Abstract
Background
This study was conducted to investigate the effects of low frequency repetitive transcranial magnetic stimulation (rTMS) of the right dorsolateral prefrontal cortex on depression and cognition in patients with traumatic brain injury.
Material/Methods
To accomplish this, 13 patients who were diagnosed with traumatic brain injury were divided into an experimental group (n=7) and a control group (n=6). The experimental group received rTMS during a 30-minute session 5 days per week for 2 weeks; the control group received sham rTMS. The patients were then evaluated for depression using the Montgomery-Asberg Depression Rating Scale (MADRS) and for cognitive function using the Trail Making Test (TMT) and the Stroop Color Word Test (SCWT).
Results
A significant decrease in MADRS, TMT, and SCWT was observed after the intervention in the experimental group (P<0.01), and there was a significant difference in the change value of MADRS, TMT, and SCWT compared to the control group (P<0.01). Moreover, the effect size for gains in the experimental group and control group was very strong for MADRS, TMT, and SCWT (effect size=1.44, 1.49, and 1.24 respectively).
Conclusions
The results of this study suggest that application of low frequency rTMS to the right dorsolateral prefrontal cortex of patients with traumatic brain injury has a positive effect on depression and cognition.
MeSH Keywords: Brain Injuries, Cognition, Depression, Transcranial Magnetic Stimulation
Background
Any impact on the skull causes physical damage to brain tissues and blood vessels, which can subsequently cause many other intracranial problems. Specifically, secondary problems can arise from physical and biochemical processes, such as changes in intracranial pressure. Such traumatic brain injury (TBI) can involve temporary or permanent damage to brain function [1]. This damage has been reported to lead to problems with motor control, cognitive function, executive function, and mood control [2]. Brain injury affects various structures depending on the location of the impact, and there is large heterogeneity among the symptoms that occur even when similar injuries occur on the surface [3]. This is because tissue damage can occur not only at the initial point of skull impact, but also in the contralateral hemisphere [1].
Diffuse axonal injury accounts for approximately one-third of TBI deaths, and TBI survivors often have cognitive and mood disorders as well as motor deficits [4]. Among the TBI reported symptoms, depression is common [5].
Transcranial magnetic stimulation (TMS) is a neuromodulatory tool that uses magnetic fields to induce nervous activity non-invasively [6]. Repeated application of TMS at regular intervals is called repetitive TMS (rTMS), which increases or decreases cortical activation based on the frequency of stimulation [7]. In this process, the magnetic field passes through the skull and produces an electrical current that induces activity of the cortical neurons. Repetitive pulses of TMS can alter the excitability of neurons: with high frequency (>5 Hz) producing neural excitability and with low frequency (~1 Hz) suppressing neural excitability. One of the main advantages of rTMS is that it is safe and has no serious side-effects [6]. rTMS has been shown to be effective at treating schizophrenia, depression, Parkinson disease, aphasia, and cognitive disorders [8–13]. The fact that the modulating effects of rTMS can last longer than the application period indicates that it is a promising treatment for various neuropsychiatric disorders [14]. These effects are considered to be prolonged after-effects cause by modulation of long-term depression and long-term potentiation between synaptic connections associated with neuroplasticity [15].
Several studies have suggested that rTMS may be applied to patients with TBI based on evidence of rTMS treatment applied to non-TBI patients [16,17].
According to previous studies, the dorsolateral prefrontal cortex is a typical target to stimulate rTMS in relation to depression and cognitive impairment [18]. One study showed improvement of cognition in response to application of rTMS to the dorsolateral prefrontal cortex of patients with Parkinson disease [19]. Fitzgerald et al. reported that depression was improved by applying rTMS to the dorsolateral prefrontal cortex of patients with TBI [20]. Some studies have shown improvements in line bisection and clock drawing task performance after applying low frequency rTMS to the contralesional parietal region of patients with visuospatial neglect [21,22]. Other studies have applied rTMS to the dorsolateral prefrontal cortex to improve the executive function of patients with TBI [23].
Reti et al. suggested that low frequency is better than high frequency when rTMS is applied to patients with TBI [24]. In a previous study, low frequency rTMS was applied to the anterior portion of Broca area, which showed a significant effect on naming accuracy, latency, and repetition compared to the sham rTMS group [25]. Taken together, previous studies suggest that low frequency rTMS is more appropriate for the treatment of depression in patients with TBI.
The effects of rTMS on the improvement of clinical symptoms in stroke patients has been actively studied, but there have been few studies on the cognitive recovery when rTMS is applied to a patient with TBI having depression and cognition problems.
This study aimed to investigate the effects of low frequency rTMS on the right dorsolateral prefrontal cortex of patients with TBI on depression and cognition.
Material and Methods
This study investigated 15 patients with TBI, who were 19 to 60 years old and who were of both genders. The sample size for this study was calculated using the G* Power program 3.1.0 (G power program Version 3.1, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany). Based on data from a pilot study, the estimated sample size required to obtain a minimum power 80% at a significant alpha of 95% was 13. Accordingly, 15 participants were recruited to account for a potential dropout rate of 20%.
All patients were recruited through the Inpatient Department of the D Rehabilitation Hospital in Daejeon. The inclusion criteria were as follows: patients who had less than 6 months TBI diagnosis and were medically stable. In addition, patients with Glasgow Coma Scale scores of 9 to 15 were included in the study [26].
The exclusion criteria were as follows: 1) history of seizure, 2) implanted devices, 3) Montgomery-Asberg Depression Rating Scale (MADRS) 35–60 score (severe depression) [29], 4) cardiac pacemaker, and 5) other rTMS contraindications.
Although 15 patients were initially recruited, 2 were excluded because of severe depression; therefore, a total of 13 patients participated in the study. Informed consent was obtained from all patients after sufficient explanation of the procedures. The study was approved by the Ethics Committee of Daegu University.
First, patients were selected and randomly divided into 2 groups. Randomization was performed using a sealed envelope containing a card labeled 1 or 2, with those receiving a 1 were assigned to the experimental group and those receiving a 2 were assigned to the control group. The experimental group (n=7) received real rTMS and the control group (n=6) received sham rTMS. All patients were blinded to their group assignment until the study was completed.
All patients received neurodevelopmental therapy (NDT) for muscle strengthening and movement re-education, and rTMS intervention was performed after NDT. The intervention was conducted for 30 minutes at a time, 5 times a week for a total of 10 days (5 consecutive weekdays, with 2 days off during weekends).
rTMS was performed using a Magstim Rapid stimulator (The Magstim Company Ltd., UK) centered over the right dorsolateral prefrontal cortex based on the F4 position of the International 10–20 system. According to Pascual-Leone et al., the right dorsolateral prefrontal cortex is located 5 cm anterior of the primary motor cortex of the right hemisphere [27]. The figure-eight coil has an external loop diameter of 70 mm that is connected to a magnetic stimulator. Low-frequency rTMS (1 Hz) was applied to each patient using an intensity of 100% of resting motor threshold. The resting motor threshold refers to the minimum stimulus intensity that can cause a 50-mV amplitude motor-evoked potential in target muscle more than 5 times in 10 consecutive attempts [18]. Each session will consist of 50 trains of 40 pulses on each train separated by 25-second pauses applied at 1 Hz. Surface electromyography electrodes were used to record from the belly of the left abductor pollicis brevis muscle. The sham rTMS was performed with a sham coil of the same size and shape as the coil used in the real rTMS without any stimulation on the cortex.
All measurements were performed before rTMS intervention and after rTMS intervention for 10 sessions.
The MADRS was developed from the Comprehensive Psychopathological Rating Scale for assessment of the emotional, physical, cognitive, and behavioral symptoms of depression [28]. The item is composed of 10 depressive symptoms rated on a 7-point Likert scale (from 0 to 6), with a total score of 0 to 60. The total score was used to classify the severity of symptoms as follows: normal or absent 0–6; mild 7–19; moderate 20–34; severe 35–60 [29]. The participants of this study included those with a MADRS score of 14 to 30 points. According to previous studies, the intraclass correlation coefficient of MADRS was ranged from 0.83 to 0.86 and was not affected by gender [30].
The Trail Making Test (TMT), which is one of the most widely used tools in neuropsychological assessment, has been shown to be a sensitive indicator of brain damage [31]. The purpose of the TMT is to evaluate the speed of processing, cognition, motor performance, and executive function [32–34]. This test consists of parts A and B. Part A requires sequentially connection of randomly distributed numbers on paper (i.e., 1–2–3–4), while TMT B requires connection of alternate numbers and letters (i.e., 1–A–2–B–3–C). In this study, TMT A used paper with 10 numbers, and TMT B used paper with 7 numbers and 7 letters. Participants were asked to perform the task as rapidly and accurately as possible, and to record the time it took to complete the task [35]. The time required for TMT A and TMT B was then summed and used for analysis. The retest reliability of TMT A and B was between 0.76 and 0.89 and between 0.86 and 0.94, respectively [36].
The Stroop Color Word Test (SCWT), which was developed by Stroop in 1935 to evaluate selective attention and cognitive flexibility, is now widely used for evaluation of executive function [37]. The stoop test, which consists of 3 components, is often used for brain damage testing. First, the participant is asked the name of a color in words (word task); second, they are asked for the color of an X bar (e.g., X in blue, red, or yellow ink, color task); third, they are asked for the ink color of the word written in ink that is a different color from the color name (e.g., the word “green” in red ink, color–word task). The color-word task is considered to evaluate both cognitive flexibility and ability to inhibit a dominant response [38]. Scores can be assessed by the number of the words (word task), number of bar colors (color task), and number of color words (color-word task) spoken within a specified time, or the time taken to complete each item [39]. In this study, the time required to complete 10 items for each of the 3 tasks was added up and used for analysis. The reliabilities of the basic scores for a single administration were W=0.88, C=0.79, and CW=0.71 [40].
The Shapiro-Wilk test was used to test the normality of the variables, but did not satisfy the normal distribution, so we used a nonparametric test.
Before intervention, differences in the general characteristics of the experimental group and the control group were compared using the Mann-Whitney test and chi-square tests.
Wilcoxon signed-rank tests were performed to assess the before- and after-effects in each group. Mann-Whitney tests were used to assess differences between real rTMS and sham rTMS. For all analyses, P values <0.05 were considered significant. Data were expressed as the mean ± standard deviation and statistical analysis was performed using SPSS version 20.0 (IBM Corporation, Armonk, NY, USA).
Results
Figure 1 shows a flow chart of this study and Table 1 presents the basic characteristics data of the study participants. Thirteen participants completed this experiment. Table 2 shows a comparison of changes in the characteristics of the 2 groups. The experimental group showed significant improvement in post-intervention MADRS, TMT, and SCWT compared to the pre-intervention (P<0.05), whereas the control group did not. In addition, a significant difference in the post-intervention MADRS, TMT, and SCWT was observed between groups (P<0.05). The effect size of gains in the experimental and control groups was also very strong for the MADRS, TMT, and SCWT (effect size=1.44, 1.49, and 1.24, respectively).
Figure 1.
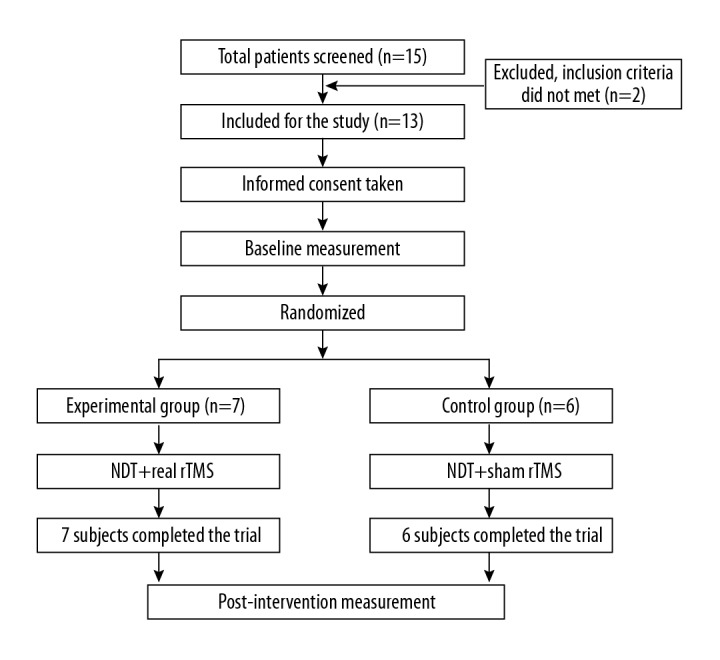
Flow chart of this study. NDT – neurodevelopmental therapy; rTMS – repetivite transcranial magnetic stimulation.
Table 1.
General characteristics of the subjects (n=13).
| Characteristic | EG (n=7) | CG (n=6) |
|---|---|---|
| Age (years) | 42.42±11.32 | 41.33±11.02 |
| Height (cm) | 171.00±4.65 | 173.00±6.72 |
| Weight (kg)a | 57.28±6.72 | 69±5.62 |
| Gender (Male/Female) | 5/2 | 4/2 |
| Duration (months) | 3.85±1.67 | 3.88±1.94 |
| MMSE-K (score) | 21.85±1.57 | 22.00±0.89 |
| GCS (score) | 13.71±1.11 | 13.66±0.81 |
EG – experimental group; CG – control group; MMSE – Mini-Mental State Examination; GCS – Glasgow Coma Scale. Values are expressed as the mean ±SD. a Significant difference in gains between two groups, p<0.05.
Table 2.
Comparison of change in characteristics of the experimental group and control group with values presented as the mean ± standard deviation.
| EG (n=7) | CG (n=6) | |||||
|---|---|---|---|---|---|---|
| Pre-test | Post-test | CWG | Pre-test | Post-test | CWG | |
| MADRS (score)a,b | 23.43±5.06 | 16.57±5.47* | 6.86±0.41 | 24.17±3.13 | 23.83±4.54 | 0.34±1.41 |
| TMT (second)a,b | 96.39±3.36 | 90.36±3.17* | 6.03±0.19 | 98.65±5.38 | 97.45±5.93 | 1.20±0.55 |
| SCWT (second)a,b | 158.03±17.37 | 138.03±13.79* | 19.99±3.48 | 160.49±12.65 | 157.49±13.94 | 3.00±1.29 |
EG – experimental group; CG – control group; CWG – changes within groups; MADRS – Montgomery-Asberg Depression Rating Scale; TMT – Trail Making Test; SCWT – Stroop Color Word Test. Values are expressed as the mean ±SD.
Significant difference from pre-test, p<0.05.
Significant difference between two groups, p<0.05;
Effect size greater than 0.70.
Discussion
Using a single-blind protocol, this study showed the effects of low frequency rTMS applied to the right dorsolateral prefrontal cortex with a figure-eight coil at a frequency of 1 Hz on depression and cognition in patients after TBI. No adverse effects on depression or other symptoms were noted. When compared to the control group, significant improvements were observed in the MADRS, TMT, and SCWT. Additionally, the real rTMS group showed a decrease of 29.29% in the MADRS post-intervention compared to pre-intervention, while the sham rTMS group showed a decrease of 1.40%.
In previous studies, low frequency rTMS was applied to the right dorsolateral prefrontal cortex and high frequency rTMS was applied to the left dorsolateral prefrontal cortex in patients with TBI, resulting in a 50% reduction in depressive symptoms [20]. In the aforementioned study, the MADRS score changed from the initial 34 points to 14 points after the intervention, consistent with improved MADRS results observed in our present study. Moreover, Mak et al. reported an improvement in depression in response to application of low frequency rTMS to the right dorsolateral prefrontal cortex [41].
Some studies have shown improvement in depression by applying high frequency rTMS to the left dorsolateral prefrontal cortex [42,43]. In another study, 20 Hz rTMS was applied to the left prefrontal area, which showed the effect of improving depression by reducing the Hamilton depression scale score [44]. That study revealed that Hamilton depression scale score of the real rTMS group decreased by 3 points, while that of the sham rTMS group increased by 5 points. We applied low frequency rTMS to the right dorsolateral prefrontal cortex. This was based on the report from the rTMS application guidelines for TBI that low frequency is safer and still results in a positive effect [45].
In our study, the real rTMS group showed a decrease of 6.25% in TMT post-intervention compared to pre-intervention, while the sham rTMS group showed a decrease of 1.21%. Moreover, the SCWT of the real rTMS group showed a decrease of 12.64% post-intervention compared to pre-intervention, while the sham rTMS group showed a decrease of 1.86%.
Many previous studies have shown the effects of rTMS on cognitive impairment. Boggio et al. reported improved cognitive function in response to application of high frequency rTMS to the left dorsolateral prefrontal cortex of patients with Parkinson disease [1]. As in the present study, their study also showed improved SCWT. In the aforementioned study, the color-word task of the SCWT was reduced by an average of 2.3 seconds in the rTMS group. The authors of another study reported improved cognitive function in response to application of 10 Hz rTMS to the left dorsolateral prefrontal cortex based on an average increase of the Lowenstein Occupational Therapy Cognitive Assessment score of 2.00±1.563 [46]. Rektorova et al. compared changes in cognitive function after applying high frequency rTMS to the left dorsolateral prefrontal cortex or left motor cortex of patients with cerebrovascular disease [47]. They found that rTMS applied to the left dorsolateral prefrontal cortex had a more positive effect on executive function than that applied to the left motor cortex.
It should be noted that this study had several limitations. First, after a certain period of time, follow-up evaluation was not conducted. Therefore, it was not possible to evaluate how long the effect lasted after discontinuing rTMS intervention. Future studies will need to investigate the duration of rTMS carryover effects on depression and cognitive functioning through follow-up evaluations. Second, it is difficult to generalize to all TBI patients because of the small sample size; therefore, future studies should provide a basis for further generalization by expanding the sample size.
Conclusions
This study was conducted to investigate the effects of low frequency rTMS on depression and cognitive function in the right dorsolateral prefrontal cortex of TBI patients.
To compare the effects of rTMS, real rTMS was applied to the experimental group and sham rTMS was applied to the control group for 10 days. As a result, the experimental group showed significant differences before and after intervention in MADRS for evaluation of depression, TMT and SCWT for cognitive function evaluation. In addition, in the MADRS, TMT, and SCWT, the experimental group showed a significant difference compared to the control group.
Therefore, we suggest that applying low frequency rTMS to the right dorsolateral prefrontal cortex is effective in improving depression and cognitive function in TBI patients. We recommend that future studies compare the rTMS application site and frequency.
Footnotes
Source of support: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT, Ministry of Science and ICT) (No. NRF-2017R1C1B5074052)
References
- 1.Parikh S, Koch RK. Traumatic brain injury. Int Anesthesiol Clin. 2007;45(3):119–35. doi: 10.1097/AIA.0b013e318078cfe7. [DOI] [PubMed] [Google Scholar]
- 2.Walker WC, Pickett TC. Motor impairment after severe traumatic brain injury: A longitudinal multicenter study. J Rehabil Res Dev. 2007;44(7):975–82. doi: 10.1682/jrrd.2006.12.0158. [DOI] [PubMed] [Google Scholar]
- 3.McAllister TW, Arciniegas D. Evaluation and treatment of postconcussive symptoms. NeuroRehabilitation. 2002;17(4):265–83. [PubMed] [Google Scholar]
- 4.Gennarelli TA, Spielman GM, Langfitt TW, et al. Influence of the type of intracranial lesion on outcome from severe head injury. J Neurosurg. 1982;56(1):26–32. doi: 10.3171/jns.1982.56.1.0026. [DOI] [PubMed] [Google Scholar]
- 5.Fann JR, Hart T, Schomer KG. Treatment for depression after traumatic brain injury: A systematic review. J Neurotrauma. 2009;26(12):2383–402. doi: 10.1089/neu.2009.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tassinari CA, Cincotta M, Zaccara G, Michelucci R. Transcranial magnetic stimulation and epilepsy. Clin Neurophysiol. 2003;114(5):777–98. doi: 10.1016/s1388-2457(03)00004-x. [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Liu TT, Song XB, et al. Comparison of different stimulation parameters of repetitive transcranial magnetic stimulation for unilateral spatial neglect in stroke patients. J Neurol Sci. 2015;359(1–2):219–25. doi: 10.1016/j.jns.2015.08.1541. [DOI] [PubMed] [Google Scholar]
- 9.Miniussi C, Cappa SF, Cohen LG, et al. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. 2008;1(4):326–36. doi: 10.1016/j.brs.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: A sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77(11):979–88. doi: 10.1016/j.biopsych.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Qu Y, Yuan M, Du T. Low-frequency repetitive transcranial magnetic stimulation for patients with aphasia after stoke: A meta-analysis. J Rehabil Med. 2015;47(8):675–81. doi: 10.2340/16501977-1988. [DOI] [PubMed] [Google Scholar]
- 12.Pallanti S, Di Rollo A, Antonini S, et al. Low-frequency rTMS over right dorsolateral prefrontal cortex in the treatment of resistant depression: cognitive improvement is independent from clinical response, resting motor threshold is related to clinical response. Neuropsychobiology. 2012;65(4):227–35. doi: 10.1159/000336999. [DOI] [PubMed] [Google Scholar]
- 13.Vonloh M, Chen R, Kluger B. Safety of transcranial magnetic stimulation in Parkinson’s disease: A review of the literature. Parkinsonism Relat Disord. 2013;19(6):573–85. doi: 10.1016/j.parkreldis.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21(1):1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8(7):559–67. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- 16.Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Bernabeu M, Tormos JM, Pascual-Leone A. Noninvasive brain stimulation in traumatic brain injury. J Head Trauma Rehabil. 2012;27(4):274–92. doi: 10.1097/HTR.0b013e318217df55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castel-Lacanal E, Tarri M, Loubinoux I, et al. Transcranial magnetic stimulation in brain injury. Ann Fr Anesth Reanim. 2014;33(2):83–87. doi: 10.1016/j.annfar.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Maruo T, Hosomi K, Shimokawa T, et al. High-frequency repetitive transcranial magnetic stimulation over the primary foot motor area in Parkinson’s disease. Brain Stimul. 2013;6(6):884–91. doi: 10.1016/j.brs.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Boggio PS, Fregni F, Bermpohl F, et al. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson’s disease and concurrent depression. Mov Disord. 2005;20(9):1178–84. doi: 10.1002/mds.20508. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald PB, Hoy KE, Maller JJ, et al. Transcranial magnetic stimulation for depression after a traumatic brain injury: A case study. J ECT. 2011;27(1):38–40. doi: 10.1097/YCT.0b013e3181eb30c6. [DOI] [PubMed] [Google Scholar]
- 21.Brighina F, Bisiach E, Oliveri M, et al. 1 Hz repetitive transcranial magnetic stimulation of the unaffected hemisphere ameliorates contralesional visuospatial neglect in humans. Neurosci Lett. 2003;336(2):131–33. doi: 10.1016/s0304-3940(02)01283-1. [DOI] [PubMed] [Google Scholar]
- 22.Lim JY, Kang EK, Paik NJ. Repetitive transcranial magnetic stimulation to hemispatial neglect in patients after stroke: An open-label pilot study. J Rehabil Med. 2010;42(5):447–52. doi: 10.2340/16501977-0553. [DOI] [PubMed] [Google Scholar]
- 23.Knoch D, Gianotti LR, Pascual-Leone A, et al. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26(24):6469–72. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reti IM, Schwarz N, Bower A, et al. Transcranial magnetic stimulation: A potential new treatment for depression associated with traumatic brain injury. Brain Inj. 2015;29(7–8):789–97. doi: 10.3109/02699052.2015.1009168. [DOI] [PubMed] [Google Scholar]
- 25.Barwood CH, Murdoch BE, Whelan BM, et al. The effects of low frequency Repetitive Transcranial Magnetic Stimulation (rTMS) and sham condition rTMS on behavioural language in chronic non-fluent aphasia: Short term outcomes. Neurorehabilitation. 2011;28(2):113–28. doi: 10.3233/NRE-2011-0640. [DOI] [PubMed] [Google Scholar]
- 26.Reith FC, Van den Brande R, Synnot A, et al. The reliability of the Glasgow Coma Scale: A systematic review. Intensive Care Medicine. 2016;42(1):3–15. doi: 10.1007/s00134-015-4124-3. [DOI] [PubMed] [Google Scholar]
- 27.Pascual-Leone A, Rubio B, Pallardó F, Catalá MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348(9022):233–37. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 28.Herrmann N, Black SE, Lawrence J, et al. The Sunnybrook Stroke Study: A prospective study of depressive symptoms and functional outcome. Stroke. 1998;29(3):618–24. doi: 10.1161/01.str.29.3.618. [DOI] [PubMed] [Google Scholar]
- 29.Asberg M, Montgomery SA, Perris C, et al. A comprehensive psychopathological rating scale. Acta Psychiatr Scand Suppl. 1978;(271):5–27. doi: 10.1111/j.1600-0447.1978.tb02357.x. [DOI] [PubMed] [Google Scholar]
- 30.Carneiro AM, Fernandes F, Moreno RA. Hamilton depression rating scale and montgomery-asberg depression rating scale in depressed and bipolar I patients: Psychometric properties in a Brazilian sample. Health Qual Life Outcomes. 2015;13:42. doi: 10.1186/s12955-015-0235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–76. [Google Scholar]
- 32.Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: Validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22(4):518–28. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- 33.Kizilbash A, Warschausky S, Donders J. Assessment of speed of processing after paediatric head trauma: Need for better norms. Pediatr Rehabil. 2000;4(2):71–74. doi: 10.1080/13638490026403. [DOI] [PubMed] [Google Scholar]
- 34.Kortte KB, Horner MD, Windham WK. The trail making test, part B: Cognitive flexibility or ability to maintain set? Appl Neuropsychol. 2002;9(2):106–9. doi: 10.1207/S15324826AN0902_5. [DOI] [PubMed] [Google Scholar]
- 35.Lezak MD. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- 36.Wagner S, Helmreich I, Dahmen N, et al. Reliability of three alternate forms of the trail making tests a and B. Arch Clin Neuropsychol. 2011;26(4):314–21. doi: 10.1093/arclin/acr024. [DOI] [PubMed] [Google Scholar]
- 37.Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–62. [Google Scholar]
- 38.Wecker NS, Kramer JH, Wisniewski A, et al. Age effects on executive ability. Neuropsychology. 2000;14(3):409–14. doi: 10.1037//0894-4105.14.3.409. [DOI] [PubMed] [Google Scholar]
- 39.Spreen O, Strauss E. A compendium of neuropsychological tests: Adminsitration, norms, and commentary, 2. NY: Oxford University Press; 1998. [Google Scholar]
- 40.Jensen AR, Rohwer WD., Jr The Stroop color-word test: A review. Acta Psychol (Amst) 1966;25(1):36–93. doi: 10.1016/0001-6918(66)90004-7. [DOI] [PubMed] [Google Scholar]
- 41.Mak ADP, Chan SSM, Lam LCW, et al. Preliminary results from a randomized sham-controlled trial of augmentative Neuro-navigated right-dorsolateral prefrontal cortex low-frequency repetitive transcranial magnetic stimulation for antidepressant non-responding Bipolar depression. Brain Stimul. 2017;10(2):378–79. [Google Scholar]
- 42.Deblieck C, Wampers M, Sienaert P, Bervoets C. Response to high-frequency repetitive transcranial magnetic stimulation over left dorsolateral prefrontal cortex is unaffected by number of treatment sessions in major depression: A systematic meta-analysis of randomized, sham-controlled trials. Brain Stimul. 2017;10(2):428. [Google Scholar]
- 43.Oliveira-Maia AJ, Press D, Pascual-Leone A. Modulation of motor cortex excitability predicts antidepressant response to prefrontal cortex repetitive transcranial magnetic stimulation. Brain Stimul. 2017;10(4):787–94. doi: 10.1016/j.brs.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.George MS, Wassermann EM, Kimbrell TA, et al. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: A placebo-controlled crossover trial. Am J Psychiatry. 1997;154(12):1752–56. doi: 10.1176/ajp.154.12.1752. [DOI] [PubMed] [Google Scholar]
- 45.Nielson DM, McKight CA, Patel RN, et al. Preliminary guidelines for safe and effective use of repetitive transcranial magnetic stimulation in moderate to severe traumatic brain injury. Arch Phys Med Rehabil. 2015;96(4):138–44. doi: 10.1016/j.apmr.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Park IS, Yoon JG. The effect of computer-assisted cognitive rehabilitation and repetitive transcranial magnetic stimulation on cognitive function for stroke patients. J Phys Ther Sci. 2015;27(3):773–76. doi: 10.1589/jpts.27.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rektorova I, Megova S, Bares M, Rektor I. Cognitive functioning after repetitive transcranial magnetic stimulation in patients with cerebrovascular disease without dementia: A pilot study of seven patients. J Neurol Sci. 2005;229–230:157–61. doi: 10.1016/j.jns.2004.11.021. [DOI] [PubMed] [Google Scholar]


