Abstract
Cancer therapies of the future will rely on synergy between drugs delivered in combination to achieve both maximum efficacy and decreased toxicity. Nanoscale drug delivery vehicles composed of highly tunable nanomaterials (‘nanocarriers’) represent the most promising approach to achieve simultaneous, cell-selective delivery of synergistic ratios of combinations of drugs within solid tumors. Nanocarriers are currently being used to co-encapsulate and deliver synergistic ratios of multiple anticancer drugs to target cells within solid tumors. Investigators exploit the unique environment associated with solid tumors, termed the tumor microenvironment (TME), to make ‘smart’ nanocarriers. These sophisticated nanocarriers exploit the pathological conditions in the TME, thereby creating highly targeted nanocarriers that release their drug payload in a spatially and temporally controlled manner. The translational and commercial potential of nanocarrier-based combinatorial nanomedicines in cancer therapy is now a reality as several companies have initiated human clinical trials.
INTRODUCTION
Despite the development of targeted chemotherapeutic drugs over the past 20 years, cancer remains among the deadliest of human diseases. Cancer is a complex genetic disease that results from normal cells that have undergone malignant mutations, many of which promote immune system evasion, allow for accelerated motility and invasion, and support cell growth and division independent of growth factor signaling.1 Cancerous tumors are genetically heterogeneous, which means that not all cancer cells in a tumor contain the same set of genetic mutations.2 The genetic heterogeneity of cancer cells composing a given tumor remains a significant obstacle in the discovery and development of the next generation of targeted anticancer drugs. In addition, the tumor microenvironment (TME) has recently started to become another major consideration in the creation and testing of novel cancer treatments today.
The TME describes all components of the tumor, which include subpopulations of genetically diverse malignant cells, healthy normal cells, endothelial cells, erythrocytes, leukocytes, and thrombocytes. Many cell types found in the TME ultimately promote cancer progression. In particular, cancer-associated fibroblasts (CAFs), tumor-associated macrophages, and endothelial cells have all been shown to promote tumor growth and aggressiveness in multiple cancer types. Recent advances in our understanding of the TME underscore the importance of expanding our search for drug targets beyond cancer cells to other, non-cancer helper cell types in TME. Indeed, preliminary studies indicate that reprogramming of the TME through nonmalignant cells can prevent and perhaps even reverse tumor growth.3 Targeting stromal tissue in the TME represents an exciting new cancer treatment paradigm.4–10
One of the most efficient ways to target multiple cell types composing a tumor involves administering multiple drugs to the patient. Current cancer treatment strategies are based on the concept that treatment with multiple drugs will lead to the greatest therapeutic benefit in most patients. For example, FOLFOXIRI, a treatment regimen consisting of folinic acid, fluorouracil, irinotecan, and oxaliplatin, is used to treat patients with metastatic colorectal cancer. Treating patients with these drugs in combination results in significantly higher 5-year survival rates compared to treatment with any of these drugs alone. A recent phase-2 clinical trial showed that administration of bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), to patients on either FOLFOXIRI or FOLFIRI (folinic acid, fluorouracil, irinotecan) led to even greater 5-year survival rates compared with FOLFOXIRI or FOLFIRI without bevacizumab.11 VEGF is a potent mitogen that stimulates angiogenesis through its actions on endothelial cells, which lends support to the continued development of combinatorial cancer treatment strategies that target multiple cellular types in the TME. Indeed, empirical evidence in oncology has shown that administration of multiple drugs to patients with mid- to late-stage malignancies is often the preferred treatment strategy. Currently, medical oncologists resort to administering combinations of unencapsulated (‘free’) drugs to their patients. However, pharmacokinetic and physicochemical interactions preclude the administration of certain classes of drugs with each other, which limits the ability of medical oncologists to use drug combinations that could prove beneficial.
Liposomes, as well as other nanoscale drug carriers currently under development, lend themselves well to the controlled intratumoral delivery of multiple drugs with the potential to target both malignant cells and nonmalignant ‘helper’ cells in the TME. The focus of this review is on nanotechnology-based combinatorial drug delivery to solid tumors through the use of liposomes and other drug carriers composed of nanomaterials (‘nanocarriers’), with an emphasis on the major opportunities and challenges associated with developing these sophisticated nanomedicines.
ACHIEVING SYNERGY IN THE TME THROUGH NANOTECHNOLOGY
The Tumor Microenvironment
Prevailing conditions in the TME guide nanocarrier design and development. The TME associated with most solid tumors is both hypoxic and acidic. Solid tumors create networks of leaky blood vessels during their growth. Nanoparticles can pass through abnormally large gaps (up to 1.5 μm) between endothelial cells that compose the leaky tumor vasculature and accumulate within the tumor.12 The phenomenon whereby nanoparticles accumulate in the tumor due to the leakiness of the vasculature is referred to as the enhanced permeability and retention (EPR) effect. One of the hallmarks of cancer is unchecked cellular proliferation,1 a process that demands significantly elevated levels of cellular energy in the form of adenoside triphosphate (ATP). Synthesis of ATP occurs through the Krebs cycle, and relies on adequate intracellular oxygen levels to serve as an electron sink. Despite increased intratumoral angiogenesis, another hallmark of cancer,1 malignant cells in the core of the tumor mass do not receive enough oxygen to efficiently drive the Krebs cycle, which means that these cells become reliant on glycolysis to produce ATP. The phenomenon whereby cancer cells rely on glycolysis instead of the Krebs cycle to produce ATP is known as the Warburg effect. ATP production through glycolysis results in the formation of lactic acid, thereby resulting in the acidification of the TME. Recent nanoparticle formulations are designed to release their drug payload within the tumor as a function of pH.13–15 These stimuli-sensitive nanocarriers have the potential to significantly limit off-target toxicity in cancer patients. The reader is directed to a recent review by Jhaveri et al. that provides an excellent discussion of stimuli-sensitive nanocarriers that can be guided and triggered through internal and external stimuli.16 The complexity of the TME demands similarly complex nanocarriers.
Stromal Reprogramming Through Combination Nanomedicine: A New Cancer Treatment Approach
Stromal cells play a number of critical roles in tumor maintenance and growth, and continuing research into the importance of intercellular crosstalk in the TME has opened the door to the potential of stromal reprogramming as a next-generation cancer treatment strategy. One recent study showed in a mouse model of pancreatic ductal adenocarcinoma that reduction of vitamin D receptor-mediated fibrosis significantly increased the bioavailability and efficacy of gemcitabine: mice treated with both a vitamin D receptor agonist and gemcitabine lived 57% longer than mice treated with gemcitabine alone.17 Scherz-Shouval et al. recently showed that increased nuclear localization of heat shock factor 1 (HSF1) in CAFs in the tumor periphery promoted malignant phenotypes in neighboring tumor cells.18 This group investigated the effects that genetically engineered Hsf1 null mouse embryonic fibroblasts (MEFs) had on cancer cells in vivo and in vitro. MCF7 human breast cancer cells were mixed with either wild-type MEFs or Hsf1 null MEFS and coinjected subcutaneously in non-obese diabetic-severe combined immunodeficiency mice. Tumors established with Hsf1 null MEFs grew significantly more slowly compared with tumors established with wild-type MEFs.18 To study the effects of stromal HSF1 on cancer cells in vitro, D2A1 mouse mammary tumor cells stably expressing dsRed were seeded on top of a layer of Hsf1 null MEFs. Compared with D2A1 cells seeded on top of wild-type MEFs, D2A1 cells co-cultured with Hsf1 null MEFs accumulated significantly less.18 These investigators further reported that Hsf1 null MEFs expressed lower levels of Tgf-β1, Tgf-β 2, Tgf-β 3, and Sdf1 compared to those of control cells. Adding Transforming growth factor beta (TGFβ) and SDF1 to D2A1, cells co-cultured with Hsf1 null MEFs reversed the inhibition of D2A1 accumulation. These studies show the importance of assessing non-cancer cells in the TME as targets for future combinatorial nanomedicines.
TGFβ family proteins are soluble factors in the TME that act on cancer cells and fibroblasts alike. Cancer cells and healthy normal fibroblasts secrete TGFβ. In healthy normal cells, TGFβ causes cell cycle arrest in G1, which stops cellular proliferation. However, many cancer cells become unresponsive to the anti-proliferative effects of TGFβ. Cancer cells exhibit uncontrolled proliferation, and continue to secrete TGFβ. Activation of TGFβ receptors on neighboring fibroblasts in the TME triggers these cells to proliferate, as well as generate fibroplasia.19 In addition, TGFβ exerts inhibitory effects on immune cells, which results in an overall blunted immune response and permissive conditions for tumor growth. Some investigators are currently developing combinatorial nanocarriers to exploit TGFβ-mediated tumor growth in the TME. Park et al. have recently demonstrated the value of nanoscale combinatorial treatment approaches in interrupting TGFβ-mediated feed forward mechanisms between tumor cells and immune cells in the TME. This group engineered biodegradable nanoscale liposomal polymeric gels to simultaneously release SB505124, a small molecule inhibitor of the TGFβ1 receptor, as well as IL-2 within the TME.20
Briefly, subcutaneous tumors were established in B6 albino mice with the B16-F10 murine melanoma cell line. Mice injected with the combinatorial nanocarrier showed significantly reduced tumor growth, increased survival, and enhanced immune system activation in the tumor, as evidenced by increased tumor-specific CD8+ T cell infiltration.20 By contrast, the effects of these polymeric liposomal gels loaded with either SB505124 or IL-2 on tumor growth, survival, and enhanced immune system activation were not as dramatic. A schematic representation of this type of combinatorial nanoparticle can be seen in Figure 1(a).
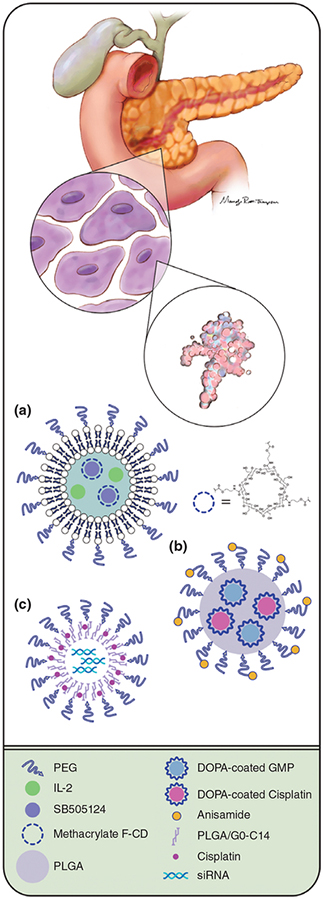
FIGURE 1 |.
Nanocarriers for combination drug delivery. The anatomical drawing in the top half of the figure shows three targeting considerations used in nanocarrier design: tissue-, cell-, and macromolecule-level targeting. The bottom half of the figure depicts three novel nanocarriers designed to deliver multiple therapeutic agents to the tumor utilizing one or more of these targeting approaches. Nanocarrier (a) shows a biodegradable nanoscale liposomal polymeric gel engineered to release SB505124, a small molecule inhibitor of the TGFβ1 receptor, as well as IL-2 within the tumor.20 Nanocarrier (b) depicts a particle-within-a-particle encapsulation strategy, whereby dioleoyl phosphatidic acid-coated drug cores were created out of gemcitabine monophosphate (GMP) and cisplatin. GMP and cisplatin drug cores were subsequently loaded into PLGA nanoparticles to generate dual-loaded nanocarriers.21 Of the three nanocarriers depicted here, this nanocarrier is the only one that uses an active (cell-level) targeting approach: Anisamide was attached to the outside of these nanoparticles to target sigma receptor-overexpressing cancer cells. Nanocarrier (c) shows PLGA-PEG/G0-C14 nanoparticles with siRNA molecules contained in their core. A pro-drug form of cisplatin was embedded in the polymeric nanoparticle shell.22
Future combinatorial therapies will increasingly utilize nanoscale drug delivery platforms to release combinations of drugs that act on cancer cells as well as on non-cancer ‘helper’ cells in the TME to inhibit or even reverse tumor growth. As an example of a combinatorial nanotechnology strategy that targets the TME, Liao et al. reported that treatment with their novel, triterpenoid-loaded targeted nanoparticle significantly inhibited STAT3 activation in the tumor, while simultaneously priming the TME for enhanced immune surveillance by activated CD8+ T cells.23 Additionally, Yokoi et al. recently reported that they were able to achieve significant targeting of tumor-associated endothelial cells in mice using porous silica nanoparticles conjugated to antibodies against Ly6C, the mouse homolog of CD59.24
Nanocarriers as a Solution to Complications Arising from Co-delivery of Free Drugs
Most research published to date on nanoparticle-mediated cancer treatments involves the use of a single encapsulated drug. However, nanocarriers are uniquely suited to deliver multiple drugs simultaneously to target cells in specific tissues. Dose timing is a critical factor in the administration of multiple drugs, as simultaneous drug delivery can result in synergy, antagonism, or neither synergy nor antagonism. For example, Abraham et al. reported significant antagonism between doxorubicin and vincristine in mice dosed with combination liposomes co-loaded with both drugs.25 A recent study by Morton et al. underscores the importance of sequential drug release to maximize tumor cell killing and growth inhibition in mice.26 Riviere et al. showed that combination fluoroorotic acid and irinotecan liposomes were more efficacious at preventing tumor growth in the C26 mouse model than fluoroorotic acid-containing liposomes administered with irinotecan-containing liposomes in the same ratio.27 The most promising way to deliver combinations of widely different classes of drugs to target cells in a patient with high spatial and temporal control is through the use of nanocarriers.
As a result of the tunability of newer generations of nanomaterials, many research groups are designing nanoscale drug carrier platforms whereby multiple drugs can be loaded into or onto the same nanocarrier and delivered simultaneously or sequentially to the target site. Spatial and temporal control of combination drug delivery represents a promising strategy to attack tumors in a way that prevents or significantly slows the development of multidrug resistant phenotypes. Prasad et al. reported that simultaneous delivery of doxorubicin and mitomycin C co-encapsulated in polymer-lipid hybrid nanoparticles increased efficacy and reduced cardiotoxicity in athymic nude mice bearing multi-drug resistant human mammary tumor xenografts.28 Subsequent studies in immunocompetent mice inoculated intramuscularly with either wild-type or doxorubicin-resistant mouse mammary sarcoma EMT6 cells and treated with these hybrid nanoparticles co-loaded with doxorubicin and mitomycin C indicated that both drugs exhibited synergy in overcoming multi-drug resistance in this model.29
Drug Synergy
Combinations of drugs that synergize with each other have the potential to show the greatest therapeutic efficacy, while minimizing toxicity. Synergy refers to the phenomenon whereby the effect of two drugs when administered together is greater than the effect of either drug alone. Accurately quantifying synergy can be problematic. The reader is directed to Chou’s review on the subject, which provides guidelines for assessing drug synergy as well as an in-depth discussion of drug synergy.30 While identifying combinations of drugs that synergize with each other in vitro is possible, a significant challenge involves determining which drugs are most likely to synergize with each other in vivo, and the doses at which those drugs synergize with each other in vivo. Because drug synergy is a function of the dose ratio of one drug to another, maintaining the doses at which the drugs exhibit synergy following administration represents another major challenge. One group at the National Center for Advancing Translational Sciences in the United States recently devised an unbiased, high-throughput in vitro assay to measure the synergy between 459 agents and ibrutinib, a small molecule inhibitor of Bruton’s tyrosine kinase.31 The assay utilized 1536-well cell culture plates, as well as acoustic dispensers for the high-throughput dispensation of drug solutions at the nanoliter scale. Within each 1536-well plate, the investigators were able to measure the cytotoxic effects of hundreds of different drugs in combination with ibrutinib. This particular high-throughput approach is advantageous because it allows for the rapid discovery of drug concentrations that show synergy in vitro. Unbiased high-throughput approaches such as this can help investigators find promising drug combinations and synergistic dose ratios early in the development of potential combination nanotherapies.
Traditional high-throughput cell-based anti-cancer drug screens rely on monolayers of cultured cancer cells to determine the effects of experimental chemical compounds on cells. However, not every drug combination that demonstrates synergy in vitro will show synergy in vivo, because therapeutic synergy between two drugs could result from the effects of the drugs on more than one cell type in the TME. At present, one of the most promising approaches to assess the effects of more than one drug on more than one cell type in vitro in a systematic, high-throughput fashion involves the use of multicellular three-dimensional spheroids. One group reported development of a method to grow tumor cells and endothelial cells together in three-dimensional spheroids suspended in a hanging drop.32 This group transferred tumor-endothelial spheroids composed of Taxol-resistant mouse 4T1 metastatic mammary epithelial cells and SV40-transformed mouse 2H11 endothelial cells to 96-well plates, treated them with Taxol, and assayed for cell death. The endothelial cells were shown to sensitize tumor cells to Taxol treatment; tumor cells grown alone in spheroids showed resistance to Taxol treatment, whereas tumor cells grown in the presence of endothelial cells underwent Taxol-dependent cell death.32 These in vitro cell-culture systems that mimic the TME have the potential to reveal drug synergies that would remain hidden in assays utilizing only one cell type.
Classes of Nanocarriers Used in Combinatorial Drug Delivery
This review focuses on liposomes as nanocarriers for combinatorial drug delivery due to the extensive work done so far to encapsulate combinations of drugs within liposomes, relative to other classes of nanocarriers. Although this review focuses on combinatorial nanocarriers used for treating cancer, multiple investigators33,34 are currently developing combination nanocarriers loaded with agents for preventing cancer. Of all the nanocarriers mentioned in this review, liposomes are currently the only nanocarrier used in nanocarrier-based drug formulations approved by the Food and Drug Administration (FDA) for marketing in the United States. There are currently 12 liposome-based drugs on the market in the United States, as outlined by Chang.35 Liposomes are nanoparticles made up of lipids arranged in a spherical bilayer. These hollow, lipid-based vesicles have an aqueous core, and are usually smaller than 100 nm in diameter. Liposomes are commonly formed through extrusion, a standard liposome production method that involves forcing lipids through a porous membrane several times in quick succession. A wide variety of drugs have been encapsulated in liposomes, and the lipid composition of liposomes can be altered to increase the encapsulation efficiency of different drugs. Liposomes can also be tuned to release their cargo in response to various stimuli, including acidic pH36–45 or hyperthermia.36,46–52 Encapsulation of toxic drugs into liposomes can significantly reduce off-target toxicity, as well as increase the half-life of drugs in the body.53 For example, doxorubicin is a highly efficacious antineoplastic drug used to treat solid tumors, but it is associated with severe, dose-limiting cardiac toxicity.54,55 Liposomal doxorubicin causes less toxicity with equivalent efficacy relative to the free drug.56,57 Several different research groups have successfully loaded more than one drug into liposomes (Table 1).
TABLE 1.
Combination Nanocarriers
| Drug combination | Liposomal nanocarrier | Author | Year | Significance | Mechanism |
|---|---|---|---|---|---|
| Paclitaxel + Epigallocatechin58 | PC:Cholesterol | Ramadass, S. | 2015 | Compared to treatment with either PTX or EGCG alone, PTX/EGCG combination treatment significantly reduced cellular viability, increased apoptosis, and decreased expression of MMP-2 and MMP-9 in MDA-MB-231 cells in vitro. | PTX inhibits the cell cycle through microtubule stabilization; EGCG inhibits matrix metalloproteinases. |
| Cytarabine + Daunorubicin59 | CPX-351 (5–20 mol% Cholesterol) | Lancet, J. | 2014 | Increased response rate (complete + incomplete remissions) by 15.5% in patients with AML. Provided rationale for a phase 3 clinical trial. | Nanoliposomal encapsulation allows the optimal molar ratio of drugs (5:1) to be maintained during delivery. |
| Doxorubicin + Omacetaxine Mepesuccinate60 | PG:PC:Cholesterol | Shim, G. | 2014 | Compared to untreated mice, the combination liposome resulted in a 98.5% reduction in tumor volume on day 35 and a 97.3% reduction on day 45 after treatment. | OMT decreases MCL1 levels, DOX inhibits topoisomerase II. MCL1 is an anti-apoptotic protein that, when inhibited in combination with DOX, has been shown to increase anti-cancer effects |
| Doxorubicin + Topotecan61 | DSPC:Cholesterol | Patankar, N. | 2013 | Mean survival time of mice receiving the combination therapy increased from 18 days (untreated) or 40 days (Topotecan) to 52 days. | Topotecan inhibits topoisomerase I, DOX inhibits topoisomerase II. |
| Irinotecan + Doxorubicin62 | DSPC:Cholesterol | Shaikh, I. | 2013 | Combo treatment resulted in a mean survival time of 52 days in ovarian tumor-bearing SCID mice, compared to 27 days with saline treatment. Encapsulation increased the mean residence time in the plasma 27-fold (DOX) or 28-fold (irinotecan). | Allowed the synergistic molar ratio of 1:1 to be kept during delivery. Used Mn2+ and pH gradients to load both drugs and reported >80% loading efficiency. |
| Irinotecan + Fluoroorotic Acid27 | DSPC:Cholesterol: mPEG-DSPE | Reviere, K. | 2011 | Combo treatment provided statistically significant differences in antitumor effects in vivo than single drug-loaded liposomes. | Irinotecan inhibits topoisomerase I, fluoroorotic acid inhibits DNA and RNA synthesis via inhibition of thymidylate synthase. |
| Irinotecan + Floxuridine63 | CPX-1 (5–20 mol% Cholesterol) | Batist, G. | 2009 | Phase I study of liposome combination therapy, ‘first clinical evaluation of fixed drug ratio dosing designed to maintain synergistic molar ratios for enhanced therapeutic benefit’. | Irinotecan and floxuridine are standard combination chemotherapies. Floxuridine inhibits DNA and RNA synthesis via inhibition of thymidylate synthase, irinotecan inhibits topoisomerase I. |
| C6 Ceramide + Sorafenib64 | C6 ceramide- containing liposome | Tran, M. | 2008 | A 30% increase in tumor inhibition in vivo (compared to sorafenib alone) and a 58% increase in tumor inhibition (compared to ceramide nanoliposome alone) occurred. Provided foundation for clinical trials. | Treatment with the combo liposome synergistically inhibited cultured cells by cooperatively targeting mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling |
Determination of suitable nanomaterials for combinations of synergistic drugs remains a critical challenge in the development of novel combination nanocarriers. Because target cells can internalize nanocarriers through mechanisms including endocytosis, encapsulation of multiple drugs within a single nanocarrier can minimize pharmacokinetic complications that arise with the simultaneous administration of multiple drugs. Therefore, novel combination nanocarriers should be developed with appropriate potential nanomaterials taken into careful consideration. Multiple factors influence the encapsulation efficiency of drugs in unexpected ways. For example, Mohan et al. indicated that addition of polyethylene glycol to their liposome formulation significantly reduced the encapsulation of resveratrol in these liposomes.65 Encapsulation efficiency, frequently expressed as a percentage, is a measure of how much of a drug becomes encapsulated by, or attached to, a nanocarrier. Co-encapsulation of drugs that differ from each other significantly with respect to their physicochemical properties can be especially difficult. Using a variety of diverse nanomaterials to their advantage, investigators are designing nanocarrier platforms for the efficient loading of combinations of diverse drugs into the same nanocarrier. These versatile drug nanocarriers can be loaded with controlled ratios of combinations of drugs. Recent successes provide promising ways to co-encapsulate and deliver controlled ratios of drugs to targets in the TME. However, caution is still warranted, as surface decoration of drug on the outside of the nanocarrier versus true encapsulation within nanocarriers does not protect the drug from unwarranted metabolism or immunogenicity.
A one-size-fits-all nanocarrier does not exist, and finding the appropriate nanocarrier still often comes down to the properties of the drugs to be encapsulated. There are a wide variety of nanocarriers under development today. These nanocarriers include liposomes, carbon nanotubes, gold nanoparticles, dendrimers, silica nanoparticles, iron oxide nanoparticles, nanoemulsions, poly(lactic-co-glycolic acid), albumin nanoparticles, DNA block copolymers, and also hybrid nanocarriers composed of two or more classes of the nanocarriers mentioned above. The National Cancer Institute’s Nanotechnology Characterization Laboratory has carried out extensive testing on nanocarriers from each class of nanocarrier mentioned here, and has outlined common pitfalls in nanocarrier development.66 The reader is also directed to another review67 that highlights safety concerns in nanocarrier development.
Next Generation Lipid-based Nanocarriers
Many drugs have physicochemical properties that limit their efficient encapsulation inside nanoscale drug carriers. Therefore, one strategy involves creating nanocarriers out of drugs using bioactive materials. One such drug is cisplatin, a highly efficacious antineoplastic agent that is poorly soluble in oil and water. A group of researchers worked around the poor solubility of cisplatin to create lipid-coated cisplatin nanoparticles that were found to have a relatively high drug-loading capacity (approximately 80% by weight).68 Whereas cisplatin is a small molecule that binds to DNA, C6 ceramide (C6) is a non-endogenous, short-chain bioactive sphingolipid that selectively induces apoptosis in cancer cells. Long-chain endogenous ceramides play an important structural role in biological membranes. Because of the structural similarities C6 shares with long-chain ceramides, C6 is easily incorporated into the lipid bilayer of liposomes during extrusion. C6 becomes a structural part of the liposomes, and confers pro-apoptotic activity on the liposomes themselves. One group incorporated C6 and paclitaxel into nonliposomal polymer-based hybrid nanoparticles.69 These C6/paclitaxel hybrid nanoparticles were engineered to temporally deliver both drugs in a sequential manner within the tumor. Delivered intravenously to athymic nude mice bearing orthotopic human breast adeno-carcinoma MCF7 or MCF7TR (a multi-drug resistant cell line) tumors, these nanoparticles increased levels of paclixel in the blood and tumors.
Creation of nanocarriers out of bioactive nano-materials minimizes complications that arise when multiple drugs are loaded into the same nanocarrier. C6-liposomes can be used as a platform to quickly assess whether a drug synergizes with C6. Once successfully loaded into C6-liposomes, the synergy of the drug with C6 can rapidly be measured in vitro. To date, the following drugs have been encapsulated in C6 liposomes: sorafenib,64 gemcitabine,70 the glucosylceramide synthase inhibitor D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP),70 curcumin,71 and doxorubicin.72 Based upon documented synergy between the C6-nanoliposome and vinblastine,73 a combinatorial vinblastine-ceramide nanoliposome has been engineered and has shown in vivo efficacy in models of solid and non-solid cancers (unpublished data, MK). It has been speculated that the effects of vinca alkaloids upon microtubules shunts ceramide-induced autophagy into ceramide-induced autophagic cell death.73 As another example of combinatorial drug delivery through nanotechnology, sorafenib-loaded C6 liposomes synergistically reduced viability of melanoma and breast cancer cells in vitro.64 Synergy between sorafenib and C6 was also observed in vivo: sorafenib-loaded C6 liposomes synergistically inhibited tumor growth in xenograft studies on athymic nude mice bearing subcutaneous melanoma and breast cancer tumors.64 The C6-ceramide nanolipo-some platform was previously physicochemically and pharmacologically characterized by the Nanotechnology Characterization Laboratory of the National Cancer Institute and shown to be non-toxic, while delivering bioactive ceramide to target cells through intrabilayer transport mechanisms.74 The C6-ceramide nanoliposome platform has been licensed by Penn State Research Foundation to Keystone Nano, Inc., who have recently scaled up production, completed preclinical testing, and are scheduled to enter the clinic with the C6-ceramide nanoliposome in the fall of 2015.
Two materials commonly used in drug encapsulation and delivery platforms are polyethylene glycol (PEG) and poly(lactic-co-glycolic acid) (PLGA). The FDA has approved both PEG and PLGA for marketing in the United States, and both are generally recognized as safe. The covalent attachment of PEG chains to small molecules or proteins is known as ‘PEGylation’, a technique developed in the 1970s.75 The FDA approved the first PEGylated drug in 1990, and has approved several more since then. PEG increases the half-life of a drug in the blood by preventing the drug from being quickly recognized and destroyed by the immune system. Many nanoscale drug encapsulation and delivery systems utilize PEG to increase the half-life of the nanocarriers in the blood. A recent study described methoxy PEG–PLGA hybrid nanocarriers that were used to co-encapsulate doxorubicin and paclitaxel using a modified double emulsion method (water/oil/water).76 This study is important because it describes how doxorubicin, a hydrophilic drug, was co-encapsulated with paclitaxel, a hydrophobic drug. These dual-loaded PEG–PLGA nanoparticles were shown to deliver both drugs simultaneously to target cells in vitro. In addition, doxorubicin and paclitaxel, when co-encapsulated in PEG–PLGA nanoparticles, showed synergy in cell-based cytotoxicity assays carried out on A549, HepG2, and B16 human cancer cells.76 Although these researchers were able to co-encapsulate two drugs in their nanocarrier, there is little evidence to suggest that they were able to precisely control drug loading. This discrepancy brings up critical questions regarding control of drug loading, and how proper dosing of drugs can be achieved, should they become encapsulated with different efficiencies. Refinement of ratiometric control of drug loading in nanoparticles should provide a means by which correct therapeutic dosing can be achieved and maintained in vivo.
Ratiometric control will be a necessary feature of next generation combination nanocarrier platforms in the future. A current strategy to achieve ratiometric control of drug loading involves encapsulating each drug into separate nanoparticles, and then loading the singly loaded nanoparticles into larger nanoparticles.21 Using this particle-within-a-particle encapsulation strategy, dioleoyl phosphatidic acid-coated drug cores were created out of gemcitabine monophosphate and cisplatin. Gemcitabine monophosphate and cisplatin drug cores were subsequently loaded into PLGA nanoparticles to generate dual-loaded PLGA nanoparticles. A schematic representation of this type of combination nanoparticle can be seen in Figure 1(b). Both gemcitabine monophosphate and cisplatin are exceedingly difficult to encapsulate in PLGA nanoparticles due to their hydrophilicity. For this reason, one of the major highlights of this research was the successful co-encapsulation of gemcitabine monophosphate and cisplatin within PLGA nanoparticles. Most important, however, was the discovery that this method could be used to successfully co-encapsulate known molar ratios of gemcitabine monophosphate with cisplatin inside PLGA nanoparticles. These nanoparticles synergistically inhibited growth of subcutaneous stroma-rich bladder cancer tumors in athymic nude mice.21 What is most exciting about this particle-within-a-particle platform is that it can be used for the co-encapsulation of known ratios of drugs that have disparate physicochemical properties.
Targeted Nanocarriers
Selective targeting of drug-loaded nanoparticles to cells within the tumor remains a challenge in the development of next-generation nanocarriers. While the EPR effect is a form of passive targeting that causes nanoparticles to accumulate in the tumor in a non-specific manner, active targeting is a strategy that could further potentially minimize toxicity and off-target effects, as well as ensure delivery of therapeutic drug doses to target cells. Several strategies exist to target nanoparticles to specific tissues or areas in the body. One of the most promising targeting methods involves the covalent attachment of protein- or nucleic-acid–based targeting motifs to the nanoparticle surface. Two of the most common classes of macromolecule used to decorate the outside of nanoparticles are antibodies and aptamers. Through interactions between their surface exposed targeting motifs and corresponding targets on the surface of target cells, decorated nanoparticles are designed to accumulate in target tissues in a highly selective manner. These targeting molecules are coupled to nanoliposomes through a variety of methods depending on their size, charge, and intended destination. The most common is by thiolation of the primary amine (−NH2) on an antibody to create sulfhydryl groups (−SH) that can then react with maleimide groups incorporated into the liposomal PEG composition. Active targeting approaches can change the biodistribution of nanoparticles in animals, as shown by Qian et al.77 This group designed PEGylated surface-enhanced Raman scattering colloidal gold nanoparticles that they targeted to tumor cells through surfaces-exposed single-chain variable fragment (ScFv) antibodies. The ScFv antibodies that decorated the outside of these nanoparticles were selective for human epidermal growth factor receptor. When injected intraveneously in athymic nude mice bearing human head-and-neck squamous cell carcinoma (Tu686) xenograft tumors, the targeted particles showed greater accumulation in the tumor compared to the untargeted particles 5 h following injection.77 Another group reported that their actively targeted immunoliposomes conjugated to anti-HER2 ScFv antibodies appeared to have a greater effect on nanoparticle internalization, not biodistribution.78 Additional research will be required to refine and enhance selective delivery of drugs to target cells within the tumor, which will be a major accomplishment in the development of the nanotechnology-mediated, smarter drug delivery platforms of the future.
Molecular-based Nanotherapies
In this review, co-delivery of pharmaceutical therapeutic agents to cells in the TME refers to the simultaneous delivery of more than one drug within the tumor to target one or more cell types. In the field of nanocarrier-mediated drug delivery, most attempts at encapsulation and delivery of drug combinations involve small molecule inhibitors. However, recent advances in nanocarrier design have allowed for the more efficient encapsulation and delivery of cutting edge molecular-based therapeutic agents such as nucleic acids. RNA interference (RNAi) of mutant genes in cancer represents a promising treatment strategy. Researchers have encapsulated small interfering RNAs (siRNAs) into the following nanocarriers: PLGA-PEG/G0-C14 hybrid nanoparticles,22 iRGD peptide-conjugated d-α-tocopheryl PEG 1000 succinate micelles,79 chitosan-based nanoparticles,80 and PEGylated liposomes.81 One reason siRNA-based anticancer strategies are so attractive is their specificity; mutant genes necessary for cancer cell survival can be knocked down, thereby killing malignant cells while sparing healthy normal cells. However, due to their size and net negative charge, unencapsulated siRNAs cannot be administered to patients. Size and charge are two major physicochemical concerns associated with siRNA pharmacokinetics that make encapsulation and delivery of siRNA therapeutics a formidable challenge. Co-encapsulation of siRNA molecules with cisplatin or other DNA crosslinkers presents its own set of challenges, as these drugs could potentially crosslink siRNA molecules. One group successfully encapsulated siRNA against survivin with cisplatin in hyaluronic acid-based nanocarriers.82 These nanocarriers also contained indocyanine green (ICG), a dye that enabled these investigators to assess the nanocarriers’ biodistribution in athymic nude mice bearing subcutaneous tumors. Following a sequestration strategy, Xu et al. used PLGA-PEG/G0-C14 to make nanoparticles that contained siRNA molecules in their core, thereby effectively shielding the siRNAs from a pro-drug form of cisplatin, which was embedded in the polymeric nanoparticle shell.22 These authors reported that these small molecule/siRNA hybrid nanoparticles reduced REV1 and REV3L gene expression in vitro, as well as synergistically suppressed tumor growth in a xenograft mouse model utilizing human Lymph Node Carcinoma of the Prostate (LNCaP) cells.22 A schematic representation of this type of combination nanoparticle can be seen in Figure 1(c).
COMMERCIALIZATION OF COMBINATORIAL NANOMEDICINES
Much work has yet to be done on identifying synergistic drug combinations and dose ratios, developing high-throughput platforms to match drug combinations with compatible nanocarriers, enhancing targeting of drug-loaded nanocarriers, and identifying and validating different populations of ‘helper’ cells in the TME that can be targeted to enhance tumor killing. Despite the many challenges that exist in the field of nanocarrier-mediated combination drug delivery, several companies were founded to translate combination nanomedicines from the laboratory bench to the clinic. BIND Therapeutics, a company headquartered in Massachusetts, USA, synthesizes and validates made-to-order polymeric nanoparticles (Accurins™) for the encapsulation and cell-specific delivery of a wide variety of drugs.
BIND utilizes a high-throughput approach to generate libraries of candidate Accurins™, which are then screened based on their effects on cells in vitro, and tested to ensure good pharmacokinetics, tolerability, biodistribution, and targeting. Accurins™ accumulate in the tumor and preferentially interact with target cells through their proprietary, surface-exposed targeting motifs, which in turn triggers release of the drug payload. Together, this “triple targeting” approach (tissue- cell- and molecule-specific targeting) improves site-specific drug delivery, thereby reducing the dose needed to achieve therapeutic efficacy and reducing toxicity. The polymers used to make Accurins™ are broken down to lactic acid in the body, thereby preventing additional toxicity associated with the particles themselves.
Celator Pharmaceuticals, a clinical-stage company based in New Jersey, USA, has developed proprietary methods to co-encapsulate known ratios of drugs into lipid-based nanocarriers. This company carried out an open-label, single-arm, dose-escalating phase I study with their CPX-1 combination liposomes, which contain controlled ratios of irinotecan HCl and floxuridine for the treatment of advanced solid tumors.63 Prior to this phase I study, Celator published proof-of-concept data supporting their rationale for loading synergistic ratios of drugs into a single nanocarrier.83,84
Celator’s phase I study utilized CPX-1 liposomes containing 1:1 molar ratios of irinotecan HCl to floxuridine to treat patients with advanced solid tumors. Irinotecan is a small molecule inhibitor of the DNA-unwinding enzyme topoisomerase I that is widely used in the treatment of colon cancer, usually in combination with other drugs. Floxuridine is a small molecule antimetabolite that is also widely used to treat colon cancer. Of the 33 patients enrolled in this study, 15 had advanced colorectal tumors. Every patient enrolled in this study had previously been treated with either oxaliplatin or irinotecan. Patients were infused with CPX-1 over 90 min every 14 days in 28-day cycles, and 30 patients were evaluated for response. Celator reported the maximum tolerated dose of CPX-1 to be 210 units/m,2 where 1 unit is equal to 1 mg of irinotecan HCl and 0.36 mg floxuridine. Analysis of irinotecan and floxuridine levels in patient plasma showed that a 1:1 molar ratio of irinotecan to floxuridine was maintained for at least 8 h following administration of 210 units/m2 CPX-1. After 48 h, the ratio of irinotecan to floxuridine in the plasma from these patients had increased approximately ninefold. Toxicity data gathered during this study indicate that CPX-1 treatment can be less toxic compared to treatment with either irinotecan or floxuridine alone.
A critical side effect in patients taking either irinotecan or floxuridine is severe, dose-limiting gastrointestinal toxicity, which manifests itself as diarrhea.85,86 The majority of patients treated with irinotecan or floxuridine experience gastrointestinal toxicity to some degree, which often precludes the use of higher doses of these drugs. In fact, nearly 25% of the patients enrolled in the CPX-1 phase I clinical trial developed gastrointestinal toxicity, and one patient died during the course of the study due to CPX-1-induced toxicity. Following this study, Celator reported that 15% of the patients receiving 210 units/m2 of CPX-1 developed grade 3 diarrhea during the course of the study. Celator cited other studies87–90 in which the incidence of diarrhea in patients treated with irinotecan or fluorouracil was anywhere from 14 to 44.4%. This comparison indicates that CPX-1 treatment causes similar levels of toxicity compared to treatments with each of these drugs alone. Of the 15 colorectal cancer patients who took part in this study, 2 achieved partial remission, 9 achieved stable disease, 2 had progressive disease, and 2 were not evaluable at the end of the study. Celator’s phase I study should help guide the development and testing of safer, more efficacious nanomedicines in the future.
Theranostics
Creation of drug nanocarriers with materials that allow them to be traced in vivo represents a promising way to assess nanoparticle biodistribution under physiologically relevant conditions. The intersection of nanomaterial design and intravital imaging has led to the advent of ‘theranostics’, which describes any drug delivery system that has dual therapeutic and diagnostic properties. Zevalin, a radioimmunotherapy approved by the FDA in 2002 for the treatment of low-grade or transformed B-cell non-Hodgkin’s lymphoma, is considered to be one of the first theranostic therapies ever created.67 This theranostic treatment involves the use of a monoclonal antibody against CD20 that is covalently conjugated to an agent that chelates yttrium-90, a radioactive isotope. The specificity of the antibody is exploited to deliver toxic doses of radiation to CD20-positive target cells. Another form of the drug uses indium-111 instead of yttrium-90 for imaging purposes to ensure high selectivity prior to administration of the antibody complex containing yttrium-90. The development of radionanomedicine-based theranostic cancer nanomedicines that can be traced in the body will allow investigators to achieve greater tissue- and cell-specific targeting.91 Iyer et al. have also reviewed image-guided theranostic nanosystems for targeted delivery of drugs in cancer.92
CONCLUSION
The administration of combinations of drugs that synergize with each other represents a promising cancer treatment approach. The goal of administering drug combinations that exhibit synergy is to reduce the effective dose of each drug in the combination, thereby achieving a therapeutic effect while minimizing dose-limiting toxicity. The simultaneous delivery of synergistic drug combinations to target cells in the TME represents a promising cancer-treatment paradigm. However, pharmacokinetic challenges prevent the site-specific, controlled delivery of combinations of drugs to target cells. Nanotechnology is well suited to meet these challenges, and many groups of investigators have reported development of novel nanomaterials to achieve the simultaneous, controlled release of synergistic combinations of drugs to target cells. To summarize, the most significant challenges in the development of combinatorial nanomedicines include: (1) identification of drugs that will synergize with each other in vitro, (2) development of accurate methods to predict whether combinations of drugs that show synergy in vitro will show synergy in vivo, (3) further optimization of nanocarriers for enhanced ratiometric control of drug loading, and (4) improved targeting of drug-loaded nanocarriers to reduce toxicity through improved biodistribution and/or enhanced uptake by target cells. The common thread linking each of these challenges is patient safety, which must inform each step in the development of a combinatorial nanomedicine as it makes its way from the laboratory bench to the clinic.
Footnotes
Conflict of interest: MK is co-founder and Chief Medical Officer of Keystone Nano. Penn State Research Foundation has licensed ceramide nanotechnology to Keystone Nano, Inc. (PA). The other authors have declared no conflicts of interest for this article.
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011, 144:646–674. [DOI] [PubMed] [Google Scholar]
- 2.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013, 19:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reisfeld RA. The tumor microenvironment: a target for combination therapy of breast cancer. Crit Rev Oncog 2013, 18:115–133. [DOI] [PubMed] [Google Scholar]
- 5.Neesse A, Krug S, Gress TM, Tuveson DA, Michl P. Emerging concepts in pancreatic cancer medicine: targeting the tumor stroma. Onco Targets Ther 2013, 7:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, Antzis M, Cotner CE, Johnson LA, Durham AC, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res 2014, 2:154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko SY, Naora H. Therapeutic strategies for targeting the ovarian tumor stroma. World J Clin Cases 2014, 2:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liss AS, Thayer SP. Therapeutic targeting of pancreatic stroma In: Grippo PJ, Munshi HG, eds. Pancreatic Cancer and Tumor Microenvironment. Trivandrum: Transworld Research Network; 2012. Chapter 9. Available from: http://www.ncbi.nlm.nih.gov/books/NBK98931/ [PubMed] [Google Scholar]
- 9.Hiscox S, Barrett-Lee P, Nicholson RI. Therapeutic targeting of tumor-stroma interactions. Expert Opin Ther Targets 2011, 15:609–621. [DOI] [PubMed] [Google Scholar]
- 10.Franco OE, Hayward SW. Targeting the tumor stroma as a novel therapeutic approach for prostate cancer. Adv Pharmacol 2012, 65:267–313. [DOI] [PubMed] [Google Scholar]
- 11.Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014, 371:1609–1618. [DOI] [PubMed] [Google Scholar]
- 12.Maeda H, Matsumura Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit Rev Ther Drug Carrier Syst 1989, 6:193–210. [PubMed] [Google Scholar]
- 13.Liu J, Huang Y, Kumar A, Tan A, Jin S, Mozhi A, Liang XJ. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv 2014, 32:693–710. [DOI] [PubMed] [Google Scholar]
- 14.Lee ES, Gao Z, Bae YH. Recent progress in tumor pH targeting nanotechnology. J Control Release 2008, 132:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Y, Tang H, Radosz M, Van Kirk E, Murdoch WJ. pH-responsive nanoparticles for cancer drug delivery. Methods Mol Biol 2008, 437:183–216. [DOI] [PubMed] [Google Scholar]
- 16.Jhaveri A, Deshpande P, Torchilin V. Stimuli-sensitive nanopreparations for combination cancer therapy. J Control Release 2014, 190:352–370. [DOI] [PubMed] [Google Scholar]
- 17.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherz-Shouval R, Santagata S, Mendillo ML, Sholl LM, Ben-Aharon I, Beck AH, Dias-Santagata D, Koeva M, Stemmer SM, Whitesell L, et al. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell 2014, 158:564–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark RA, McCoy GA, Folkvord JM, McPherson JM. TGF-beta 1 stimulates cultured human fibrob-lasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J Cell Physiol 1997, 170:69–80. [DOI] [PubMed] [Google Scholar]
- 20.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, et al. Combination delivery of TGF-beta inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater 2012, 11:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao L, Guo S, Zhang J, Kim WY, Huang L. Nanoparticles with precise ratiometric co-loading and co-delivery of gemcitabine monophosphate and cisplatin for treatment of bladder cancer. Adv Funct Mater 2014, 24:6601–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Xie K, Zhang XQ, Pridgen EM, Park GY, Cui DS, Shi J, Wu J, Kantoff PW, Lippard SJ, et al. Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug. Proc Natl Acad Sci USA 2013, 110: 18638–18643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao D, Liu Z, Wrasidlo WJ, Luo Y, Nguyen G, Chen T, Xiang R, Reisfeld RA. Targeted therapeutic remodeling of the tumor microenvironment improves an HER-2 DNA vaccine and prevents recurrence in a murine breast cancer model. Cancer Res 2011, 71:5688–5696. [DOI] [PubMed] [Google Scholar]
- 24.Yokoi K, Godin B, Oborn CJ, Alexander JF, Liu X, Fidler IJ, Ferrari M. Porous silicon nanocarriers for dual targeting tumor associated endothelial cells and macrophages in stroma of orthotopic human pancreatic cancers. Cancer Lett 2013, 334:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham SA, McKenzie C, Masin D, Ng R, Harasym TO, Mayer LD, Bally MB. In vitro and in vivo characterization of doxorubicin and vincristine coencapsulated within liposomes through use of transition metal ion complexation and pH gradient loading. Clin Cancer Res 2004, 10:728–738. [DOI] [PubMed] [Google Scholar]
- 26.Morton SW, Lee MJ, Deng ZJ, Dreaden EC, Siouve E, Shopsowitz KE, Shah NJ, Yaffe MB, Hammond PT. A nanoparticle-based combination chemotherapy delivery system for enhanced tumor killing by dynamic rewiring of signaling pathways. Sci Signal 2014, 7:ra44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riviere K, Kieler-Ferguson HM, Jerger K, Szoka FC Jr. Anti-tumor activity of liposome encapsulated fluoroorotic acid as a single agent and in combination with liposome irinotecan. J Control Release 2011, 153: 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad P, Shuhendler A, Cai P, Rauth AM, Wu XY. Doxorubicin and mitomycin C co-loaded polymer-lipid hybrid nanoparticles inhibit growth of sensitive and multidrug resistant human mammary tumor xenografts. Cancer Lett 2013, 334:263–273. [DOI] [PubMed] [Google Scholar]
- 29.Shuhendler AJ, Prasad P, Zhang RX, Amini MA, Sun M, Liu PP, Bristow RG, Rauth AM, Wu XY. Synergistic nanoparticulate drug combination overcomes multidrug resistance, increases efficacy, and reduces cardiotoxicity in a nonimmunocompromised breast tumor model. Mol Pharm 2014, 11:2659–2674. [DOI] [PubMed] [Google Scholar]
- 30.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 2010, 70:440–446. [DOI] [PubMed] [Google Scholar]
- 31.Mathews Griner LA, Guha R, Shinn P, Young RM, Keller JM, Liu D, Goldlust IS, Yasgar A, McKnight C, Boxer MB, et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci USA 2014, 111:2349–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Upreti M, Jamshidi-Parsian A, Koonce NA, Webber JS, Sharma SK, Asea AA, Mader MJ, Griffin RJ. Tumor-endothelial cell three-dimensional spheroids: new aspects to enhance radiation and drug therapeutics. Transl Oncol 2011, 4:365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grandhi BK, Thakkar A, Wang J, Prabhu S. A novel combinatorial nanotechnology-based oral chemopreventive regimen demonstrates significant suppression of pancreatic cancer neoplastic lesions. Cancer Prev Res (Phila) 2013, 6:1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanthamneni N, Chaudhary A, Wang J, Prabhu S. Nanoparticulate delivery of novel drug combination regimens for the chemoprevention of colon cancer. Int J Oncol 2010, 37:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine 2012, 7:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kheirolomoom A, Lai CY, Tam SM, Mahakian LM, Ingham ES, Watson KD, Ferrara KW. Complete regression of local cancer using temperature-sensitive liposomes combined with ultrasound-mediated hyperthermia. J Control Release 2013, 172:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, Hu M, Yu X, Li Y, Fu Y, Zhou X, Zhang D, Li J. Design and evaluation of pH-sensitive liposomes constructed by poly(2-ethyl-2-oxazoline)-cholesterol hemisuccinate for doxorubicin delivery. Eur J Pharm Biopharm 2015, 91:66–74. [DOI] [PubMed] [Google Scholar]
- 38.Adhikari C, Das A, Chakraborty A. Controlled release of a sparingly water-soluble anticancer drug through pH-responsive functionalized gold-nanoparticle-decorated liposomes. Chemphyschem 2015, 16:866–871. [DOI] [PubMed] [Google Scholar]
- 39.Yuba E, Tajima N, Yoshizaki Y, Harada A, Hayashi H, Kono K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials 2014, 35:3091–3101. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Geng D, Su H. Safe and efficient pH sensitive tumor targeting modified liposomes with minimal cytotoxicity. Colloids Surf B Biointerfaces 2014, 123: 395–402. [DOI] [PubMed] [Google Scholar]
- 41.Ghanbarzadeh S, Arami S, Pourmoazzen Z, Khorrami A. Improvement of the antiproliferative effect of rapamycin on tumor cell lines by poly (monomethylitaconate)-based pH-sensitive, plasma stable liposomes. Colloids Surf B Biointerfaces 2014, 115: 323–330. [DOI] [PubMed] [Google Scholar]
- 42.Chiang YT, Lo CL. pH-responsive polymer-liposomes for intracellular drug delivery and tumor extracellular matrix switched-on targeted cancer therapy. Biomaterials 2014, 35:5414–5424. [DOI] [PubMed] [Google Scholar]
- 43.Bersani S, Vila-Caballer M, Brazzale C, Barattin M, Salmaso S. pH-sensitive stearoyl-PEG-poly (methacryloyl sulfadimethoxine) decorated liposomes for the delivery of gemcitabine to cancer cells. Eur J Pharm Biopharm 2014, 88:670–682. [DOI] [PubMed] [Google Scholar]
- 44.Bertrand N, Fleischer JG, Wasan KM, Leroux JC. Pharmacokinetics and biodistribution of N-isopropylacrylamide copolymers for the design of pH-sensitive liposomes. Biomaterials 2009, 30:2598–2605. [DOI] [PubMed] [Google Scholar]
- 45.Garg A, Kokkoli E. pH-sensitive PEGylated liposomes functionalized with a fibronectin-mimetic peptide show enhanced intracellular delivery to colon cancer cell. Curr Pharm Biotechnol 2011, 12:1135–1143. [DOI] [PubMed] [Google Scholar]
- 46.Staruch RM, Hynynen K, Chopra R. Hyperthermia-mediated doxorubicin release from thermosensitive liposomes using MR-HIFU: therapeutic effect in rabbit Vx2 tumours. Int J Hyperthermia 2015, 31:118–133. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Noriega A, Hastings CL, Ozbakir B, O’Donnell KE, O’Brien FJ, Storm G, Hennink WE, Duffy GP, Ruiz-Hernandez E. Hyperthermia-induced drug delivery from thermosensitive liposomes encapsulated in an injectable hydrogel for local chemotherapy. Adv Healthc Mater 2014, 3:854–859. [DOI] [PubMed] [Google Scholar]
- 48.Al-Ahmady ZS, Chaloin O, Kostarelos K. Monoclonal antibody-targeted, temperature-sensitive liposomes: in vivo tumor chemotherapeutics in combination with mild hyperthermia. J Control Release 2014, 196:332–343. [DOI] [PubMed] [Google Scholar]
- 49.Mannaris C, Efthymiou E, Meyre ME, Averkiou MA. In vitro localized release of thermosensitive liposomes with ultrasound-induced hyperthermia. Ultrasound Med Biol 2013, 39:2011. –2020. [DOI] [PubMed] [Google Scholar]
- 50.Li L, ten Hagen TL, Hossann M, Suss R, van Rhoon GC, Eggermont AM, Haemmerich D, Koning GA. Mild hyperthermia triggered doxorubicin release from optimized stealth thermosensitive liposomes improves intratumoral drug delivery and efficacy. J Control Release 2013, 168:142–150. [DOI] [PubMed] [Google Scholar]
- 51.Grull H, Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J Control Release 2012, 161:317–327. [DOI] [PubMed] [Google Scholar]
- 52.Smith B, Lyakhov I, Loomis K, Needle D, Baxa U, Yavlovich A, Capala J, Blumenthal R, Puri A. Hyperthermia-triggered intracellular delivery of anti-cancer agent to HER2(+) cells by HER2-specific affibody (ZHER2-GS-Cys)-conjugated thermosensitive liposomes (HER2(+) affisomes). J Control Release 2011, 153:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin Pharmacokinet 2003, 42:419–436. [DOI] [PubMed] [Google Scholar]
- 54.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003, 97:2869–2879. [DOI] [PubMed] [Google Scholar]
- 55.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 1998, 339:900–905. [DOI] [PubMed] [Google Scholar]
- 56.O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 2004, 15:440–449. [DOI] [PubMed] [Google Scholar]
- 57.Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol 2000, 11:1029–1033. [DOI] [PubMed] [Google Scholar]
- 58.Ramadass SK, Anantharaman NV, Subramanian S, Sivasubramanian S, Madhan B. Paclitaxel/Epigallocatechin gallate coloaded liposome: a synergistic delivery to control the invasiveness of MDA-MB-231 breast cancer cells. Colloids Surf B Biointerfaces 2015, 125:65–72. [DOI] [PubMed] [Google Scholar]
- 59.Lancet JE, Cortes JE, Hogge DE, Tallman MS, Kovacsovics TJ, Damon LE, Komrokji R, Solomon SR, Kolitz JE, Cooper M, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood 2014, 123:3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shim G, Lee S, Choi J, Lee S, Kim CW, Oh YK. Liposomal co-delivery of omacetaxine mepesuccinate and doxorubicin for synergistic potentiation of antitumor activity. Pharm Res 2014, 31:2178–2185. [DOI] [PubMed] [Google Scholar]
- 61.Patankar NA, Pritchard J, van Grinsven M, Osooly M, Bally MB. Topotecan and doxorubicin combination to treat recurrent ovarian cancer: the influence of drug exposure time and delivery systems to achieve optimum therapeutic activity. Clin Cancer Res 2013, 19:865–877. [DOI] [PubMed] [Google Scholar]
- 62.Shaikh IM, Tan KB, Chaudhury A, Liu Y, Tan BJ, Tan BM, Chiu GN. Liposome co-encapsulation of synergistic combination of irinotecan and doxorubicin for the treatment of intraperitoneally grown ovarian tumor xenograft. J Control Release 2013, 172:852–861. [PubMed] [Google Scholar]
- 63.Batist G, Gelmon KA, Chi KN, Miller WH Jr, Chia SK, Mayer LD, Swenson CE, Janoff AS, Louie AC. Safety, pharmacokinetics, and efficacy of CPX-1 lipo-some injection in patients with advanced solid tumors. Clin Cancer Res 2009, 15:692–700. [DOI] [PubMed] [Google Scholar]
- 64.Tran MA, Smith CD, Kester M, Robertson GP. Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clin Cancer Res 2008, 14:3571–3581. [DOI] [PubMed] [Google Scholar]
- 65.Mohan A, Narayanan S, Sethuraman S, Krishnan UM. Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. Biomed Res Int 2014, 2014:424239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crist RM, Grossman JH, Patri AK, Stern ST, Dobrovolskaia MA, Adiseshaiah PP, Clogston JD, McNeil SE. Common pitfalls in nanotechnology: lessons learned from NCI’s nanotechnology characterization laboratory. Integr Biol (Camb) 2013, 5:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol Pharm 2011, 8:2101–2141. [DOI] [PubMed] [Google Scholar]
- 68.Guo S, Wang Y, Miao L, Xu Z, Lin CM, Zhang Y, Huang L. Lipid-coated Cisplatin nanoparticles induce neighboring effect and exhibit enhanced anticancer efficacy. ACS Nano 2013, 7:9896–9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Vlerken LE, Duan Z, Little SR, Seiden MV, Amiji MM. Biodistribution and pharmacokinetic analysis of Paclitaxel and ceramide administered in multifunctional polymer-blend nanoparticles in drug resistant breast cancer model. Mol Pharm 2008, 5:516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang Y, DiVittore NA, Kaiser JM, Shanmugavelandy SS, Fritz JL, Heakal Y, Tagaram HR, Cheng H, Cabot MC, Staveley-O’Carroll KF, et al. Combinatorial therapies improve the therapeutic efficacy of nanoliposomal ceramide for pancreatic cancer. Cancer Biol Ther 2011, 12:574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dhule SS, Penfornis P, He J, Harris MR, Terry T, John V, Pochampally R. The combined effect of encapsulating curcumin and C6 ceramide in liposomal nanoparticles against osteosarcoma. Mol Pharm 2014, 11:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fonseca NA, Gomes-da-Silva LC, Moura V, Simoes S, Moreira JN. Simultaneous active intracellular delivery of doxorubicin and C6-ceramide shifts the additive/antagonistic drug interaction of non-encapsulated combination. J Control Release 2014, 196:122–131. [DOI] [PubMed] [Google Scholar]
- 73.Adiseshaiah PP, Clogston JD, McLeland CB, Rodriguez J, Potter TM, Neun BW, Skoczen SL, Shanmugavelandy SS, Kester M, Stern ST, et al. Synergistic combination therapy with nanoliposomal C6-ceramide and vinblastine is associated with autophagy dysfunction in hepatocarcinoma and colorectal cancer models. Cancer Lett 2013, 337:254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zolnik BS, Stern ST, Kaiser JM, Heakal Y, Clogston JD, Kester M, McNeil SE. Rapid distribution of liposomal short-chain ceramide in vitro and in vivo. Drug Metab Dispos 2008, 36:1709–1715. [DOI] [PubMed] [Google Scholar]
- 75.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem 1977, 252:3582–3586. [PubMed] [Google Scholar]
- 76.Wang H, Zhao Y, Wu Y, Hu YL, Nan K, Nie G, Chen H. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials 2011, 32:8281–8290. [DOI] [PubMed] [Google Scholar]
- 77.Qian X, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol 2008, 26:83–90. [DOI] [PubMed] [Google Scholar]
- 78.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res 2006, 66:6732–6740. [DOI] [PubMed] [Google Scholar]
- 79.Shen J, Meng Q, Sui H, Yin Q, Zhang Z, Yu H, Li Y. iRGD conjugated TPGS mediates codelivery of paclitaxel and survivin shRNA for the reversal of lung cancer resistance. Mol Pharm 2014, 11:2579–2591. [DOI] [PubMed] [Google Scholar]
- 80.Wei W, Lv PP, Chen XM, Yue ZG, Fu Q, Liu SY, Yue H, Ma GH. Codelivery of mTERT siRNA and paclitaxel by chitosan-based nanoparticles promoted synergistic tumor suppression. Biomaterials 2013, 34:3912–3923. [DOI] [PubMed] [Google Scholar]
- 81.Qu MH, Zeng RF, Fang S, Dai QS, Li HP, Long JT. Liposome-based co-delivery of siRNA and docetaxel for the synergistic treatment of lung cancer. Int J Pharm 2014, 474:112–122. [DOI] [PubMed] [Google Scholar]
- 82.Ganesh S, Iyer AK, Gattacceca F, Morrissey DV, Amiji MM. In vivo biodistribution of siRNA and cisplatin administered using CD44-targeted hyaluronic acid nanoparticles. J Control Release 2013, 172:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, Ramsay EC, Bally MB, Janoff AS. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther 2006, 5:1854–1863. [DOI] [PubMed] [Google Scholar]
- 84.Tardi PG, Dos Santos N, Harasym TO, Johnstone SA, Zisman N, Tsang AW, Bermudes DG, Mayer LD. Drug ratio-dependent antitumor activity of irinotecan and cisplatin combinations in vitro and in vivo. Mol Cancer Ther 2009, 8:2266–2275. [DOI] [PubMed] [Google Scholar]
- 85.Hecht JR. Gastrointestinal toxicity or irinotecan. Oncology (Williston Park) 1998, 12:72–78. [PubMed] [Google Scholar]
- 86.von Roemeling R, Hrushesky WJ. Circadian patterning of continuous floxuridine infusion reduces toxicity and allows higher dose intensity in patients with widespread cancer. J Clin Oncol 1989, 7:1710–1719. [DOI] [PubMed] [Google Scholar]
- 87.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000, 355:1041–1047. [DOI] [PubMed] [Google Scholar]
- 88.Douillard JY, Sobrero A, Carnaghi C, Comella P, Diaz-Rubio E, Santoro A, Van Cutsem E. Metastatic colorectal cancer: integrating irinotecan into combination and sequential chemotherapy. Ann Oncol 2003, 14(Suppl 2):ii7–ii12. [DOI] [PubMed] [Google Scholar]
- 89.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004, 22:23–30. [DOI] [PubMed] [Google Scholar]
- 90.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004, 22:229–237. [DOI] [PubMed] [Google Scholar]
- 91.Lee DS, Im HJ, Lee YS. Radionanomedicine: widened perspectives of molecular theragnosis. Nanomedicine 2015, 11:795–810. [DOI] [PubMed] [Google Scholar]
- 92.Iyer AK, He J, Amiji MM. Image-guided nanosystems for targeted delivery in cancer therapy. Curr Med Chem 2012, 19:3230–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
FURTHER READING
- http://www.bindtherapeutics.com/
- http://www.celatorpharma.com/
- Cosco D, Paolino D, Cilurzo F, Casale F, Fresta M. Gemcitabine and tamoxifen-loaded liposomes as multidrug carriers for the treatment of breast cancer diseases. Int J Pharm 2012, 422:229–237. [DOI] [PubMed] [Google Scholar]
- Harasym TO, Tardi PG, Harasym NL, Harvie P, Johnstone SA, Mayer LD. Increased preclinical efficacy of irinotecan and floxuridine coencapsulated inside liposomes is associated with tumor delivery of synergistic drug ratios. Oncol Res 2007, 16:361–374. [DOI] [PubMed] [Google Scholar]
- Kim HP, Gerhard B, Harasym TO, Mayer LD, Hogge DE. Liposomal encapsulation of a synergistic molar ratio of cytarabine and daunorubicin enhances selective toxicity for acute myeloid leukemia progenitors as compared to analogous normal hematopoietic cells. Exp Hematol 2011, 39:741–750. [DOI] [PubMed] [Google Scholar]
- Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, Harvie P, Bermudes D, Mayer L. In vivo maintenance of synergistic cytarabine: daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res 2009, 33:129–139. [DOI] [PubMed] [Google Scholar]