Abstract
Gene-gene (GXG) and gene-environment (GXE) interactions play important roles in pharmacogenetics study. Simultaneously incorporating multiple single nucleotide polymorphisms (SNPs) and clinical factors is needed to explore the association of their interactions with drug response and toxicity phenotypes. We genotyped 504 SNPs in a total of 490 Chinese non-small cell lung cancer (NSCLC) patients, and the correlation of GXG and GXE interactions with platinum-based chemotherapeutic efficacy and safety were analyzed. In this data descriptor, we shared our data set which could help others to reuse them. All kinds of file types needed for GXG and GXE analysis were supplied. The process of genotyping and data analysis was also introduced step by step.
Subject terms: Genotype, Cancer genetics
Background and summary
Univariate analysis strategy examining the association of genetic polymorphisms with complex quantitative traits is widely used1–3. However, a number of studies show that single variation is far from satisfactory to explain complex phenotypic characteristics4–6. For pharmacogenomics investigation, analyzing a single polymorphism at a time is also not sufficient to explain drug efficacy and safety4,7. To explore the multivariate contribution to drug response and toxicity phenotypes, we conducted an association study by incorporating multiple single nucleotide polymorphisms (SNPs) and clinical factors simultaneously.4 Platinum-based chemotherapeutic response and toxicity in the non-small cell lung cancer (NSCLC) was selected as our investigated phenotypes. Lung cancer ranks one of the tumors with high mortality rate in the world, it consists of NSCLC and small cell lung cancer (SCLC) in histology according to World Health Organization (WHO) classification.8,9 Although immune and molecular target therapy is developing rapidly, chemotherapy is still a major treatment choice for NSCLC patients with platinum-based doublet as the first-line regimen10,11. One of the challenges for successful treatment is the remarkable inter-individual difference of drug response and toxicity 12–14. Only a part of patients can benefit from platinum-based chemotherapy, unfortunately, the contribution of genetic and environment factors to them still remains largely unknown15,16. We proposed that the combination of multiple genetic and environmental factors could contribute to the inter-individual variation.
We genotyped 504 SNPs in a total of 490 Chinese NSCLC patients.4 Based on these SNPs, we explored the effects of gene-gene (GXG) and gene-environment (GXE) interactions on platinum-based chemotherapeutic response and toxicity. Our results showed that some SNPs previously identified as “negative” were in fact significantly related to the phenotype when conducting interaction analysis. In addition, environment also plays an important role in the drug response through GXE interactions. Some significant results were validated in another cohort. The main purpose of this data descriptor is to share our data set. All kinds of file types needed for GXG and GXE analysis were supplied. Moreover, we provided an analytical method to analyze the correlation of GXG and GXE interaction to complex phenotype in pharmacogenomic research.
Methods
Study design
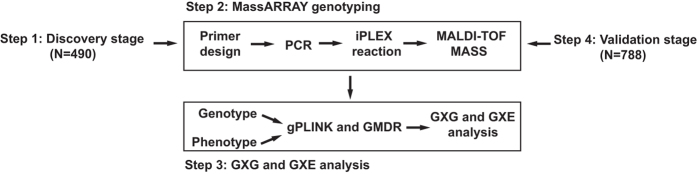
As our previous study introduced, the discovery stage enrolled 490 platinum-based treatment NSCLC patients4. To enrolled genes which may contribute to platinum-based chemotherapy, 504 SNPs located in 185 genes which are mainly involved in DNA repair, drug transport, apoptosis and detoxification pathways were genotyped.4 Then, the correlations of GXG and GXE interaction with drug response were analyzed. 9 pairs of SNP-SNP interactions and 15 groups of multiple gene-gene and gene-environment interactions were found significantly associated with platinum-based chemotherapy in the discovery stage. 16 SNPs were included among these interactions. They were further genotyped in the validation stage including another 788 platinum-based treatment NSCLC patients4. All genotyping was conducted by using Sequenom’s MassARRAY system (Sequenom, San Diego, California, USA). The correlation of GXG and GXE interactions in both discovery and validation stage were analyzed by using gPLINK (version 2.050, available http://zzz.bwh.harvard.edu/plink/gplink.shtml#down) and GMDR (available http://www.ssg.uab.edu/gmdr/) software4,17,18. Registration Number of ChiCTR-ROC-14005699 was acquired after meeting the clinical admission in the Chinese Clinical Trial Register. All patients provided written informed consent in compliance with the code of ethics of the World Medical Association (Declaration of Helsinki). The protocol used in this study was approved by the Ethics Committee of Xiangya School of Medicine, Central South University (Registration Number: CTXY-110008-1).4 The study design is summarized in Fig. 1.
Figure 1. Flow diagram of study design.
DNA extraction
5 ml fresh peripheral blood samples were obtained from patients using EDTA anticoagulant tubes. The plasma was removed from the whole blood by centrifuging at 2100 g for 10 min at room temperature. Wizard® Genomic DNA Purification kits (Promega, Madison, WI, USA) were utilized for genomic DNA extraction. Primarily, blood cells were lysed and white blood cell nucleus was collected. Then, the nuclear membrane was broken and the nucleic acid was released after adding the nucleic acid lysis solution. The proteins in the mixed system were precipitated using protein precipitation solution and all the precipitation was discarded. Finally, isopropanol was added to the supernatant at an equal volume to precipitate the genomic DNA. Agarose gel electrophoresis was used for the quality control of these DNA samples, and the DNA samples that reached the quality control standard were stored in a −80 ultra-low temperature freezer before genotyping (QC of DNA, Data Citation 1). These methods are expanded versions of descriptions in our related work.4
Genotyping
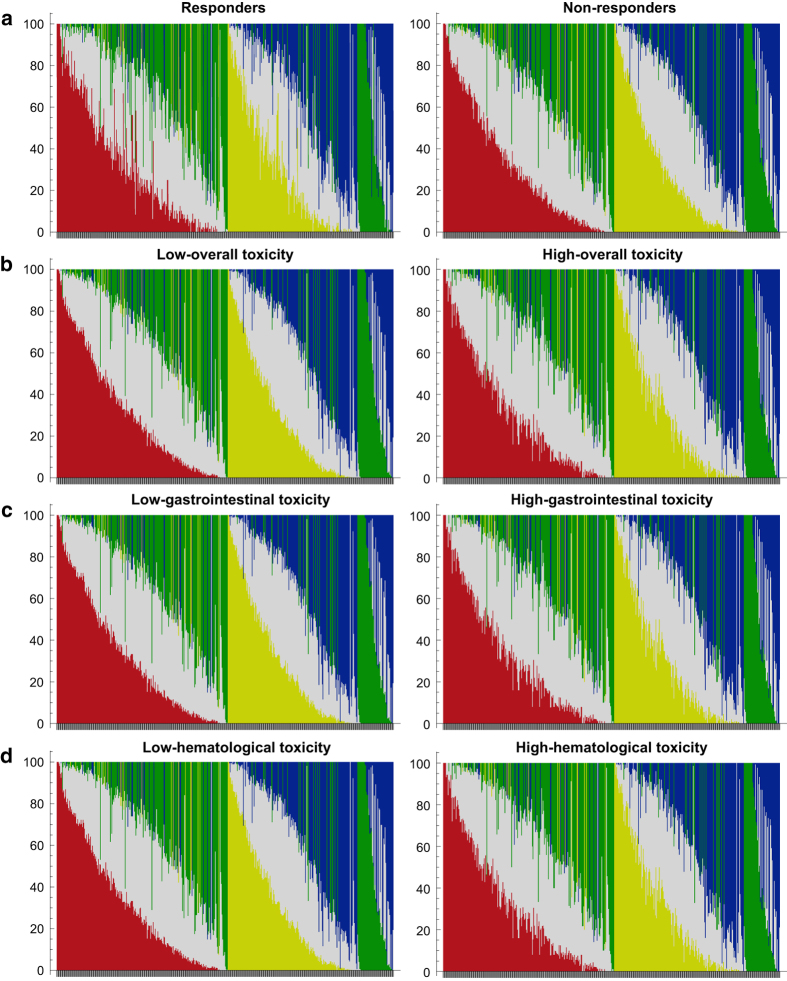
All genotyping experiments were conducted by Sequenom’s MassARRAY® system (Sequenom, San Diego, California, USA) as our related work briefly described.4 In detail, both amplification and genotyping primers were designed using MassARRAY Assay Design 3.1 software (SEQUENOM, San Diego, California, USA). At first, a 300–400 bp length sequence covering detected SNPs need to be prepared. It should be noted that the alleles of all other known SNPs must be replaced by “N”, while detected SNPs need to be provided as “wildtype allele/mutant allele”. Then, this sequence can be loaded by the software directly. In this study, the primers of 16 SNPs identified from discovery stage were provided in Table 1. To perform the multiple PCR, DNA samples were loaded into 384 well plates with reaction system containing dNTPs, primers, reaction buffer and DNA polymerase. After treating with shrimp alkaline phosphatase (SAP), the multiple PCR production can be subjected to single base extension using special mass-modified ddNTPs and primers. Finally, the productions purified with cationic resin were transferred into SpectroChip to perform genotyping. The raw data was interpreted by MassARRAY TYPER 4.0 software (pre-installed in the MassARRAY® System), and genotyping data was provided as both scatter plots, spectrums. To improve their reusability, additional accompanied quantitative tsv files and txt files were also provided. The landscapes of genotyping results were showed in Fig. 2. These methods are expanded versions of descriptions in our related work.4
Table 1. Primers of 16 SNPs for MassARRAY identified from discovery stage.
| Chr. | SNP | Location | Gene | Primer-EP | Primer-F | Primer-R |
|---|---|---|---|---|---|---|
| 1 | rs4658 | 43392250 | SLC2A1 | TCCAGGCCAGCAGAA | ACGTTGGATGAAAGCTTCTATCCCAGGAGG | ACGTTGGATGAATCCTAATGGAGCCTGACC |
| 2 | rs17730989 | 198362524 | HSPD1 | ACCATCAAGGCAAGTAG | ACGTTGGATGTATGTTGCGTGAACCTGGAA | ACGTTGGATGGTGACTTGTTTTAAAATCCG |
| 3 | rs2633851 | 4403817 | SUMF1 | GTGGACTGGGGAAGACT | ACGTTGGATGGCCCACTATGGACTGACAAC | ACGTTGGATGAGAAAAGCCCAATGTAGGTC |
| 4 | rs2231142 | 89052323 | ABCG2 | TTTAGAAGAGCTGCTGAGAACT | ACGTTGGATGTGATGTTGTGATGGGCACTC | ACGTTGGATGCGTCATAGTTGTTGCAAGCC |
| 5 | rs3776332 | 142441514 | ARHGAP26 | TTATGTCACATCTCATT GGTC | ACGTTGGATGCCCACAAGTGGCTCAGATAA | ACGTTGGATGTCCAGCACACTTTATGTCAC |
| 6 | rs28688207 | 32628660 | HLA-DQB1 | GAGGCCCTTGAGGTC | ACGTTGGATGAATATTACCTGCTGGTGGAG | ACGTTGGATGTGAGAGAGTGGCTGTTTGTG |
| 6 | rs462779 | 111695887 | REV3L | TTATTTTTTCATCCTTAAGTGTT | ACGTTGGATGACTTAACCTCAGCACCAGAC | ACGTTGGATGAATGAGAAAGGTACATCGAG |
| 7 | rs2070804 | 75933712 | HSPB1 | CCGAAACCTACACCAGTGTACCC | ACGTTGGATGCAGGAGTCATCTTTGCTCAG | ACGTTGGATGATGTGAGTCAGCCTGTGTCC |
| 8 | rs2977530 | 134215112 | WISP1 | CCTCTGAGTCAGCCA | ACGTTGGATGAGGTGTTGGGAAAAGAGGTG | ACGTTGGATGTCCCCACGCTTGTTTCAAAG |
| 8 | rs2929970 | 134241137 | WISP1 | AGGAAGATGGAGGTTTACC | ACGTTGGATGGCTTCAACCTCTTCAGCTTT | ACGTTGGATGTTCTGGTAGGAAGATGGAGG |
| 8 | rs2977549 | 134242033 | WISP1 | ATATTAAATGTCTCTTTTGCTAAG | ACGTTGGATGCATACATATGCATTTCTTTG | ACGTTGGATGCCAAAGCTACATGAAAATAG |
| 10 | rs2228528 | 50732280 | ERCC6 | CTTCAGCTCATAGTCAGTA | ACGTTGGATGAGGAAGATGACGAGGTGGAG | ACGTTGGATGGCAGAGGCTTCAGCTCATAG |
| 11 | rs228589 | 108093208 | NPAT | GGTCCAATAACCCTCC | ACGTTGGATGCTTGTATTGGGTAAGCGCGG | ACGTTGGATGTTTGGCCTCAAAGGTCCTTC |
| 11 | rs189037 | 108093833 | ATM | TCTCGCCTCCTCCCG | ACGTTGGATGGCTAACGGAGAAAAGAAGCC | ACGTTGGATGGTCAAAGTAGTATCAACCGC |
| 16 | rs3859104 | 55895249 | CES5A | TTCTATGTTCCAAATTCAAATAAATA | ACGTTGGATGCTTCGAACGGAGAGATGAAC | ACGTTGGATGCAAGTACCTCAATCCTCCTC |
| 17 | rs799917 | 41244936 | BRCA1 | AAGCGCCAGTCATTTGCTC | ACGTTGGATGAAGGTTTCAAAGCGCCAGTC | ACGTTGGATGAGAGTGGGCAGAGAATGTTG |
Figure 2. Landscape of genotyping in the discovery stage.
(a) The genotyping results of 504 SNPs in the discovery stage of response analysis. (b) The genotyping results of 504 SNPs in the discovery stage of overall toxicity analysis. (c) The genotyping results of 504 SNPs in the discovery stage of gastrointestinal toxicity analysis. (d) The genotyping results of 504 SNPs in the discovery stage of hematological toxicity analysis. Each bar showed the percentage distribution of genotypes for each SNP. Red, yellow, blue, green and gray referred as the homozygous of T, A, C, G and heterozygous, respectively.
Data pre-process
All data should be pre-processed before analysis using gPLINK and GMDR. For gPLINK, ped and map files were the basic requirement, while covar files were required for adjusting the effect of covariate. In detail, ped file recorded the patients and genotyping information. The first six columns were family ID, individual ID, paternal ID, maternal ID, gender and phenotype, followed by the genotypes. map file recorded the information of SNPs. The four columns were located chromosome, identifier, genetic distance and base-pair positions. It should be noted that the order of “identifier” (from top to bottom) in the Map file and the order of “SNP 1 to N” (from left to right) in the Ped file must be the same. Otherwise, the calculated results would be incorrect. covar files recorded all covariates, for example, age, smoke stage and histology of patients. All covariates should be converted to binary form and each column recorded one covariate. In our study, all toxicity was evaluated according to the National Cancer Institute Common Toxicity Criteria 3.0 (NCI-CTC 3.0). In detail, we classified toxicity into hematologic toxicity (anemia, leukopenia, neutropenia and thrombocytopenia) and gastrointestinal toxicity. Each one was further scored from 0 to 4. Grade 0–2 was considered as low-toxicity and grade 3–4 was considered as high-toxicity. The response to chemotherapy was evaluated following the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines. The curative effect was classified as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). We defined CR and PR as platinum-sensitive phenotypes, SD and PD as platinum-resistant phenotypes.4 The tumor stage and PS stage was evaluated according to the TNM Classification of Malignant Tumor (TNM) and Eastern Cooperative Oncology Group performance score (ECOG-PS) respectively. The smoking stage of these patients were defined as “yes” or “no”. The smoking stage of patients was defined as “no” only when the patient had never been reported smoked. Smoking stage of “yes” included both current and ever smokers. Available pack-years of patients were also provided in Genotyping Data (Genotyping Data, Data Citation 1).
For GMDR, txt files containing SNP information (for GXG analysis) or both SNP information and clinical information (for GXE analysis) were needed. Different SNPs and covariates should be listed in different columns. The phenotype of each patient needed to be provided in the last column. Headers, which describe the details of each column should be created for all genotypes, covariates and the phenotype in the first row. Subsequent analyses were all based on these files.
Hardy-Weinberg equilibrium (HWE) and Linkage Disequilibrium (LD) test
Both HWE and LD test were performed using Haploview (version 4.2, available https://www.broadinstitute.org/haploview/haploview).20 It should be noted that ped and map files were also needed as described previously for this software.
GXG and GXE interaction analysis
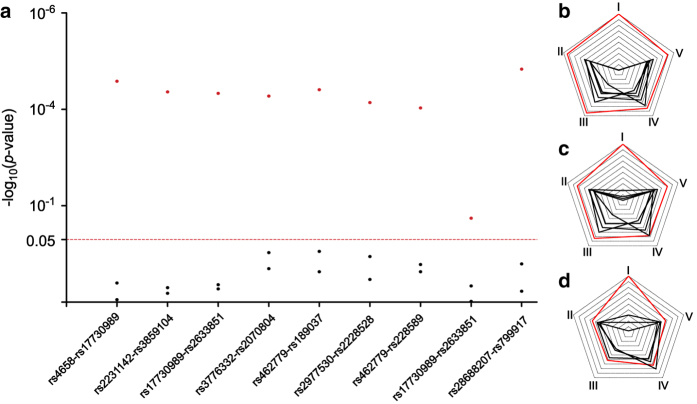
The pre-processed data can be directly recognized by both gPLINK and GMDR. For gPLINK, the command we used for GXG interaction analysis was: plink --map “xxx.map” --ped “xxx.ped” --epistasis --epi1 0.05 --covar “xxx.txt” --covar-number 3 --out “xxx” --gplink. The “xxx” referred to the filename. The command “--epistasis” was utilized for epistasis analysis, “--epi1 0.05” was used to define 0.05 as the threshold value of significant statistical difference. “--covar-number 3” was utilized for adjusting the covariate in the third column in covar file. “-- out” was utilized for results output. “plink” and “--gplink” were necessary for commands to be recognized by this software. For GMDR, pre-processed genotypes files were loaded using “Load Marker” in the “Analysis” Tab. To define the number of dimensions included in the final model, “Marker Count Range” function in the “Configuration” Tab could be used. Then, GXG and GXE analysis was completed by clicking “Run Analysis” button in the “Analysis” Tab. Our results showed that GXG and GXE models incorporating multiple variates performed better than univariate analysis, which were showed in Fig. 3.
Figure 3. The performances of GXG and GXE analysis.
(a) Correlation results of 16 SNPs which showed statistically significant association with platinum-based chemotherapy in the discovery stage analyzed by gPLINK. Red and black dot represented the p-value of paired and single SNP analysis, respectively. (b) Illustrative performances of GMDR models of rs3776332-rs2228528-rs228589 -rs2977549-Histology. (c) Illustrative performances of GMDR models of rs3776332-rs2228528-rs4658-Histology. (d) Illustrative performances of GMDR models of rs2228528-rs2929970-Histology. Red and black line represented the statistical significance (I), accuracy (II), sensitivity (III), specificity (IV) and precision (V) of model and univariate analysis.
Data records
Genotype
Scatter plots in both discovery and validation stages was provided (Scatter Plots, Data Citation 1). In addition, each spectral data of 788 patients was indicated in Spectrums Data (Spectrums Data, Data Citation 1). Accompanied additional tsv files for Scatter Plots and Spectrums Data were also provided. For Scatter Plots (Scatter Plots, Data Citation 1), tsv files contained patients’ ID and the MASSArray quantity of major and minor allele for markers of each patient. For Spectrums Data (Spectrums Data, Data Citation 1), tsv files contained intensity at specific mass-to-charge value in spectrums. A “Master Mass List” file was also provided as an accompaniment for tsv files of Spectrums Data (Spectrums Data, Data Citation 1). This “Master Mass List” file showed the spectrum files’ name and the m/z value from which each scatter plot value was retrieved for each marker. Each patient’s genotype and their clinical characteristics were provided as txt files in Genotyping Data (Genotyping Data, Data Citation 1). Information about all designed primers for multiple PCR and single base extended were provided in Supplementary Table S1.
Pre-processed data files
In the previous study, all pre-processed data files were generated from Genotyping Data (Genotyping Data, Data Citation 1). Ped, map and txt files for gPLINK and GMDR were all presented based on phenotypes of sensitivity, overall toxicity, hematological toxicity and gastrointestinal toxicity. All clinical variates showed significant association with given phenotype should be adjusted, therefore, we adjusted different clinical variates for different phenotypes. For example, in the discovery stage, the histology was adjusted when we performed GXG analysis for overall toxicity, while the age was adjusted when we performed GXG analysis for gastrointestinal toxicity.
To make this process clear, we provided all files utilized in GXG and GXE analysis for overall toxicity. For gPLINK, Ped.ped and Map.map were used for GXG interaction analysis. Covar.txt was used for covariates adjusting. For GMDR, GXG.txt and GXE.txt were used for GXG and GXE interaction analysis. These files can be found in Pre-processed Data Files (Pre-processed Data Files, Data Citation 1).
In Data Citation files, the “1” referred low-toxicity, platinum-sensitive phenotypes, male, age <60, non-smokers, adenocarcinoma cancer, stage III and PS stage = 0 respectively; In the contrary, the “2” referred high-toxicity, platinum-resistant phenotypes, female, age ≥60, smokers, squamous cell cancer, stage IV and PS stage > 0 respectively. The “0” referred the empty value.
Results of interaction analysis
For gPLINK, result files with an extension of .epi.cc were generated by software automatically. For GMDR, results of each model could be outputted separately and opened as txt files. All raw data generated by gPLINK and GMDR was packaged in Raw Analysis Data (Raw Analysis Data, Data Citation 1).
Technical Validation
Quality control of genomic DNA
Genomic DNA concentration was measured in NanoDrop 2000c spectrophotometers (Thermo Fisher, Wilmington, Delaware, USA). The ratios of absorbance in the length of 260 nm/280 nm were recorded, 1.8–2.0 was qualified for genotyping. In addition, integrality of genomic DNA was examined in agarose gels according to the location of bands.19 All low-quality samples were removed in this step.
Quality control of MassARRAY genotyping
Productions of multiple PCR were observed in agarose gel. Briefly, 1 μl PCR productions were diluted in 1 μl 2× loading buffer and analyzed using 1% agarose gel. Bands’ specificity was used to evaluate PCR quality. TYPER 4.0 software was utilized to evaluated the quality of all SNPs. Genotypes with low signal-to-noise ratio or in the interface of two genotype were identified as low probability SNP calls.
Usage Notes
DNA extraction
When the genomic DNA was extracted from frozen blood, washing the whole blood with red cell lysed agent twice may be essential to ensure that red cells were full lysed. To obtain enough high quality genomic DNA, nuclei should be lysed sufficiently and protein should be precipitated thoroughly as well. To guarantee the genotyping accuracy, quality of each patient’s genomic DNA should be carefully examined.
MassARRAY genotyping
Primer design was the most important step for genotyping. To get reliable results from mass spectrometer, mass of extended primers should be between 4.5 to 9.0 KDa. A difference of 30 Da among different primers was necessary for distinguishing various extended productions. It was noted that the concentration of primer should be diluted into 4 different levels according to their un-extended mass. Primers powder should be centrifuged at 3,000 rpm for 3 min before they were dissolved to avoid inaccurate final concentration. All steps should be performed carefully on ice. 384 well plates should be sealed in some steps to prevent the evaporation of productions. When prepare the mix reagents for multiple PCR, SAP reaction and primer extension, using a 30% excess volume of each reagent to ensure its sufficiency for the next step is important.
Data analysis
Correct data format was the fundamental requirement for successfully running software. For gPLINK, the missing data should be recorded as value “0 0”, while that was “..” in GMDR. All covariates should be converted to binary format which helps them to be recognized by software easier. If errors occurred in the process, the format and the locations of these files should be checked carefully to ensure that these files could be recognized by software.
Additional information
How to cite this article: Wang, L. Y. et al. Gene-gene and gene-environment interaction data for platinum-based chemotherapy in non-small cell lung cancer. Sci. Data. 5:180284 doi: 10.1038/sdata.2018.284 (2018).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81773823, 81573463, 81402968), Hunan Provincial Natural Science Foundation of China Grant (2015JJ1024), National Science and Technology Major Project (2017ZX09304014, 2016YFC0905000) and Key Research and Development Program of Hunan Province (No. 2016JC2039).
Footnotes
The authors declare no competing interests.
Data Citations
- Wang L. Y., et al. . 2018. figshare. https://doi.org/10.6084/m9.figshare.c.4135967
References
- Yin J. Y. et al. Association of positively selected eIF3a polymorphisms with toxicity of platinum-based chemotherapy in NSCLC patients. Acta. Pharmacol. Sin. 36, 375–384 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian C. Y. et al. Associations of genetic polymorphisms of the transporters organic cation transporter 2 (OCT2), multidrug and toxin extrusion 1 (MATE1), and ATP-binding cassette subfamily C member 2 (ABCC2) with platinum-based chemotherapy response and toxicity in non-small cell lung cancer patients. Chin. J. Cancer 35, 85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day F. R. et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet. 49, 834–841 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J. J. et al. Gene-gene and gene-environment interactions influence platinum-based chemotherapy response and toxicity in non-small cell lung cancer patients. Sci. Rep 7, 5082 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan S. & Joe B. Towards Precision Medicine for Hypertension: A Review of Genomic, Epigenomic, and Microbiomic Effects on Blood Pressure in Experimental Rat Models and Humans. Physiol. Rev. 97, 1469–1528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell H. J. Detecting gene-gene interactions that underlie human diseases. Nat. Rev. Genet. 10, 392–404 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J. Y. et al. Prediction models for platinum-based chemotherapy response and toxicity in advanced NSCLC patients. Cancer. Lett. 377, 65–73 (2016). [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D. & Jemal A. Cancer Statistics, 2017. CA. Cancer. J. Clin. 67, 7–30 (2017). [DOI] [PubMed] [Google Scholar]
- Travis W. D. et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac. Oncol. 10, 1243–1260 (2015). [DOI] [PubMed] [Google Scholar]
- Tan W. L. et al. Novel therapeutic targets on the horizon for lung cancer. Lancet. Oncol. 17, e347–e362 (2016). [DOI] [PubMed] [Google Scholar]
- Rosell R. & Karachaliou N. Lung cancer in 2014: optimizing lung cancer treatment approaches. Nat. Rev. Clin. Oncol. 12, 75–76 (2015). [DOI] [PubMed] [Google Scholar]
- Shiraishi K. et al. Association of DNA repair gene polymorphisms with response to platinum-based doublet chemotherapy in patients with non-small-cell lung cancer. J. Clin. Oncol. 28, 4945–4952 (2010). [DOI] [PubMed] [Google Scholar]
- Perez-Ramirez C. et al. Contribution of genetic factors to platinum-based chemotherapy sensitivity and prognosis of non-small cell lung cancer. Mutat. Res. 771, 32–58 (2017). [DOI] [PubMed] [Google Scholar]
- Liu J. Y. et al. Association between DNA mismatch repair gene polymorphisms and platinum-based chemotherapy toxicity in non-small cell lung cancer patients. Chin. J. Cancer 36, 12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J. Y., Li X., Zhou H. H. & Liu Z. Q. Pharmacogenomics of platinum-based chemotherapy sensitivity in NSCLC: toward precision medicine. Pharmacogenomics. 17, 1365–1378 (2016). [DOI] [PubMed] [Google Scholar]
- NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 383, 1561–1571 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. M. et al. GMDR: Versatile Software for Detecting Gene-Gene and Gene-Environ- ment Interactions Underlying Complex Traits. Curr. Genomics. 17, 396–402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R. & Li S. Extraction of human genomic DNA from whole blood using a magnetic microsphere method. Int. J. Nanomedicine. 9, 3781–3789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Fry B., Maller J. & Daly M. J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 21, 263–265 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Wang L. Y., et al. . 2018. figshare. https://doi.org/10.6084/m9.figshare.c.4135967