Abstract
Pistacia atlantica (P. atlantica) subsp. mutica has been used in traditional medicine and is famous for its medicinal properties. The aim of this study was to evaluate the effect of methanol (MeOH), n-hexane, dichloromethane (CH2Cl2), n-butanol (BuOH), ethyl acetate (EtOAc), water extracts and essential oil of P. atlantica subsp. mutica on melanin synthesis and oxidative stress in B16F10 melanoma cell line. The B16F10 cells viability after treatment with increasing concentrations of different extracts of the plant (0.2-200 μg/mL) was measured using resazurin. Essential oil composition was identified by gas-chromatography-mass spectrometry (GC-MS) analysis and inhibitory effect on synthesis of melanin, mushroom tyrosinase activity, cellular tyrosinase, and oxidative stress were evaluated by the colorimetric and fluorometric methods. The data showed extracts at concentrations 0.2-200 μg/mL, did not show significant toxicity on melanoma cells but concentrations of 200 μg/mL of essential oil had cytotoxic effect. Pistacia atlantica subsp. mutica could inhibit the mushroom tyrosinase activity. Also the amount of melanin in B16F10 cells declined. In addition, the ability of P. atlantica subsp. mutica extracts in decreasing the amount of reactive oxygen species in melanoma cells revealed remarkable antioxidant activity. In addition, all concentrations of essential oil had no significant effect in this study. The melanogenesis inhibitory and antioxidant effects of P. atlantica subsp. mutica on B16F10 cells may suggest the potential whitening activity of the plant for using in dermatological skin care products and for prevention of skin aging in cosmetic industry.
Keywords: Anti-tyrosinase, Melanogenesis, P. atlantica subsp. mutica
INTRODUCTION
Melanin is a skin pigment which is synthesized in melanosomes and is transferred to keratinocytes throughout the physiological process called melanogenesis. Melanin plays an important role in the protection against UV damage and determines the color of skin, hair and eyes. Excess production of melanin is attributed in melanoma and abnormal pigmentation of the skin(1,2,3). The key enzyme in the melanin biosynthesis is tyrosinase which catalyzes two separate reactions, the oxidation of 3,4-dihydroxy-phenylalanine (DOPA) to dopaquinone and hydroxylation of L-tyrosine to the DOPA(4). Tyrosinase or polyphenol oxidase is a copper containing mixed-function enzyme found in microorganisms, animals, and plants(5). Tyrosinase is the most influencing factor for the browning of fruits and vegetables. Overproduction and accumulation of melanin may result in a large number of skin disorders including melasma, freckles, solar melanosis, and age spots(6). Therefore, tyrosinase inhibitors have attracted much interest in food and cosmetic industries.
In many living organisms, oxidation is essential for the production of energy to fuel biological processes.
However, hydrogen peroxide (H2O2) and other -reactive oxygen species (ROS)- cause cell death and tissue damage in many physiologic and pathologic phenomena including ROS increment following UV irradiation during melanogenesis process. Also it is well known that UV-induced production of ROS is involved in the pathogenesis of several skin conditions including aging, wrinkles, photosensitivity, and malignancy(7). Thus, finding natural sources of antioxidants with anti-tyrosinase activity helps to modify skin damages related to excess melanogenesis(4).
The genus Pistacia is a member of the Anacardiaceae family comprising about 9 species and is mostly distributed in Mediterranean region, Europe, and some parts of Asia(8,9). Pistacia atlantica (P. atlantica) Desf. has 3 subspecies namely: P. atlantica subsp. kurdica (Zohary) Rech. f., P. atlantica subsp. mutica (Fischer & C. A. Meyer) Rech. f. and P. atlantica subsp. cabulica (Stocks) Rech. f. The three mentioned subspecies of P. atlantica, P. khinjuk Stocks, and P. vera L. are grown in Iran(10). The fruits of P. atlantica subsp. mutica namely Baneh, is round to oval with 0.5-0.7 cm in diameter. The antioxidant and anticancer activity of the hull of the P. atlantica has been related to high total phenolic content of the plant. P. atlantica has traditionally been used for relieving upper abdominal discomfort and pain, dyspepsia, and peptic ulcer(11,12,13). However, there are no studies on melanogenesis inhibitory activity of P. atlantica subsp. mutica. So, in this project we choose B16F10 melanoma cells for studying the antioxidant and anti-melanogenic properties of P. atlantica subsp. mutica extracts. The aim of this study was to investigate the inhibitory effect of methanol (MeOH), n-hexane, dichloromethane (CH2Cl2), n-butanol (BuOH), ethyl acetate (EtOAc), water (H2O) extracts and essential oil of P. atlantica subsp. mutica fruit on melanogenesis and to evaluate the potential antioxidant capacity of the plant on B16F10 melanoma cells.
MATERIALS AND METHODS
Chemicals
Mushroom tyrosinase from the Agaricus bisporus, kojic acid, resazurin, L-3,4-dihydroxyphenylalanine (L-DOPA), dichloro-dihydro-fluorescein diacetate (DCFH-DA), egtazic acid (EGTA), dimethyl sulfoxide (DMSO), phenylmethylsulfonyl fluoride, β-glycerophosphate, β-mercaptoethanol, phosphate buffered saline (PBS), sodium orthovanadate, tris-buffered saline tween 20 (TBST) purchased from Sigma (USA). Fetal bovine serum (FBS), penicillin, streptomycin, and trypsin-EDTA were obtained from GibcoBRL (Grand Island, USA). Melanoma cell line (B16F10, Cat. No C540) was purchased from the Pasteur Institute of Iran (Tehran, I.R. Iran). All other chemicals and solvents were from Merck (Germany).
Preparation of extracts
P. atlantica subsp. mutica was collected in May 2014 from Bardaskan Mountains, Khorasan Razavi province, northeast of Iran and identified by Mrs. M. Souzani. A voucher specimen (No:13069) was deposited in the herbarium of School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, I.R. Iran. Unripe fruit of P. atlantica subsp. mutica (200 g) were grounded by a blender (Toos chekan Co, I.R. Iran) and then percolated with MeOH at room temperature for 24 h according to the previously reported protocol(14). After extraction, the solvent was evaporated using rotary evaporator and then freeze-dried. Methanol extract (94 g) was further fractionated by solvent-solvent partition to give five different fractions including MeOH, n-hexane, CH2Cl2, BuOH, EtOAc, and H2O.
Isolation of the essential oil
The unripe fruit of P. atlantica subsp. mutica (150 g) was subjected to hydro-distillation using a Clevenger-type apparatus for 3 h. The colorless oil was obtained with a yield of 0.8% (v/w). The obtained essential oil was dried over anhydrous sodium sulphate and stored at 4 °C in dark until further testing.
Gas-chromatography and gas-chromatography-mass spectrometry
The gas-chromatography (GC) analysis was performed using a Varian CP-3800 equipped with a FID detector, interfaced with a fused-silica column (CP-Sil 8CB, 50 m × 0.25 mm, film thickness 0.12 μm) under following condition: oven temperature 50-250 °C with the rate of 3 °C/min; injector temperature 260 °C, split ratio 1:5, with carrier gas, N2 flow rate 2 mL/min; detector temperature 280 °C.
Gas-chromatography-mass (GC-MS) analyses were performed using an Agilent 5975 apparatus equipped with a HP-5 MS column (30 m × 0.25 mm i.d., 0.25 μm film thicknesses) interfaced with a quadruple mass detector and a computer equipped with Wiley 7n.L library. Oven temperature 50-250 °C with the rate of 3 °C/min, injector temperature 250 °C, injection volume: 0.1 μL, split injection with split ratio of 1:50, with the carrier gas (helium) flow rate 1 mL/min, ion source: 70 eV, ionization current: 150 μA, and scan range: 35-465. Identification of the components of the essential oil was based on retention gas chromatography obtained with reference to n-alkanes series (C6-C20) on HP-5MS column, comparison of their mass spectra and fragmentation patterns reported in literature, and computer matching with the Wiley 7n.L library(15). Quantification of the relative amount of the individual components of unripe fruit was performed according to the area percentage method without consideration of calibration factor.
Cell culture
Melanoma cell line, B16F10, maintained at 37 °C in a humidified atmosphere (90%) containing 5% CO2. Cells were cultured in RPMI-1640 (Bioidea, Iran) with 10% (v/v) FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin. The stock solution of the P. atlantica subsp. mutica was prepared at 50 μg/mL in DMSO and kept at -40 °C. Kojic acid (2 and 4 mM) was used as positive control in all experiments. Cells were cultured in 96-well plates in a density of 105 cells/mL. The activity of the extract in each experiment was calculated using the following equation:
Cell viability assay
Resazurin is a cell health indicator uses the reducing power of live cell and converts to resorufin. Resazurin is a blue, non-toxic, non-fluorescent and cell permeable compound that converts to red in color and highly fluorescent resorufin in live cells(16). About 104 B16F10 melanoma cells were seeded in each well of 96-microwell plate and treated with various concentrations of extracts and essential oil of P. atlantica subsp. mutica (0.2-200 μg/mL). After 4 h incubation, the absorbance of resazurin and resorufin was measured at 570 nm and 600 nm using H4 Hybrid Multi-Mode microplate reader (BioTek, Winooski, USA). Each experiment was done in triplicate. The half maximal inhibitory concentration (IC50) was calculated using GraphPad software from the concentration-effect curve: log concentration vs response.
Mushroom tyrosinase activity assay
The activity of mushroom tyrosinase in oxidation of L-DOPA was measured spectrophotometrically as described previously(17), with some modifications. Briefly, 160 μL of 5 mM L-DOPA (in 100 mM sodium phosphate buffer pH 6.8) and 20 μL of the same buffer with and without P. atlantica subsp. mutica extracts and essential oil (10-1000 μg/mL) were mixed with 20 μL of mushroom tyrosinase (200 units/mL) and then incubated at 37 °C for 30 min. The absorbance was measured at 475 nm with Synergy H4 Hybrid Multi-Mode microplate reader (BioTek, Winooski, USA).
Determination of melanin content in melanoma cells
Melanoma cells, B16F10, were seeded at a density of 105 cells per well in 96-well culture plates and incubated for 24 h. They were then incubated with different concentrations (0.2-200 μg/mL) of P. atlantica subsp. mutica extracts and essential oil for 24 h. The melanin content was measured as described previously(18). After treatment, the cells were collected using trypsin. They washed with PBS. The cell pellets were solubilized in 50 μL solution of sodium hydroxide (2 M) for 60 min at 60 °C. The melanin content was measured by measuring the absorbance at 405 nm with Synergy H4 Hybrid Multi-Mode microplate reader (BioTek, Winooski, USA).
Cellular tyrosinase activity assay
The oxidation of DOPA to DOPA chrome was analyzed by spectrophotometry as indicator of tyrosinase activity(18). 106 cells of B16F10 melanoma were plated in each well of 96-well plate overnight. After treating of cell with different concentrations (0.2-200 μg/mL) of P. atlantica subsp. mutica extracts and essential oil for 24 h, The cells were detached using trypsin; washed with PBS, and lysed with 50 μL sodium phosphate buffer (pH 6.8, 100 mM) containing 1% triton X-100 and 0.1 mM phenylmethylsulfonyl fluoride. The lysates were centrifuged at 10,000 rpm for 20 min at 4 °C. 100 μL of each lysate (each containing 100 mg protein) was mixed with 30 μL of 5 mM DOPA in 96 well plate and incubated at 37 °C for 2 h, the absorbance was measured at 475 nm with Synergy H4 Hybrid Multi-Mode microplate reader (BioTek, Winooski, USA).
Determination of cellular reactive oxygen species level
Reactive oxygen species level was measured as described previously with minor modifications(19). Melanoma cells, B16F10, (2 × 104) were seeded in 96-well plates overnight and then were treated with different concentrations (0.2-200 μg/mL) of P. atlantica subsp. mutica extracts and essential oil for 24 h. Then cells incubated with 50 μL H2O2(24 mM) at 37 °C for 30 min. Then 50 μL of DCFH-DA were added to the cells and the fluorescence intensity of DCF was measured at 504 nm emission and 524 nm excitation using a Synergy H4 microplate reader (BioTek, USA).
Western blotting analysis
Melanoma cells, B16F10, were cultured in 75 cm3 flasks with and without methanol extract (0.5-100 μg/mL) for 24 h. The cells were then lysed in a buffer (50 mM tris-HCl, pH 7.4, 2 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 10 mM β-glycerophosphate, 10 mM β-mercaptoethanol, 1 mM sodium orthovanadate, 0.1% deoxycholic acid sodium salt). Equal amount of proteins (50 μg) were loaded on 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked for 2 h in 10% skim milk in TBST (20 mM tris-HCl pH 7.4, 100 mM NaCl, and 0.1% tween 20) buffer at room temperature. After washing with TBST buffer, the membrane was then incubated overnight with a primary antibody: 1:300 (rabbit anti-tyrosinase antibody (Santa Cruz Biotechnology, CA)). After rinsing 3 times with TBST buffer, the membranes were then incubated for 2 h with anti-rabbit IgG (1:2000) as secondary antibody (cell signaling). Rinsing 3 times with TBST buffer was repeated and the protein bands were detected using the enhanced chemiluminescent (ECL) prime western blotting detection system (BioRaD, USA)(20). Anti-β-actin antibody was used as the loading control.
Statistical analysis
The relative results of the experiments were presented as the mean ± SD of the three independent measurements. Analysis of variance and IC50 calculation were performed with GraphPad Prism 6.0 using one-way ANOVA test and the means were compared by Dunnett tests. P < 0.05 stands for statistically significant difference between extract-treated cells and control. Two-way ANOVA test and bonferroni comparison post-test was used to compare the effect of different extracts in each assay.
RESULTS
Essential oil composition
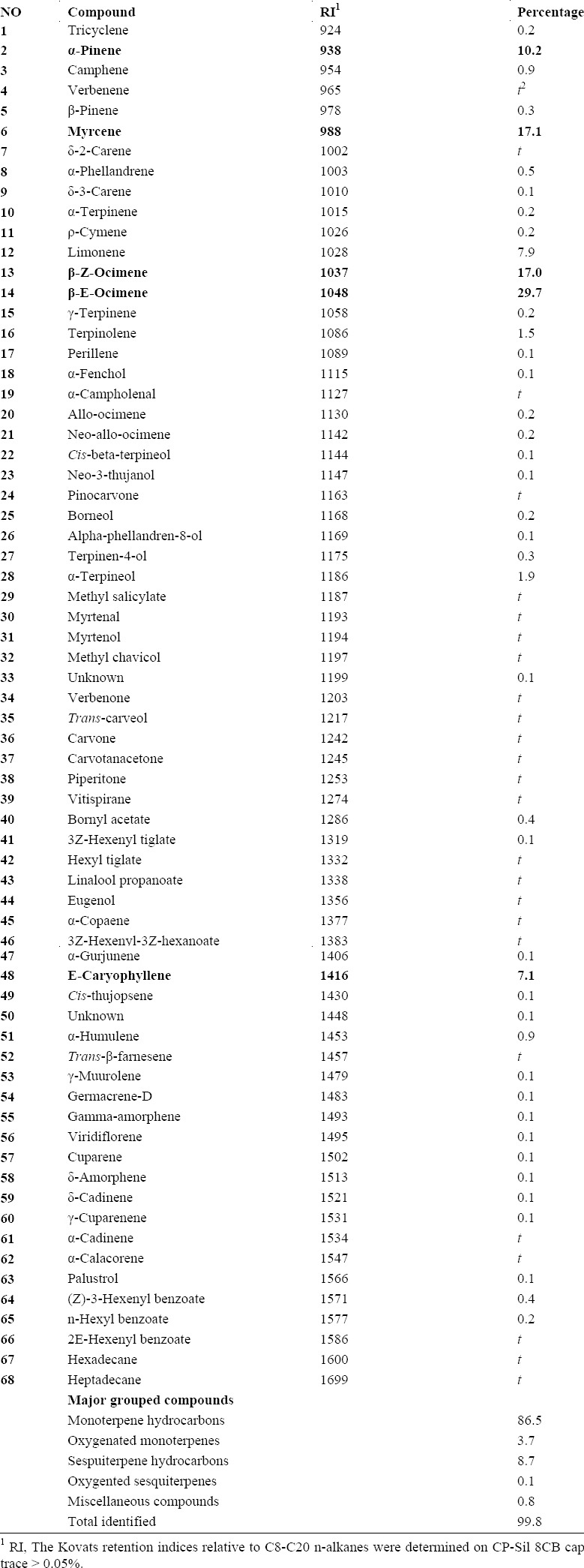
Gas-chromatography has been used for quantification and Gc-Mass for identification of the compounds. The results of both GC and GC-MS analysis are presented in the quantitative and qualitative identification table of the compounds. In the case of P. atlantica subsp. mutica, 68 constituents were identified in the essential oil and accounted for 99.8% of the total oil composition (Table 1). The grouped compounds of the essential oil was determined as monoterpene hydrocarbons 86.5%, oxygenated monoterpenes 3.7%, sesquiterpene hydrocarbons 8.7%, oxygenated sesquiterpens 0.1%, and the miscellaneous 0.8%. The major components of the essential oil were β-E-ocimene 29.7%, myrcene 17.1%, β-Z-ocimene 17.0%, α-pinene 10.2%, and E-caryophyllene 7.1%.
Table 1.
Chemical compositions of volatile oil of oleoresins of Pistacia atlantica subsp. mutica obtained by hydro-distillation.
Effect of extracts on cell survival
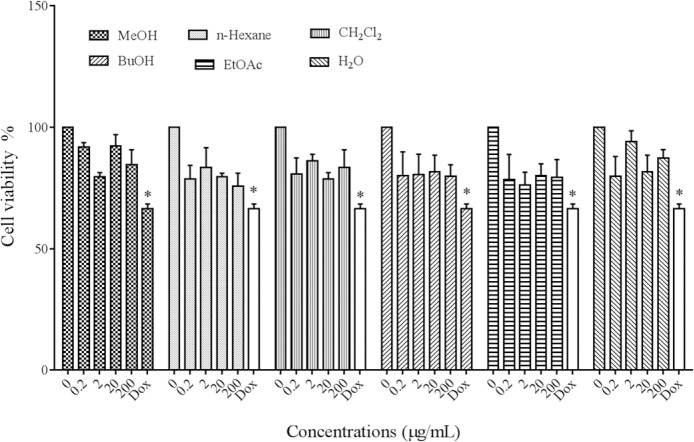
The viability of cells was monitored with resazurin. Melanoma cells, B16F10, were seeded in 96-well plate. After 24 h cells treated with different concentrations (0.2-200 μg/mL) of P. atlantica subsp. mutica extracts, results showed that the extracts had no significant cytotoxic effect on B16F10 cells at the concentrations used in this study (Fig. 1). Doxorubicin as the positive control significantly induced cell death (P < 0.05).
Fig. 1.
Cytotoxic effects of Pistacia atlantica subsp.mutica extracts on melanoma cell viability. *P< 0.05, as compared to control.
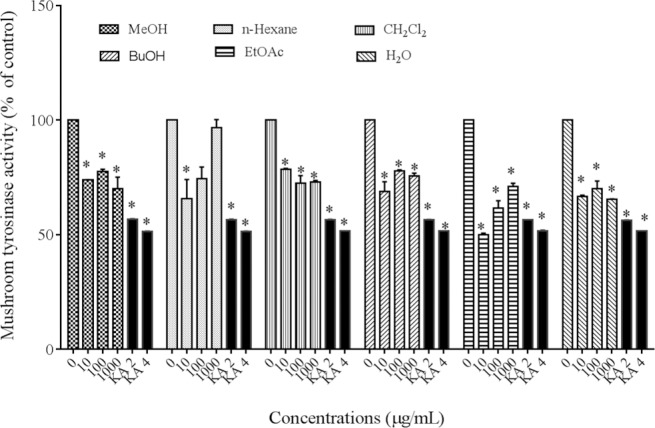
Effect of extracts on mushroom tyrosinase activity
The effect of P. atlantica on inhibition of the mushroom tyrosinase in oxidation of L-DOPA was assessed. The results indicated that mushroom tyrosinase activity was inhibited by all different extracts, but the concentration of 1000 μg/mL of n-hexane extract had no significant effect in this test. Kojic acid (2 and 4 mM) was used as positive control (P < 0.05) (Fig. 2).
Fig. 2.
Effect of Pistacia atlantica subsp.mutica extracts on mushroom tyrosinase activity. *P< 0.05, as compared to control.
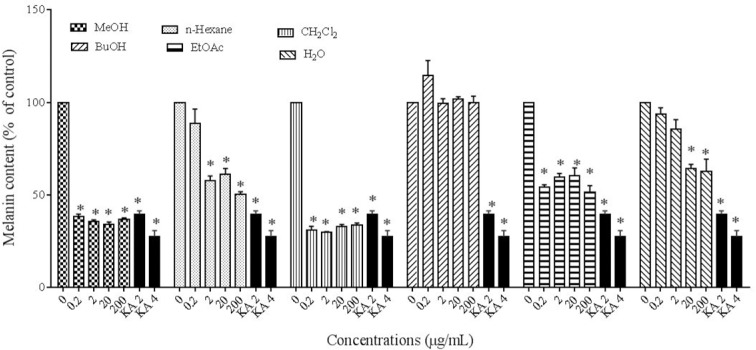
Effect of extracts and essential oil on the synthesis of melanin
To study the effect of different extracts and essential oil of P. atlantica subsp. mutica on melanin synthesis, the melanin content of extract-treated B16F10 melanoma cells was assessed. Kojic acid (2 and 4 mM) was utilized as a positive control.
The results showed that MeOH, CH2Cl2, and EtOAc extracts (0.2-200 μg/mL), n-hexane (2-200 μg/mL), and H2O extract (20 and 200 μg/mL) had inhibitory effect on melanin synthesis (P < 0.05) (Fig. 3). However, all of the concentrations of essential oil and BuOH extract had no significant inhibitory effect on melanin synthesis.
Fig. 3.
Effect of Pistacia atlantica subsp.mutica extracts on melanin content. *P< 0.05 as compared to control.
The results of essential oil are not shown in the figures.
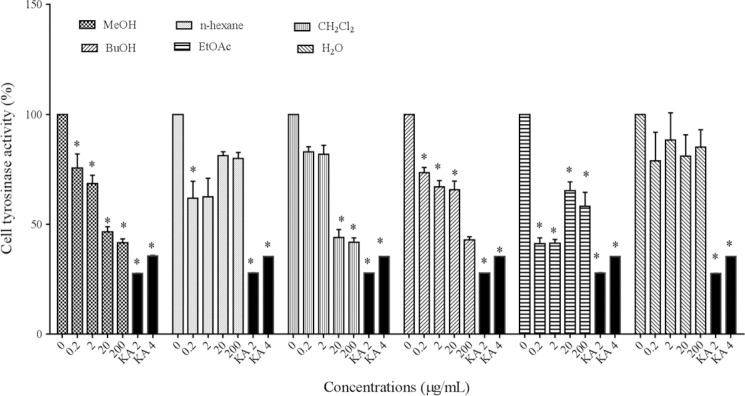
Effect of extracts on cellular tyrosinase activity
To evaluate the mechanism of the inhibitory effect of P. atlantica subsp. mutica extract on melanogenesis in particular, we evaluated the intracellular tyrosinase activity in B16F10 melanoma cells. The results indicated that MeOH, EtOAc, and BuOH extracts (0.2-200 μg/mL), n-hexane (0.2 μg/mL), and CH2Cl2(20 and 200 μg/mL) extracts of P. atlantica subsp. mutica could significantly inhibit cellular tyrosinase activity (P < 0.05) (Fig. 4) but water extract had no inhibitory effect on cellular tyrosinase activity.
Fig. 4.
Effect of Pistacia atlantica subsp.mutica extracts on cellular tyrosinase activity. *P< 0.05 as compared to control.
Effect of extracts on cellular reactive oxygen species level
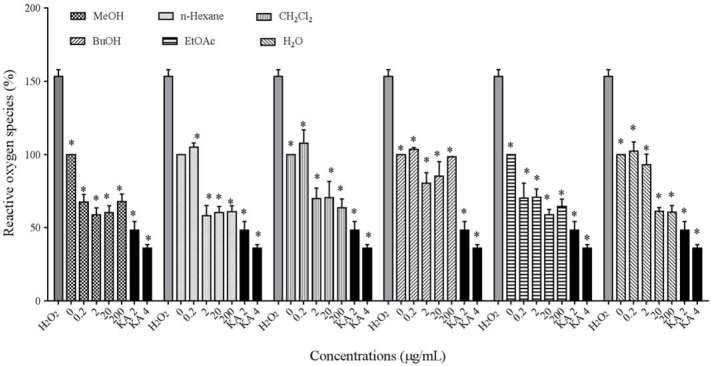
The intracellular ROS level as indicative of antioxidant capacity of P. atlantica subsp. mutica was measured in cells treated with 24 mM H2O2 alone or with different extracts in B16F10 melanoma cells. The results indicated that all extracts concentrations of P. atlantica subsp. mutica except for the 0.2 μg/mL of CH2Cl2 extract could significantly suppress the oxidative stress induced by H2O2(P < 0.05) (Fig. 5).
Fig. 5.
Antioxidant effect of Pistacia atlantica subsp.mutica extracts on cellular reactive oxygen species (ROS) levels.*P< 0.05 as compared to control.
Effect of extracts on cellular tyrosinase protein level
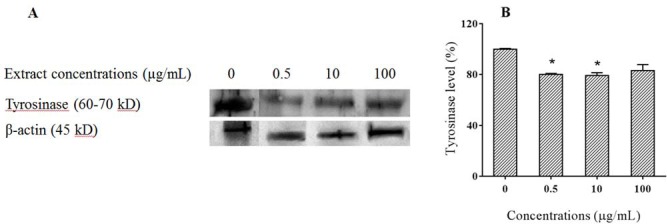
The intracellular effect of P. atlantica subsp. mutica on melanogenic related proteins such as tyrosinase as indicator of melanogenesis was evaluated by western blot.
As shown in Fig. 6, tyrosinase protein levels were significantly decreased by P. atlantica subsp. mutica extract treatment at the concentration of 0.5 and 10 μg/mL (P < 0.05). β-Actin was used as internal control.
Fig. 6.
Effect of Pistacia atlantica subsp.mutica on cellular tyrosinase protein level. A, Western blotting analysis of tyrosinase and β-actin proteins expression in B16F10 cells; B, the level of tyrosinase in cells normalized to the related β-actin band in comparision with control, *P< 0.05.
Effects of essential oil on different parameters
Essential oil at 0.2-200 μg/mL revealed no significant effect on all above-mentioned parameters, except at concentration 200 μg/mL, which exhibited cytotoxic effect on melanoma cells. Hence, the results of essential oil are not included in the result section as no inhibitory effects were observed.
DISCUSSION
Natural based cosmetic products are widely advertised by commercial agencies for their safety and multifunctional activity. Finding new anti-melanogenic agents with antioxidant activity from natural sources is one of the interests in formulation of the products used for hyperpigmentation disorders. In medicinal and cosmetic products used as skin whitening agents, inhibition of the melanin formation, scavenging free radicals and inhibition of the tyrosinase activity that important for treating related skin disorders(21).
In this study the antioxidant activity of different extracts of P. atlantica subsp. mutica was evaluated and their effects on the tyrosinase activity and melanin synthesis were analyzed. The MeOH and EtOAc extracts of P. atlantica subsp. mutica showed significant antioxidant activity at 2, 20 μg/mL and 20, 200 μg/mL which may be attributed to the polyphenols and flavonoids widely found in the plant. The results showed all of the extracts especially MeOH, EtOAc, and H2O extracts could significantly inhibit the mushroom tyrosinase activity. Also, The MeOH, EtOAc, and BuOH extracts were able to decrease the cellular tyrosianse activity which was in accordance with reduction in the melanin production in cells. In this study, all of concentration of essential oil had no significant inhibitory effect against cellular tyrosianse, melanin synthesis, and antioxidant activity. Due to the more activity of the MeOH extract in different experiment in comparison with other extracts we have chosen the mentioned extract for western blot analysis. To sum up, the anti-melanogenic effect of this extract was confirmed in all assays in B16F10 cells, being comparable to the positive control kojic acid (Table 2).
Table 2.
The half maximal inhibitory concentration (IC50) values (μg/mL) for different extracts of Pistacia atlantica subsp. mutica.
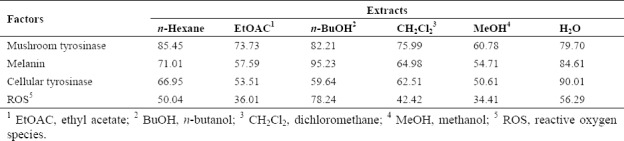
The MeOH, CH2Cl2, and EtOAc extracts have decreased cellular tyrosinase activity, melanin content, and ROS. Semipolar nature fractions may extract phytochemicals which are responsible for antimelanogenic activity such as polyphenols and flavonoids. The n-hexane fraction and essential oil were less active indicating that plant phytochemicals with nonpolar and volatile nature have not significant effect on the melanogenesis process(22,23).
Polyphenols and flavonoids act as inhibitors of ROS production and could be responsible for the anti-melanogenic properties of plant extracts(24,25,26,27). The major compounds in the extract of P. atlantica subsp. mutica are quercetin, luteolin, isoquercetin, rutin, luteolin 7-lactate, and phenolic compounds such as p-coumaric acid, caffeic acid and gallic acid which are responsible for antioxidant activity(28). Rutin has been reported to inhibit the activity of tyrosinase and is a potent anti-pigment agent due to the presence of hydroxyl groups(29). Interestingly quercetin, luteolin, and isoquercetin also have many hydroxyl groups in their structure making them suitable for interaction with tyrosinase resulting in the anti-tyrosinase activity(21,30). Recently it has been shown that effect of flavonoids like luteolin and quercetin are mainly mediated via the modulation of transcriptional factor of melanogenesis associated transcription factor (MITF) and/or the melanogenesis enzymes tyrosinase, DCT or tyrosinase-related protein 1 (TYRP-1)(31). P-coumaric acid and caffeic acid inhibited the mushroom tyrosinase activity and were 10- and 3-fold more potent than kojic acid(32).
Gallic acid displays tyrosinase inhibitory activity and reduces dopaquinone back to L-DOPA through a redox cycling, similar to ascorbic acid(33). Similarly, in the present study P. atlantica subsp. mutica decreased the level of tyrosinase protein in cells which is strongly correlated with the presence of quercetin, luteolin, isoquercetin, rutin, and other polyphenols in the plant.
There are many reports about plants to have the anti-melanogenic and antioxidant activity that decrease oxidative stress, which may have a high potential for the treatment of skin disorder. For example, the different extracts of aerial parts of P. atlantica reduced oxidative stress, which may be attributed to the polyphenols, flavonoids, and anthocyanin widely found in the plant(34). In another study, the MeOH extract of P. vera decreased the melanin secretion which was attributed to some antioxidant compounds in this plant. This study indicated the kojic acid, as a positive control, showed an IC50 value of 0.05 mg/mL and higher concentrations of P. vera can be used as an effective agent for skin disorders treatment such as melanoma cancer(35). Gourine, et al. showed the aerial parts of P. atlantica have the powerful antioxidant properties(36). Also MeOH and CH2Cl2 fractions of Nepeta satureioides indicated potential effects against melanin production in B16F10 melanoma cells and may contribute in cosmetic formulations of skin care products(37). The derivatives of kojic acid had inhibitory activity on tyrosinase. It seems that the presence of free hydroxyl group and methyl substitute in this compound confirmed inhibitory activity. In this study kojic acid (positive control) indicated an IC50 value of 0.28 mM(38). The phenylpropanoid glycosides and flavonoids isolated from Teucrium polium L. var. gnaphalodes showed antioxidant and tyrosinase inhibitory activities. The authors exhibited jaranol showed the highest tyrosinase inhibitory activity (IC500.041 mM) and poliumoside was the best antioxidant among the tested compounds. Kojic acid showed an IC50 value of 0.02 mM(39).
Decrease in the protein level of tyrosinase by P. atlantica subsp. mutica and antioxidant properties and reduced ROS were confirmed the use of this agent as anti-melanogenesis agent. This study for the first time has determined the inhibitory effect of P. atlantica subsp. mutica on melanogenesis in B16F10 melanoma cells. In order to prevent accumulation and overproduction of melanin in skin, inhibition of tyrosinase has an important role. Hence, respect to the reported anti-oxidant effect of P. atlantica subsp. mutica, flavonoid and phenolic compounds are important components of the plant responsible for anti-melanogenesis and antityrosinase activities of P. atlantica subsp. mutica. Both antioxidant and anti-melanogenic activity of P. atlantica subsp. mutica may offer suitable agent for skin whitening formulations and could be included in cosmetic product for skin care formulations.
CONCLUSION
In conclusion, this study for the first time showed that MeOH and EtOAc extracts of P. atlantica subsp. mutica has strong tyrosinase inhibitory activity which is in accordance with melanin reduction. Moreover, P. atlantica subsp. mutica also demonstrated high scavenging activity and antioxidant properties, which could be attributed mainly to its high levels of flavonoids and total polyphenols. The results suggested that the ability of P. atlantica subsp. mutica to reduce melanin production may correlate to depletion of cellular ROS and its inhibitory action on the signaling pathway regulating tyrosinase activity. Since the semipolar extract had significant anti-tyrosinase and anti-melanogenic effects, therefore, we conclude the compounds responsible for these effects are accumulated in the extract, not in essential oils.
ACKNOWLEDGEMENT
This study was part of a Pharm. D thesis (Grant No. 930209) which was financially supported by Mashhad University of Medical Sciences, Mashhad, I.R. Iran.
REFERENCES
- 1.Chung KW, Park YJ, Choi YJ, Park MH, Ha YM, Uehara Y, et al. Evaluation of in vitro and in vivo anti-melanogenic activity of a newly synthesized strong tyrosinase inhibitor (E)-3-(2,4 dihydroxybenzylidene)pyrrolidine-2,5-dione (3-DBP) Biochim Biophys Acta. 2012;1820(7):962–969. doi: 10.1016/j.bbagen.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Riley PA. Melanogenesis and melanoma. Pigment Cell Res. 2003;16(5):548–552. doi: 10.1034/j.1600-0749.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Daim M, Funasaka Y, Komoto M, Nakagawa Y, Yanagita E, Nishigori C. Pharmacogenomics of metabotropic glutamate receptor subtype 1 and in vivo malignant melanoma formation. J Dermatol. 2010;37(7):635–646. doi: 10.1111/j.1346-8138.2010.00833.x. [DOI] [PubMed] [Google Scholar]
- 4.Agar N, Young AR. Melanogenesis: a photoprotective response to DNA damage. Mutat Res? 2005;571(1-2):121–132. doi: 10.1016/j.mrfmmm.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Hashemi-Shahri SH, Golshan A, Mohajeri SA, Baharara J, Amini E, Salek F, Sahebkar A, Tayarani-Najaran Z. ROS-scavenging and anti-tyrosinase properties of crocetin on B16F10 murine melanoma cells. Anticancer Agents Med Chem. 2017 doi: 10.2174/1871520618666171213143455. Epub ahead of print. DOI: 10.2174/1871520618666171213143455. [DOI] [PubMed] [Google Scholar]
- 6.Ortonne JP, Bissett DL. Latest insights into skin hyperpigmentation. J Investig Dermatol Symp Proc. 2008;13(1):10–4. doi: 10.1038/jidsymp.2008.7. [DOI] [PubMed] [Google Scholar]
- 7.Safaeian L, Sajjadi SE, Haghjooy Javanmard S, Montazeri H, Samani F. Protective effect of Melissa officinalis extract against H2O2-induced oxidative stress in human vascular endothelial cells. Res Pharm Sci. 2016;11(5):383–389. doi: 10.4103/1735-5362.192488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mabberley DJ. The Plant-Book. 2rd ed. Cambridge: Cambridge University Press; 2008. p. 42. [Google Scholar]
- 9.Gardeli C, Vassiliki P, Athanasios M, Kibouris T, Komaitis M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008;107(3):1120–1130. [Google Scholar]
- 10.Khatamsaz M. Assadi M. Flora of Iran. Tehran: Research Institute of Forests and Rangelands; 1988. Anacardiaceae; p. 11. [Google Scholar]
- 11.Fathi Rezaei P, Fouladdel S, Ghaffari SM, Amin G, Azizi E. Induction of G1 cell cycle arrest and cyclin D1 down-regulation in response to pericarp extract of Baneh in human breast cancer T47D cells. Daru. 2012;20(1):101–105. doi: 10.1186/2008-2231-20-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozorgi M, Memariani Z, Mobli M, Salehi Surmaghi MH, Shams-Ardekani MR, Rahimi R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): a review of their traditional uses, phytochemistry, and pharmacology. Sci World J. 2013;2013 doi: 10.1155/2013/219815. Article ID:219-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanideh N, Davarmanesh M, Andisheh-Tadbir A, Ranjbar Z, Mehriar P, Koohi-Hosseinabadi O. Healing acceleration of oral mucositis induced by 5-fluorouracil with Pistacia atlantica (bene) essential oil in hamsters. J Oral Pathol Med. 2016;46(9):725–730. doi: 10.1111/jop.12540. [DOI] [PubMed] [Google Scholar]
- 14.Tayarani-Najaran Z, Mousavi SH, Tajfard F, Asili J, Soltani S, Hatamipour M, et al. Cytotoxic and apoptogenic properties of three isolated diterpenoids from Salvia chorassanica through bioassay-guided fractionation. Food Chem Toxicol. 2013;57:346–351. doi: 10.1016/j.fct.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 15.Adams RP. Identification of Essential Oil Components by Gas Chromatography/ Quadrupole Mass Spectroscopy. 4th ed. Allured Publishing Corporation; 2007. p. 104.p. 125.p. 146.p. 150.p. 423. [Google Scholar]
- 16.O'Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267(17):5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim YM, Yun J, Lee CK, Lee H, Min KR, Kim Y. Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J Biol Chem. 2002;277(18):16340–16344. doi: 10.1074/jbc.M200678200. [DOI] [PubMed] [Google Scholar]
- 18.Qiao Z, Koizumi Y, Zhang M, Natsui M, Flores MJ, Gao L, et al. Anti-melanogenesis effect of Glechoma hederacea L. extract on B16 murine melanoma cells. Biosci Biotechnol Biochem. 2012;76(10):1877–1883. doi: 10.1271/bbb.120341. [DOI] [PubMed] [Google Scholar]
- 19.Huang HC, Hsieh WY, Niu YL, Chang TM. Inhibition of melanogenesis and antioxidant properties of Magnolia grandiflora L. flower extract. BMC Complement Altern Med. 2012;12:72–80. doi: 10.1186/1472-6882-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tayarani-Najaran Z, Akaberi M, Vatani M, Emami SA. Evaluation of antioxidant and anti-melanogenic activities of different extracts from aerial parts of Nepeta binaludensis Jamzad in murine melanoma B16F10 cells. Iran J Basic Med Sci. 2016;19(6):662–669. [PMC free article] [PubMed] [Google Scholar]
- 21.Chen QX, Kubo I. Kinetics of mushroom tyrosinase inhibition by quercetin. J Agric Food Chem. 2002;50(14):4108–1412. doi: 10.1021/jf011378z. [DOI] [PubMed] [Google Scholar]
- 22.Boozari M, Mohammadi A, Asili J, Emami SA, Tayarani-Najaran Z. Growth inhibition and apoptosis induction by Scutellaria pinnatifida A. Ham. on HL-60 and K562 leukemic cell lines. Environ Toxicol Pharmacol. 2015;39(1):307–312. doi: 10.1016/j.etap.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Mälkiä A, Murtomäki L, Urtti A, Kontturi K. Drug permeation in biomembranes: in vitro and in silico prediction and influence of physicochemical properties. Eur J Pharm Sci. 2004;23(1):13–47. doi: 10.1016/j.ejps.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Shan J, Fu J, Zhao Z, Kong X, Huang H, Luo L, et al. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-κB and JNK/AP-1 activation. Int J Immunopharmacol. 2009;9(9):1042–1048. doi: 10.1016/j.intimp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Bouzaiene NN, Chaabane F, Sassi A, Chekir-Ghedira L, Ghedira K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016;144:80–85. doi: 10.1016/j.lfs.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Ye Y, Chou GX, Wang H, Chu JH, Yu ZL. Flavonoids, apigenin and icariin exert potent melanogenic activities in murine B16 melanoma cells. Phytomedicine. 2010;18(1):32–35. doi: 10.1016/j.phymed.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Choi MY, Song HS, Hur HS, Sim SS. Whitening activity of luteolin related to the inhibition of cAMP pathway in alpha-MSH-stimulated B16 melanoma cells. Arch Pharm Res. 2008;31(9):1166–1171. doi: 10.1007/s12272-001-1284-4. [DOI] [PubMed] [Google Scholar]
- 28.Rezaie M, Farhoosh R, Pham N, Quinn RJ, Iranshahi M. Dereplication of antioxidant compounds in Bene (Pistacia atlantica subsp. mutica) hull using a multiplex approach of HPLC-DAD, LC-MS and (1)H NMR techniques. J Pharm Biomed Anal. 2016;117:352–362. doi: 10.1016/j.jpba.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Si YX, Yin SJ, Oh S, Wang ZJ, Ye S, Yan L, et al. An integrated study of tyrosinase inhibition by rutin: progress using a computational simulation. J Biomol Struct Dyn. 2012;29(5):999–1012. doi: 10.1080/073911012010525028. [DOI] [PubMed] [Google Scholar]
- 30.Liu-Smith F, Meyskens FL. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol Nutr Food Res. 2016;60(6):1264–1274. doi: 10.1002/mnfr.201500822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwak JY, Seok JK, Suh HJ, Choi YH, Hong SS, Kim DS, et al. Antimelanogenic effects of luteolin 7-sulfate isolated from Phyllospadix iwatensis Makino. Br J Dermatol. 2016;175(3):501–511. doi: 10.1111/bjd.14496. [DOI] [PubMed] [Google Scholar]
- 32.Lee HS, Shin KH, Ryu GS, Chi GY, Cho IS, Kim HY. Synthesis of small moleculepeptide conjugates as potential whitening agents. Bull Korean Chem Soc. 2012;33(9):3004–3008. [Google Scholar]
- 33.Lee SY, Baek N, Nam TG. Natural, semisynthetic and synthetic tyrosinase inhibitors. J Enzyme Inhib Med Chem. 2015;31(1):1–13. doi: 10.3109/14756366.2015.1004058. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed ZB, Yousfi M, Viaene J, Dejaegher B, Demeyer K, Mangelings D, et al. Antioxidant activities of Pistacia atlantica extracts modeled as a function of chromatographic fingerprints in order to identify antioxidant markers. Microchem J. 2016;128:208–217. [Google Scholar]
- 35.Sarkhail P, Salimi M, Sarkheil P, Mostafapour H. Anti-melanogenic activity and cytotoxicity of Pistacia vera hull on human melanoma SKMEL-3 cells. Acta Med Iran. 2017;55(7):422–428. [PubMed] [Google Scholar]
- 36.Gourine N, Yousfi M, Bombarda I, Nadjemi B, Stocker P, Gaydou E. Antioxidant activities and chemical composition of essential oil of Pistacia atlantica from Algeria. Ind Crops Prod. 2010;31(2):203–208. [Google Scholar]
- 37.Emami SA, Yazdian-Robati R, Sadeghi M, Baharara J, Amini E, Salek F, et al. Inhibitory effects of different fractions of Nepeta satureioides on melanin synthesis through reducing oxidative stress. Res Pharm Sci. 2017;12(2):160–167. doi: 10.4103/1735-5362.202455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saghaie L L, Pourfarzam M, Fassihi A, Sartippour B. Synthesis and tyrosinase inhibitory properties of some novel derivatives of kojic acid. Res Pharm Sci. 2013;8(4):233–242. [PMC free article] [PubMed] [Google Scholar]
- 39.Boghrati Z, Naseri M, Rezaie M, Pham N, Quinn RJ, Tayarani-Najaran Z, et al. Tyrosinase inhibitory properties of phenylpropanoid glycosides and flavonoids from Teucrium polium L. var. gnaphalodes. Iran J Basic Med Sci. 2016;19(8):804–811. [PMC free article] [PubMed] [Google Scholar]