Abstract
Background
There is a need to better define the epidemiology of sepsis in intensive care units (ICUs) around the globe.
Methods
The Intensive Care over Nations (ICON) audit prospectively collected data on all adult (>16 years) patients admitted to the ICU between May 8 and May 18, 2012, except those admitted for less than 24 hours for routine postoperative surveillance. Data were collected daily for a maximum of 28 days in the ICU, and patients were followed up for outcome data until death, hospital discharge, or for 60 days. Participation was entirely voluntary.
Results
The audit included 10069 patients from Europe (54.1%), Asia (19.2%), America (17.1%), and other continents (9.6%). Sepsis, defined as infection with associated organ failure, was identified during the ICU stay in 2973 (29.5%) patients, including in 1808 (18.0%) already at ICU admission. Occurrence rates of sepsis varied from 13.6% to 39.3% in the different regions. Overall ICU and hospital mortality rates were 25.8% and 35.3%, respectively, in patients with sepsis, but it varied from 11.9% and 19.3% (Oceania) to 39.5% and 47.2% (Africa), respectively. After adjustment for possible confounders in a multilevel analysis, independent risk factors for in-hospital death included older age, higher simplified acute physiology II score, comorbid cancer, chronic heart failure (New York Heart Association Classification III/IV), cirrhosis, use of mechanical ventilation or renal replacement therapy, and infection with Acinetobacter spp.
Conclusions
Sepsis remains a major health problem in ICU patients worldwide and is associated with high mortality rates. However, there is wide variability in the sepsis rate and outcomes in ICU patients around the globe.
Keywords: critically ill, international, mortality, septic shock
Sepsis is a major cause of morbidity and mortality in modern intensive care units (ICUs). Although several studies have provided epidemiological data on sepsis in ICU patients in the developed world [1–6], there is limited information on the global burden of sepsis worldwide [7, 8]. Yet, such data are crucially important to (1) increase awareness of the global impact of sepsis, (2) highlight the need for continued research into potential preventive and therapeutic interventions, and (3) help guide resource allocation [9]. Information on patterns of sepsis around the globe is also of interest, including causative microorganisms, primary source of infection, and associated outcomes.
In 2012, the World Federation of Societies of Intensive and Critical Care Medicine conducted a worldwide audit of data from ICUs around the world, providing a large database from which to extract information. We used these data to explore the characteristics of patients with sepsis around the world, including international differences in occurrence rates, causative microorganisms, and outcomes. We also evaluated some factors associated with in-hospital mortality in these patients.
METHODS
The worldwide Intensive Care over Nations (ICON) audit recruited ICUs by open invitation, through national scientific societies, national and international meetings, e-mail lists, and individual contacts. Participation was entirely voluntary, with no financial incentive. Ethics committee approval was obtained by the participating institutions according to local ethical regulations. Informed consent was not required for this observational and anonymous audit. Of the 730 ICUs contributing to the study from 84 countries (see the participants list in Appendix 1), 419 (57.4%) were in university/academic hospitals. The organizational characteristics of these centers have been described previously [10].
Each ICU was asked to prospectively collect data on all adult (>16 years) patients admitted to their ICU between May 8 and May 18, 2012, except those who stayed in the ICU for <24 hours for routine postoperative surveillance. Readmissions of previously included patients were not included. Data were collected daily for a maximum of 28 days in the ICU. Outcome data were collected at the time of ICU and hospital discharge or at 60 days. Data were entered anonymously using electronic case report forms via a secured internet-based website. Data collection on admission included demographic data and comorbidities. Clinical and laboratory data for simplified acute physiology (SAPS) II [11] and Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II [12] scores were reported as the worst values within the first 24 hours after admission. A daily evaluation of organ function was performed according to the sequential organ failure assessment (SOFA) score [13]; organ failure was defined as a SOFA subscore >2 for the organ in question. Clinical and microbiologic infections were reported daily as well as antimicrobial therapy.
Infection was defined according to the criteria of the International Sepsis Forum [14]. Sepsis was defined as the presence of infection with associated organ failure [15]. Septic shock was defined as sepsis associated with cardiovascular failure requiring vasopressor support (SOFA cardiovascular of 3 or 4). Intensive care unit-acquired infection was defined as infection identified at least 48 hours after ICU admission. Non-ICU acquired infection was defined as infection present on admission or within the first 48 hours after ICU admission. Only the first episode of infection was considered in the analysis.
Detailed instructions and definitions were available through a secured website for all participants before starting data collection and throughout the study period. Any additional queries were answered on a per case basis. Validity checks were made at the time of electronic data entry, including plausibility checks within each variable and between variables. Data were further reviewed by the coordinating center for completeness and plausibility, and any doubts were clarified with the participating center. There was no on-site monitoring. We did not attempt to verify the pathogenicity of the microorganisms, including the relevance of Staphylococcus epidermidis or the distinction between colonization and infection.
For the purposes of this audit, we divided the world into 8 geographic regions: North America, South America, Western Europe, Eastern Europe, South Asia, East and Southeast Asia, Oceania, and Africa. Individual countries were also classified into 3 income groups according to the 2011 gross national income (GNI) per capita, calculated using the World Bank Atlas method [16]: GNI <$4035 = low and lower middle income; GNI $4036–12475 = upper middle income; and GNI >$12476 = high income.
Statistical Analysis
Data are shown as means with standard deviation (SD), mean and 95% confidence intervals (CIs), medians and interquartile ranges (IQRs), numbers, and percentages. Differences between groups in distribution of variables were assessed using analysis of variance, Kruskal-Wallis test, Student’s t test, Mann-Whitney test, χ2 test, or Fisher’s exact test as appropriate.
To identify the risk factors associated with in-hospital mortality in septic patients, we used a 3-level multilevel technique with the structure of an individual patient (level 1) admitted to a hospital (level 2) within a country (level 3). The explanatory variables considered in the model were as follows:
Individual-level factors: age, sex, SAPS II score, type of admission, source of admission, mechanical ventilation or renal replacement therapy at any time during the ICU stay, comorbidities, onset of infection, site of infection, and the most common microorganisms
Hospital-level factors: type of hospital; ICU specialty; total number of ICU patients in 2011; number of staffed ICU beds
Country-level factors: GNI
Individual-level variables to be included in the final model were selected on the basis of a multilevel model including country-level and hospital-level factors and each of the individual-level factors; variables with P < .2 were considered in the final model. Collinearity between variables was checked by inspection of the correlation between them, by looking at the correlation matrix of the estimated parameters, and by looking at the change of parameter estimates and at their estimated standard errors (SEs) [17]. Q-Q plots were drawn to check for normality in the residuals. The results of fixed effects (measures of association) are given as odds ratios (ORs) with their 95% CIs and the 80% interval OR. Random effects (measures of variation) measures included the variance (var) and its SE and the median OR. The statistical significance of covariates was calculated using the Wald test.
Data were analyzed using IBM SPSS Statistics software, version 22 for Windows and R software, version 2.0.1 (CRAN project). All reported P values are 2-sided, and P < .05 was considered to indicate statistical significance. The results of fixed effects are given as OR with 95% CIs.
RESULTS
Characteristics of the Study Group
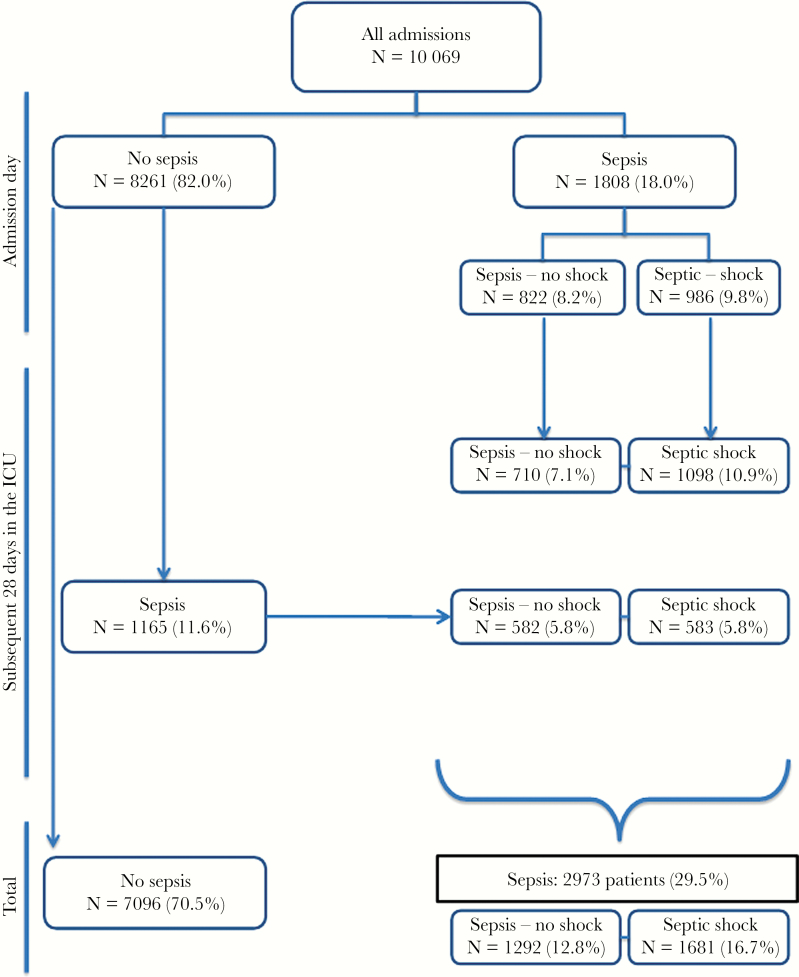
A total of 10069 patients were included in the audit; 2973 patients (29.5%) had sepsis, including 1808 (18.0%) with sepsis at admission to the ICU (Figure 1). In the whole cohort, antimicrobials were given to 5975 (59.3%) patients during their ICU stay. Patients with sepsis were older, had higher severity scores on admission to the ICU, had more comorbidities, and were more commonly receiving mechanical ventilation and renal replacement therapy on admission to the ICU than patients without sepsis (Table 1). Patients with sepsis also had more organ failures than the other patients (median [IQ]: 3 [1–4] vs 1 [0–2] organs; P < .001).
Figure 1.
Distribution of patients according to the presence or absence of sepsis on admission and during the intensive care unit (ICU) stay.
Table 1.
Characteristics of the Study Cohort on Admission to the ICU According to the Presence of Sepsisa
| Characteristics | All Patients N = 10069 |
No Sepsis N = 7096 |
Sepsis N = 2973 |
P Value |
|---|---|---|---|---|
| Age, years, mean ± SD | 60.0 ± 18.0 | 59.4 ± 18.4 | 61.5 ± 17.0 | <.001 |
| Male, n (%) | 5973 (60.1) | 4177 (59.7) | 1796 (61.0) | .21 |
| Severity scores, mean ± SD | ||||
| SAPS II score | 40.2 ± 18.2 | 36.4 ± 17.4 | 49.2 ± 16.6 | <.001 |
| SOFA score | 5 [3–9] | 4 [2–7] | 8 [6–11] | <.001 |
| Type of admission, n (%) | <.001 | |||
| Surgical | 3432 (36.0) | 2475 (37.0) | 957 (33.7) | |
| Medical | 5382 (56.5) | 3646 (54.6) | 1736 (61.1) | |
| Trauma | 643 (6.8) | 512 (7.7) | 131 (4.6) | |
| Other | 66 (0.7) | 49 (.7) | 17 (.6) | |
| Source of admission, n (%) | <.001 | |||
| ER/ambulance | 3814 (37.9) | 2780 (39.2) | 1034 (34.8) | |
| Hospital floor | 2625 (26.1) | 1664 (23.4) | 961 (32.3) | |
| OR/recovery | 1811 (18.0) | 1363 (19.2) | 448 (15.1) | |
| Other hospital | 981 (9.7) | 652 (9.2) | 329 (11.1) | |
| Other | 838 (8.3) | 637 (9.0) | 201 (6.8) | |
| Comorbidities, n (%) | ||||
| COPD | 1240 (12.3) | 788 (11.1) | 452 (15.2) | <.001 |
| Cancer | 1049 (10.4) | 710 (10.0) | 339 (11.4) | .04 |
| Diabetes mellitus, insulin-dependent | 972 (9.7) | 664 (9.4) | 308 (10.4) | .12 |
| Heart failure, NYHA III/IV | 921 (9.1) | 588 (8.3) | 333 (11.2) | <.001 |
| Chronic renal failure | 912 (9.1) | 582 (8.2) | 330 (11.1) | <.001 |
| Cirrhosis | 349 (3.5) | 217 (3.1) | 132 (4.4) | <.001 |
| Immunosuppression | 346 (3.4) | 177 (2.5) | 169 (5.7) | <.001 |
| Metastatic cancer | 332 (3.3) | 221 (3.1) | 111 (3.7) | .11 |
| Haematologic cancer | 212 (2.1) | 99 (1.4) | 113 (3.8) | <.001 |
| HIV infection | 71 (.7) | 37 (.5) | 34 (1.1) | <.001 |
| Number of comorbidities, n (%) | <.001 | |||
| None | 5512 (54.7) | 4145 (58.4) | 1367 (46.0) | |
| 1 | 2800 (27.8) | 1880 (26.5) | 920 (30.9) | |
| 2 | 1207 (12.0) | 740 (10.4) | 467 (15.7) | |
| 3 | 416 (4.1) | 249 (3.5) | 167 (5.6) | |
| ≥4 | 134 (1.3) | 82 (1.2) | 52 (1.7) | |
| Procedures, n (%) | ||||
| Mechanical ventilation | 4776 (47.4) | 2755 (38.8) | 2021 (68.0) | <.001 |
| Renal replacement therapy | 537 (5.3) | 264 (3.7) | 273 (9.2) | <.001 |
| Antimicrobials, n (%) | 5975 (59.3) | 3002 (42.3) | 2973 (100) | <.001 |
| Antibiotic | 5935 (58.9) | 2979 (42.0) | 2956 (99.4) | <.001 |
| Antifungal | 784 (7.8) | 202 (2.8) | 582 (19.6) | <.001 |
| Antiviral | 273 (2.7) | 80 (1.1) | 193 (6.5) | <.001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; ER, emergency room; HIV, human immunodeficiency virus; ICU, intensive care unit; NYHA, New York Heart Association Classification; OR, operating room; SAPS, simplified acute physiology; SOFA, sequential organ assessment; SD, standard deviation.
aValid percentages are given after exclusion of missing values (data missing from 546 patients for type of admission).
Patterns of Infections
The most common source of sepsis was the respiratory tract (67.4%) followed by the abdomen (21.8%) (Supplementary Table E1). Positive isolates were retrieved in 69.6% (n = 2069) of patients with sepsis; two thirds of these patients had Gram-negative microorganisms isolated and half had Gram-positive microorganisms; 1068 (51.6%) of the sepsis patients with positive isolates had more than 1 microorganism isolated (Table 2). Patients with urinary tract (82.6% vs 43.9%), abdominal (77.1% vs 50.8%), and respiratory tract (70.0% vs 51.4%) infections were more likely to have Gram-negative than Gram-positive isolates (Supplementary Table E1). Microbiological patterns varied around the globe (Table 2), with Gram-positive isolates being much less frequent (21.4%) in South Asia than in other regions. Methicillin-resistant Staphylococcus aureus (MRSA) was more common in the Middle East (14.4%) and North America (12.8%) than in Western Europe (6.1%). Klebsiella spp isolates were most commonly reported in Africa (31.3%), Eastern Europe (28.5%), and South America (24.7%), and Pseudomonas spp was most frequent in Eastern Europe (21.1%) and South America (20.4%). Fungal organisms contributed to 14.5% and 14.8% of isolates in Western and Eastern Europe, respectively, but to only 5.1% of isolates in North America.
Table 2.
Distribution of the Most Common Microorganisms in Patients With Positive Isolates and Mortality Rates According to Geographic Region
| Characteristic | All Patients | Western Europe |
Eastern Europe |
South America |
North America |
East and Southeast Asia |
South Asia |
Oceania | Middle East |
Africa |
|---|---|---|---|---|---|---|---|---|---|---|
| Total number of patients, n | 10 069 | 4335 | 1110 | 993 | 730 | 946 | 982 | 439 | 393 | 141 |
| Patients with sepsis, n (%) | 2973 (29.5) | 1357 (31.3) | 336 (30.3) | 303 (30.5) | 147 (20.1) | 372 (39.3) | 134 (13.6) | 135 (30.8) | 151 (38.4) | 38 (27.0) |
| Patients with positive isolates, n (%)a | 2069 (69.6) | 947 (69.8) | 256 (76.2) | 186 (61.4) | 117 (79.6) | 240 (64.5) | 84 (62.7) | 105 (77.8) | 118 (78.1) | 16 (42.1) |
| Gram-positive, n (%) | 1030 (49.8) | 517 (54.6) | 144 (56.3) | 84 (45.2) | 59 (50.4) | 86 (35.8) | 18 (21.4) | 57 (54.3) | 58 (49.2) | 7 (43.8) |
| Methicillin-sensitive Staphylococcus aureus | 257 (12.4) | 120 (12.7) | 37 (14.5) | 29 (15.6) | 14 (12.0) | 25 (10.4) | 6 (7.1) | 14 (13.3) | 10 (8.5) | 2 (12.5) |
| Methicillin-resistant S aureus | 151 (7.3) | 58 (6.1) | 20 (7.8) | 15 (8.1) | 15 (12.8) | 14 (5.8) | 2 (2.4) | 9 (8.6) | 17 (14.4) | 1 (6.3) |
| Coagulase-negative Staphylococcus | 500 (24.2) | 269 (28.4) | 79 (30.9) | 39 (21.0) | 25 (21.4) | 25 (10.4) | 11 (13.1) | 19 (18.1) | 27 (22.9) | 6 (37.5) |
| Streptococcus, D group | 84 (4.1) | 52 (5.5) | 10 (3.9) | 5 (2.7) | 5 (4.3) | 3 (1.3) | - | 6 (5.7) | 3 (2.5) | - |
| Streptococcus, Others | 222 (10.7) | 109 (11.5) | 25 (9.8) | 12 (6.5) | 15 (12.8) | 27 (11.3) | 1 (1.2) | 15 (14.3) | 16 (13.6) | 2 (12.5) |
| Other Gram-positive cocci | 46 (2.2) | 19 (2.0) | 11 (4.3) | - | 3 (2.6) | 7 (2.9) | - | 4 (3.8) | 2 (1.7) | - |
| Gram negative, n (%) | 1389 (67.1) | 610 (64.4) | 189 (73.8) | 140 (75.3) | 65 (55.6) | 159 (66.3) | 67 (79.8) | 66 (62.9) | 84 (71.2) | 9 (56.3) |
| Escherichia coli | 470 (22.7) | 236 (24.9) | 57 (22.3) | 51 (27.4) | 22 (18.8) | 35 (14.6) | 22 (26.2) | 25 (23.8) | 19 (16.1) | 3 (18.8) |
| Klebsiella spp | 356 (17.2) | 124 (13.1) | 73 (28.5) | 46 (24.7) | 13 (11.1) | 45 (18.8) | 18 (21.4) | 13 (12.4) | 19 (16.1) | 5 (31.3) |
| Pseudomonas spp | 337 (16.3) | 147 (15.5) | 54 (21.1) | 38 (20.4) | 18 (15.4) | 35 (14.6) | 13 (15.5) | 12 (11.4) | 20 (16.9) | - |
| Acinetobacter spp | 243 (11.7) | 39 (4.1) | 55 (21.5) | 36 (19.4) | 5 (4.3) | 51 (21.3) | 24 (28.6) | 1 (1.0) | 29 (24.6) | 3 (18.8) |
| Enterobacter spp | 188 (9.1) | 91 (9.6) | 45 (17.6) | 13 (7.0) | 3 (2.6) | 11 (4.6) | 10 (11.9) | 8 (7.6) | 5 (4.2) | 2 (12.5) |
| Proteus spp | 121 (5.8) | 63 (6.7) | 25 (9.8) | 6 (3.2) | 5 (4.3) | 7 (2.9) | 1 (1.2) | 5 (4.8) | 6 (5.1) | 3 (18.8) |
| Gram-negative, others | 320 (15.5) | 174 (18.4) | 35 (13.7) | 20 (10.8) | 14 (12.0) | 32 (13.3) | 4 (4.8) | 17 (16.2) | 22 (18.6) | 2 (12.5) |
| Anaerobes, n (%) | 79 (3.8) | 45 (4.8) | 12 (4.7) | 3 (1.6) | 8 (6.8) | 4 (1.7) | - | 4 (3.8) | 2 (1.7) | 1 (6.3) |
| Other bacteria, n (%) | 18 (0.9) | 4 (0.4) | 2 (0.8) | 2 (1.1) | 1 (0.9) | 5 (2.1) | 1 (1.2) | 2 (1.9) | 1 (0.8) | - |
| Fungi, n (%) | 266 (12.9) | 137 (14.5) | 38 (14.8) | 18 (9.7) | 6 (5.1) | 31 (12.9) | 8 (9.5) | 10 (9.5) | 16 (13.6) | 2 (12.5) |
| Candida albicans | 185 (8.9) | 93 (9.8) | 31 (12.1) | 9 (4.8) | 3 (2.6) | 23 (9.6) | 6 (7.1) | 9 (8.6) | 10 (8.5) | 1 (6.3) |
| Candida non-albicans | 89 (4.3) | 47 (5.0) | 8 (3.1) | 8 (4.3) | 2 (1.7) | 11 (4.6) | 4 (4.8) | 4 (3.8) | 4 (3.4) | 1 (6.3) |
| Fungi, others | 44 (2.1) | 28 (3.0) | 4 (1.6) | 2 (1.1) | 1 (0.9) | 5 (2.1) | - | - | 4 (3.4) | - |
| Viruses and parasites, n (%) | 59 (2.9) | 31 (3.3) | 5 (2.0) | 6 (3.2) | 2 (1.7) | 10 (4.2) | - | 1 (1.0) | 3 (2.5) | 1 (6.3) |
| Mortality rates in patients with sepsis, n (%) | ||||||||||
| Intensive care unit | 753 (25.8) | 309 (22.9) | 118 (35.3) | 102 (36.3) | 27 (18.5) | 76 (21.2) | 37 (28.9) | 16 (11.9) | 53 (35.6) | 15 (39.5) |
| Hospital | 1004 (35.3) | 439 (33.3) | 146 (44.8) | 119 (45.4) | 37 (25.2) | 108 (31) | 44 (35.2) | 26 (19.3) | 68 (46.6) | 17 (47.2) |
aIn patients with sepsis.
Patients with ICU-acquired infections (n = 764) were younger, more likely to be surgical admissions, and had lower SAPS II and SOFA scores on admission to the ICU, compared with those who had infections within the first 48 hours on the ICU (Table 3 and Supplementary Table E2). Respiratory and catheter-associated infections were more frequent and abdominal infections less frequent in patients with ICU-acquired than in those with non-ICU-acquired infections (Supplementary Table E2). Patients with ICU-acquired infections were more likely to have positive isolates than patients with non-ICU-acquired infections (79.5% vs 66.2%, P < .001) (Supplementary Table E3).
Table 3.
Severity Scores on Admission to the ICU, Maximum Number of Organ Failure, and Mortality Rates According to Sepsis Status
| Severity Scores, Mean ± SD |
Mortality Rates, % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Category | n | SAPS II | SOFA | ICU LOSa, Median [IQR] | No. of Organ Failures Median [IQR] | ICUb | In-Hospitalc |
| Onset of Sepsis | |||||||
| Within the first 48 hoursd | 2209 | 50.5 ± 16.8 | 9.2 ± 3.9 | 5 [2–10] | 3 [2–4] | 26.0 (24.2–27.9) | 35.8 (33.8–37.9) |
| Later | 764 | 45.4 ± 15.5e | 7.5 ± 3.8e | 12 [6–19]e | 3 [2–4] | 25.1 (22.0–28.3) | 33.8 (30.3–37.2) |
| Severity of Sepsis on ICU Admission | |||||||
| No sepsisd | 8261 | 37.8 ± 17.5 | 5.3 ± 4.1 | 3 [1–5] | 1 [0–2] | 13.6 (12.9–14.4) | 19.0 (18.1–19.9) |
| Sepsis without shock | 822 | 46.2 ± 15.4e | 7.4 ± 2.9e | 5 [2–9]e | 2 [1–3]e | 20.1 (17.4–22.9)e | 30.3 (27.1–33.6)e |
| Septic shock | 986 | 55.1 ± 17.2e | 11.3 ± 3.6e | 5 [2–11]e | 3 [2–4]e | 33.7 (30.7–36.7)e | 43.0 (39.9–46.2)e |
| Severity of Sepsis During ICU Stay | |||||||
| No sepsisd | 7096 | 36.4 ± 17.4 | 4.9 ± 4.0 | 2 [1–4] | 1 [0–2] | 12.1 (11.3–12.8) | 16.7 (15.8–17.6) |
| Sepsis without shock | 1292 | 44.6 ± 15.3e | 7.0 ± 3.2e | 6 [3–11]e | 2 [1–3]e | 14.3 (12.4–16.2)f | 23.6 (21.3–26.0)e |
| Septic shock | 1681 | 52.7 ± 16.7e | 10.1 ± 3.9e | 7 [3–14]e | 3 [2–4]e | 34.6 (32.3–36.9)e | 44.2 (41.7–46.6)e |
Abbreviations: CI, confidence interval; ICU, intensive care unit; IQR, interquartile; LOS, length of stay; SAPS, simplified acute physiology score; SD, standard deviation; SOFA, sequential organ failure assessment score.
Missing observations: a489, b401, c797.
dThe reference group.
e P < .01 among groups.
f P < .05 among groups.
Outcomes
Intensive care unit mortality rates were 25.8% in patients with sepsis and 12.1% in those without (P < .001); hospital mortality rates were 35.3% vs 16.7%, P < .001). Intensive care unit and hospital mortality rates varied from 11.9% and 19.3% (Oceania) to 39.5% and 47.2% (Africa), respectively (Table 2). Intensive care unit length of stay was longer (6 [3–13] vs 2 [1–4] days, P < .001) in patients with than in those without sepsis. As expected, there was a stepwise increase in ICU and hospital mortality rates according to the severity of sepsis (Table 3). Although patients with ICU-acquired sepsis had longer ICU stays than those who had sepsis within 48 hours of admission to the ICU, they did not have higher mortality rates (Table 3).
The crude risk of in-hospital death was higher in patients with infections caused by Pseudomonas spp, Acinetobacter spp, and fungi (Table 4). In the multilevel analysis, independent risk factors for in-hospital death in patients with sepsis were older age, higher SAPS II score, cirrhosis, metastatic cancer, chronic heart failure (NYHA III/IV), use of mechanical ventilation or renal replacement therapy at any time during the ICU stay, and infection with Acinetobacter spp (Supplementary Table E4). The use of mechanical ventilation and presence of comorbid cirrhosis more than doubled the risk of death. The relative risk of death was higher in patients admitted to ICUs in countries with upper middle GNI than in those with high GNI (1.77 [1.31–2.39], P < .001). However, although the model suggested significant between-hospital variation (var = 0.28, P = .001) in the individual risk of in-hospital death, the between-country variation was not significant.
Table 4.
Outcome According to Isolated Microorganisms in Patients With Sepsis (n = 2973)
| Risk Factor | ICU Mortality, n (%) |
Hospital Mortality, n (%) | Crude Risk of In- Hospital Death OR (95% CI) |
P Value |
|---|---|---|---|---|
| Gram-positive | 267 (26.2) | 360 (36.0) | 1.05 (0.89–1.23) | .55 |
| Staphylococcus aureus methicillin sensitive | 71 (28.0) | 89 (36.0) | 1.04 (0.79–1.36) | .80 |
| MRSA | 36 (24.2) | 51 (34.7) | 0.97 (0.69–1.38) | .87 |
| Staphylococcus, coagulase negative | 129 (26.0) | 184 (37.9) | 1.14 (0.93–1.40) | .20 |
| Streptococcus, D group | 16 (19.3) | 22 (27.5) | 0.69 (0.42–1.13) | .14 |
| Streptococcus, others | 57 (26.1) | 77 (35.8) | 1.02 (0.77–1.37) | .87 |
| Other Gram-positive cocci | 9 (19.6) | 13 (29.5) | 0.77 (0.40–1.47) | .42 |
| Gram negative | 364 (26.6) | 492 (37.0) | 1.15 (0.99–1.34) | .07 |
| Escherichia coli | 114 (24.7) | 162 (36.0) | 1.04 (0.84–1.28) | .74 |
| Enterobacter spp | 45 (24.1) | 67 (36.8) | 1.07 (0.79–1.46) | .66 |
| Klebsiella spp | 92 (26.4) | 128 (37.9) | 1.13 (0.90–1.43) | .29 |
| Acinetobacter spp | 88 (37.0) | 110 (48.0) | 1.78 (1.36–2.33) | <.01 |
| Proteus spp | 28 (23.1) | 40 (33.6) | 0.92 (0.63–1.36) | .69 |
| Pseudomonas spp | 100 (30.1) | 131 (40.4) | 1.28 (1.01–1.62) | .04 |
| Gram negative, others | 82 (25.9) | 110 (35.7) | 1.02 (0.80–1.31) | .87 |
| Anaerobes | 23 (29.1) | 31 (39.7) | 1.22 (0.77–1.93) | .41 |
| Other bacteria | 6 (33.3) | 7 (38.9) | 1.17 (0.45–3.02) | .75 |
| Fungi | 77 (29.2) | 107 (41.6) | 1.34 (1.04–1.75) | .03 |
| Candida albicans | 49 (26.8) | 71 (39.9) | 1.23 (0.90–1.68) | .19 |
| Candida non-albicans | 26 (29.2) | 38 (43.7) | 1.44 (0.93–2.21) | .10 |
| Fungi, others | 16 (36.4) | 20 (45.5) | 1.54 (0.85–2.80) | .16 |
| Viruses and parasites | 16 (28.1) | 21 (36.8) | 1.07 (0.62–1.84) | .81 |
Abbreviations: CI, confidence interval; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; OR, odds ratio.
DISCUSSION
The present audit confirms the considerable burden that sepsis presents in modern ICUs. This large study, including more than 10000 patients from 730 ICUs, indicates that approximately 30% of all ICU patients have sepsis, as defined by the presence of infection and organ dysfunction. This percentage is identical to that (29.5%) reported in the earlier Sepsis Occurrence in Acutely Ill Patients (SOAP) study [1], a large European study that used the same methodology, and in a recent analysis of a large United Kingdom database [18], but somewhat higher than in some other large studies [4, 5, 19, 20]. In addition to possible differences associated with different definitions of sepsis used in the various studies, 2 other major elements may account for these apparent inconsistencies. First, we did not include all patients admitted to the ICU, but only critically ill patients, excluding patients admitted to the ICU for postoperative surveillance without complications. Second, some studies focused on admission data [20]; if we consider only the patients who had sepsis on admission in our study, the rate of sepsis was 18%. More importantly, the percentage of ICU patients with sepsis varied around the globe, with particularly high rates in East and Southeast Asia, confirming the high disease burden in this area [21, 22]. Although these data were collected in 2012, we believe they are still relevant, especially given the general lack of global data in this regard.
A strength of the present study compared with studies assessing only sepsis on admission or prevalence studies (eg, EPIC II [2]) is that patients were followed throughout the ICU course, enabling evaluation of sepsis that developed during the ICU stay as well as sepsis present on admission. It is interesting to note that patients with ICU-acquired sepsis had similar outcomes to those of patients with sepsis on admission, and ICU-acquired sepsis was not independently associated with a higher risk of mortality after adjusting for confounders in the multilevel analysis. Although we were unable to assess this specifically, van Vught et al [23] recently reported a low attributable mortality of ICU-acquired infections. Shankar-Hari et al [24] reported that the inferred causal link between sepsis and long-term mortality was significantly confounded by age, comorbidity, and preacute illness trajectory. More importantly, in our multivariable regression analysis, all the above-mentioned factors were found to be significant determinants of mortality, suggesting that ICU-acquired sepsis may not on its own be a causative factor for mortality. Nevertheless, nosocomial infections are responsible for prolonged stays in the ICU and increased costs [25, 26].
Positive isolates were obtained in 70% of the patients with sepsis, a similar finding to that reported in other studies [1, 19, 27, 28]. Two thirds of these patients had Gram-negative organisms isolated and one half had Gram-positive organisms isolated. The most common Gram-negative microorganisms recovered were E coli, Klebsiella spp, Pseudomonas spp, and Acinetobacter spp, as in previous studies [1, 27, 28]. It is interesting to note that Gram-positive organisms were more common in North America than in other parts of the world; MRSA was also more common in North America than in other parts of the world except the Middle East. These findings are important when using guidelines for management of infection and sepsis, because guidelines developed in one part of the world, for example North America, may not be relevant to other areas. The results also underline the ongoing importance of fungal infections, which were involved in 13% of cases of sepsis overall, although the frequency was lower in the United States (5%), perhaps because more stringent criteria are used to characterize fungal infections in the United States. Finally it is noteworthy that approximately 42% of patients without sepsis received antimicrobial agents. The reasons for this are unclear, but antimicrobials may still be prescribed despite sepsis resolution or exclusion. In a retrospective analysis of 269 patients who were diagnosed with suspected sepsis in the emergency department and started on antibiotic therapy, 29% of the patients were found not to have bacterial disease, but the median duration of antibiotics in these patients was still 7 days (IQR, 4–10) [29].
Intensive care unit mortality rates in patients with sepsis were approximately 26% and were twice as high as those in nonseptic patients. This percentage is lower than the 32% observed in the SOAP study (using their “severe sepsis” definition that is equivalent to our current definition of sepsis) [1] and in other studies [1, 19, 27, 28]. Intensive care unit mortality rates in patients with septic shock were approximately 35%, a percentage that is also lower than that reported in earlier studies [1, 5]. Increased awareness of sepsis diagnosis and improved early management may have contributed to improved outcomes over time. Mortality rates varied around the globe, but in multivariable analysis, the between-country variation was not significant. These findings are in contrast to those from the International Multicenter Prevalence Study on Sepsis (IMPreSS) study of 1794 patients with sepsis from 62 countries, in which mortality rates were higher in East Europe and Central/South America compared with North America after adjustment for adjusted for ICU admission, sepsis status, location of diagnosis, origin of sepsis, APACHE II score, and country [30].
As expected, nonsurvivors were older and had more comorbidities. As in previous ICU studies [1, 2], Pseudomonas and fungal infections were associated with worse outcomes, although only Acinetobacter infection was an independent predictor for hospital death in the multilevel analysis. More importantly, our data do not infer a cause-effect relationship, and the presence of Acinetobacter may simply be a marker of severity. In a systematic review of 6 matched case-control and cohort studies, Falagas et al [31] reported that Acinetobacter infection was associated with increased attributable mortality, although others have suggested no independent link between Acinetobacter infection and increased risk of death [32].
Mechanical ventilation at any time during the ICU stay and pre-existing liver cirrhosis were also important prognostic factors, more than doubling the risk of death. Use of renal replacement therapy at any time during the ICU stay was also associated with increased mortality. We also identified significant between-center variation, suggesting that differences in local ICU organization may have an impact on outcomes of patients with sepsis. Some of the potential factors associated with between-center outcomes differences have been identified in the literature. In an international cohort of 13796 ICU patients, Sakr et al [33] reported that a high nurse/patient ratio was independently associated with a lower risk of in-hospital death. Gaieski et al [34] reported that sepsis outcomes were improved in centers with higher sepsis case volumes. In a multicenter study in Canada, Yergens et al [35] reported that ICU occupancy>90% was associated with an increase in hospital mortality in patients with sepsis admitted from the emergency department. We are unable to identify which particular organizational factors may have influenced outcomes from our data, and this is an area that needs further study.
Our database was very large, including considerable data on demographics, organ function, and outcomes. Nevertheless, to successfully collect a large amount of data in many ICUs requires some limitations in the level of detail of the collected data; therefore, we did not collect precise information on all subtypes of microorganisms or their resistance patterns or on the appropriateness of antimicrobial coverage. Moreover, data were collected by ICU doctors or research nurses who may not have specific expertise in infectious diseases, although the significance of this is uncertain. Our study has other limitations. First, although the audit included a large number of ICUs, the purely voluntary nature of the participation may have an impact on the representativeness of the data. Second, data collection was not monitored so small errors could not be corrected; only obvious incongruous data were verified. Third, in some countries, identification of microorganisms may have been incomplete because of the limited availability of microbiological testing. Moreover, the quality of the antimicrobials used in the treatment of infection has also been questioned in low-resource countries [36]. Fourth, there was no means of differentiating between colonization and infection for some organisms, including Acinetobacter and coagulase-negative staphylococci. Therefore, microorganisms were weighted equally in the multilevel analysis. The absence of comparative large epidemiologic data that address this issue makes it difficult to judge whether the estimates of microorganisms provided in our study overestimate the frequency of these infections. Fifth, data were collected for the same period in all regions and therefore do not take into account any possible influence of seasonal variation. Sixth, we did not use the exact recent Sepsis-3 definitions [37], which were published after our study, partly because we had no data on the evolution of SOFA scores before ICU admission and blood lactate levels were not available in all patients. Nevertheless, we used a definition based on the presence of organ dysfunction, a key feature of Sepsis-3. Finally, despite adjusting for a large number of variables that may influence outcome, the results of the multilevel analysis could not take into account other unmeasured variables that may have been of potential significance.
CONCLUSIONS
Sepsis, as defined by infection with organ dysfunction, remains a major health problem in ICU patients worldwide, associated with high mortality. There is wide variation in sepsis rates, causative microorganisms, and outcome in ICU patients around the world. A history of liver cirrhosis or metastatic cancer, use of mechanical ventilation or renal replacement therapy, and Acinetobacter infection were independently associated with an increased risk of in-hospital death. Global epidemiological data such as these help increase awareness of sepsis and provide crucial information for future healthcare planning. Further studies in this field should be done on a regular basis with standardized methodology to ensure the comparability of the results.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Dr. Hassane Njimi (Department of Intensive Care, Erasme University Hospital, Brussels, Belgium) for help with the statistical analyses.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006; 34:344–53. [DOI] [PubMed] [Google Scholar]
- 2. Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302:2323–9. [DOI] [PubMed] [Google Scholar]
- 3. Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012; 40:754–61. [DOI] [PubMed] [Google Scholar]
- 4. SepNet Critical Care Trials Group. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med 2016; 42:1980–9. [DOI] [PubMed] [Google Scholar]
- 5. Sakr Y, Elia C, Mascia L, et al. Epidemiology and outcome of sepsis syndromes in Italian ICUs: a muticentre, observational cohort study in the region of Piedmont. Minerva Anestesiol 2013; 79:993–1002. [PubMed] [Google Scholar]
- 6. Yébenes JC, Ruiz-Rodriguez JC, Ferrer R, et al. Epidemiology of sepsis in Catalonia: analysis of incidence and outcomes in a European setting. Ann Intensive Care 2017; 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet 2010; 376:1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193:259–72. [DOI] [PubMed] [Google Scholar]
- 9. Finfer S, Machado FR. The global epidemiology of sepsis. Does it matter that we know so little?Am J Respir Crit Care Med 2016; 193:228–30. [DOI] [PubMed] [Google Scholar]
- 10. Vincent JL, Marshall JC, Namendys-Silva SA, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med 2014; 2:380–6. [DOI] [PubMed] [Google Scholar]
- 11. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993; 270:2957–63. [DOI] [PubMed] [Google Scholar]
- 12. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–29. [PubMed] [Google Scholar]
- 13. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–10. [DOI] [PubMed] [Google Scholar]
- 14. Calandra T, Cohen J. The International Sepsis Forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med 2005; 33:1538–48. [DOI] [PubMed] [Google Scholar]
- 15. Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet 2013; 381:774–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The World Bank. GNI per capita, Atlas method (current US$) Available at: http://data.worldbank.org/indicator/ny.gnp.pcap.cd. Accessed 10 May 2016.
- 17. Martin-Loeches I, Njimi H, Vincent JL. Collinearity and multivariable analysis: response to comments by Claret et al. Intensive Care Med 2016; 42:1835. [DOI] [PubMed] [Google Scholar]
- 18. Shankar-Hari M, Harrison DA, Rubenfeld GD, Rowan K. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br J Anaesth 2017; 119:626–36. [DOI] [PubMed] [Google Scholar]
- 19. Brun-Buisson C, Meshaka P, Pinton P, Vallet B. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med 2004; 30:580–8. [DOI] [PubMed] [Google Scholar]
- 20. Padkin A, Goldfrad C, Brady AR, et al. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med 2003; 31:2332–8. [DOI] [PubMed] [Google Scholar]
- 21. Zhou J, Qian C, Zhao M, et al. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS One 2014; 9:e107181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Southeast Asia Infectious Disease Clinical Research Network. Causes and outcomes of sepsis in Southeast Asia: a multinational multicentre cross-sectional study. Lancet Glob Health 2017; 5:e157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Vught LA, Klein Klouwenberg PM, Spitoni C, et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA 2016; 315:1469–79. [DOI] [PubMed] [Google Scholar]
- 24. Shankar-Hari M, Ambler M, Mahalingasivam V, et al. Evidence for a causal link between sepsis and long-term mortality: a systematic review of epidemiologic studies. Crit Care 2016; 20:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salgado Yepez E, Bovera MM, Rosenthal VD, et al. Device-associated infection rates, mortality, length of stay and bacterial resistance in intensive care units in Ecuador: International Nosocomial Infection Control Consortium’s findings. World J Biol Chem 2017; 8:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brunelli SM, Turenne W, Sibbel S, et al. Clinical and economic burden of bloodstream infections in critical care patients with central venous catheters. J Crit Care 2016; 35:69–74. [DOI] [PubMed] [Google Scholar]
- 27. Vincent JL, Rello J, Marshall J. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302:2323–9. [DOI] [PubMed] [Google Scholar]
- 28. Vincent JL, Bihari DJ, Suter PM, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA 1995; 274:639–44. [PubMed] [Google Scholar]
- 29. Minderhoud TC, Spruyt C, Huisman S, et al. Microbiological outcomes and antibiotic overuse in emergency department patients with suspected sepsis. Neth J Med 2017; 75:196–203. [PubMed] [Google Scholar]
- 30. Rhodes A, Phillips G, Beale R, et al. The Surviving Sepsis Campaign bundles and outcome: results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study). Intensive Care Med 2015; 41:1620–8. [DOI] [PubMed] [Google Scholar]
- 31. Falagas ME, Bliziotis IA, Siempos II. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care 2006; 10:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 2006; 42:692–9. [DOI] [PubMed] [Google Scholar]
- 33. Sakr Y, Moreira CL, Rhodes A, et al. The impact of hospital and ICU organizational factors on outcome in critically ill patients: results from the Extended Prevalence of Infection in Intensive Care study. Crit Care Med 2015; 43:519–26. [DOI] [PubMed] [Google Scholar]
- 34. Gaieski DF, Edwards JM, Kallan MJ, et al. The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med 2014; 190:665–74. [DOI] [PubMed] [Google Scholar]
- 35. Yergens DW, Ghali WA, Faris PD, et al. Assessing the association between occupancy and outcome in critically Ill hospitalized patients with sepsis. BMC Emerg Med 2015; 15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. World Health Organization. Who global surveillance and monitoring system for substandard and falsified medical products Available at: http://apps.who.int/medicinedocs/documents/s23373en/s23373en.pdf. Accessed 12 July 2017.
- 37. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.