Abstract
Background
The disequilibrium of T helper (Th) cells play an important role in the occurrence and development of immune thrombocytopenic purpura (ITP). Th22 cells, as a newly discovered subset of T lymphocytes, plays an important role in autoimmune disorders and inflammatory diseases.
Material/Methods
This study explored the role of different lymphocyte subsets in chronic ITP. To explore the value of Th22 cells in the diagnosis of ITP, the numbers of Th1, Th17, and Th22 cells were detected by a 4-color flow cytometric in 32 chronic ITP patients and 30 healthy controls.
Results
Our data showed that, compared with healthy controls, the numbers of circulating Th1, Th17, and Th22 (p<0.05) cells increased significantly in ITP patients, and Th22 cells were correlated positively with Th1 cells (r=0.4041, p<0.01) and Th17 cells (r=0.4637, p<0.05). Moreover, a positive relationship was found between Th1/Th22 cells and Th1 cells (r=0.7696, p<0.001).
Conclusions
A disequilibrium expression profile of Th22 cells in peripheral blood was associated with pathogenesis of ITP, possibly through cooperatively working with Th17 and Th1, which may provide a novel approach for diagnosis of ITP.
MeSH Keywords: Cytokines; Purpura, Thrombocytopenic, Idiopathic; T-Lymphocytes, Helper-Inducer
Background
Immune thrombocytopenic purpura (ITP) is an acquired autoimmune hematologic disorder defined as a peripheral blood platelet count less than 100×109/L, with the characteristic of isolated thrombocytopenia, which is different from other causes or diseases that can result in thrombocytopenia [1], such as systemic lupus erythematosus (SLE), human immunodeficiency virus (HIV), drug exposure, heparin-induced thrombocytopenia, antiphospholipid syndrome (APS), or chronic lymphocytic leukemia (CLL) [2]. The main clinical manifestation of ITP is bleeding [3], most commonly mucocutaneous bleeding, menorrhagia, visceral bleeding, and even intracranial hemorrhage, which seriously affects quality of life. However, the pathogenesis of ITP remains unclear.
In recent years, the exploration of ITP pathogenesis has gradually improved, from discovery of platelet autoantibodies in the initially destruction of platelets in the spleen or megakaryocyte inhibition/destruction in the born marrow [4–7] to the study of autoimmune mechanism [8]. Glycoproteins (GPs) IIb/IIIa and Ib/IX are major types of platelet antibody. Many studies have reported that only approximately 60% of ITP patients showed positive platelet antibody detection [9,10] with T helper 1/T helper 2(Th1/Th2) cell subsets disequilibrium [11,12], suggesting that T cell abnormality plays an important role in the occurrence of ITP [13,14].
Studies have shown that T helper 17 (Th17) cells, a proinflammatory CD4+ T lymphocyte subset that secretes many kinds of cytokines, including interleukin (IL) 17, IL-17F, IL-21, tumor necrosis factor (TNF), and IL-22 [15,16], can also mediate inflammatory responses and autoimmune diseases, such as experimental autoimmune encephalomyelitis (EAE), rheumatoid arthritis (RA), and allergen-specific responses [17,18]. IL-22, a family of IL-10, exhibits specific biological activities of tissue repair and wound healing [19]. Research shows that Th17 can secret IL-22 and Th22 lymphocyte subsets and natural killer (NK) cells can also secrete IL-22. However, unlike other types of CD4+ T cell subsets, Th22 cells do not secrete IL-17A and IFN-γ [20]. In this study, we explored the relationship between IL-22 and proinflammatory cytokines, including IL-17 and IFN-γ. Moreover, we improved the sensitivity and specificity for ITP diagnosis and explored the pathogenesis of ITP, including the timing of treatment and the choice of treatment options.
Material and Methods
Patients and controls
The ITP patients were diagnosed by a process of exclusion: first, there are no blood abnormalities other than a low platelet count less than 100×109/L, and no physical signs other than bleeding. Then, secondary causes such as leukemia, medications (e.g., quinine and heparin), lupus erythematosus, cirrhosis, HIV, hepatitis C, congenital causes, antiphospholipid syndrome, von Willebrand factor deficiency, onyalai, and others should be excluded [2,3]. We enrolled 32 patients diagnosed with ITP (14 males and 18 females) at Qilu Hospital of Shandong University in this study, with a mean age of 49.16±2.86 years old (range from 28 to 76). Meanwhile, 30 healthy people (14 males and 16 females) were enrolled as controls (HC), with a mean age of 45.87±1.93 years old. There were no significant differences between the ITP group and control group regarding basic characteristics. Diagnosis of ITP was based on thrombocytopenia (platelet count <100×109/L) without any evidence of red or white blood cell abnormalities, normal or increased number of megakaryocytes with normal myeloid in bone marrow smears, absence of splenomegaly or other known causes to thrombocytopenia such as connective tissue disease, malignancy, and drug-induced thrombocytopenia. The study protocol was approved by the local ethics committee. All patients and controls were subjected to a full history-taking including disease duration, drug intake, preceding viral infection, and bleeding manifestations. A thorough clinical examination and a full blood panel were conducted. All enrolled patients were chronic ITP patients with no effect of hormonal treatment and were currently or intended to be treated with decitabine. All patients signed informed consent.
Flow cytometric analysis
The effect of intracellular cytokines on cytokine production was studied by flow cytometry. We added 200 uL of heparin anticoagulant peripheral blood to 200 uL of Roswell Park Memorial Institute (RPMI) – 1640 medium (Thermo Fisher Scientific, Inc.) and incubated at 37°C for 4 h in 5% CO2, together with 100 ng/mL of phorbol myristate acetate (PMA), 2 μg/mL of ionomycin, and 4 μg/mL monensin (all purchased from Alexis Biochemicals, San Diego, CA, USA). PMA and ionomycin worked as pharmacological T cell-activating agents to mimic signals generated by the T cell receptor (TCR) complex, with the advantage of stimulating T cells of any antigen specificity. Monensin was used to block intracellular transport to accumulate cytokines in the cell [21]. After incubation, the mixture was removed. PE-Cy5-conjugated anti-CD4 monoclonal antibodies (mAbs) were used to stain the cells in the dark at room temperature for 15 min. After fixation and permeabilization, FITC-conjugated anti-interferon (IFN)-γ monoclonal antibodies were applied to stain the cells, including PE-conjugated anti-IL-17 monoclonal antibodies and APC-conjugated anti-IL22 monoclonal antibodies in that sequence (all antibodies were purchased from eBioscience, San Diego, CA, USA). Isotype control was applied for correct compensation and checking antibody specificity. Flow cytometry was used to detect the stained cells using a FACScan II cytometer equipped with BD FACSDiva™ software (BD Bioscience, Franklin Lakes, NJ, USA).
Statistical analysis
Data analysis was performed using SPSS 19 software. All results are presented as mean ±SD (standard deviation). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to analyze statistical significance. Newman-Keels’ multiple comparison test (q test) was applied to assess differences between 2 groups. If the data were abnormally distributed, the Kruskal-Wallis test (H test) and Nemenyi test were used. Correlation analysis depending on data distribution was analyzed by Pearson or Spearman correlation test. Statistical significance was set at p<0.05.
Results
Increased circulation of CD4+IFN-γ+ cells, CD4+IL-17+cells, and CD4+IL-22+ cells
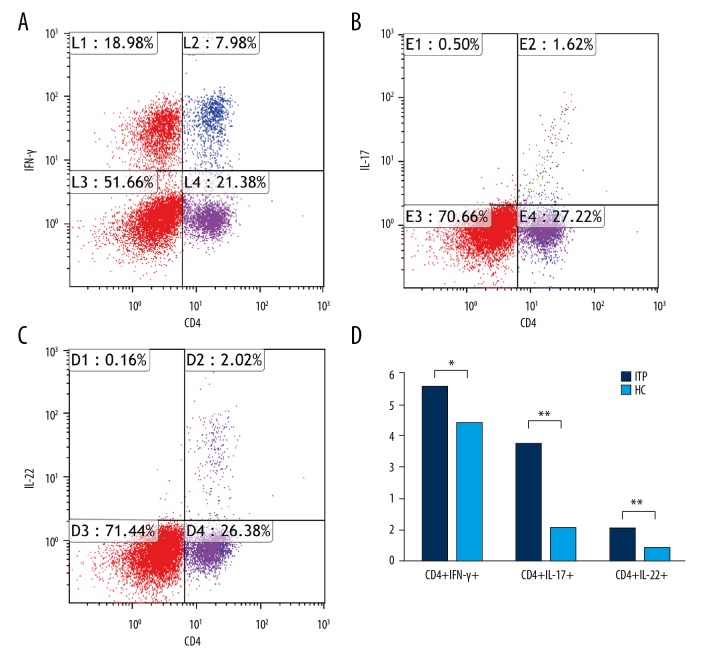
After gating of lymphocytes, we quantified CD4+IFN-γ+ cells, CD4+IL-17A+ cells, and CD4+IL-22+ cells (Figure 1A–1C). X and Y axes are cells of different types. The patients with ITP had a higher level of CD4+IFN-γ+ cells (5.50±0.32 vs. 4.40±0.16, p=0.0022), CD4+IL-17A+ cells (3.72±0.22 vs. 1.02±0.037, p<0.001), and CD4+IL-22+ cells (1.02±0.066 vs. 0.40±0.023, p<0.001) when compared with the control group (Figure 1D).
Figure 1.
Quantification of circulating CD4+IFN-γ+, CD4+IL-17A+, and IL-22+ cells in each group. (A–C) Representative four-color dot plot analyses of CD4+IFN-γ+, CD4+IL-17A+, and IL-22+ cells after stimulation with PMA and ionomycin. (D) Frequency of CD4+IFN-γ+, CD4+IL-17A+, and IL-22+ cells on the gated lymphocytes in the FSC/SSC plot in each group. Results are represented as the mean ±SD (** p<0.05). ANOVA followed by Tukey’s post hoc test was used to analyze statistical significance.
Enhanced circulation of Th1 (IFN-γ+IL-17A−IL-22−), Th17 (CD4+IFN-γ−IL-17A+IL-22−, CD4+IFN-γ−IL-17A+IL-22+), Th22 Cells (IFN-γ−IL-17A−IL-22+), and Th1/Th17 cells
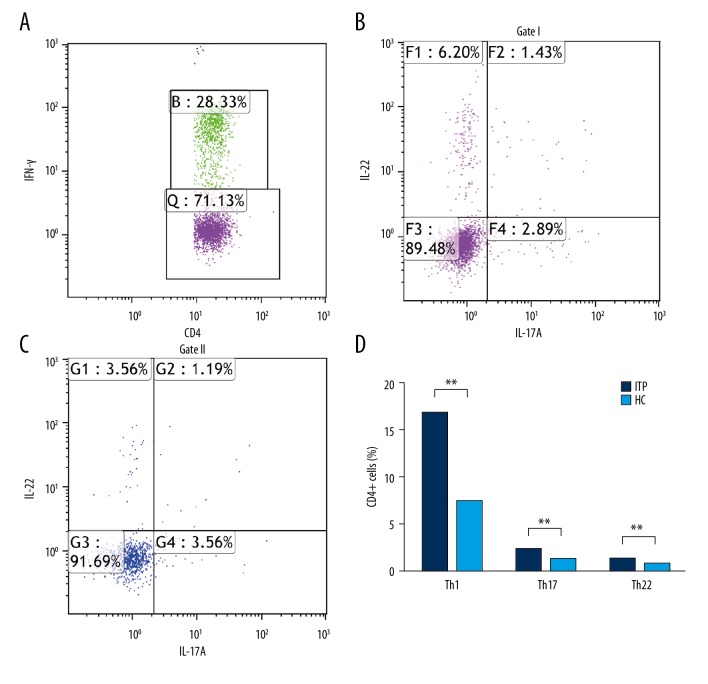
After gating of CD4+ cells, we detected the percentages of Th1, Th1/Th17 (IFN-γ+IL-17A+IL-22−), Th17 (IFN-γ−IL-17A+IL-22−, IFN-γ−IL-17A+IL-22+), and Th22 (IFN-γ−IL-17A−IL-22+) cells (Figure 2A–2C). X and Y axes are cells of different cell types. The compared data of circulating cells revealed that the patients with ITP had a higher percentage of Th22 cells (IFN-γ−IL-17A−IL-22+) (1.28±0.12 vs. 0.84±0.033, p<0.001), a significantly higher frequency of Th1 cells (16.59±0.80 vs. 7.30±0.267, p<0.001), and a higher frequency of Th1/Th17 cells than HC (1.68±0.15 vs. 0.22±0.0074, p<0.001) (Figure 2D).
Figure 2.
Percentages of circulating Th1 (IFN-γ+IL-17A−IL-22−), Th1/Th17 (IFN-γ+IL-17A+IL-22−), Th17 (IFN-γ−IL-17A+IL-22−, IFN-γ−IL-17A+IL-22+), and Th22 (IFN-γ−IL-17A−IL-22+) cells on CD4+cells of ITP and HC. (A–C) Representative four-color dot plot analyses of Th1, Th17, Th22, Th1/Th22, and Th1/Th17 cells. (D) Percentages of Th1 (IFN-γ+IL-17A−IL-22−), Th17 (IFN-γ−IL-17A+IL-22−, IFN-γ−IL-17A+IL-22+), and Th22 (IFN-γ−IL-17A−IL-22+) cells, Th1/Th22, and Th1/Th17 cells. Results are represented as the mean ±SD (** p<0.05). ANOVA followed by Tukey’s post hoc test was used to analyze statistical significance.
Correlations between Th22, Th17, and Th1 cells in ITP patients
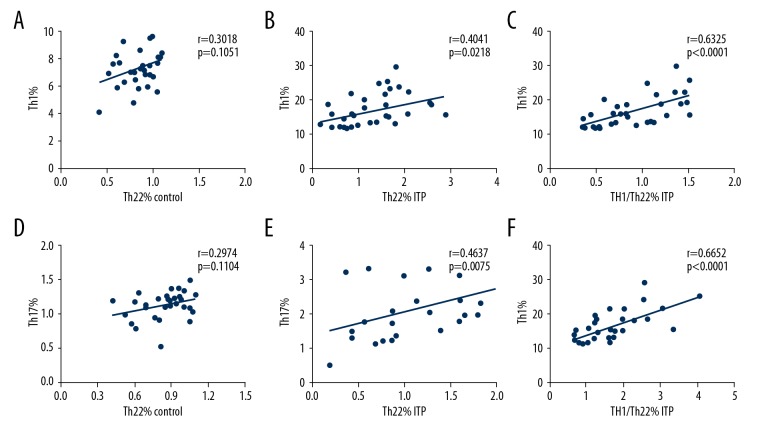
The relationship between Th22, Th17, and Th1 cells in ITP patients and healthy people were analyzed (Figure 3A–3F). We found a positive correlation between Th22 cells and Th17 cells (r=0.4637, P=0.0075; Figure 3B); Th22 cells and Th1 cells (r=0.4041, p=0.0218; Figure 3A) were found in ITP patients. Moreover, a positive relationship between Th1/Th22 cells and Th1 cells (r=0.7696, p<0.001; Figure 3C) was found.
Figure 3.
Correlations between Th22 cells Th17 cells, and Th1 cells in ITP patients. (A, B) A positive correlation was found between Th22 cells and Th1 cells (r=0.4041, p=0.0218) in the ITP patients but not in the controls. (C) A positive correlation was found between Th1/Th22and Th1 cells (r=0.7696, p=0.0002). (D, E) A positive correlation was found between the percentages of Th22 cells and the percentages of Th17 cells in the ITP patients (r=0.4637, p=0.0075) but not in the controls. (F) Th1/Th17 percentage was positively correlated with Th1 percentage (r=0.6652, p<0.0001). The q test was applied to determine the difference between 2 groups. If the data were abnormally distributed, H test and Nemenyi test were used. Correlation analysis depending on data distribution was analyzed by Pearson or Spearman correlation test.
Correlations between Th22 cells, platelet counts, severity of bleeding, and frequency and duration of disease in ITP patients
Due to condition restrictions, we followed up observation of the disease development process of the 18 ITP patients, and found that the incidence was higher in patients with higher levels of Th22 cells. At the onset, a higher the frequency of Th22 cells was associated with lower platelet count and worse effect of treatment was associated with a large dose of decitabine.
Discussion
CD4+ T lymphocytes were stimulated by PMA and differentiated into distinct effector T cell subsets that secreted cytokines with immune regulatory functions. Previous reports have shown that ITP is the predominant disease associated with Th1 cells [12]. There has been growing research interest in the imbalance of Th1/Th2 ratios in peripheral blood in immunotherapy for ITP [12]. Th17 cells are a proinflammatory distinct lineage of CD4+ T lymphocyte subsets, but their mediation of pathogenesis of ITP remains controversial. Cao et al. [22] demonstrated that Th17 was not involved in the pathogenesis of ITP. On the contrary, studies have shown that Th17 was elevated in ITP disease [23], which is consistent with our present results. This may be related to the different stages of disease development and the diversity of populations. In our study, Th17 contributed to ITP diseases and Th1/Th17 cells also had a similar effect. Although the literature suggests that the expression of IL-17 by CD4 T cells does not require IFN-γ or the transcription factors T-bet or STAT4, all of which are essential regulators for the differentiation of Th1 [24], our results indicate that Th17 is also elevated in patients with elevated Th1 in ITP disease, which means that Th17 showed a positive correlation with Th1 in chronic ITP patients.
The peripheral blood Th22 cells in our experiments were significantly increased. Elevated Th1/Th22 cells were correlated to Th1 and Th22 cells, might work collaboratively in the immunopathogenesis of ITP. These results are consistent with Cao et al. and Eyerich et al. [25,26]. Th1 and Th22 cells may not be the only unique pathogenic cells in an intricate network of inflammatory molecules in the pathogenesis of ITP. Similar to Th17 cells and Th1 cells, Th22 cells are proinflammatory and take part in the pathogenesis of inflammatory and autoimmune diseases. Previous studies showed that Th22 plays an important role in the pathogenesis of ITP, but the mechanism of Th22 regulation in ITP is unclear [26]. Our future research will focus on whether lowering the abnormally high levels of Th22 is helpful in the treatment of ITP patients. We conjectured that the interaction between IFN-γ and IL-22 functioned more importantly in the pathogenesis of ITP. As the proportion of cells in the blood is very small, it is difficult to conclude the mechanism involved in these cells. Blockade of the cytokine IL-22 may be a potential therapeutic option in patients with chronic ITP.
Conclusions
During our research, we gated on lymphocytes previously, founding that CD4+IL-22+ was increased. For further precise research, we gated on CD4+ cells as well. The results demonstrated that Th22 (CD4+IFN-γ−IL-17A−IL-22+) cells were also increased. In the same way, the circulating Th1, Th17, and Th22 cells were obviously elevated in ITP patients. Moreover, the correlation between Th1/Th22 cells and Th1 cells in the ITP patients was significantly positive. We found a disequilibrium expression profile of Th22 cells in peripheral blood was associated with pathogenesis of ITP, possibly through cooperatively working with Th17 and Th1, which might provide a novel approach in the diagnosis of ITP.
Footnotes
Source of support: This study was supported by the National Program on Key Basic Research Project (973 Program) (2015CB755402), the Wu Jieping Medical Fund (320.6750.17181), and the Scientific Research Foundation of Qilu Hospital of Shandong University (Qingdao) (QDKY2015ZD07)
Conflict of interests
None.
References
- 1.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood. 2009;113:2386–93. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 2.Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The IPT syndrome: Pathogenic and clinical diversity. Blood. 2009;113:6511–21. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujimura K. Pathogenesis and management of immune thrombocytopenia. Rinsho Ketsueki. 2014;55:83–92. [PubMed] [Google Scholar]
- 4.Aslam R, Kapur R, Segel GB, et al. The spleen dictates platelet destruction, anti-platelet antibody production, and lymphocyte distribution patterns in a murine model of immune thrombocytopenia. Exp Hematol. 2016;44:924–930. doi: 10.1016/j.exphem.2016.07.004. e921. [DOI] [PubMed] [Google Scholar]
- 5.Brighton TA, Evans S, Castaldi PA, et al. Prospective evaluation of the clinical usefulness of an antigen-specific assay (MAIPA) in idiopathic thrombocytopenic purpura and other immune thrombocytopenias. Blood. 1996;88:194–201. [PubMed] [Google Scholar]
- 6.Chang M, Nakagawa P, Williams S, et al. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood. 2003;102:887–95. doi: 10.1182/blood-2002-05-1475. [DOI] [PubMed] [Google Scholar]
- 7.Mcmillan R, Wang LA, Nichol J, Pistillo J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood. 2004;103:1364–69. doi: 10.1182/blood-2003-08-2672. [DOI] [PubMed] [Google Scholar]
- 8.Olsson B, Andersson PO, Jernas MS, et al. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9:1123–24. doi: 10.1038/nm921. [DOI] [PubMed] [Google Scholar]
- 9.He R, Reid DM, Jones CE, Shulman NR. Extracellular epitopes of platelet glycoprotein ib alpha reactive with serum antibodies from patients with chronic idiopathic thrombocytopenic purpura. Blood. 1995;86:3789–96. [PubMed] [Google Scholar]
- 10.Kiefel V, Freitag E, Kroll H, et al. Platelet autoantibodies (IgG, IgM, IgA) against glycoproteins IIb/IIIa and Ib/IX in patients with thrombocytopenia. Ann Hematol. 1996;72:280–85. doi: 10.1007/s002770050173. [DOI] [PubMed] [Google Scholar]
- 11.Panitsas F, Theodoropoulou M, Kouraklis A, et al. Adult chronic idiopathic thrombocytopenic purpura (ITP) is the manifestation of a type-1 polarized immune response. Blood. 2004;103:2645–47. doi: 10.1182/blood-2003-07-2268. [DOI] [PubMed] [Google Scholar]
- 12.Wang T, Zhao H, Ren H, et al. Type 1 and type 2 T-cell profiles in idiopathic thrombocytopenic purpura. Haematologica. 2005;90:914–23. [PubMed] [Google Scholar]
- 13.Semple JW, Provan D. The immunopathogenesis of immune thrombocytopenia: T cells still take center-stage. Curr Opin Hematol. 2012;19:357–62. doi: 10.1097/MOH.0b013e3283567541. [DOI] [PubMed] [Google Scholar]
- 14.Zhou B, Zhao H, Yang RC, Han ZC. Multi-dysfunctional pathophysiology in ITP. Crit Rev Oncol Hematol. 2005;54:107–16. doi: 10.1016/j.critrevonc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Wilson N, Boniface K, Chan J, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–57. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 16.Yang XO, Pappu B, Nurieva R, et al. TH17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORα. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Infante-Duarte C, Horton HF, et al. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 18.Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolk K, Sabat R. Interleukin-22: A novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 2006;17:367–80. doi: 10.1016/j.cytogfr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Duhen T, Geiger R, Jarrossay D, et al. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–63. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Li J, Liu X, et al. Elevated Th22 cells correlated with Th17 cells in patients with rheumatoid arthritis. J Clin Immunol. 2011;31:606. doi: 10.1007/s10875-011-9540-8. [DOI] [PubMed] [Google Scholar]
- 22.Cao J, Chen C, Zeng L, et al. Elevated plasma IL-22 levels correlated with Th1 and Th22 cells in patients with immune thrombocytopenia. Clin Immunol. 2011;141:121–23. doi: 10.1016/j.clim.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Ma D, Zhu X, et al. Elevated profile of Th17, Th1 and Tc1 cells in patients with immune thrombocytopenic purpura. Haematologica. 2009;94:1326–29. doi: 10.3324/haematol.2009.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park HL, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao J, Li L, Chen C, et al. Expression of interleukin-22 and relative CD4(+) T cell subsets in patients with immune thrombocytopenia. J Exp Hematol. 2012;20:1432–35. [PubMed] [Google Scholar]
- 26.Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]