Abstract
Over the past two decades, neurophysiological responses in the lateral intraparietal area (LIP) have received extensive study for insight into decision-making. In a parallel manner, inferred cognitive processes have enriched interpretations of LIP activity. Because of this bidirectional interplay between physiology and cognition, LIP has served as fertile ground for developing quantitative models that link neural activity with decision-making. These models stand as some of the most important frameworks for linking brain and mind, and they are now mature enough to be evaluated in finer detail and integrated with other lines of investigation of LIP function. Here, we focus on the relationship between LIP responses and known sensory and motor events in perceptual decision-making tasks, as assessed by correlative and causal methods. The resulting sensorimotor-focused approach offers an account of LIP activity as a multiplexed amalgam of sensory, cognitive, and motor-related activity, with a complex and often indirect relationship to decision processes. Our data-driven focus on multiplexing (and de-multiplexing) of various response components can complement decision-focused models and provides more detailed insight into how neural signals might relate to cognitive processes such as decision-making.
Keywords: decision-making, visual motion, visual perception, parietal, lateral intraparietal cortex
INTRODUCTION: THE INTERPLAY BETWEEN LIP PHYSIOLOGY AND DECISION-MAKING
In this review, we focus on LIP neural responses during various versions of a perceptual decision-making task that involves discriminating the direction of visual motion (Newsome & Pare 1988, Shadlen & Newsome 1996) (see the sidebar titled Direction Discrimination Protocols for Studying LIP and Decision-making). LIP activity has long been regarded as an explicit neural representation of evidence accumulation during these decisions (Shadlen & Kiani 2013). Here, we put forth an analysis that considers how numerous sensory, cognitive, and motor events are related to patterns of LIP activity. While sensory and motor events are typically controlled or measured by the experimenter, cognitive components are almost by definition inferred without direct observation. We therefore adopt a sensorimotor perspective to explore how much of LIP responses can be explained by simple sensory and motor factors. After reviewing a number of correlational and causal investigations, we propose that LIP dynamics during decision-making are very well explained by a mixture of visual and premotor signals, with some remaining response components dissociable as decision related. In explicating this perspective, we hope to offer a general framework for interpreting neural correlates of cognitive processes in LIP and elsewhere.
Perceptual decision-making is easily conceived of as a two-stage process in which a decision variable evolves until a stopping mechanism commits the process to a particular choice (Schall 2001). The motivation for recording from LIP is guided by the assumption that somewhere in the brain, the decision variable is represented explicitly in the spike rates of single neurons, and that recording from those neurons would effectively let biology constrain models of decision-making. LIP has been taken as a likely candidate, and the spike rates of single LIP neurons have been interrogated as direct neural correlates of an evolving decision variable (Gold & Shadlen 2007). Later progress has sometimes reversed the direction of inference, relying on particular decision-making models to constrain (or at least focus) the interpretation of responses in LIP. This work stands as a critical domain for interpreting neural correlates of cognition, in which models of decision-making can constrain the interpretation of biology and biology can constrain models of decision-making. At times, this bi-directionality can pose a challenge for refining theories, if and when physiology and behavior provide ambiguous or contradictory descriptions of the decision process. Thus, to complement decision-focused explanations of what LIP activity encodes during perceptual decision-making, we propose the adoption of a firm sensorimotor starting point. This simplifying perspective derives from the long history of interpreting neural activity in many brain areas (including LIP) in terms of known variables such as visual and oculomotor factors (Colby et al. 1996, Gnadt & Andersen 1988).
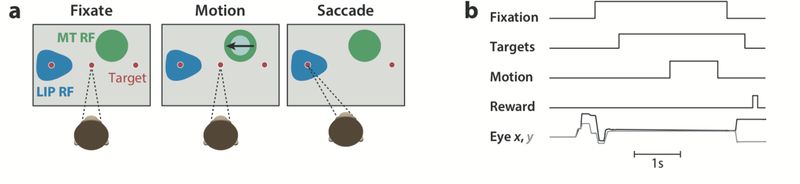
In the following sections, we focus on studies of LIP activity in the brains of rhesus monkeys while they perform a visual motion direction discrimination task (Figure 1). This review is not comprehensive, even within the LIP motion discrimination literature, but we have attempted to provide a thorough treatment of studies most relevant to disentangling sensory, cognitive, and motor factors. Similarly, although we discuss specific insights from complementary studies in other brain areas, other task frameworks, and other species, each of these topics is deserving of longer consideration and has recently been the focus of excellent reviews (Ding & Gold 2013, Freedman & Assad 2016, Hanks & Summerfield 2017).
FIGURE 1.
Generic direction discrimination task and neural recording geometry. (a) In a standard version of the direction discrimination task, monkeys are trained to discriminate the net direction of visual motion and communicate their decision with a saccadic eye movement to one of two diametrically opposite choice targets. When electrophysiological measurements are taken, task elements are positioned in reference to the receptive field (RF) of the neuron under study. For lateral intraparietal area (LIP) recordings, one of the saccade targets is typically placed in the LIP RF (blue patch). For middle temporal (MT) recordings, the motion stimulus is placed in the MT RF ( green patch). (b) Sequence of task events. In this example, the motion epoch is a fixed duration (FD) of approximately 1 s. Timings of individual events can be temporally jittered to decorrelate components. Other task permutations may include motion that has a variable duration (VD) between trials or with a duration determined by the animal’s response time (RT).
WHAT WE LEARN ABOUT LIP FROM DECISON-MAKING
The first characterization of LIP activity during visual motion decision-making was performed in the Newsome lab, following their classic and extensive studies of the middle temporal (MT) area’s role in the discrimination of motion direction (Britten et al. 1992, 1993, 1996; Newsome & Pare 1988, Salzman et al. 1992). Having established MT’s sensory role in the discrimination ´ of a noisy moving dot stimulus, Shadlen & Newsome (1996, 2001) ventured beyond this key sensory representation to record from macaque LIP during the direction discrimination task. They laid out a compelling set of reasons why LIP was well poised to integrate noisy sensory signals from MT in favor of an oculomotor decision: LIP receives anatomical input from MT (Lewis & Van Essen 2000a,b; Maunsell & Van Essen 1983; Ungerleider & Desimone 1986), projects to oculomotor centers such as the frontal eye fields and superior colliculus (Andersen et al. 1985, 1990; Ferraina et al. 2002; Pare & Wurtz 1997; Wurtz et al. 2001), and displays persistent activity during sensorimotor delay periods (Gnadt & Andersen 1988), reminiscent of the sustained activity in prefrontal cortex during working memory (Goldman-Rakic 1995). Thus, based on both anatomy and physiology, area LIP stood as a potential sensorimotor nexus for the physiological linkage of sensory to motor events (Felleman & Van Essen 1991, Lennie 1998).
LIP was studied using the same motion direction discrimination task used to study MT, in which monkeys view a random dot motion stimulus and communicate their choice about motion direction with a saccadic eye movement to one of two choice targets. For LIP, instead of placing the motion stimulus in the response field (RF) of the cell, the experimenters placed one of the two choice targets in the RF of the LIP neuron under study (Figure 1). When the monkey communicated its choice with a saccade into the RF of the neuron, neural activity gradually increased, long before the actual saccade was made. Motor buildup signals are often observed in many oculomotor structures (including LIP) during saccade preparation ( Janssen & Shadlen 2005, Wurtz et al. 2001), but this ramping activity had a unique characteristic: The slope of the ramping response was proportional to the strength of the motion stimulus. Thus, the dynamics of the LIP response mimicked what one would expect from the accumulation of motion evidence (i.e., the time integral). These early observations were made in a fixed-duration (FD) version of the random dot task (Shadlen & Newsome 2001), but related observations were made in a response-time (RT) version of the task (Roitman & Shadlen 2002). (The two paradigms are complementary, and we discuss their tradeoffs in the sidebar titled Direction Discrimination Protocols for Studying LIP and Decision-making.)
LIP’s ramping responses led to the hypothesis that this brain area reflects the accumulation of evidence, and a quantitative model showed that LIP responses were qualitatively similar to the time integral of differential MT activity (Mazurek et al. 2003). Such temporal integration could be the neural basis of evidence accumulation, as formulated in a variety of sequential sampling models in statistical and psychological work, most notably, the drift-diffusion model (Palmer et al. 2005, Ratcliff 1978). This was a powerful instantiation of connecting physiology to an existing mathematical model of decision-making, broadly analogous to efforts put forth by others in more rapidly performed oculomotor tasks (Carpenter & Williams 1995; Hanes & Schall 1995, 1996). To test this apparent correspondence, Huk & Shadlen (2005) used brief pulses of motion to test whether LIP firing rates matched the predictions of an integrator. They predicted that if LIP reflected the accumulated motion evidence, then these additional pulses of evidence should affect LIP firing in a sustained (as opposed to transient) manner. In line with this prediction, LIP responses reflected the brief added pulses for many hundreds of milliseconds after the motion pulse had ended—and the length of these effects corresponded quantitatively to the ending of the decision process, as might be implemented by a decision bound. This result reinforced the notion that the accumulation of motion evidence believed to underlie decisions was reflected explicitly in the spike rates of individual LIP neurons. Because the quantitative relation between motion stimuli and the time-varying LIP responses is at the heart of what computations LIP might reflect during decision-making, we revisit repeatedly this apparent mapping between LIP activity and the temporal accumulation of motion evidence.
Although these foundational studies bolstered interest in LIP responses as a neural correlate of evidence accumulation, it has long been appreciated that LIP activity during decision-making comprises a number of non-decision-related response components as well. One such component is the onset of the choice targets preceding the motion stimulus (one of which is placed within the LIP RF), which elicit strong responses in LIP. Strong, time-locked responses to sensory stimuli in the LIP RF have been well documented (Bisley et al. 2004, Blatt et al. 1990, Robinson et al. 1978), but it is not clear whether the sensory-driven responses are punctate (and effectively over by the time of motion onset) or whether a more persistent, target-driven response component bleeds into the motion viewing epoch in which the decision about motion direction is made. A second component is the saccade used to communicate the decision, which is likely preceded by a premotor response that may emerge during the motion viewing epoch as well. Although initial analyses assumed that only the final 75–100 ms of LIP activity during decisions are affected by a premotor burst (Roitman & Shadlen 2002), pre-saccadic activity often builds up gradually over hundreds of milliseconds across multiple areas of the oculomotor system in simpler tasks (Mountcastle et al. 1975, Wurtz et al. 2001). Despite these considerations, early experimental paradigms and analyses were not designed to isolate either persistent responses to the visual targets or premotor buildup responses to the saccade, both of which could temporally overlap and complexify the interpretation of responses that occur during motion evidence accumulation.
For LIP activity to provide clear insight into the decision process not available from the behavioral responses themselves, it is important to characterize and quantify how these secondary sensory and motor signals are encoded in LIP spike rate and, ideally, to isolate them from the decision formation signals. This is a challenging endeavor because in the standard motion discrimination paradigm, the spatial and motor aspects of the task are often inextricably linked to the decision about the sensory stimulus (Gold & Shadlen 2007, Roitman & Shadlen 2002, Shadlen & Newsome 1996). Simply put, in standard versions of the task, there are correlations between the motion stimulus, the decision about motion direction, and the motor response used to indicate the decision. This structure calls for a more targeted dissection of the response components in order to isolate decision-making signals; we next describe both empirical and analytic attempts to do so.
Dissection of LIP’s Correlations with Decision-making and Identification of Sensorimotor Multiplexing
The isolation of sensory and motor responses from decision-correlated activity has recently received renewed consideration. In one especially powerful variant of the motion discrimination task, the impending direction of the saccade was decoupled from the directional decision using a color-coded mapping between motion direction and saccade targets, revealed at different times relative to decision formation (Bennur & Gold 2011). LIP neurons were found to distinctly represent the onset of targets, the direction of motion, and the premotor plan to saccade, mixed in their spike rates. Importantly, the signals associated with saccadic choices (and even the color coding instruction) were substantial in magnitude relative to the direction-selective motion response, and their time course appeared to begin to emerge as soon as the stimulus-response mapping was revealed to the subject. Furthermore, the direction-selective motion response could be present irrespective of whether or not the mapping to the saccade target locations had been revealed (and could even be inconsistent with the preferred direction of the choice-related response), raising the question as to whether motion-driven responses (dissociable from saccadic choice signals) might be a sensory phenomenon, akin to observations of direction selectivity in other LIP studies (Fanini & Assad 2009).
In another empirical sensorimotor dissociation, Meister et al. (2013) manipulated the presence of the choice targets to isolate the target-driven visual response from the decision- correlated activity.Monkeys performed the standard direction discrimination task, but on a fraction of trials— instead of leaving the visual targets on for the course of the trials—the targets appeared for only 100 ms and then quickly extinguished. This simple manipulation revealed that the visual drive from the target exerted prolonged effects on firing rates throughout the trial, including the decision-making epoch. Thus, LIP spikes during motion viewing were, at the least, a function of both the decision process and the simple presence of a target in the RF. Meister et al. (2013) additionally highlighted substantial heterogeneity in the response dynamics and sensorimotor multiplexing of individual LIP neurons. A similar multiplexed encoding of task and saccade components in LIP has been observed in a perceptual categorization task (Rishel et al. 2013).
These sensorimotor manipulations paint a picture of multiple, temporally overlapping response components that contribute to the neural response during the putative decision phase of the trial. Although such empirical investigations were valuable, these first steps involved coarse analyses, such as comparison of the mean responses across a small number of differential experimental conditions. In focusing on such simple comparisons, analyses do not access all the factors that might be driving neurons, nor do they provide insight into single-trial response dynamics. It struck us as potentially useful to characterize LIP’s multiple response components by leveraging the temporal dissociation between events on individual trials that are typically present in experimental designs (i.e., jittered timings between experimenter-controlled events, and variable onsets of behavioral responses) to provide detailed and data-driven characterizations of the sensory and motor components, and to quantify how such response components might interact.
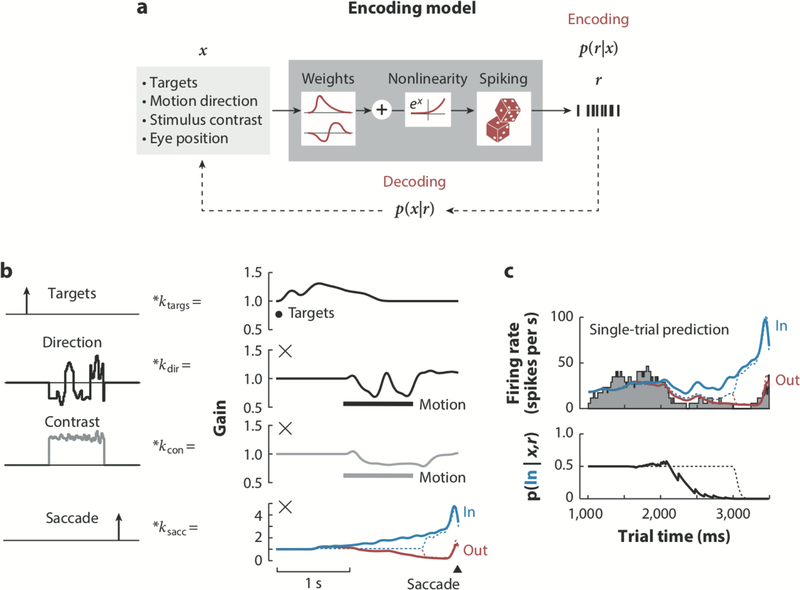
To dissect LIP’s multiplexed encoding in such a general-purpose analytic framework, Park et al. (2014) applied a generalized linear model (GLM) to statistically characterize the multiple factors that drive spiking in LIP during decision-making. The GLM is a form of the linear-nonlinear-Poisson model often used to describe sensory and motor responses as a function of external variables (Brown et al. 1998; Fernandes et al. 2014; Jacobs et al. 2009; Mayo et al. 2015, 2016; Paninski et al. 2004; Pillow et al. 2008; Ramkumar et al. 2016; Yu et al. 2009). These encoding models describe the probability of spiking for a single LIP neuron at a particular time as a nonlinear function of a weighted combination of the stimulus and task components, whose contributions can overlap in time or space (Figure 2a) (Pillow et al. 2005, Truccolo et al. 2005).
FIGURE 2.
Generalized linear model (GLM) applied to lateral intraparietal area (LIP). (a) The GLM describes the probability of a spike train r given external variables x. This relationship, p(r|x), is given by a Poisson process with a rate that is generated by filtering the external variables linearly and then passing the summed output through a static nonlinearity. The conventional exponential nonlinearity implies that all linear terms interact multiplicatively. (b) The separate contribution of the targets, direction of motion, contrast of the stimulus, and saccade of the animal (left column) to spike rate are depicted as spike rate gains. The individual component gains (right column) are produced by convolving the stimulus covariates with their respective linear filters and exponentiating them. The × indicates that these gains are multiplied together to produce the spike rate for the neuron, a result of the exponential nonlinearity. The saccade kernel exerts a choicedependent effect on spike rate with rate increasing for choices in the response field (RF) (blue) and decreasing for choices out of the RF (red ). The dashed lines represent the effect the saccade would have if it affected spike rate only up to 500 ms before the saccade. (c) An example single-trial prediction for an LIP neuron (top). The predicted rates for a choice into the RF (blue) and out of the RF (red ) are overlaid with the binned spike count for this neuron ( gray). The probability of a choice into the RF is derived from the two predicted rates (black). Predicted responses using more punctate (truncated) saccade kernels are shown by the dashed lines, and clearly fail to account for the observed response. Figure modified with permission from Park et al. (2014) using data from Katz et al. (2016) and Yates et al. (2017).
Applied to LIP, the GLM analysis revealed that activity throughout the trial reflected responses to the choice targets, the visual motion, and the impending oculomotor choice, each with their own shape and time course (Figure 2b). These multiple components overlapped over long timescales, meaning that LIP spikes, even during the period of motion viewing (and hence, during decision-making), could be described by a combination of factors (including a decaying visual response to the onset of the choice targets and a growing response corresponding to the impending saccade), as opposed to a pure neural correlate of evidence accumulation. Within this framework, these contributions were characterized as the output of linear filters (convolved with the time course of corresponding events). Importantly, although these components are summed before being passed through a static nonlinearity, the particular form of the nonlinearity can capture multiplicative interactions between them (Figure 2b). In LIP, many of the neurons were better described by multiplicative interactions between different sensory and motor components than by linear combinations (Park et al. 2014). These nonlinear interactions between task and stimulus variables, combined with substantial encoding heterogeneity across the population of LIP neurons, reflects an instance of mixed selectivity, for which theoretical treatments have highlighted computational benefits (Fusi et al. 2016, Pagan & Rust 2014, Raposo et al. 2014, Rigotti et al. 2013). Thus, the multiplexed nature of LIP responses may be purely accidental but could also carry information-processing benefits, wherein the heterogeneous population can still be read out with a simple mechanism (Park et al. 2014).
One limitation of Park et al.’s (2014) analysis was that the motion stimulus was modeled with a single fixed coherence value throughout each trial. It therefore characterized LIP’s motion-related response component in reference to the idealized stimulus (in the statistical sense, the expectation of the stimulus), as opposed to the actual noisy output of MT neurons, which Newsome’s work has compellingly demonstrated provides a critical source of sensory data to decision-making stages. This simplified formulation obscures the degree to which LIP reflected a particular computation as opposed to inheriting it from MT’s sensory processing stages, especially at the scale of single trials. This motivated us to characterize the responses of both LIP and MT neurons using a reverse correlation stimulus (see the sidebar titled Direction Discrimination Protocols for Studying LIP and Decision-making) while simultaneously recording from neurons in both MT and LIP (Yates et al. 2017).
The first notable observation from these multi-area recordings was that MT responses to motion exhibited direction selectivity that was initially strong and decreased over the course of the motion epoch. Such dynamics have important implications for interpreting the time-varying effects of motion in later cortical stages such as LIP and in the psychophysical behavior. By analyzing LIP with respect to MT’s time-varying output (instead of with respect to the stimulus), the oft-observed decay of motion-driven responses in LIP could largely be explained by inheritance of temporally decaying weighting already evident in MT. Isolating this stimulus-related term then revealed the second most notable observation: that LIP’s ramping responses were more closely linked to the upcoming saccade than to the integration of motion. The motion integration response was small, and a larger saccade-related response buildup was necessary to quantitatively explain the underlying steepness of the response ramps (Figure 2c). These motion-integration and saccade-linked responses were distinguishable by virtue of each trial having a distinctly variable (and known) time course of motion, thus improving the statistical dissection of decision from saccadic response. In fact, many single LIP units exhibited only the saccade-linked response component, further supporting the notion that the majority of LIP’s ramping is dissociable from motion integration.
Regardless of the source of the motion response in LIP, the primary take-home message from this analysis is simply that LIP responses may be based on a correlation with the impending saccade more than with the accumulated evidence per se. When viewed through a sensorimotor lens, LIP’s responses do not require an appeal to a primary status as a direct neural correlate of decision formation. Instead, LIP is well explained as a combination of decision-irrelevant sensory responses, a large premotor component, and a substantially smaller component related to the integrated motion signal, which itself inherits important dynamics from the MT stage of processing. The isolated motion response (i.e., the representation of the putative decision variable) is certainly deserving of more study. Based on the sensorimotor multiplexing perspective, however, one might suspect that the small motion responses in LIP—despite having been isolated from sensory and motor events—could at least in part result from the motion stimulus grazing the RF of some LIP neurons. This conjecture is consistent with two established aspects of LIP responses: (a) that LIP RFs are quite large (Blatt et al. 1990) and (b) that when a motion stimulus is intentionally placed within the RF, LIP neurons exhibit direction-selective responses (Fanini & Assad 2009).
Causal Interrogations of LIP and Distributed Processing for Decision-making
Although LIP has received extensive focus, decision-related ramping activity has been observed in many brain areas, even during motion direction discrimination tasks. These areas include the frontal eye fields (Ding & Gold 2012a, Gold & Shadlen 2000, Kim & Shadlen 1999), the parietal reach region (de Lafuente et al. 2015), and subcortical structures such as the superior colliculus (Horwitz & Newsome 1999) and the caudate nucleus (Ding & Gold 2010, 2012b). Decision-related activity has even been observed outside the central nervous system, in muscle stretch reflexes (Selen et al. 2012) and inferred from overt arm movements (Song & Nakayama 2009, Spivey et al. 2005). Even saccades, which exhibit considerably more ballistic dynamics, can reflect evidence accumulation during a motion discrimination task ( Joo et al. 2016). In light of the complexities in interpreting LIP’s ramping responses (described in the previous section), this constellation of results is a reminder that thorough physiological insight into the mechanisms of decision-making will likely require recording from areas beyond LIP and also calls for more nuanced dissections of their respective decision-correlated activity patterns.
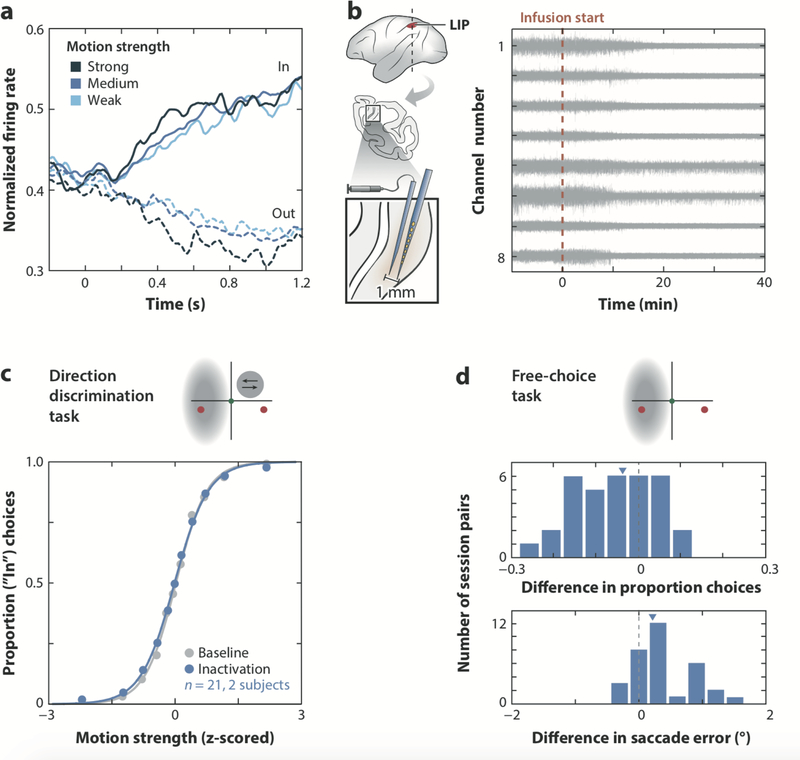
To explore the functional relevance of one node of this larger network, we recently tested LIP’s role in decisions by inactivating it reversibly during a direction discrimination task (Katz et al. 2016). The muscimol infusions targeted the very clusters of LIP cells that exhibited substantial decision-related activity in preceding electrophysiological recordings, and silencing of these clusters was confirmed electrophysiologically on every session. Despite having exhibited strong decision-related activity prior to muscimol infusion, inactivation of neurons in LIP resulted in no clear impact on the measured decision-making performance, indicated by virtually indistinguishable behavior between the baseline and LIP inactivation conditions (Figure 3). This held for both a reverse-correlation direction discrimination task and an FD version of the classic moving dots task. Behavior in a free-choice control task, in contrast, was significantly disrupted by the LIP inactivations. In this control task, monkeys chose freely between two saccade targets in the absence of a motion stimulus. Inactivation in LIP shifted choices away from the target in the contralesional hemifield, consistent with previous inactivation studies in monkeys, rodents, and humans (Balan & Gottlieb 2009, Erlich et al. 2015, Kerkhoff 2001, Kubanek et al. 2015, Wardak et al. 2002, Wilke et al. 2012, Zirnsak et al. 2015), and indicating that neural activity in LIP—although necessary for guiding free choices—is not necessary for the type of perceptual decision traditionally used to elicit decision-correlated activity in LIP.
FIGURE 3.
Inactivation of decision-correlated activity in lateral intraparietal area (LIP) does not have a significant effect on decision making. (a) Average response of 113 LIP neurons as a function of motion strength and direction (in versus out of the cell’s response field, solid and dashed lines, respectively), aligned to motion onset. (b) Schematic of the inactivation protocol (left). A multielectrode array was lowered alongside the infusion cannula to identify the targeted cortical location, verify neural selectivity before infusion, and confirm neural silencing after. On the right, continuous voltage traces are shown from an example inactivation session in which neural silencing is evident approximately 10 min after infusion start. (c) Psychophysical data for the direction discrimination task, averaged over pairs of baseline and muscimol treatment sessions in area LIP. Upper panel shows the experimental geometry along with the estimated inactivated field ( gray cloud ). (d ) Distribution of behavioral data in the free-choice task in which the animal chose between two targets that flashed in variable locations in the absence of a motion stimulus. Histograms show baseline and inactivation differences in proportion contralateral choices (top) and saccade error (bottom); positive numbers indicate an increase following inactivation. Figure modified with permission from Katz et al. (2016).
Do these results mean that LIP is not used for decision-making? Interpreting the results of a causal manipulation is challenging when the manipulation produces a positive result but is all the more so for a null result. A lack of measurable impact of inactivation is inconsistent with the notion that clusters of decision-correlated neurons in LIP play some sort of large and stable role in decision-making. However, it could also be the case that these LIP neurons are in fact usually relied upon, but that other structures are recruited in compensation. This is an intriguing, but we think unlikely, hypothesis, given that inactivation of LIP in other cognitive tasks (e.g., attentional selection, visual search, free choices) does result in reliable behavioral deficits (Balan & Gottlieb 2009, Kubanek et al. 2015, Liu et al. 2010, Wardak et al. 2004, Wilke et al. 2012). Such an account would require that LIP be compensated for by other structures in a decision-making task but not in these related tasks—including the free-choice control task in which behavioral disruption was observed (in the same experimental sessions as the motion discrimination task inactivations).
A related issue is the unilateral nature of the inactivations performed. Could bilateral inactivation of LIP have revealed an effect that is unobservable in the unilateral condition? Along similar lines of reasoning, it seems unlikely that unilateral inactivation in LIP is sufficient to elicit clear behavioral deficits in the studies mentioned above but not in a direction discrimination task. Additionally, Katz et al. (2016) tested for this possibility by placing both saccadic choice targets within contralesional space and still found no effect (i.e., instead of bilateralizing the inactivations, they unilateralized the task geometry). Thus, although LIP is critical for other cognitive functions, it is unlikely to be a critical node, and definitely not a fixed bottleneck for the behavior under study in the decision-making task. Of course, it remains possible that more nimble perturbation techniques (e.g., temporally precise and trial-interleaved optogenetic silencing) stand to reveal more nuanced contributions of LIP to decisions in the future (Dai et al. 2014, Li et al. 2016). If such techniques generate results not easily reconcilable with those of pharmacological inactivation, that would be particularly exciting, because they would contrast starkly with the history of causal perturbations in MT—for which slow, coarse techniques (lesion, pharmacological inactivation) and fast, localized techniques (microstimulation) paint a fairly cohesive picture with little need to appeal to fast or middle time frames of compensation (Chowdhury & DeAngelis 2008; Fetsch et al. 2014; Katz et al. 2016; Murasugi et al. 1993; Newsome & Pare 1988; Salzman et al. 1990, 1992).
These LIP inactivation results weigh against a critical role for LIP in the accumulation of sensory evidence, but a previous study may appear superficially at odds: Hanks et al. (2006) performed microstimulation of LIP neurons during the motion viewing epoch of the direction discrimination task. They found that microstimulation shortened response times in both monkeys, and subtly shifted the proportions of choices in favor of the target in the microstimulated LIP RF. Reduction in RT and increased choices to the target in the RF of the microstimulated LIP neurons is indeed consistent with an offset of the integrated signal. However, the results from this experiment should be interpreted with an open mind for several reasons. First, microstimulation may recruit areas other than the targeted area (LIP) via antidromic stimulation (Histed et al. 2009, Tehovnik et al. 2006), consistent with a distributed network. Second, the reduction in RT and subtle increase in choices can be explained in multiple forms outside the evidence accumulation framework, for example, as an increase in attention to one choice target over the other. In studies that focus on target selection in the presence of distractor targets, both microstimulation and inactivation in LIP produce behavioral deficits consistent with the results reported by Hanks et al. (2006) (Balan & Gottlieb 2009, Cutrell & Marrocco 2002, Dai et al. 2014, Kubanek et al. 2015, Wardak et al. 2002, Zirnsak et al. 2015).
Attempts to elucidate the differential role of decision-related activity have recently emerged in the rat model system, with a particular focus on posterior parietal cortex (PPC) (Erlich et al. 2015, Hanks et al. 2015, Harvey et al. 2013, Licata et al. 2016, Raposo et al. 2014). Despite an incomplete case for homology between rodent and primate PPC (Belmonte et al. 2015, Cooke et al. 2014), one clear result is that inactivations of rat PPC did not affect behavior in an auditory evidence accumulation task (Erlich et al. 2015), whereas similar inactivations in PPC by a different group did have an impact in a visual decision-making task (Licata et al. 2016, Raposo et al. 2014). Although one way to reconcile these results with the macaque results reported above is to posit that rodent PPC and macaque LIP are fundamentally different, another is that the visual deficits observed by the Churchland group may be more sensory in nature and correspond to the motion-driven responses observed in macaques when motion is placed in the LIP RF (Fanini & Assad 2009, Freedman & Assad 2006, Licata et al. 2016).
In light of the above correlational and causal observations, our perspective is that the neural basis of evidence accumulation requires considerable additional investigation. Temporal accumulation could be performed in a single cortical area, but it could just as well be performed in a subcortical region or within a more distributed network. Regardless of how one might map a cognitive process onto a brain area or multi-area circuit, LIP’s complex and perhaps indirect relation to decision formation may soften focus on recording from this area to draw inferences about nuanced neural mechanisms of decision formation, a topic we turn to in the following section.
APPLYING THE SENSORIMOTOR MULTIPLEXING PERSPECTIVE TO OTHER ASPECTS OF LIP RESPONSES
In the previous section, we focused on how LIP responses can be described in terms of experimenter-controlled or experimenter-observed external variables, such as visual stimuli or saccadic eye movements. This is not the approach that has generated so much interest in LIP, however. Instead, the appeal of LIP recordings is the prospect of access to internal (cognitive) dynamics that are not directly experimentally controlled or observed. These dynamics are traditionally interpreted under the umbrella of drift-diffusion models (Ratcliff et al. 2016), where a number of model parameters describe the relationship between LIP responses and decision formation. In contrast, our statistical dissections and causal interrogations of LIP support the notion of LIP as carrying a combination of sensory and motor signals that are related to the task but less directly correlated with decision-making. In the current section, to test the viability of our complementary focus on sensorimotor multiplexing, we ask whether simple sensory and motor components might also account for neural activity that has typically been taken as an impetus to append the drift-diffusion model of LIP.
Perhaps the most obvious mismatch between LIP responses and generic forms of drift-diffusion models of evidence accumulation is the initial phase of the neural responses. LIP motion-related responses often begin with a nonselective phase that lasts ∼200 ms and then evolve into the classic ramping dynamics, which follow the motion stimulus with the same latency. Within those first ∼200 ms, LIP responses often follow a dip-and-rise pattern that is not an inherent part of abstract drift-diffusion models (Churchland et al. 2008; de Lafuente et al. 2015; Huk & Shadlen 2005; Katz et al. 2016; Kiani & Shadlen 2009; Kiani et al. 2008; Meister et al. 2013; Roitman & Shadlen 2002; Shadlen & Newsome 1996, 2001). In the past, it has been posited that the dip and rise reflects the brain’s way of resetting the integration process (Huk & Shadlen 2005), a transient attentional shift to the motion stimulus (Wong 2007), or both, although neither cognitive explanation has direct empirical support. Alternatively, a parsimonious account of the dip and rise derives from a sensory account. In an experiment in which we presented the motion stimulus very soon (∼200 ms) after the onset of the response targets (Meister et al. 2013), we found that the dip and rise now exhibited a coherence dependence that was nondirectional: The stronger the motion, the lower the LIP response dipped, independent of motion direction. This would be quite perplexing if one were to take the LIP response as a neural correlate of a decision variable, because a decision-framework explanation would posit that the animal had an early hunch about the strength of motion and then lowered the starting point of evidence accumulation on easier trials.
Instead, the nondirectional coherence-dependent dip-and-rise pattern of responses can be explained parsimoniously as an instance of wide-field divisive normalization, as suggested in other LIP studies (Falkner et al. 2010, Louie et al. 2014). In this sensory-based explanation, the response of the LIP neuron under study (which, in the idealized case, has a single response target in its RF) is divided by the net response of other neurons in the area. If the response of other LIP neurons (which have the motion stimulus in their RFs) is larger for higher motion strengths [as it is in many other areas, including MT and medial superior temporal area (MST)], then the normalization term would be stronger and the response would be reduced more. This idea is further supported given the temporal match between maximal dipping time of the LIP dip and rise (when the target is in the RF, ∼200 ms) and the time when directional selectivity of LIP neurons is maximal (when motion is in the RF, also ∼200 ms) (Fanini & Assad 2009). The only difference between this scheme and the normalization that is better understood in earlier visual areas is that it would require broad spatial tuning of the normalization pool. This seems reasonable given the large RF sizes and messy retinotopy in LIP (Ben Hamed et al. 2001, 2002; Blatt et al. 1990; Janssen et al. 2008; Platt & Glimcher 1997; but see Patel et al. 2010) and the flexible tuning observed in LIP during learning (Sarma et al. 2015), but more direct analyses are required.
In other experiments, the number of targets has been manipulated to change the decision-making context. In one important study, the number of directions to be judged was increased to four, and so was the number of choice targets (Churchland et al. 2008). Responses in LIP started lower in the dip-and-rise phase when more choice alternates were allowed (and when more targets were present). This response baseline could be interpreted cognitively, as a lower starting point for a drift-diffusion process when more options are contemplated. Alternatively, the sensorimotor framework expanded above highlights that more targets would drive the full-field normalization pool more strongly and thus reduce the level of activity in a particular LIP neuron being recorded from. Similar target-number effects have been observed in other oculomotor structures (Basso & Wurtz 1997, 1998). Such normalization could also contribute to response dynamics when a third target is presented in variants of the perceptual decision-making task to study confidence (Kiani & Shadlen 2009). Although such studies have included some controls, the strongest isolation of sensorimotor/normalization contributions would involve always presenting the same number (and geometric layout) of targets while changing only their instructive meaning (by virtue of color coding or similar manipulations). Such controls are difficult to implement in practice (Churchland et al. 2008), but the sensorimotor framework will hopefully motivate such considerations in related future work.
Under some conditions, modulations of the dip and rise not easily ascribed to simple stimulus components have been observed. When there are asymmetric rewards or prior probabilities associated with one direction relative to the other, the dip and rise exhibits modest but sometimes measurable offsets in response—slightly higher activity in neurons that correspond to the more preferred target (Hanks et al. 2011, Rao et al. 2012, Rorie et al. 2010). This could be interpreted in terms of a changing starting point of evidence accumulation. However, because decisions and motor responses were linked in these studies, it is not possible to identify whether the observed bias is an offset in the decision variable or an increased level of motor preparation. In our work, the statistical relation between LIP activity at one point in time and the eventual saccade spans a wide time range. Thus, when manipulations of bias affect behavior, they are likely to affect the neural responses in commensurate ways, and the exact form (i.e., whether they offset the starting level or have more complex time-varying effects) likely depends on the timing of the saccade (or the saccade planning) relative to the motion viewing period (Rao et al. 2012). However, it is certainly possible that other types of decision-related variables (e.g., payoffs, rewards) could be directly reflected in LIP activity, and the sorts of dissections performed in some studies (Park et al. 2014, Rorie et al. 2010) are likely to continue to provide insights on this issue.
Analogous to the difficulties in interpreting the beginning of LIP responses as the start of evidence accumulation, the final level of activity is equally difficult to interpret as reflecting the final level of accumulated evidence. The sensorimotor approach taken by Meister et al. (2013), for example, showed that the level of LIP response around the time of the saccade—even though it was separated from the motion viewing by a delay period—was lower in trials in which the visual targets had been extinguished at the beginning of the trial compared to those in which the targets stayed on (despite no obvious difference in psychophysical performance). This indicates that the final level of LIP activity cannot be interpreted as a singular correlate (e.g., a decision bound). A visual manipulation occurring at the start of a trial affecting the response level at the end of the trial highlights the limitations in interpreting any phase of the LIP response in uniquely decision-related terms.
LIP responses sometimes exhibit a global upward trend that is not consistent with a pure diffusion to bound mechanism. This pattern of residuals has been cast in decision-related terms as an added urgency signal that serves to push the accumulated evidence closer to the bound (Carland et al. 2016, Churchland et al. 2008, Cisek et al. 2009, Hanks et al. 2014). In LIP experiments, this urgency signal was originally defined as the residuals between the observed LIP response and the predicted time course based on symmetric drift-diffusion. Although this upward trend could indeed be an added cognitive signal, the fact that the urgency signal is the mathematical difference between LIP responses and pure accumulation could also be couched in a sensorimotor manner: The same premotor buildup terms we have described can capture this overall positive tendency, because the saccade component related to saccades into the RF is larger than the corresponding term for out-of-RF saccades. It would therefore be interesting to see manipulations of urgency that do not manipulate the timing of motor responses, although such a dissociation is challenging to envision in practice.
One other feature of LIP responses that has recently garnered interest is the dynamics of single trial spike trains during decision-making. A recent application of advanced statistical modeling argued that discrete transitions (steps) provided a better description of single-trial LIP activity than noisy accumulation trajectories (ramps), the latter being the core component of drift-diffusion models (Latimer et al. 2015). Although these conclusions have been followed by extensive and ongoing debate, the sensorimotor multiplexing hypothesis highlights that single-trial responses are a function of multiple overlapping features with their own dynamics. Just as this perspective tempers attempts to treat averaged response ramps as direct correlations of decisions, it seems only fair to also apply this caveat to single-trial assessments of either stepping or ramping. We propose that if isolatable decision-related dynamics exist at the single-neuron or single-trial level, a more definitive analysis scheme will need to be built upon single-trial isolation of both sensory and motor factors. Otherwise, such finer-grained analyses of the statistics of LIP responses may reflect differential dynamics that are in part due to sensory responses, pre-saccadic activity, or both (see also Churchland et al. 2011). If we are to draw a constructive take-home message from the step-ramp debate at this time, it is simply that the time is right to specify models of decisions and their relation to physiology with greater precision, and that this level of precision requires simultaneous consideration of a multitude of sensory, cognitive, and motor signals. Of course, given the lack of a clear causal role of LIP in motion decisions, recordings from areas (or circuits) more clearly involved in decisions may provide more direct insight into the dynamics of the decision-making process (Brody & Hanks 2016, Pesaran & Freedman 2016, Pisupati et al. 2016).
Taken together, dynamics in LIP activity during decision formation could still potentially map onto evidence accumulation, though in a complex and indirect manner. We hope to have highlighted the challenges in doing so, with a constructive emphasis on sensory and motor factors. We put forth that renewed interest in multiplexed sensorimotor factors will allow for less controversial and more powerful isolation of cognitive signals.
CONCLUSIONS
In this review, we have highlighted a complementary framework for interpreting LIP activity with respect to perceptual decision-making. The two basic ideas behind the sensorimotor multiplexing framework are that (a) LIP (and many other higher brain areas) responds to a multiplicity of sensory, cognitive, and motor factors, and (b) in the motion discrimination task (and essentially every other perceptual decision-making task), there are a multitude of sensory and motor task elements present. These sensorimotor task elements can have varying degrees of relation to the decision process; at one end, saccadic buildup is typically tightly related to the decisions, whereas at the other, visual responses to the choice targets are an indirect by-product of the way the task is implemented. Such signals, even during simple tasks, are multiplexed, distributed, and not necessarily isomorphic with psychological theories. At an extreme, the close correspondence between LIP spike rates and theories of decision-making could result primarily from an amalgam of partially correlated factors overlapping. Based on the evidence summarized above, we believe that a moderate form of this implication is warranted—that significant components of LIP activity are sensorimotor correlates of task performance, and that direct correlates of decision formation should be isolated only after taking sensorimotor multiplexing into account, either in experimental design or in data analysis.
Although the overlap and possible mixture of decision and motor signals are always a theoretical possibility, this review has suggested that in practice, a substantial part of LIP activity is easily thought of as premotor buildup activity, as distinct from the temporal accumulation of evidence. Although a small component of LIP activity does indeed carry the signature of the motion time course on each trial, the majority of decision-correlated activity can be described as a response component that builds up (or not) if the animal is forming a decision that corresponds to an eye movement into (or out of ) the RF of the neuron under study. More direct tests of this point are called for, especially in light of category-specific responses in LIP that also appear to be dissociable from eye movement signals (Rishel et al. 2013). We propose that LIP activity will continue to be inextricably linked with choices across a wide range of tasks whenever the decisions are communicated with geometrically consistent saccades (Kira et al. 2015, Yang & Shadlen 2007), and that many instances of decision-correlated LIP dynamics will remain ambiguous until experimental and analytic approaches facilitate the disentangling of its multiplexed response components.
The sensorimotor framework we have proposed emphasizes an aspect inherent to many studies and thus initially aligns with an intentional framework: that decision-making signals and premotor activity are often commensurate and mixed together in many experimental designs and brain areas. However, the framework also comprises both clever experimental designs and sensorimotor demultiplexing analyses, which suggest that such signals are potentially dissociable (Chowdhury & DeAngelis 2008, Freedman & Assad 2011, Shadlen et al. 2008). Decision and premotor signals are multiplexed and intertwined to the extent that decision-making signals may even be observed outside the central nervous system, in the motor periphery ( Joo et al. 2016, Selen et al. 2012, Song & Nakayama 2009, Spivey et al. 2005). However, not all task variants produce this multiplexing phenomenon (Bennur & Gold 2011, Gold & Shadlen 2003), presumably because the mixed nature of decision and premotor responses may only be present once the mapping between abstract decision and appropriate motor action is predictable and has been learned (Law & Gold 2008, Sarma et al. 2015). Thus, our perspective is that rather than study particular tasks in which LIP resembles a neural correlate of decisions, it will be particularly illuminating to seek and identify when and how these multiplexed signals are (and are not) present to understand how the brain computes decisions and plans corresponding actions.
It is intuitive to raise a philosophical objection here—that pre-saccadic buildup in and of itself is a neural correlate of the evolving decision. However, the sensorimotor (de-)multiplexing focus on dissecting multiple decision-correlated response components is important to appreciate. If LIP primarily represented a decision variable, its activity would be well explained by the transformation of the motion stimulus, whereas responses related to the saccade would be of smaller amplitude and precede the saccade by a brief period of time (as is commonly posited by analyses that simply censor the ∼75 ms before a saccade). Instead, statistical analyses suggest that the motion-related response is relatively tiny, and that the dominating aspect of LIP response is a slow buildup of saccade-related activity. Of course, the response buildup is also correlated with the passage of time preceding a saccade, which LIP activity is known to track (in a choice-selective manner) even without ongoing perceptual decisions (Ipata et al. 2006, Janssen & Shadlen 2005, Jazayeri & Shadlen 2015, Leon & Shadlen 2003).
We have hypothesized that saccade-correlated activity emerges before decisions are complete, and that this premotor component interacts with a small stimulus-related signal. If one drops the assumption that LIP signals must map onto decisions explicitly, the potentially unintuitive nature of this sensorimotor interaction dissolves, as these signals may in fact be irrelevant to aspects of task performance upon which we focus, or even epiphenomenal. Of course, what we have called a pre-saccadic signal might not be inherently motoric but could reflect computations and signals only correlated with saccade planning (e.g., attention) and that are difficult to dissociate in the tasks we have discussed. Finally, we also note that our statistical identification of this pre-saccadic signal was performed within a GLM framework that included multiplicative interactions. As described above, this form of interaction is a basic component of all linear-nonlinear models of neural processing (Chichilnisky 2001). If such interactions are reflected in LIP’s computations, this would imply that as the visual target response fades and the pre-saccadic signal increases, any true motion-driven responses will have changing gains. This last point also relates our perspective to models that assert that a time-varying signal gates a low-passed version of the stimulus (Carland et al. 2016, Cisek et al. 2009).
Although we have focused on the relations between decision formation and motor preparation, the sensorimotor framework also calls for renewed focus on the sensory representations that feed the decision mechanism. In particular, a detailed understanding of the sensory stimulus and neural representation of that stimulus have important implications for inferences about evidence accumulation derived from behavior. The classical motion discrimination experiments have used an algorithm for modulating motion strength of a moving dot display with strong stochastic elements (Newsome & Pare 1988). This means that although each trial is designed to have a ´ particular coherence and direction, the actual motion strength presented can be quite different from the statistical expectation, both in the moment-by-moment fluctuations within a trial and in the sense of the cumulative net motion the subject is supposed to discriminate. Thus, accuracy in the dots task is limited by a combination of both internal neural noise as well as external noise (i.e., variability of the noisy stimulus as presented on a particular trial, point in time within a trial, or both). The discrepancy between motion expectation and cumulative net motion presented in a particular trial is mitigated for longer viewing durations (as the net motion approaches the expectation in the limit of time). Improvements in accuracy as a function of duration thus could reflect both internal evidence accumulation and the external process of the stimulus coming closer to its statistical expectation. Both types of temporal integration could play out similarly, and simultaneously, during the time course of decisions. Given that MT’s encoding of motion is sensitive to variability in the motion stimulus and has significant temporal dynamics, it is important to frame decision-related signals in higher brain regions with respect to the MT response, as opposed to with respect to the stimulus, and especially not with respect to the expected or average stimulus. This is why some of our recent studies have used a stimulus and generation algorithm with more direct control over the stochastic elements—an approach motivated by elegant designs in other lines of decision-making research (Katz et al. 2016, Raposo et al. 2014, Brunton et al. 2013, Wyart et al. 2012).
Importantly, LIP does not function in isolation, and the sensorimotor perspective offered here is similarly applicable across the entire relevant network. In the case of motion decisions, we have already argued that the sensory filtering reflected in MT outputs is an important element in the proper interpretation of how later brain areas and decisions process motion information (which is not the stimulus, but the relevant neural signals encoding the stimulus with only partial veracity). Tasks that involve changing the urgency of responses (such as manipulations of speed-accuracy tradeoffs) could also change the MT representation via the same attentional mechanisms observed in other tasks (Cook & Maunsell 2004, Ghose & Bearl 2010, Ghose & Maunsell 2002). Direct measurements of the sensory signals will continue to be important. Likewise, many other brain areas have been implicated in carrying ramping responses during motion discrimination (de Lafuente et al. 2015, Ding & Gold 2010, Horwitz & Newsome 1999, Kim & Shadlen 1999, Mante et al. 2013, Shadlen et al. 1996) and are also likely to contain a mixture of overlapping, decision-relevant and -irrelevant sensory and motor response components. Partitioning out these elements may reveal greater functional distinctions across this complex and distributed network.
SUMMARY
The sensorimotor framework is simple but still speculative, and we hope it evolves to complement and enrich interpretation of neural recordings during decision-making. Ideally, it will provide a useful basis for interpreting complex patterns of response, clarify apparent neuronal or cross-area heterogeneity, and allow the remaining dynamics of decision-related activity to directly inform models of how the brain approximates mathematical theories of decision-making. Perhaps most importantly, the sensorimotor multiplexing framework should allow decision-making studies of LIP to connect more tightly with other lines of work investigating LIP function. Similarly, this approach could facilitate comparisons across brain areas (Ding & Gold 2012a, 2013; Horwitz et al. 2004) and allow for the isolation of factors of focus in various lines of work (e.g., attention, motor intention, categorization, and abstract decisions) (Freedman & Assad 2016, Gottlieb & Balan 2010, Snyder et al. 2000). Complementing experimental designs that explicitly try to dissociate one or two factors that drive LIP, we have argued for the value of comprehensive regression-based analyses that decompose all possible impacts of external variables on the response of an LIP neuron. This approach is far from a deep functional theory but, as an exploratory tool, would provide an organizational scheme for allowing the data to speak to the formation of new ideas. Finally, sensorimotor multiplexing reminds us that many aspects of a response might not be decision relevant or decision critical and thus calls for increasingly nimble and powerful techniques for perturbing these signals. It would be exciting if some of the simpler conclusions we have put forth were fine-tuned by insights from future methodologies.
Whether or not LIP proves a strongly informative neural correlate of decision formation, it is absolutely the case that recording from LIP has generated many important ideas for relating neural activity and decisions (Shadlen & Kiani 2013). These ideas remain incontrovertibly historically groundbreaking for understanding how neural signal, noise, and correlation could relate to cognition and behavior. A core assumption of these exercises is that the brain must somehow accumulate evidence. However, this need not imply that a particular brain area or a special set of neurons must integrate sensory evidence in a manner that is directly instantiated in the firing rates of individual neurons on individual trials. The sensorimotor multiplexing perspective on LIP thus motivates a broader search for the cellular and network-level factors that contribute to such fundamental cognitive computations. This is likely to remain a topic that the entire field remains deeply enthusiastic about, even if the weight of evidence ultimately shifts neurophysiological recording choices away from LIP.
BOX 1: DIRECTION DISCRIMINATION PROTOCOLS FOR STUDYING LIP AND DECISION-MAKING.
The classical paradigms used to study evidence accumulation are the response-time (RT) and fixed-duration (FD) tasks. In the FD task, the sensory evidence is presented for a fixed amount of time regardless of whether the subject has reached a decision or not. The RT task is different in that the sensory evidence is presented for an indefinite amount of time and it is up to the observer to communicate their decision at any given time (Laming 1968). Thus, whereas the FD task produces one data point for every trial—accuracy—the RT task produces two: accuracy and response time.
The response time on every trial in the RT task is thought to reflect the internal process of accumulation and decision formation. The richness of the RT data has appealed to LIP researchers who want to gain more traction on single trials. Although the RT task provides a temporal upper bound on how long a decision took, there is no direct knowledge of all components that constitute the total reaction time. Although some portion of the reaction time is indeed related to the accumulation of sensory evidence, the remainder (termed non-decision time) includes a number of other components (Palmer et al. 2005). The study of neurons in the RT task is challenging because the link between neural activity and evidence accumulation could be mixed with neural responses linked to response preparation.
The FD task thus complements the RT paradigm in this regard, as the motor response is prompted only after the stimulus has been extinguished, such that neural activity related to accumulation is at least separated in time from response preparation, at least to a greater degree than in RT tasks. Of course, the stimulus duration can be varied across trials in a variable-duration (VD) version of the task such that subject accuracy may be probed at different times, providing the researcher with the average state of the evolving decision at any probed time. However, both the FD and VD approaches, in which the experimenter (and not the subject) determines the motion viewing period, are more limited in providing constrained insight into the time frame of individual decisions and might themselves change the strategic weighting of evidence over time without providing a direct means of assessing these effects.
Perceptual decisions can also be probed in a reverse correlation framework. In reverse correlation analysis, it is possible to compute the (average) influence of each stimulus epoch in order to discriminate between different temporal weighting schemes and infer subject strategy. Recent years have seen an increased use of stimuli that are directly amenable to this type of analysis (Brunton et al. 2013, Katz et al. 2016, Raposo et al. 2014, Wyart et al. 2012).
Such reverse-correlation stimuli contrast with the classic random dot motion stimulus, for which reverse correlation calculations must be done post hoc by filtering the moving dot stimulus (Kiani et al. 2008). These analyses should be viewed with caution, as such filtering exercises typically work with simplified simulations of the sensory signals, and any analysis based purely on behavior can miss the temporal dynamics actually present in neural representations of the stimulus (see main text for more discussion of this point). Missing some of the realistic dynamics, sensory filtering could in turn complicate estimates of the time course of temporal integration. More generally, although these new tasks offer a finer level of detail in mapping the relationship between the stimulus and behavior, it is important to note that the resulting kernels are the result of averaging over trials and do not necessarily by themselves indicate the strategy employed on each individual trial.
BOX 2: FUTURE ISSUES.
How does LIP interact with other areas that exhibit similar decision-correlated activity (e.g., frontal eye fields, superior colliculus, basal ganglia)? Can we arrive at a neural circuit model of decision-making that takes new data into account and moves beyond MT-LIP? What, if anything, is LIP’s causal role in the decision-making circuit? Can additional permutations of tasks be used to isolate or expose a functional contribution to well-studied tasks? Can additional techniques that allow for more precise and nuanced manipulations of neural activity provide greater insight?
How do neurons in LIP come to reflect temporally integrated representations with time constants considerably longer than those evident in single neurons in sensory areas? Are there unique cellular properties? What are the network-scale contributions to integration, and do these arise within a brain area, across areas, or both?
Can behavioral paradigms be refined to glean more direct insight into the underlying strategies of subjects at the scale of individual trials, or at least experimental sessions? As analytic tools become more adept at interrogating single-trial responses, it becomes critical to have parallel single-trial insight into the behavior that does not rely on averaging or on using the physiological responses to make inferences.
Can behavioral paradigms be extended to directly test the posited extensions of the driftdiffusion model that have been hinted at by LIP physiology? Can urgency be separated from motor preparation—i.e., are the dynamics of LIP that are not directly linked to the stimulus, but that do evolve over time in a decision-correlated manner, the reflection of decision formation per se, or are they simply an evolving motor plan that is downstream of a critical decision-making process?
What are the functional roles of LIP neurons that are driven by visual motion, as opposed to saccade targets, in their RFs? This has been explored in a categorization task with suprathreshold motion stimuli, but it is unknown if such conditions might reveal LIP’s contributions to temporal integration.
What is the functional structure of LIP? Our understanding of its basic functional architecture is nascent. More precise knowledge of multiple subregions, the nature and extent of maps, and cell types should allow for more detailed insights into what LIP does.
What is the homology between macaque LIP and regions in PPC in other species, many of which would allow for more immediate deployment of cutting-edge neurophysiological tools? Is rodent PPC functionally similar to LIP, or is it better thought of as a more sensory visual area? Does the marmoset model system have an LIP that might be a complementary sweet spot between highly trainable macaques and cutting-edge tool availability?
Can these studies benefit from current efforts to standardize research procedures?Would broader and less focused sampling of LIP neurons help or hurt our ability to infer its functional contributions to decision-making? Would preregistering numbers of trials or cells paint a different picture than those from more flexible procedures? Can standard training procedures and pre hoc criteria for behavior be shared and adapted broadly? Can a full set of models be developed and then used to decide on specific experiments to select between these established options? Would these lead to conclusions different than more exploratory practices that interpret deviations from models after data collection?
ACKNOWLEDGMENTS
This research was supported by a Howard Hughes Medical Institute International Student Research Fellowship to L.N.K., a National Eye Institute grant (R01-EY017366) to A.C.H. and J. Pillow, a Ruth L. Kirschstein National Research Service Award (T32DA018926) from the National Institute on Drug Abuse, and grant number T32EY021462 from the National Eye Institute.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Andersen RA, Asanuma C, Cowan WM. 1985. Callosal and prefrontal associational projecting cell populations in area 7A of the macaque monkey: a study using retrogradely transported fluorescent dyes. J. Comp. Neurol 232(4):443–55 [DOI] [PubMed] [Google Scholar]
- Andersen RA, Asanuma C, Essick G, Siegel RM. 1990. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule 296(1):65–113 [DOI] [PubMed] [Google Scholar]
- Balan PF, Gottlieb J. 2009. Functional Significance of Nonspatial Information in Monkey Lateral Intraparietal Area. Journal of Neuroscience 29(25):8166–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. 1998. Modulation of neuronal activity in superior colliculus by changes in target probability. Journal of Neuroscience 18(18):7519–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. 1997. Modulation of neuronal activity by target uncertainty. Nature 389(6646):66–69 [DOI] [PubMed] [Google Scholar]
- Belmonte JCI, Callaway EM, Churchland P, Caddick SJ, Feng G, et al. 2015. Brains, Genes, and Primates. Neuron 86(3):617–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Hamed S, Duhamel JR, Bremmer F, Graf W. 2002. Visual receptive field modulation in the lateral Intraparietal area during attentive fixation and free gaze. CerebralCortex 12(3):234–45 [DOI] [PubMed] [Google Scholar]
- Bennur S, Gold JI. 2011. Distinct Representations of a Perceptual Decision and the Associated Oculomotor Plan in the Monkey Lateral Intraparietal Area. Journal of Neuroscience 31(3):913–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Krishna BS, Goldberg ME. 2004. A rapid and precise on-response in posterior parietal cortex. J. Neurosci 24(8):1833–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. 1990. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J.Comp. Neurol 299(4):421–45 [DOI] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. 1996. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis.Neurosci 13:87–100 [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. 1992. The analysis of visual motion: a comparison of neuronal and psychophysical performance. Journal of Neuroscience 12(12):4745–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. 1993. Responses of neurons in macaque MT to stochastic motion signals. Vis. Neurosci 10(6):1157–69 [DOI] [PubMed] [Google Scholar]
- Brody CD, Hanks TD. 2016. Neural underpinnings of the evidence accumulator. Current Opinion in Neurobiology 37:149−−57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Frank LM, Tang D, Quirk MC, Wilson MA, Wilson MA. 1998. A statistical paradigm for neural spike train decoding applied to position prediction from ensemble firing patterns of rat hippocampal place cells. Journal of Neuroscience 18(18):7411–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland MA, Marcos E, Thura D. 2016. Evidence against perfect integration of sensory information during perceptual decision making. Journal of Neurophysiology 115(2): 915–30 [DOI] [PubMed] [Google Scholar]
- Carpenter RH, Williams ML. 1995. Neural computation of log likelihood in control of saccadic eye movements. Nature 377(6544):59–62 [DOI] [PubMed] [Google Scholar]
- Chichilnisky EJ. 2001. A simple white noise analysis of neuronal light responses. Network (Bristol, England) 12(2):199–213 [PubMed] [Google Scholar]
- Chowdhury SA, DeAngelis GC. 2008. Fine discrimination training alters the causal contribution of macaque area MT to depth perception. Neuron 60(2):367–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland AK, Kiani R, Chaudhuri R, Wang X-J, Pouget A, Shadlen MN. 2011. Variance as a signature of neural computations during decision making. Neuron 69(4):818–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland AK, Kiani R, Shadlen MN. 2008. Decision-making with multiple alternatives. Nat. Neurosci 11(6):693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Puskas GA, El-Murr S. 2009. Decisions in changing conditions: The urgency-gating model. Journal of Neuroscience 29(37):11560 –11571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. 1996. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. Journal of Neurophysiology 76(5):2841–52 [DOI] [PubMed] [Google Scholar]
- Cook EP, Maunsell JHR. 2004. Attentional modulation of motion integration of individual neurons in the middle temporal visual area. Journal of Neuroscience 24(36):7964–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke DF, Goldring A, Recanzone GH, Krubitzer L. 2014. The evolution of parietal areas associated with visuomanual behavior: from grasping to tool use. In the New Visual Neurosciences, ed. Werner JS, Chalupa LM, pp. 1049–63. Cambridge, MA: MIT Press [Google Scholar]
- Cutrell EB, Marrocco RT. 2002. Electrical microstimulation of primate posterior parietal cortex initiates orienting and alerting components of covert attention. Experimental Brain Research 144(1):103–13 [DOI] [PubMed] [Google Scholar]
- Dai J, Brooks DI, Sheinberg DL. 2014. Optogenetic and electrical microstimulation systematically bias visuospatial choice in primates. Curr. Biol 24(1):63–69 [DOI] [PubMed] [Google Scholar]
- de Lafuente V, Jazayeri M, Shadlen MN. 2015. Representation of Accumulating Evidence for a Decision in Two Parietal Areas. Journal of Neuroscience 35(10):4306–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Gold JI. 2010. Caudate encodes multiple computations for perceptual cecisions. Journal of Neuroscience 30(47):15747–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Gold JI. 2012a. Neural correlates of perceptual decision making before, during, and after decision commitment in monkey frontal eye field. Cerebral Cortex 22(5): 1052–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Gold JI. 2012b. Separate, Causal roles of the caudate in saccadic choice and execution in a perceptual decision task. Neuron 75(5):865–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Gold JI. 2013. The basal ganglia’s contributions to perceptual decision making. Neuron 79(4):640–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich JC, Brunton BW, Duan CA, Hanks TD, Brody CD. 2015. Distinct effects of prefrontal and parietal cortex inactivations on an accumulation of evidence task in the rat. Elife 4:e05457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner AL, Krishna BS, Goldberg ME. 2010. Surround suppression sharpens the priority map in the lateral intraparietal area. J. Neurosci 30(38):12787–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanini A, Assad JA. 2009. Direction selectivity of neurons in the macaque lateral intraparietal area. Journal of Neurophysiology 101(1):289–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex 1(1):1–47 [DOI] [PubMed] [Google Scholar]
- Fernandes HL, Stevenson IH, Phillips AN, Segraves MA, Körding KP. 2014. Saliency and saccade encoding in the frontal eye field during natural scene search. Cereb.Cortex 24(12):3232–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraina S, Paré M, Wurtz RH. 2002. Comparison of cortico-cortical and cortico-collicular signals for the generation of saccadic eye movements. Journal of Neurophysiology 87(2):845–58 [DOI] [PubMed] [Google Scholar]
- Fetsch CR, Kiani R, Newsome WT, Shadlen MN. 2014. Effects of cortical microstimulation on confidence in a perceptual decision. Neuron 83(4):797–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, Assad JA. 2006. Experience-dependent representation of visual categories in parietal cortex. Nature 443(7107):85–88 [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Assad JA. 2011. A proposed common neural mechanism for categorization and perceptual decisions. Nat. Neurosci 14(2):143–46 [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Assad JA. 2016. Neuronal mechanisms of visual categorization: An abstract view on decision making. Annu. Rev. Neurosci 39:129–47 [DOI] [PubMed] [Google Scholar]
- Fusi S, Miller EK, Rigotti M. 2016. Why neurons mix: high dimensionality for higher cognition. Current Opinion in Neurobiology 37:66–74 [DOI] [PubMed] [Google Scholar]
- Ghose GM, Bearl DW. 2010. Attention directed by expectations enhances receptive fields in cortical area MT. Vision Research 50(4): 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose GM, Maunsell J. 2002. Attentional modulation in visual cortex depends on task timing. Nature 419(6907):616–20 [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. 1988. Memory related motor planning activity in posterior parietal cortex of macaque. Experimental Brain Research 70(1):216–20 [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. 2000. Representation of a perceptual decision in developing oculomotor commands. Nature 404(6776):390–94 [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. 2003. The influence of behavioral context on the representation of a perceptual decision in developing oculomotor commands. Journal of Neuroscience 23(2):632–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. 2007. The neural basis of decision making. Annu. Rev. Neurosci 30:535–74 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. 1995. Neuron 14(3):477–85. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Balan P. 2010. Attention as a decision in information space. rends in Cognitive Sciences 14(6):240–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed SB, Duhamel JR, Bremmer F, Graf W. 2001. Representation of the visual field in the lateral intraparietal area of macaque monkeys: a quantitative receptive field analysis. Experimental Brain Research 140(2):127–44 [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. 1995. Countermanding saccades in macaque. Vis. Neurosci 12(5):929–37 [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. 1996. Neural control of voluntary movement initiation. Science 274(5286):427–30 [DOI] [PubMed] [Google Scholar]
- Hanks T, Kiani R, Shadlen MN. 2014. A neural mechanism of speed-accuracy tradeoff in macaque area LIP. Elife 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks TD, Ditterich J, Shadlen MN. 2006. Microstimulation of macaque area LIP affects decision-making in a motion discrimination task. Nat. Neurosci 9(5):682–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks TD, Kopec CD, Brunton BW, Duan CA, Erlich JC, Brody CD. 2015. Distinct relationships of parietal and prefrontal cortices to evidence accumulation. Nature 520(7546):220–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks TD, Mazurek ME, Kiani R, Hopp E, Shadlen MN. 2011. Elapsed decision time affects the weighting of prior probability in a perceptual decision task. J. Neurosci 31(17):6339–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks TD, Summerfield C. 2017. Perceptual decision making in rodents, monkeys, and humans. Neuron 93(1):15–31 [DOI] [PubMed] [Google Scholar]
- Harvey CD, Coen P, Tank DW. 2013. Choice-specific sequences in parietalcortex during a virtual-navigation. Nature 484(7392):62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Histed MH, Bonin V, Reid RC. 2009. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 63(4):508–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GD, Batista AP, Newsome WT. 2004. Representation of an abstract perceptual decision in macaque superior colliculus. Journal of Neurophysiology 91(5):2281–96 [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. 1999. Separate signals for target selection and movement specification in the superior colliculus. Science 284(5417):1158–61 [DOI] [PubMed] [Google Scholar]
- Huk AC, Shadlen MN. 2005. Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making 25(45): 10420–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Goldberg ME, Bisley JW. 2006. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. J. Neurosci 26(14):3656–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AL, Fridman G, Douglas RM, Alam NM, Latham PE, et al. 2009. Ruling out and ruling in neural codes. Proceedings of the National Academy of Sciences 106(14): 5936–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen P, Shadlen MN. 2005. A representation of the hazard rate of elapsed time in macaque area LIP. Nat. Neurosci 8(2):234–41 [DOI] [PubMed] [Google Scholar]
- Janssen P, Srivastava S, Ombelet S, Orban GA. 2008. Coding of shape and position in macaque lateral intraparietal area. Journal of Neuroscience 28(26):6679–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri M, Shadlen MN. 2015. A neural mechanism for sensing and reproducing a time interval. Curr. Biol 25(20):2599–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo SJ, Katz LN, Huk AC. 2016. Decision-related perturbations of decision-irrelevant eye movements. Proceedings of the National Academy of Sciences 113(7):1925–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LN, Yates JL, Pillow JW, Huk AC. 2016. Dissociated functional significance of decision-related activity in the primate dorsal stream. Nature 535(7611):285–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff G 2001. Spatial hemineglect in humans. Progress in Neurobiology 63(1):1–27 [DOI] [PubMed] [Google Scholar]
- Kiani R, Hanks TD, Shadlen MN. 2008. Bounded integration in parietal cortex underlies decisions even when viewing duration is dictated by the environment. Journal of Neuroscience 28(12):3017–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani R, Shadlen MN. 2009. Representation of confidence associated with a decision by neurons in the parietal cortex. Science 324(5928):759–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JN, Shadlen MN. 1999. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat. Neurosci 2:176–85 [DOI] [PubMed] [Google Scholar]
- Kira S, Yang T, Shadlen MN. 2015. A neural implementation of Wald’s sequential probability ratio test. Neuron 85(4):861–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubanek J, Li JM, Snyder LH. 2015. Motor role of parietal cortex in a monkey model of hemispatial neglect. Proceedings of the National Academy of Sciences 112(16):E2067–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laming D 1968. Information theory of choice-reaction times New York: Acad. Press [Google Scholar]
- Latimer KW, Yates JL, Meister MLR, Huk AC, Pillow JW. 2015. NEURONAL MODELING. Single-trial spike trains in parietal cortex reveal discrete steps during decision-making. Science 349(6244):184–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law C-T, Gold JI. 2008. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat. Neurosci 11(4):505–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P 1998. Single units and visual cortical organization. Perception 27(8):889–935 [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. 2003. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron 38(2):317–27 [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. 2000a. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J.Comp. Neurol 428(1):112–37 [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. 2000b. Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto-occipital cortex. J.Comp. Neurol 428(1):79–111 [DOI] [PubMed] [Google Scholar]
- Li N, Daie K, Svoboda K, Druckmann S. 2016. Robust neuronal dynamics in premotor cortex during motor planning. Nature 532(7600):459–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata AM, Kaufman MT, Raposo D, Ryan MB. 2016. Posterior parietal cortex guides visual decisions in rats. bioRxiv 066639 http://dx.doi.org/10.1101/066639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yttri EA, Snyder LH. 2010. Intention and attention: different functional roles for LIPd and LIPv. Nat. Neurosci 13(4):495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, LoFaro T, Webb R, Glimcher PW. 2014. Dynamic divisive normalization predicts time-varying value coding in decision-related circuits. J. Neurosci 34(48): 16046–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mante V, Sussillo D, Shenoy KV, Newsome WT. 2013. Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature 503(7474):78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. 1983. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. Journal of Neurophysiology 49(5):1127–47 [DOI] [PubMed] [Google Scholar]
- Mayo JP, DiTomasso AR, Sommer MA, Smith MA. 2015. Dynamics of visual receptive fields in the macaque frontal eye field. Journal of Neurophysiology 114(6):3201–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo JP, Morrison RM, Smith MA. 2016. A probabilistic approach to receptive field mapping in the frontal eye fields. Front Syst Neurosci 10:10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. 2003. A role for neural integrators in perceptual decision making. Cerebral Cortex 13(11):1257–69 [DOI] [PubMed] [Google Scholar]
- Meister MLR, Hennig JA, Huk AC. 2013. Signal multiplexing and single-neuron computations in lateral intraparietal area during decision-making. J. Neurosci 33(6): 2254–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. 1975. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. Journal of Neurophysiology 38(4):871–908 [DOI] [PubMed] [Google Scholar]
- Murasugi CM, Salzman CD, Newsome WT. 1993. Microstimulation in visual area MT: effects of varying pulse amplitude and frequency. Journal of Neuroscience 13(4): 1719–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. 1988. A selective impairment of motion perception following lesions of the middle temporal visual area (MT). Journal of Neuroscience 8(6): 2201–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan M, Rust NC. 2014. Quantifying the signals contained in heterogeneous neural responses and determining their relationships with task performance. Journal of Neurophysiology 112(6):1584–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J, Huk AC, Shadlen MN. 2005. The effect of stimulus strength on the speed and accuracy of a perceptual decision. Journal of Vision 5(5):376−404 [DOI] [PubMed] [Google Scholar]
- Paninski L, Shoham S, Fellows MR, Hatsopoulos NG, Donoghue JP. 2004. Superlinear population encoding of dynamic hand trajectory in primary motor cortex. J. Neurosci 24(39):8551–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare M, Wurtz RH. 1997. Monkey posterior parietal cortex neurons antidromically activated from superior colliculus. Journal of Neurophysiology 78(6):3493–97 [DOI] [PubMed] [Google Scholar]
- Park IM, Meister MLR, Huk AC, Pillow JW. 2014. Encoding and decoding in parietal cortex during sensorimotor decision-making. Nat. Neurosci 17(10):1395–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel GH, Shulman GL, Baker JT, Akbudak E, Snyder AZ, et al. 2010. Topographic organization of macaque area LIP. Proceedings of the National Academy of Sciences 107(10):4728–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Freedman DJ. 2016. Where are perceptual decisions made in the brain? Trends in Neurosciences 39(10):642–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillow JW, Paninski L, Uzzell VJ, Simoncelli EP, Chichilnisky EJ. 2005. Prediction and decoding of retinal ganglion cell responses with a probabilistic spiking model. J. Neurosci 25(47):11003–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillow JW, Shlens J, Paninski L, Sher A, Litke AM, et al. 2008. Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature 454(7207):995–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisupati S, Chartarifsky L, Churchland AK. 2016. Decision activity in parietal cortex – Leader or follower? Trends in Cognitive Sciences 20(11):788–89 [DOI] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. 1997. Responses of intraparietal neurons to saccadic targets and visual distractors. Journal of Neurophysiology 78(3):1574–89 [DOI] [PubMed] [Google Scholar]
- Ramkumar P, Dekleva B, Cooler S, Miller L, Kording K. 2016. Premotor and motor cortices encode reward. PLoS ONE 11(8):e0160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V, DeAngelis GC, Snyder LH. 2012. Neural correlates of prior expectations of motion in the lateral intraparietal and middle temporal areas. J. Neurosci 32(29): 10063–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo D, Kaufman MT, Churchland AK. 2014. A category-free neural population supports evolving demands during decision-making. Nat. Neurosci 17(12):1784–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R 1978. A theory of memory retrieval. Psychological Review 85(2):59–108 [Google Scholar]
- Ratcliff R, Smith PL, Brown SD, McKoon G. 2016. Diffusion decision model: Current issues and history. Trends in Cognitive Sciences 20(4):260–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton BW, Botvinick MM, & Brody CD (2013). Rats and humans can optimally accumulate evidence for decision-making. Science, 340(6128), 95–98. [DOI] [PubMed] [Google Scholar]
- Rigotti M, Barak O, Warden MR, Wang X-J, Daw ND, et al. 2013. The importance of mixed selectivity in complex cognitive tasks. Nature 497(7451):585–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishel CA, Huang G, Freedman DJ. 2013. Independent category and spatial encoding in parietal cortex. Neuron 77(5):969–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Goldberg ME, Stanton GB. 1978. Parietal association cortex in the primate: sensory mechanisms and behavioral modulations. Journal of Neurophysiology 41(4):910–32 [DOI] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. 2002. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. Journal of Neuroscience 22(21):9475–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorie AE, Gao J, McClelland JL, Newsome WT. 2010. Integration of sensory and reward information during perceptual decision-making in lateral intraparietal cortex (LIP) of the macaque monkey. PLoS ONE 5(2):e9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman CD, Britten KH, Newsome WT. 1990. Cortical microstimulation influences perceptual judgements of motion direction. Nature [DOI] [PubMed]
- Salzman CD, Murasugi CM, Britten KH, Newsome WT. 1992. Microstimulation in visual area MT: effects on direction discrimination performance. Journal of Neuroscience 12(6):2331–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma A, Masse NY, Wang X-J, Freedman DJ. 2015. Task-specific versus generalized mnemonic representations in parietal and prefrontal cortices. Nat. Neurosci 19(1): 143–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD. 2001. Neural basis of deciding, choosing and acting. Nat Rev Neurosci 2(1):33–42 [DOI] [PubMed] [Google Scholar]
- Selen LPJ, Shadlen MN, Wolpert DM. 2012. Deliberation in the motor system: Reflex gains track evolving evidence leading to a decision. Journal of Neuroscience 32(7): 2276–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Britten KH, Newsome WT, Movshon JA. 1996. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. Journal of Neuroscience 16(4):1486–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Kiani R. 2013. Decision making as a window on cognition. Neuron 80(3):791–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Kiani R, Hanks TD, Churchland AK. 2008. Neurobiology of decision making: An intentional framework Better Than Conscious? Decision Making, the Human Mind, and Implications for Institutions Engel C and Singer W, Editors. 2008, MIT Press: Cambridge. [Google Scholar]
- Shadlen MN, Newsome WT. 1996. Motion perception: seeing and deciding. Proceedings of the National Academy of Sciences 93(2):628–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. 2001. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. Journal of Neurophysiology 86(4):1916–36 [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. 2000. Intention-related activity in the posterior parietal cortex: a review. Vision Research [DOI] [PubMed]
- Song J-H, Nakayama K. 2009. Hidden cognitive states revealed in choice reaching tasks. Trends in Cognitive Sciences 13(8):360–66 [DOI] [PubMed] [Google Scholar]
- Spivey MJ, Grosjean M, Knoblich G. 2005. Continuous attraction toward phonological competitors. Proceedings of the National Academy of Sciences 102(29):10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehovnik EJ, Tolias AS, Slocum WM, Logothetis NK. 2006. Direct and indirect activation of cortical neurons by electrical microstimulation. Journal of Neurophysiology 96(2):512–21 [DOI] [PubMed] [Google Scholar]
- Truccolo W, Eden UT, Fellows MR, Donoghue JP, Brown EN. 2005. A point process framework for relating neural spiking activity to spiking history, neural ensemble, and extrinsic covariate effects. Journal of Neurophysiology 93(2):1074–89 [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Desimone R. 1986. Projections to the superior temporal sulcus from the central and peripheral field representations of V1 and V2. J. Comp. Neurol 248(2): 147–63 [DOI] [PubMed] [Google Scholar]
- Wardak C, Olivier E, Duhamel J-R. 2002. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. Journal of Neuroscience 22(22):9877–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardak C, Olivier E, Duhamel J-R. 2004. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron 42(3):501–8 [DOI] [PubMed] [Google Scholar]
- Wilke M, Kagan I, Andersen RA. 2012. Functional imaging reveals rapid reorganization of cortical activity after parietal inactivation in monkeys. Proceedings of the National Academy of Sciences 109(21):8274–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K-F. 2007. Neural circuit dynamics underlying accumulation of time-varying evidence during perceptual decision making. Front. Comput. Neurosci 1: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH, Sommer MA, Pare M, Ferraina S. 2001. Signal transformations from cerebral cortex to superior colliculus for the generation of saccades. Vision Research 41(25–26):3399–3412 [DOI] [PubMed] [Google Scholar]
- Wyart V, De Gardelle V, Scholl J, Summerfield C. 2012. Rhythmic fluctuations in evidence accumulation during decision making in the human brain. Neuron [DOI] [PMC free article] [PubMed]
- Yang T, Shadlen MN. 2007. Probabilistic reasoning by neurons. Nature 447(7148):1075– 80 [DOI] [PubMed] [Google Scholar]
- Yates JL, Katz L, Park IM, Pillow JW, Huk A. 2014. Choice probabilities and correlations in simultaneously recorded MT and LIP neurons. Presented at Soc. Neurosci, Prog. No. 16.06, Nov. 15, Washington, DC [Google Scholar]
- Yu BM, Cunningham JP, Santhanam G, Ryu SI, Shenoy KV, Sahani M. 2009. Gaussian-process factor analysis for low-dimensional single-trial analysis of neural population activity. J Neurophysiol 102(1):1881–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirnsak M, Chen X, Lomber SG, Moore T. 2015. Effects of reversible inactivation of parietal cortex on the processing of visual salience in the frontal eye field. Presented at Soc. Neurosci., Prog. No. 747.06, Oct. 21, Chicago [Google Scholar]