. isora, the highest numbers of duct formation appeared at 100 μg/mL of the extract. The positive control, MTA gave the best ability in duct formation at 200 μg/mL (Fig. 1).
Table 1.
Differentiation-inducing effects of Helicteres isora fruit extracts and methylthio acetic acid on human rectum adenocarcinoma cells cells.
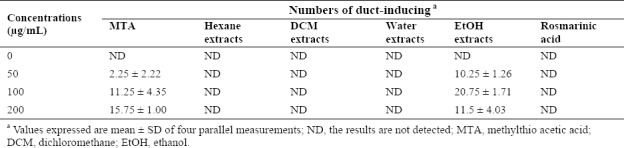
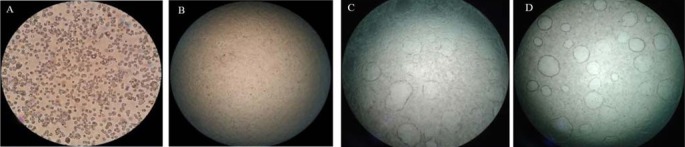
Fig. 1.
Effects of Helicteres isora fruit extracts on the duct formation. (A) Morphology of RCM-1 cells in a well at 1 × 105 cells. (B) RCM-1 cells treated with the medium and 0.1% of DMSO as a negative control. (C) Duct formation of RCM-1 cells treated with 200 μg/mL methylthio acetic acid as a positive control. (D) Duct formation of RCM-1 cells treated with 80% ethanol of Helicteres isora fruit extract at 100 μg/mL. Values expressed are mean ± SD of four parallel measurements.
Cell proliferative assay
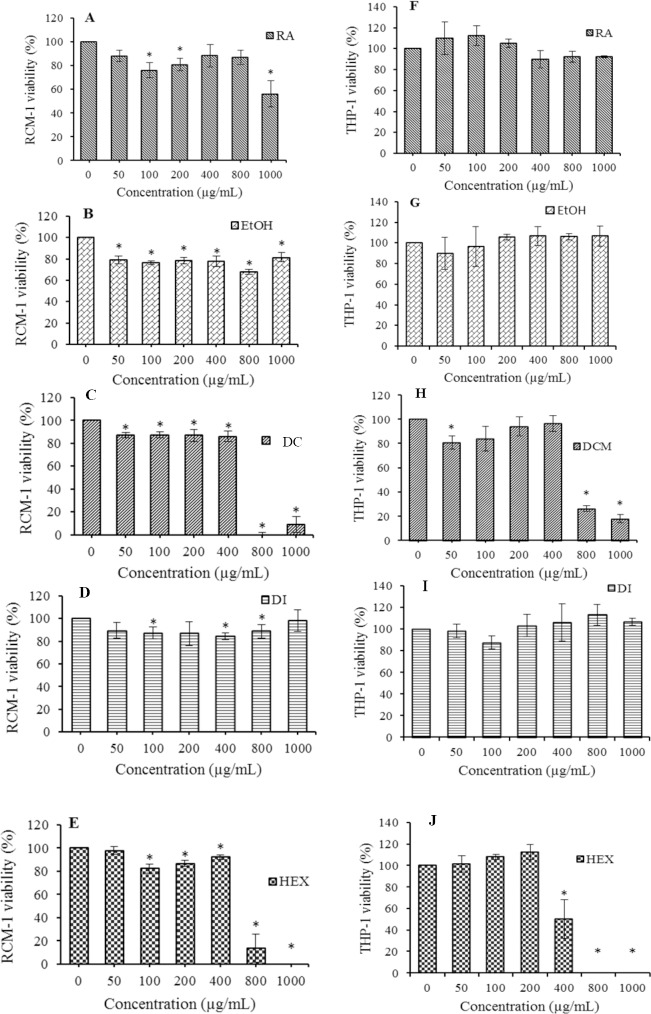
Cell proliferative effect of H. isora dried fruit extracts on RCM-1 and THP-1 cells were evaluated using MTT assay. The results demonstrated that all H. isora extracts and rosmarinic acid showed no cytotoxic effect on both cell types at the highest concentrations used except dichloromethane and hexane extracts which affected cell growth at IC50500.32 and 633.33 μg/mL on THP-1 cells and IC50508.19 and 508.46 μg/mL on RCM-1 cells, respectively (Fig. 2).
Fig. 2.
Effects of various concentrations of Helicteres isora fruit extracts on (A-E) RCM-1 and (F-J) THP-1cell viabilities. EtOH, ethanol extract; DCM, dichloromethane extract; DI, distilled water extract; HEX, hexane extract; and RA, rosmarinic acid. Values expressed are mean ± SD of at least three parallel measurements. * The significant difference was assumed at 0.05 level when compared with the untreated group.
Anti-inflammatory activity
The effect of H. isora fruit extracts on proinflammatory mediators such as PGE-2, COX-2, and TNF-α were further investigated. The inhibitory effects of H. isora fruit extracts on LPS-stimulated PGE-2 production in THP-1cells were measured by ELISAs. The results revealed that hexane extract showed the strongest activity on PGE-2 production with 69.68 ± 0.017% inhibition followed by 80% ethanol extracts with 57.17 ± 0.021% inhibition compared to celecoxib, the drug acted as COX-2 inhibitor. Furthermore, the effect of H. isora fruit extracts on LPS-stimulated COX-2 production in THP-1 cells was examined. It was demonstrated that dichloromethane extracts possessed high inhibitory activity on COX-2 production at 106.58 ± 0.003% followed by 80% ethanol extracts with 56.58 ± 0.003% inhibition compared to celecoxib. Anti-inflammatory activities of H. isora fruit extracts were further examined on TNF-α production in LPS-stimulated THP-1 cells.
The results of this study revealed that hexane extract of H. isora fruit exhibited activity against TNF-α production with 51.61 ± 0.79% inhibition at 100 μg/mL and all crude extracts showed lower effect than dexamethasone, an anti-inflammatory drug (Fig. 3).
Fig. 3.
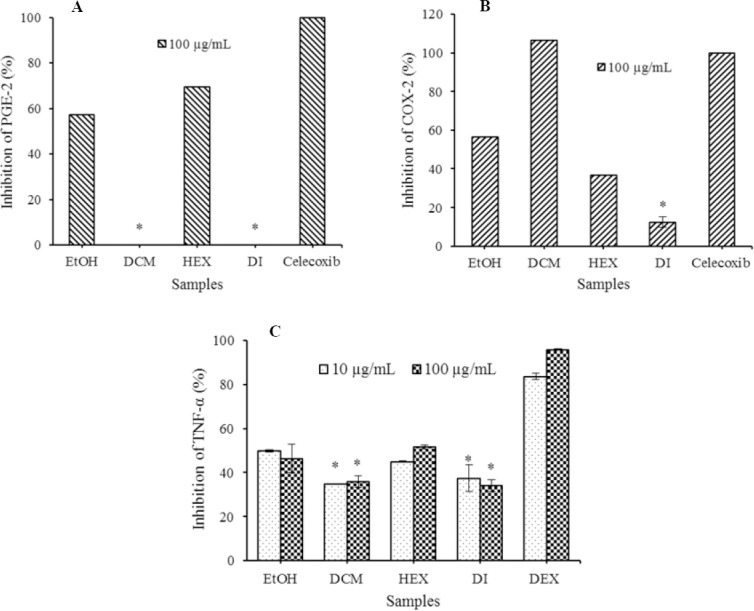
Inhibitory effect of Helicteres isora fruit extracts on lipo-polysaccharide (LPS)-stimulated (A) prostaglandin E2 (PGE-2), (B) cyclooxygenase-2 (COX-2), and (C) tumor necrosis factor-alpha (TNF-α) production in THP-1 cell line compared with 10 and 100 μg/mL dexamethasone and 0.02 μg/mL celecoxib. Each value indicates the mean ± SD of three parallel measurements. EtOH, ethanol extract; DCM, dichloromethane extract; DI, distilled water extract; HEX, hexane extract. *The significant difference was assumed at 0.05 level when compared with other extracts.
Antioxidant activity
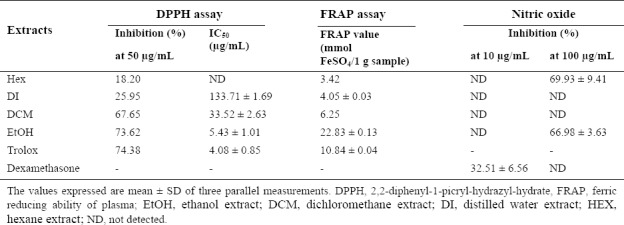
H. isora fruit extracts were also examined for antioxidant effect by reducing NO production in LPS-stimulated human monocytic cells. Hexane extract of this plant could inhibit NO production at about 69.93 ± 9.41% followed by the ethanol extract with 66.98 ± 5.63% inhibition which showed higher effect than dexamethasone. In addition, the DPPH radical scavenging assay showed that all crude extracts of H. isora fruit exhibited free radical scavenging activity at different degrees. It was demonstrated that 80% ethanol extracts exhibited the strongest activity with IC50 value of 5.43 ± 1.01 μg/mL, followed by dichloromethane extracts with IC50 value of 33.52 ± 2.63 μg/mL. Trolox was used as the positive control with IC50 value of 4.08 ± 0.85 μg/mL. The reducing properties of the crude extracts of H. isora fruit were evaluated by FRAP assay. Ethanol extracts possessed the highest FRAP value of 22.83 ± 0.13 mmol FeSO4/g sample. The FRAP value of ethanol extracts was higher than trolox with FRAP value of 10.84 ± 0.04 mmol FeSO4/g sample (Table 2).
Table 2.
Antioxidant activity of Helicteres isora fruit extracts.
DISCUSSION
Chemoprevention may involve perturbation of a variety of steps in tumor initiation, promotion, and progression. Numerous potential mechanisms have been described and attempts have been made to broadly classify agents according to the effects they have on different stages of carcinogenesis. Biological properties such as antimicrobial, antioxidant, and anticancer activities have been reported for H. isora. In the present study, the experiments demonstrated antioxidant, anti-inflammatory, and chemopreventive activities of the fruit extracts of H. isora and rosmarinic acid. All of the extracts and rosmarinic acid showed no cytotoxicity to RCM-1 and THP-1 cells at high concentration. The 80% ethanol extracts of H. isora dried fruit showed the highest number of duct formation at 100 μg/mL. It is in the range of 3-(methylthio) propanoic acid ethyl ester (MTPE were derived from fully ripened Japanese pickling melon) that have been reported to inhibit or prevent the activity of a carcinogen properties by duct induced differentiation of RCM-1 cells at 0.25 to 2 mM or 37.055 μg/mL to 296.44 μg/mL(27). Later, the new compound found in fully ripened Japanese pickling melon, methylthioacetic acid ethyl ester (MTAE), has been reported as anticarcinogen as determined by the duct formation of 50% of effective ose (ED50) at 0.61 mM or 81.86 μg/mL(28).
In the present study, anti-inflammatory activities of H. isora fruit extracts were also tested for inhibitory activities on TNF-α, NO, PGE-2 and COX-2. Pro-inflammatory cytokines such as TNF-α, NO, PGE-2, and COX-2 can be produced by LPS stimulation causing chronic inflammatory diseases(29). Stem bark of H. isora has shown slight anti-inflammatory activities. This study demonstrated that the hexane extract of H. isora fruit exhibited the strongest activity against TNF-α, PGE-2, and NO production. The dichloromethane extracts showed the strongest activity in the inhibition of COX-2 production followed by ethanol extracts. Generally, when the cells are triggered by stimuli, they will produce pro-inflammatory mediators. In this study, H. isora fruit extracts exert their anti-inflammatory effects via suppressing the induction of TNF-α, NO, and COX-2 enzyme in macrophages via LPS influence. These results revealed that H. isora fruit extracts could be a natural COX-1 and COX-2 inhibitors. There have been many studies indicating the activity of natural products on inflammation such as Dracocephalum kotschyi extract and apigenin as one of the major active component of the extract responsible for anti-inflammatory effect(30). The oxidative damage can cause several diseases including cancers, cardiovascular diseases, cataract, atherosclerosis, diabetes, arthritis, immune deficiency diseases, and aging. Antioxidants are important in the prevention to reduce the oxidative damage. Previous studies have reported antioxidant activity by using DPPH assay, β-carotenelinoleate model and microsomal lipid peroxidation or thiobarbituric acid reactive species assay(31,32). The present study showed that 80% ethanol extracts of H. isora dried fruit demonstrated potential antioxidant activity indicating the strong effect in reducing oxidative damage. The ethanol extracts from H. isora fruits probably contained phenolic compounds as evidenced by related antioxidant activity with plant phenolic compounds(33). Taken together, the results of this study provide further evidence for the implication of the H. isora dried fruit extracts which showed chemo-preventive, anti-inflammatory, and antioxidant responses also represents a potential therapeutic target for aging-associated diseases. Although the active compound might not be its major compound, the crude extract itself demonstrated significant activities suggesting that many compounds might act in concert.
CONCLUSIONS
H. isora fruit extracts possessed potential chemo-preventive properties by inducing differentiation in human colon cancer cells and showed no cytotoxic effect at high amount. Moreover, the extracts possess strong antioxidant activity and some anti-inflammatory activity. However, further in vivo study of this plant is still needed.
ACKNOWLEDGEMENT
This project was supported by Faculty of Pharmacy, Mahidol University, Thailand. The authors wish to thank all staffs in the Department of Microbiology and Department of Pharmaceutical botany for their help and suggestions on this work.
REFERENCES
- 1.Kumar N, Singh AK. Plant profile, phytochemistry and pharmacology of Avartani (Helicteres isora Linn.): A review. Asian Pac J Trop Biomed. 2014;4(Suppl 1):S22–S26. doi: 10.12980/APJTB.4.2014C872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesh S, Reddy DG, Reddy YS, Sathyavathy D, Reddy MB. Effect of Helicteres isora root extracts on glucose tolerance in glucose-induced hyperglycemic rats. Fitoterapia. 2004;75(3-4):364–367. doi: 10.1016/j.fitote.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Kumar TM, Christy AMV, Ramya RCS, Malaisamy M, Sivaraj C, Arjun P, et al. Antioxidant and anticancer activity of Helicteres isora dried fruit solvent extracts. J Acad Indus Res. 2012;1(3):148–152. [Google Scholar]
- 4.Manke MB, Dhawale SC, Jamkhande PG. Anthelmintic potential of Helicteres isora bark extract against Pheretima posthuma. Asian Pac J Trop Dis. 2015;5(4):313–315. [Google Scholar]
- 5.Vikrant A, Arya ML. A review on anti-inflammatory plant barks. Int J Pharmtech Res. 2011;3(2):899–908. [Google Scholar]
- 6.Shaikh R, Pund M, Dawane A, Iliyas S. Evaluation of anticancer, antioxidant, and possible anti-inflammatory properties of selected medicinal plants used in Indian traditional medication. J Tradit Complement Med. 2014;4(4):253–257. doi: 10.4103/2225-4110.128904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Cancer Society. Cancer Facts & Figures 2016. Atlanta, Georgia: American Cancer Society; 2016. p. 12. [Google Scholar]
- 8.Imsamran W, Chaiwerawattana A, Wiangnon S, Pongnikorn D, Suwanrungrung K, Sangrajrang S, editors. Cancer in Thailand. Vol 8. Bangkok: New Thammada Press (Thailand) Co.; 2015. p. 10. [Google Scholar]
- 9.Majid A, Ali S, Iqbal M, Kausar N. Prediction of human breast and colon cancers from imbalanced data using nearest neighbor and support vector machines. Comput Methods Programs Biomed. 2014;113:792–808. doi: 10.1016/j.cmpb.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Gaston D, Giacomantonio C. Genomics of Colorectal Cancer. In: Dellaire G, Berman JN, Arceci RJ, editors. Cancer Genomics: From Bench to Personalized Medicine. California: Academic Press; 2014. pp. 247–264. [Google Scholar]
- 11.Raffoul JJ, Kucuk O, Sarkar FH, Hillman GG. Dietary Agents in Cancer Chemoprevention and Treatment. J Oncol. 2012 doi: 10.1155/2012/749310. DOI: 10.1155/2012/749310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsao AS, Kim ES, Hong WK. Chemoprevention of Cancer. CA Cancer J Clin. 2004;54(3):150–180. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- 13.Lee BM, Park KK. Beneficial and adverse effects of chemopreventive agents. Mutat Res. 2003;523-524:265–278. doi: 10.1016/s0027-5107(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 14.Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, et al. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000;130(2S Suppl):467S–471S. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- 15.Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215(2):129–140. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Lea MA, Ibeh C, Han L, Desbordes C. Inhibition of growth and induction of differentiation markers by polyphenolic molecules and histone deacetylase inhibitors in colon cancer cells. Anticancer Res. 2010;30(2):311–318. [PubMed] [Google Scholar]
- 17.Phengklai C. Sterculiaceae. In: Santisuk T, Larsen K, editors. Flora of Thailand, Volume 7 part 3. Bangkok: Prachachon Co. Ltd.; 2001. pp. 539–654. [Google Scholar]
- 18.Kataoka H, Nabeshima K, Komada N, Koono M. New human colorectal carcinoma cell lines that secrete proteinase inhibitors in vitro. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(3):157–165. doi: 10.1007/BF02899077. [DOI] [PubMed] [Google Scholar]
- 19.Drummond EM, Harbourne N, Marete E, Martyn D, Jacquier J, O'Riordan D, et al. Inhibition of proinflammatory biomarkers in THP1 macrophages by polyphenols derived from chamomile, meadowsweet and willow bark. Phytother Res. 2013;27(4):588–594. doi: 10.1002/ptr.4753. [DOI] [PubMed] [Google Scholar]
- 20.Sylvester PW. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol. 2011;716:157–168. doi: 10.1007/978-1-61779-012-6_9. [DOI] [PubMed] [Google Scholar]
- 21.Rattanamaneerusmee A, Thirapanmethee K, Nakamura Y, Chomnawang MT. Differentiation-inducing effect in human colon cancer cells of essential oils. Pharm Sci Asia. 2018;45(3):154–160. [Google Scholar]
- 22.Eo HJ, Park JH, Park GH, Lee MH, Lee JR, Koo JS, et al. Anti-inflammatory and anti-cancer activity of mulberry (Morus alba L.) root bark. BMC Complement Altern Med. 2014;14:200–208. doi: 10.1186/1472-6882-14-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong Y, Xue B, Jiao J, Jing L, Wang X. Triptolide inhibits COX-2 expression and PGE2 release by suppressing the activity of NF-kappaB and JNK in LPS-treated microglia. J Neurochem. 2008;107(3):779–788. doi: 10.1111/j.1471-4159.2008.05653.x. [DOI] [PubMed] [Google Scholar]
- 24.O'Leary KA, de Teresa PS, Needs PW, Bao YP, O'Brien NM, Williamson G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat Res. 2004;551(1-2):245–254. doi: 10.1016/j.mrfmmm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174(1):27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant ower”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura Y, Nakayama Y, Ando H, Tanaka A, Matsuo T, Okamoto S, et al. 3-Methylthiopropionic acid ethyl ester, isolated from Katsura-uri (Japanese pickling melon, Cucumis melo var. conomon), enhanced differentiation in human colon cancer cells. J Agric Food Chem. 2008;56(9):2977–2984. doi: 10.1021/jf072898i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura Y, Watanabe S, Kageyama M, Shirota K, Shirota K, Amano H, et al. Antimutagenic; differentiation-inducing; and antioxidative effects of fragrant ingredients in Katsura-uri (Japanese pickling melon; Cucumis melo var. conomon) Mutat Res Genet Toxicol Environ Mutagen. 2010;703(2):163–168. doi: 10.1016/j.mrgentox.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Wen CL, Chang CC, Huang SS, Kuo CL, Hsu SL, Deng JS, et al. Anti-inflammatory effects of methanol extract of Antrodia cinnamomea mycelia both in vitro and in vivo. J Ethnopharmacol. 2011;137(1):575–584. doi: 10.1016/j.jep.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Sadraei H, Asghari G, Khanabadi M, Minaiyan M. Anti-inflammatory effect of apigenin and hydroalcoholic extract of Dracocephalum kotschyi on acetic acid-induced colitis in rats. Res Pharm Sci. 2017;12:322–329. doi: 10.4103/1735-5362.212050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basniwal PK, Suthar M, Rathore GS, Gupta R, Kumar V, Pareek A, et al. In-vitro antioxidant activity of hot aqueous extract of Helicteres isora Linn. fruits. Indian J Nat Prod Resour. 2009;8:483–487. [Google Scholar]
- 32.Suthar M, Rathore GS, Pareek A. Antioxidant and antidiabetic activity of Helicteres isora (L.) fruits. Indian J Pharm Sci. 2009;71(6):695–699. doi: 10.4103/0250-474X.59557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conforti F, Sosa S, Marrelli M, Menichini F, Statti GA, Uzunov D, et al. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J Ethnopharmacol. 2008;116(1):144–151. doi: 10.1016/j.jep.2007.11.015. [DOI] [PubMed] [Google Scholar]