Abstract
Background
An inpatient antimicrobial stewardship program is vital for judicious antimicrobial use. We began a hospital-wide, postprescription review with feedback (PPRF) in 2014; the present study evaluated its impact on antimicrobial consumption and clinical outcomes over 4 years.
Methods
Once-weekly PPRF for carbapenems and piperacillin/tazobactam was implemented. We tracked the data on each antimicrobial use as days of therapy (DOT) per 1000 patient-days (PD). Changes in the incidence of drug-resistant organisms, in-hospital mortality, and length of hospital stay per month were analyzed by an interrupted time series.
Results
Carbapenem use continued to decline in the preintervention and intervention periods (−0.73 and −0.003 DOT/1000 PD, respectively), and although monthly average use remained low in the intervention period (8.3 DOT/1000 PD), more importantly, the postintervention change in the slope diminished significantly. Piperacillin/tazobactam use showed a steeper decline in the intervention period, but the change in the slope was not statistically significant (change in slope: −0.20 DOT/1000 PD per month [P = .16]). Postintervention use of narrower-spectrum antimicrobials including ampicillin/sulbactam (change in slope: +0.58 DOT/1000 PD per month [P < .001]) increased.
The antimicrobial cost and the monthly average length of hospital stay also declined (−37.4 USD/1000 PD per month [P < .001] and −0.04 days per month [P < .001], respectively), whereas few postintervention changes in the incidence of drug-resistant organisms were observed.
Conclusions
In our study, the 4-year PPRF for broad-spectrum antimicrobials coincided with a reduction in the use of targeted antimicrobials and resulted in an improvement in 1 patient-centered outcome, thus conferring the additional benefit of reducing expenditures for antimicrobials.
Keywords: antimicrobial stewardship program, broad-spectrum antimicrobials, postprescription review and feedback
Judicious use of antimicrobial agents is strongly advocated in healthcare settings, and the antimicrobial stewardship program (ASP) is considered vital for optimizing antimicrobial use in healthcare facilities [1, 2]. Among various interventions with an antimicrobial stewardship component, postprescription review with feedback (PPRF) is one of the most effective, especially for the inpatient setting. Although it is time consuming and labor intensive, PPRF is commonly implemented because its efficacy is supported by strong evidence [3].
Although hospitals worldwide have already developed an ASP, there are significant variations in its quality among healthcare settings and countries [4]. The reason for these variations is likely to be multifactorial, including staffing constraints, insufficient funding, and lack of education [5, 6]. As in other countries, healthcare institutions in Japan have been facing issues related to antimicrobial resistance, and the optimizing antimicrobial use has become an urgent need. In 2016, the Japanese government issued an antimicrobial resistance action plan and in 2017 published guidelines for outpatient antimicrobial stewardship to promote judicious use of antimicrobial agents [7, 8]. However, limited resources and demographic factors such as an increasing elderly population continue to pose challenges to ASP implementation [9, 10].
Tokyo Metropolitan Tama Medical Center implemented its own ASP for broad-spectrum antimicrobial agents in April 2014 [11]. The purpose of our study was to compare the impact of a 4-year (April 2014 to March 2018), hospital-based antimicrobial stewardship with PPRF of broad-spectrum antimicrobials in terms of antimicrobial use, patient-related outcomes, and the incidence of drug-resistant pathogens in a 2-year preintervention period (from April 2012 to March 2014).
METHODS
Study Setting
This before-after study was conducted at Tokyo Metropolitan Tama Medical Center, a 790-bed tertiary care center with 29 subspecialties in Tokyo, Japan with a division of infectious diseases and an ASP. All the physicians in the division of infectious diseases were also actively involved in antimicrobial stewardship activities.
Study Design
We evaluated the impact of our ASP on antimicrobial use, patient-related outcomes, and changes in antimicrobial resistance in the inpatient setting between the 2 periods (the preintervention period [April 2012 to March 2014] and the intervention period [April 2014 to March 2018]). The efficacy of the ASP was assessed by trends in overall antimicrobial use, antipseudomonal antimicrobial use (each antipseudomonal agent including carbapenems, piperacillin/tazobactam, cefepime, fluoroquinolones, ceftazidime, and aztreonam), other intravenous antimicrobial use (ie, vancomycin, ceftriaxone, cefmetazole, first-generation cephalosporin, ampicillin, and ampicillin/sulbactam) expressed as days of therapy (DOT) per 1000 patient-days (PD), incidence density of drug-resistant organisms including methicillin-resistant Staphylococcus aureus (MRSA), and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae per 1000 PD, respectively. We also reviewed the trends in annual antibiogram data for certain antimicrobial-resistant organisms including S aureus, Pseudomonas aeruginosa, and selected Enterobacteriaceae. For patient-related outcomes, we evaluated the trends in the average length of stay, monthly in-hospital mortality, and the incidence density of Clostridium difficile infection (CDI) per 10000 PD. More importantly, there was no change in hospital or infection control policy to reduce the length of hospital stay during the study period.
Antimicrobial Stewardship Program
An ASP run by a multidisciplinary team was officially implemented in April 2014. The program was staffed by 2 infectious diseases physicians (0.2 full-time equivalent), an infectious diseases fellow (0.1 full-time equivalent), clinical pharmacist (0.2 full-time equivalent), microbiology laboratory technician (0.1 full-time equivalent), and infection control nurse (0.1 full-time equivalent). Before implementation, there were no interventions contributing to antimicrobial stewardship except for an infectious disease consultation service, which was begun in July 2013 by an American Board of Internal Medicine-Infectious Diseases-certified physician [11]. A clinical pharmacist routinely monitored all inpatient antimicrobial consumption. Postprescription review with feedback was the main activity in our ASP, and all the members had a once-weekly PPRF meeting. We focused on broad-spectrum antimicrobial agents (ie, carbapenems and piperacillin/tazobactam) due to time and staffing constraints.
From April 2014, we started PPRF for patients who were on carbapenems more than 72 hours. The formulary of carbapenem antimicrobials in the study institution included imipenem/cilastatin, meropenem, and doripenem. Before the once-weekly antimicrobial stewardship meeting, our clinical pharmacist identified patients eligible for PPRF. Carbapenem use was considered appropriate for the treatment for febrile neutropenia, infections only susceptible to carbapenem antimicrobials, and infections for which carbapenems were conventionally considered to be first-line agents. We also implemented PPRF for piperacillin/tazobactam in May 2015. As in our carbapenem PPRF, we audited patients who were on piperacillin/tazobactam more than 72 hours. Piperacillin/tazobactam use was considered appropriate for the following: treatment of febrile neutropenia, empiric therapy for healthcare-associated infection, definitive therapy for healthcare-associated infections for which piperacillin-tazobactam was considered the best choice based on culture results and clinical conditions, and polymicrobial infections (eg, intra-abdominal infections) for which piperacillin/tazobactam was the preferred therapy. All antimicrobial stewardship program members discussed each case to determine the appropriateness of carbapenem or piperacillin/tazobactam use based on a comprehensive assessment of the patients’ clinical condition, culture results, and the prespecified indications as noted above. We classified the appropriateness of the use of these antimicrobial agents following the method prescribed by Kunin et al [12], after a slight modification to fit our local practice (see the definition in the NOTE in Appendix 1).
Once we determined the appropriateness of these 2 agents, we documented their use in each patient’s electronic medical records. For all patients with inappropriate antimicrobial use, we modified or stopped the antimicrobial therapy by directly contacting the primary care team providers by telephone. The primary care team accepted the recommendations at their own discretion. There was no penalty for disagreement with the recommendations. We considered a recommendation to be accepted whether the primary care team providers modified/stopped their antimicrobial therapy based on our recommendations within 72 hours.
Data Collection
A clinical pharmacist identified patients who were eligible for PPRF and collected their demographic characteristics, indications for antimicrobial use, and duration of therapy to discuss their case at the weekly meeting. The information was recorded in the data collection form. A clinical pharmacist also prospectively tracked the data on all antimicrobial use and cost outlays on a monthly basis.
Statistical Analysis
We use segmented regression of interrupted time series analysis [13] to assess the changes in the overall use of antimicrobials, antipseudomonal agents (ie, total use of carbapenems, piperacillin/tazobactam, and cefepime), and individual antimicrobial agents and to assess the trends in the incidence of CDI, MRSA, and ESBL-producing Enterobacteriaceae, the monthly average length of stay, and the monthly in-hospital mortality rate. We used Stata version 15.2 (StataCorp, College Station, TX) for the analysis. The institutional review board at Tokyo Metropolitan Tama Medical Center approved the study.
RESULTS
Overall Appropriateness of Antimicrobial Use
Since the implementation of once-weekly PPRF for carbapenems and piperacillin/tazobactam, there were 733 episodes of carbapenem use in 469 patients and 981 episodes of piperacillin/tazobactam use in 725 patients. Overall appropriate carbapenem and piperacillin/tazobactam use was 61.9% and 56.7%, respectively. The rate of acceptance of ASP recommendations ranged from 87.7% to 96.4% (Appendix 1).
Antimicrobial Use
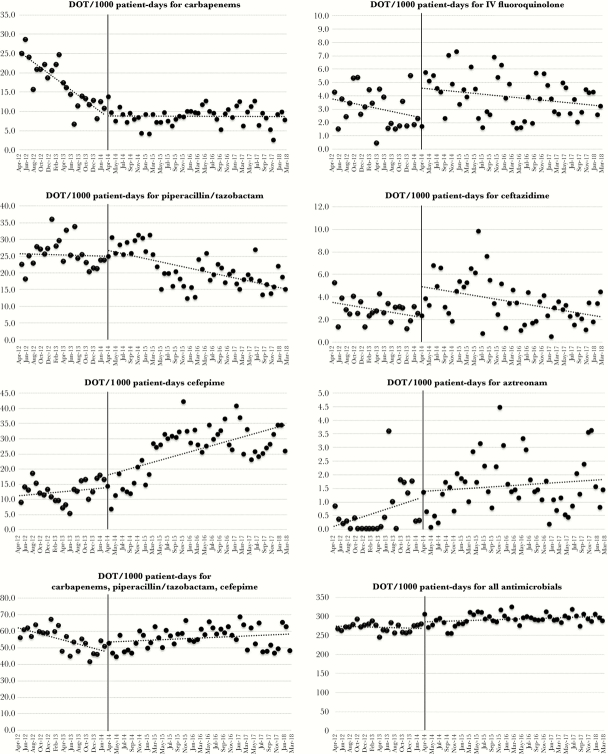
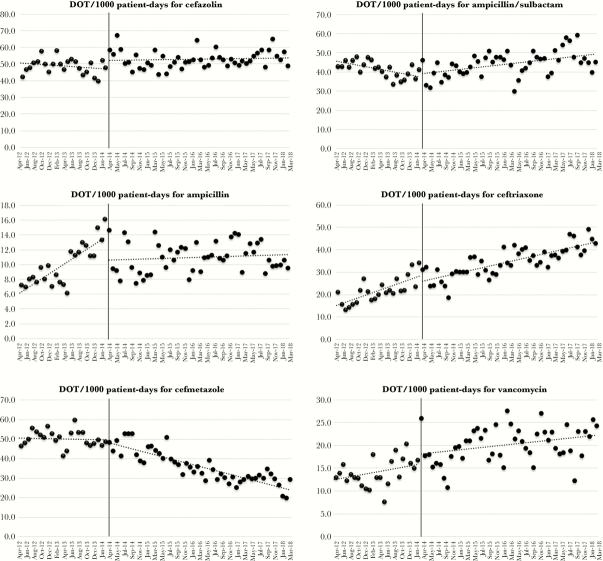
Table 1 shows the changes in the overall and individual use of each antipseudomonal agent before and after PPRF implementation as determined by interrupted time series (ITS) analysis. Figures 1 and 2 show the changes in the absolute monthly use of antimicrobials between the 2 periods. Appendix 2 shows the average monthly use of antimicrobials for both periods. Although the monthly average use of both carbapenems and piperacillin/tazobactam was lower in the intervention period (8.67 and 20.95 DOT per 1000 PD), the change in the slope for carbapenem use significantly diminished, showing a leveling-off effect (from −0.73 to −0.003 DOT per 1000 PD [P < .001]), and that for piperacillin/tazobactam did not reach statistical significance (−0.04 to −0.24 DOT per 1000 PD [P = .16]). Although the monthly, average use of some narrower-spectrum antimicrobials was higher in intervention period, only the slope for ampicillin/sulbactam use reflected a statistically significant change (+0.58 DOT per 1000 PD [P < .001]).
Table 1.
Changes in Antimicrobial Use Before and After the Implementation of Postprescription Review and Feedback Analyzed by Interrupted Time Series Analysisa
| Days of Therapy per 1000 Patient-Days per Month | ||||||
|---|---|---|---|---|---|---|
| Antimicrobials | Baseline Trend in the Preintervention Period (95% CI) |
P | Slope in the Intervention Period (95% CI) |
P | Change in Slope | P |
| Carbapenems | −0.73 (−0.89 to −0.57) | <.001 | −0.003 (−0.06 to 0.06) | .92 | 0.73 (0.55–0.91) | <.001 |
| Piperacillin/tazobactam | −0.04 (−0.31 to 0.24) | .80 | −0.24 (−0.33 to −0.15) | <.001 | −0.20 (−0.49 to 0.08) | .16 |
| Cefepime | 0.12 (−0.10 to 0.34) | .29 | 0.35 (0.18–0.52) | <.001 | 0.23 (−0.04 to 0.51) | .19 |
| 3 antipseudomonal agents | −0.63 (−0.91 to −0.36) | <.001 | 0.10 (−0.05 to 0.26) | .21 | 0.73 (0.42–1.06) | <.001 |
| Fluoroquinolones | −0.04 (−0.11 to 0.02) | .21 | −0.02 (−0.05 to 0.01) | .07 | 0.02 (−0.05 to 0.09) | .63 |
| Ceftazidime | −0.05 (−0.12 to 0.14) | .29 | −0.06 (−0.10 to −0.02) | .001 | −0.01 (−0.08 to 0.06) | .82 |
| Cefazolin | −0.16 (−0.45 to 0.13) | .76 | 0.03 (−0.11 to 0.17) | .63 | 0.19 (−0.13 to 0.52) | .24 |
| Ampicillin | 0.33 (0.26–0.41) | <.001 | 0.02 (−0.03 to 0.07) | .54 | −0.32 (−0.42 to −0.22) | <.001 |
| Ampicillin/sulbactam | −0.36 (−0.53 to −0.18) | <.001 | 0.22 (0.08–0.35) | .002 | 0.58 (0.35–0.80) | <.001 |
| Cefamycins | −0.04 (−0.29 to 2.13) | .76 | −0.51 (−0.62 to −0.41) | <.001 | −0.47 (−0.75 to −0.20) | <.001 |
| Ceftriaxone | 0.59 (0.36–0.82) | <.001 | 0.37 (0.28–0.47) | <.001 | −0.22 (−0.47 to 0.04) | .10 |
| Vancomycin | 0.15 (−0.01 to 0.30) | .05 | 0.09 (−0.01 to 0.18) | .06 | −0.06 (−0.24 to 0.12) | .49 |
| All antimicrobials | −0.27 (−0.91 to 0.37) | .40 | 0.29 (−0.04 to 0.62) | .09 | 0.56 (−0.18 to 1.30) | .13 |
| Overall antimicrobial cost, $ | 9.5 (−12.9 to 31.8) | .84 | −27.9 (−36.7 to −19.1) | <.001 | −37.4 (−61.6 to −13.2) | .003 |
Abbreviations: CI, confidence interval; USD, US dollar.
aOverall antimicrobial cost was calculated at the rate of 100 Yen = 1 USD.
Figure 1.
Use of antimicrobials (antipseudomonal agents) after the implementation of postprescription review and feedback.
Figure 2.
Use of antimicrobials (non-antipseudomonal agents) after the implementation of postprescription review and feedback.
Antimicrobial Resistance and Patient-Centered Outcomes
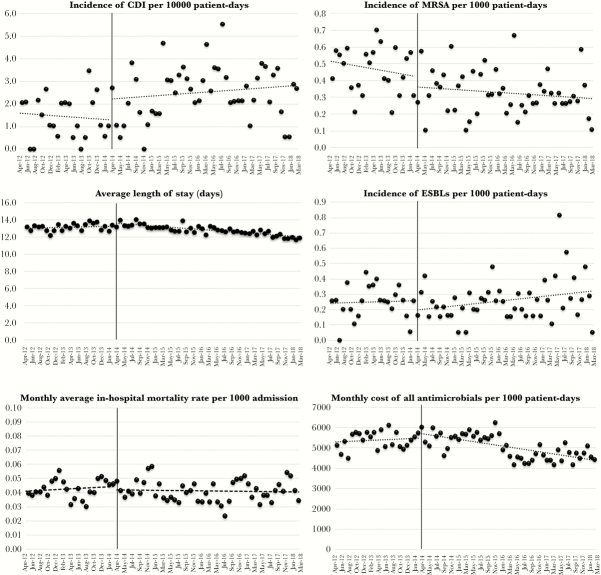
Table 2 shows the antimicrobial susceptibility for the target pathogens in the preintervention and intervention periods. We observed improved susceptibility in the organisms to certain antimicrobials. Figure 3 shows changes in the incidence of selected multidrug-resistant organisms, patient-centered outcomes, and overall antimicrobial costs after PPRF implementation. Table 3 shows the trends in the incidence density of selected multidrug-resistant organisms and patient-centered outcomes after PPRF implementation based on the ITS. The incidence density of MRSA, ESBL-producing organisms, and CDI was relatively stable throughout the study period, whereas the average monthly length of hospital stay significantly decreased after PPRF implementation (from 0.01 to −0.03, change in slope −0.04 days per month [P = .002]).
Table 2.
Changes in Antimicrobial Susceptibility in Selected Organisms Between the Preintervention and the Intervention Periodsa
| Organisms and Antimicrobials | Isolates Resistant in the Preintervention Period (%) |
Isolates Resistant in the Intervention Period (%) | P |
|---|---|---|---|
| Escherichia coli | |||
| Cefepime | 101/866 (11.7)b | 579/3822 (15.1) | .01 |
| Cefotaxime | 201/1601 (12.6) | 635/3822 (16.6) | <.001 |
| Levofloxacin | 395/1601 (24.7) | 1113/3822 (29.1) | <.001 |
| Enterobacter cloacae | |||
| Meropenem | 0/136 (0)b | 2/551 (0.7) | N/A |
| Piperacillin/tazobactam | 40/136 (29.4)b | 90/551 (16.3) | <.001 |
| Cefepime | 16/136 (11.8)b | 44/551 (8.0) | .16 |
| Pseudomonas aeruginosa | |||
| Imipenem-cilastatin | 80/995 (8.0) | 147/2344 (6.3) | .06 |
| Piperacillin/tazobactam | 90/995 (9.5) | 251/2344 (10.7) | .15 |
| Cefepime | 156/995 (15.7) | 250/2344 (10.7) | <.001 |
| Staphylococcus aureus | |||
| Methicillin | 386/1527 (25.3) | 804/3348 (24.0) | 0.34 |
Abbreviations: N/A, not available.
aThe preintervention period was from April 2012 to March 2014, and the intervention period was from April 2014 to March 2018.
bSusceptibility data from April 2012 to March 2013 were not available.
Figure 3.
Changes in incidence of infection by selected multidrug-resistant organisms, patient-centered outcomes, and overall antimicrobial cost after the implementation of postprescription review and feedback.
Table 3.
Changes in Patient-Centered Outcomes and Incidence of Selected Multidrug-Resistant Organisms After the Implementation of Postprescription Review and Feedback
| Patient-Centered Outcome | Baseline Trend in the Preintervention Period (95% CI) |
P | Slope Change in the Intervention Period (95% CI) |
P | Change in slope (95% CI) |
P |
|---|---|---|---|---|---|---|
| Incidence of CDI per 10000 patient-days | −0.01 (−0.08 to 0.05) | .67 | 0.01 (−0.02 to 0.04) | .41 | 0.03 (−0.05 to 0.10) | .46 |
| Average length of hospital stay | 0.01 (−0.02 to 0.36) | .46 | −0.03 (−0.04 to −0.02) | <.001 | −0.04 (−0.07 to −0.17) | .002 |
| Average monthly mortality rate | 0.15 (−0.15 to 0.45) | .15 | −0.01 (−0.02 to 0.01) | .73 | −0.02 (−0.05 to 0.17) | .32 |
| Incidence of MRSA per 1000 patient-days | −0.004 (−0.01 to 0.003) | .25 | −0.001 (−0.004 to 0.001) | .28 | 0.002 (−0.005 to 0.01) | .50 |
| Incidence of ESBL per 1000 patient-days | 0.001 (−0.006 to 0.007) | .89 | 0.003 (−0.001 to 0.006) | .09 | 0.002 (−0.005 to 0.009) | .59 |
Abbreviations: CDI, Clostridium difficile infection; ESBL, extended-spectrum β lactamase; MRSA, methicillin-resistant Staphylococcus aureus.
DISCUSSION
The present study described the impact of a 4-year ASP on antimicrobial use and patient-centered outcomes at a Japanese tertiary care center. A number of favorable results in antimicrobial use and a change in 1 patient-centered outcome were observed, although the changes may not solely be the result of the intervention.
The current study differs from previous reports of PPRF in the acute care setting in several respects. We performed PPRF just once weekly with relatively limited personnel. Each participant engaging in PPRF had a full-time equivalent of approximately 0.1 to 0.2. Although an ASP ideally has adequate, dedicated infrastructure and personnel [14], personnel constraints are common throughout the world including in Japan [5, 9, 15]. However, this study demonstrated that strategies can be devised to overcome such limitations. Another highlight of this study is that the PPRF directly or indirectly influenced other outcomes including the use of other antimicrobials, cost outlays, and one of the patient-centered outcomes, although the PPRF targeted only 2 broad-spectrum antimicrobials.
Although the change in slope showed that carbapenem use only slightly declined in the postintervention period, the decrease in absolute terms was consistent and sustained. Similarly, although piperacillin/tazobactam use failed to reach statistical significance, sustained reduction continued to be observed in the intervention period. A gradual decline in carbapenem use was observed even in the preintervention period, possibly due to infectious diseases consultation, which began before the intervention. In contrast, the use of fourth-generation cephalosporins and a number of narrower-spectrum agents increased due to the shift towards cefepime in empiric therapy combined with the de-escalation in the use of nonantipseudomonal agents. Because of the increased use of cefepime, we were unable to demonstrate a decrease in the total use of 3 antipseudomonal agents (ie, carbapenems, pipercillin/tazobactam, and cefepime). A similar increase in cefepime or narrower-spectrum antimicrobial use in response to decreased carbapenem or piperacillin/tazobactam use was observed in another study of PPRF [16]. Increasing use of narrower-spectrum antimicrobials and decreasing use of broader-spectrum antimicrobials during the study period suggest that hospital-wide de-escalation in antimicrobials use was achieved.
Regarding patient-centered outcomes, PPRF showed a decrease in postintervention length of hospital stay. Although this may not be an ideal patient-centered outcome for assessing the effectiveness of an ASP, it is commonly used presumably because it is generally tracked as a quality measure in acute care settings [17, 18]. Improved antimicrobial susceptibility in certain pathogens, especially P aeruginosa and Enterobacter cloacae, might be associated with decreased carbapenem and piperacillin/tazobactam use. The present study succeeded in demonstrating that the ASP is instrumental in achieving the cardinal goals of improving antimicrobial susceptibility and preventing the emergence of extensive resistance, especially in hospital-acquired pathogens.
Moreover, the present study revealed that overall cost outlays for antimicrobials decreased. During the study period, generic piperacillin/tazobactam only began to be used in the middle of the intervention period (since February 2016) and may have contributed somewhat to reducing the cost of antimicrobials. However, the overall change in cost cannot be explained only by this single change, because piperacillin/tazobactam use comprised a small proportion of overall antimicrobial use. The changes were likely due to the greater use of cheaper agents resulting from the intervention.
In this study, overall antimicrobial use increased slightly after the PPRF implementation, although the difference was not statistically significant. Although PPRF has been shown to decrease overall antimicrobial use, one previous study apparently demonstrated increased overall antimicrobial use after ASP implementation at institutions with no pre-existing ASP [19]. Moreover, improvements in the standard of care may have contributed to an intermittent increase in overall antimicrobial use after PPRF implementation. For instance, vancomycin was more frequently administered in standard empiric therapy for healthcare-associated infections during the intervention period. It is worth noting that in the last year of the intervention, overall antimicrobial use started to decline; thus, it is important to track long-term trends in antimicrobial use in inpatient settings.
During the intervention period, the incidence of CDI and ESBL-producing Enterobacteriaceae increased slightly without reaching statistical significance. Although almost all antimicrobials can conduce to the development of CDI, some studies demonstrated that cephalosporin may be more strongly associated with its development than other antimicrobials [20, 21]. Other studies also showed that cephalosporin exposure may contribute more to the emergence of ESBL-producing Entereobacteriaceae [22, 23]. Long-term trends in the incidence of these organisms relative to the use of particular antimicrobials need to be clarified in future studies especially because the incidence of these organisms can be influenced by other factors such as a history of previous healthcare exposure and the degree of infection prevention activities.
This study has some limitations. Although the study showed the effectiveness of PPRF performed by a relatively limited number of antimicrobial stewardship team members, the findings at a single tertiary care center have limited generalizability. Nonetheless, previous studies assessing the impact of antimicrobial stewardship even under personnel constraints corroborate our findings [6, 9]. Due to the before-after protocol, it was difficult to determine whether the observed changes, especially in the patient-centered outcome, were due solely to PPRF, and other outcomes may require longer observation to assess the full impact of PPRF. Although the changes in cost appear to be favorable, our study only assessed antimicrobial cost but not those associated with decreased length of stay, incidence of CDI, or treatment against drug-resistant pathogens. Future studies comprehensively analyzing these costs will doubtless corroborate the financial feasibility of ASP.
CONCLUSIONS
Although the trends in antimicrobial use seen in the current study failed to demonstrate statistical significance, the results do suggest that PPRF for broad-spectrum antimicrobials can contribute to a sustained reduction in the use of antimicrobials and conduce to a hospital-wide de-escalation in antimicrobial use without compromising patients’ clinical outcomes. Our experience suggests that PPRF can be implemented even when there are staffing constraints. The time required for changing outcomes associated with antimicrobial stewardship varies, depending on the type of outcome measure. Long-term monitoring of the outcomes is important for accurate assessment of the efficacy of an inpatient ASP.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the staff of the Department of Microbiology at Tokyo Metropolitan Tama Medical Center for management of the antimicrobial susceptibility data. We are indebted to James R. Valera for assistance with editing the manuscript.
Author contributions. H. H. designed the study protocol. H. H., S. M., Y. Ta., Y. U., K. G., A. T., and S. H. ran the antimicrobial stewardship program. S. M. collected the data. Y. To. and H. H. performed the data analysis. H. H. drafted the first version of the manuscript. Y. Ta., A. T., S. M., Y. To., and S. H. performed the critical review. H. H. revised the manuscript, and all the authors contributed to the final version of manuscript.
Financial support. This work was funded by the Japan Society for the Promotion of Science (JSPS) (Grant Number 16K09196). H. H. was the principle researcher in this study.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44:159–77. [DOI] [PubMed] [Google Scholar]
- 2. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tamma PD, Avdic E, Keenan JF, et al. What is the more effective antibiotic stewardship intervention: preprescription authorization or postprescription review with feedback?Clin Infect Dis 2017; 64:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karanika S, Paudel S, Grigoras C, et al. Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrob Agents Chemother 2016; 60:4840–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doron S, Nadkarni L, Lyn Price L, et al. A nationwide survey of antimicrobial stewardship practices. Clin Ther 2013; 35:758–765.e20. [DOI] [PubMed] [Google Scholar]
- 6. Apisarnthanarak A, Lapcharoen P, Vanichkul P, et al. Design and analysis of a pharmacist-enhanced antimicrobial stewardship program in Thailand. Am J Infect Control 2015; 43:956–9. [DOI] [PubMed] [Google Scholar]
- 7. The Government of Japan and Ministry of Health Labour and Welfare Japan. National Action Plan on Antimicrobial Resistance (AMR) 2016-2020. April 5, 2016. Available at: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000138942.pdf Accessed 23 November 2018.
- 8. The Government of Japan, Ministry of Health Labour and Welfare Health Service Bureau Tuberculosis and Infectious Diseases Control Division. Manual of Antimicrobial Stewardship (1st Edition). June 1, 2017. Available at https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000193504.pdf Accessed 23 Novmber 2018. [Google Scholar]
- 9. Kimura T, Uda A, Sakaue T, et al. Long-term efficacy of comprehensive multidisciplinary antibiotic stewardship programs centered on weekly prospective audit and feedback. Infection 2018; 46:215–24. [DOI] [PubMed] [Google Scholar]
- 10. Honda H, Ohmagari N, Tokuda Y, et al. Antimicrobial stewardship in inpatient settings in the Asia Pacific Region: a systematic review and meta-analysis. Clin Infect Dis 2017; 64:119–26. [DOI] [PubMed] [Google Scholar]
- 11. Tagashira Y, Horiuchi M, Tokuda Y, et al. Antimicrobial stewardship for carbapenem use at a Japanese tertiary care center: an interrupted time series analysis on the impact of infectious disease consultation, prospective audit, and feedback. Am J Infect Control 2016; 44:708–10. [DOI] [PubMed] [Google Scholar]
- 12. Kunin CM, Tupasi T, Craig WA. Use of antibiotics. A brief exposition of the problem and some tentative solutions. Ann Intern Med 1973; 79:555–60. [DOI] [PubMed] [Google Scholar]
- 13. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27:299–309. [DOI] [PubMed] [Google Scholar]
- 14. Pollack LA, Plachouras D, Sinkowitz-Cochran R, et al. A concise set of structure and process indicators to assess and compare antimicrobial stewardship programs among EU and US hospitals: results from a multinational expert panel. Infect Control Hosp Epidemiol 2016; 37:1201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Howard P, Pulcini C, Levy Hara G. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother 2015; 70:1245–55. [DOI] [PubMed] [Google Scholar]
- 16. Jenkins TC, Knepper BC, Shihadeh K, et al. Long-term outcomes of an antimicrobial stewardship program implemented in a hospital with low baseline antibiotic use. Infect Control Hosp Epidemiol 2015; 36:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wagner B, Filice GA, Drekonja D, et al. Antimicrobial stewardship programs in inpatient hospital settings: a systematic review. Infect Control Hosp Epidemiol 2014; 35:1209–28. [DOI] [PubMed] [Google Scholar]
- 18. Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2017; 2:CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehta JM, Haynes K, Wileyto EP, et al. Comparison of prior authorization and prospective audit with feedback for antimicrobial stewardship. Infect Control Hosp Epidemiol 2014; 35:1092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 2014; 69:881–91. [DOI] [PubMed] [Google Scholar]
- 21. Muldoon EG, Epstein L, Logvinenko T, et al. The impact of cefepime as first line therapy for neutropenic fever on Clostridium difficile rates among hematology and oncology patients. Anaerobe 2013; 24:79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zerr DM, Miles-Jay A, Kronman MP, et al. Previous antibiotic exposure increases risk of infection with extended-spectrum-β-lactamase- and AmpC-producing Escherichia coli and Klebsiella pneumoniae in pediatric patients. Antimicrob Agents Chemother 2016; 60:4237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boix-Palop L, Xercavins M, Badía C, et al. Emerging extended-spectrum β-lactamase-producing Klebsiella pneumoniae causing community-onset urinary tract infections: a case-control-control study. Int J Antimicrob Agents 2017; 50:197–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.