Abstract
Introduction:
Need for procedural sedation and analgesia (PSA) is felt in emergency department (ED) more and more each day. This study aimed to compare the effectiveness of low-dose fentanyl, propofol, midazolam, ketamine and lidocaine combination with regular dose of propofol and fentanyl combination for induction of deep sedation.
Methods:
In this single-blind clinical trial, candidate patients for sedation and analgesia aged more than 15 and less than 60 years old, with pain score ≥6 were allocated to one of the groups using block randomization and were compared regarding onset of action, recovery time, and probable side effects.
Results:
125 patients with the mean age of 37.8 ± 14.3 years were randomly allocated to each group. 100% of the patients in group 1 (5 drugs) and 56.5% of the patients in group 2 (2 drugs) were deeply sedated in the 3rd minute after injection. The 2 groups were significantly different regarding onset of action (p = 0.440), recovery time (p = 0.018), and treatment failure (p < 0.001).
Conclusion:
Low-dose fentanyl, propofol, midazolam, ketamine and lidocaine combination was more successful in induction of deep sedation compared to regular dose of propofol and fentanyl combination. Recovery time was a little longer in this group and both groups were similar regarding drug side effects and effect on vital signs.
Key Words: Clinical trial, deep sedation, emergency service, hospital, ketamine, propofol, analgesia
Introduction:
Pain and excessive agitation are usually big obstacles for providing medical services (1, 2). Therefore, need for procedural sedation and analgesia (PSA) is felt in emergency department (ED) more and more each day. It is estimated that 10% of ED patients need some kind of sedative (3-5). PSA is a technique used to induce a level of anesthesia and analgesia for patients in order to undergo unpleasant and painful procedures without changes in cardiopulmonary function. Drugs used for sedation induction can be classified in 2 general groups of sedatives such as propofol, ketamine, etomidate, and midazolam and analgesics such as fentanyl, remifentanil, morphine, and ketamine (6). These drugs have the potential for causing dangerous side effects such as respiratory, cardiac and vascular depression, especially in higher doses (7). Finding new compounds that have few side effects in addition to effective and deep PSA with rapid recovery of patients from PSA is of great interest. Numerous studies have evaluated and compared drug compounds. Both propofol-ketamine and propofol-fentanyl have provided sufficient sedation and analgesia during dressing change for burn patients (8). Both compounds have also provided sufficient analgesia for doing endoscopy of the upper digestive system but propofol-ketamine compound has led to more stable hemodynamics and deeper analgesia (9). Using low-dose drugs can prevent manifestation of drug side effects. However, there is no accurate data regarding the effects of using low-dose sedative and analgesic drugs. Therefore, this study aimed to compare the effectiveness and safety of using low-dose sedative and analgesic drugs (including propofol, ketamine, midazolam, fentanyl, and lidocaine) with regular dose of propofol and fentanyl in patients in need of PSA presenting to ED.
Methods:
Study design and setting
The present study is a clinical trial carried out aiming to compare the effectiveness of low-dose 5 drug combination (fentanyl, propofol, midazolam, ketamine, and lidocaine) with regular dose of 2 drug combination (propofol-fentanyl) for induction of deep sedation in patients presenting to the ED of Imam Hossein Teaching Hospital, during 6 months in 2015.
In line with the Declaration of Helsinki, the researchers adhered to ethical principles and kept patient data confidential. Before entering the study, all participants were given explanations regarding the study protocol and then written informed consent was obtained from them. The protocol of the study was registered on the Iranian registry of clinical trials under the number IRCT2015112525235N1 and was approved by the ethics committee of Shahid Beheshti University of Medical Sciences under the license number: sbmu.rec.13930717.
Participants
In this study, candidate patients for PSA, according to the decision of a senior emergency medicine specialist, whose age was over 15 and under 60 years and had a pain score equal to or higher than 7 (based on numeric analog scale: NAS) were included. In cases of patients not wanting to participate, previous allergy to drugs used in the study, allergy to protein products such as egg and soy, hemodynamic instability, increased intracranial pressure and lactating and pregnant women, they were excluded.
Intervention
In this study, patients were randomly allocated to 2 groups via block randomization. Group 1 consisted of patients receiving a low-dose fentanyl (0.5 -1 µg/kg), propofol (0.5 mg/kg), midazolam (0.1–0.02 mg/kg), ketamine (0.2–0.25 mg/kg), and lidocaine (0.5 mg/kg) combination and group 2 included patients receiving regular dose of propofol (1 mg/kg) and fentanyl (1 mg/kg). Drug injection was done during 20 - 30 seconds using syringes with a similar volume and color.
All the patients were under close cardiac, respiratory, blood pressure, and blood O2 saturation monitoring during the procedure.
Time interval between receiving drug and deep PSA induction was considered as the drug’s onset of action. In addition, the time interval between deep PSA and complete recovery was considered as recovery time.
For measuring pain severity, NAS was used and for measuring depth of PSA, Ramsay system was applied. Ramsey score of 6 was considered as deep PSA and score of 2 was considered as recovery. Pain score equal to or higher than 6 was considered severe pain.
Data gathering
To gather data, a checklist consisting of demographic data (age, sex, weight); vital signs (blood pressure, respiratory rate, heart rate, O2 saturation) before and during procedure; reason for requiring PSA (upper/lower limb injury, soft tissue injury); and final studied outcome (onset of drug action, recovery time, probable side effects, and patient and physician’s satisfaction) was designed and used. Data gathering was done by a senior emergency medicine resident in charge of carrying out the study. Patients and data analyzer were blind to the administered drugs.
Outcome
Onset of action and recovery time were considered as primary outcomes and drug side effects such as nausea and vomiting, apnea and hemodynamic instability in addition to patient and physician’s satisfaction were considered secondary outcomes.
Statistical analysis
Considering 17 minutes standard deviation in recovery time, 5% error, 10% desired precision, and 90% power, the minimum sample size required for each group was estimated to be 61 cases (10). Data were analyzed using SPSS 20. Qualitative variables were reported as frequency and percentage, and quantitative ones as mean ± standard deviation. Chi squared and Fisher’s exact tests were applied for analytical comparisons. Non-parametric test of chi-square for trend was used for evaluating the frequency of successful PSA cases and assessing recovery time. Significance level was considered to be p < 0.05.
Results:
Baseline characteristics
125 patients with the mean age of 37.8 ± 14.3 (15-60) years were studied (75.2% male). 63 (50.4%) patients were randomly allocated to group 1 and 62 (49.6%) to group 2. Table 1 compares the baseline characteristics of the studied patients. The 2 groups were not significantly different regarding demographic data.
Table 1.
Baseline characteristics of the studied patients
| Variable |
Group 1
(n = 63) |
Group 2
(n = 62) |
P |
|---|---|---|---|
| Sex | |||
| Male | 45 (71.4) | 46 (74.2) | 0.80 |
| Female | 18 (28.6) | 16 (25.8) | |
| Age (year) | |||
| Mean ± standard deviation | 37.2 ± 13.2 | 38.2 ± 15.6 | 0.70 |
| Weight (kg) | |||
| Mean ± standard deviation | 73.4 ± 12.4 | 70.1 ± 11.1 | 0.12 |
| History of PSA* | |||
| No | 56 (91.8) | 54 (87.1) | 0.40 |
| Yes | 5 (8.2) | 8 (12.9) | |
| Reason for requiring PSA* | |||
| Upper limb injury | 45 (71.4) | 44 (71.0) | 0.99 |
| Lower limb injury | 15 (23.8) | 16 (25.8) | |
| Soft tissue injury | 1 (1.6) | 1 (1.6) | |
| Other | 2 (3.2) | 1 (1.6) | |
| History of underlying illness | |||
| Yes | 5 (8.2) | 8 (12.9) | 0.29 |
| No | 58 (92.1) | 54 (87.1) | |
| Vital signs | |||
| Heart rate (/minute) | 84.5 ± 11.6 | 88.0 ± 11.2 | 0.09 |
| Respiratory rate (/minute) | 15.6 ± 1.8 | 16.1 ± 1.9 | 0.23 |
| Systolic blood pressure (mmHg) | 123.4 ± 12.0 | 124.8 ± 12.7 | 0.52 |
| Diastolic blood pressure (mmHg) | 79.9 ± 9.4 | 79.3 ± 19.6 | 0.73 |
| Arterial O2 saturation (%) | 97.3 ± 1.6 | 98.3 ± 13.3 | 0.63 |
PSA: procedural sedation and analgesia; Data are shown as mean ± standard deviation or frequency (%).
Comparison of outcomes
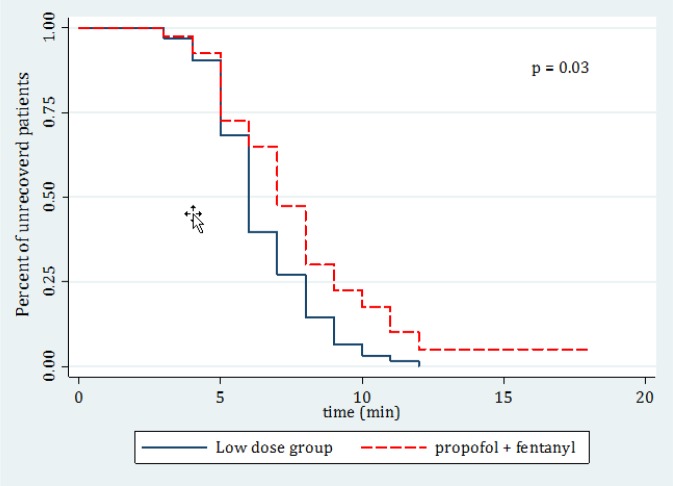
Table 2 compares the outcome of treatment in the 2 groups. The 2 groups were significantly different regarding onset of action (p = 0.440), recovery time (p = 0.018), treatment failure (p < 0.001), physician’s satisfaction (p < 0.001), and patient’s satisfaction (p < 0.001). Kaplan-Meier curve of recovery time for the 2 studied groups is shown in figure 1. 100% of the patients in group 1 and 56.5% of the patients in group 2 were deeply sedated in the 3rd minute after injection. In total, 5 cases of side effect due to PSA were detected that included 3 (2.4%) vertigo and 2 (1.6%) vomiting cases. The cost of the treatment regimen for a 70-kg patient was 33750 Rials (1.7 US dollar) in group 2 (2 drug combination) and 37400 Rials (1.9 US Dollar) in group 1 (5 drug combination).
Table 2.
Comparison of treatment characteristics between low-dose multi-drug group (group 1) and propofol-fentanyl combination group (group 2)
| Variable | Group 1 | Group 2 | P |
|---|---|---|---|
| Onset of action (minute) | |||
| Mean ± standard deviation | 1.73 ± 0.62 | 1.36 ± 1.29 | 0.044 |
| Treatment failure | |||
| Number (%) | 0 (0) | 22 (37.1) | < 0.001 |
| Recovery time (minute) | |||
| Mean ± standard deviation | 6.48 ± 1.84 | 5.02 ± 4.47 | 0.03 |
| Vital signs | |||
| Heart rate (/minute) | 78.2 ± 8.8 | 83.1 ± 9.8 | 0.006 |
| Systolic blood pressure (mmHg) | 119.0 ± 12.1 | 121.0 ± 11.6 | 0.36 |
| Diastolic blood pressure (mmHg) | 74.8 ± 9.0 | 76.25 ± 9.7 | 0.73 |
| Arterial O2 saturation (%) | 95.5 ± 3.1 | 91.9 ± 6.8 | 0.00003 |
| Side effects | |||
| None | 61 (96.8) | 59 (95.2) | 0.836 |
| Vertigo | 1 (1.6) | 2 (3.2) | |
| Vomiting | 1 (1.6) | 1 (1.6) | |
| Patient’s satisfaction | |||
| Dissatisfied | 1 (1.6) | 1 (1.6) | < 0.001 |
| Partial | 19 (30.2) | 56 (90.3) | |
| Complete | 43 (68.3) | 5 (8.1) | |
| Physician’s satisfaction | |||
| Dissatisfied | 1 (1.6) | 1 (1.6) | < 0.001 |
| Partial | 19 (30.2) | 53 (86.9) | |
| Complete | 43 (68.3) | 7 (11.5) |
Data are shown as mean ± standard deviation or frequency (%).
Figure 1.
Kaplan-Meier curve of recovery time (in minutes) for the 2 studied groups.
Discussion:
Based on the findings of the present study, low-dose propofol, ketamine, midazolam, fentanyl, and lidocaine combination had a higher success rate in inducing deep sedation, and patient and physician’s satisfaction compared to fentanyl and propofol combination with regular dose. Recovery time in this group was slightly longer and the 2 groups did not differ significantly regarding drug side effects and influence on vital signs.
As mentioned before, finding new drugs that have few side effects in addition to effective and deep PSA with rapid recovery is of great interest among physicians. In line with the current study, Ebrahimi et al. aimed to compare propofol-midazolam and propofol-fentanyl regimens in patients undergoing microlaryngeal surgery and showed that mean arterial O2 saturation during laryngoscopy was further reduced in fentanyl group, while recovery time was shorter in midazolam group (11). Comparison of propofol-ketamine and propofol-fentanyl compounds for PSA induction in pediatric burn patients during change of dressing showed that there is no significant difference between these combinations regarding heart rate, systolic blood pressure, arterial O2 saturation, respiratory rate, and sedation score during PSA (8). Erden et al. showed that adding a low dose of ketamine to propofol-fentanyl combination leads to decreased risk of drop in arterial O2 saturation and less need for additional propofol for induction of analgesia (12). Using propofol-ketamine combination results in more stable hemodynamics and deeper analgesia in PSA induction for pediatrics during upper gastrointestinal tract endoscopy compared to propofol-fentanyl. However, it had more side effects (9). Akin et al. also revealed that propofol-fentanyl and propofol-ketamine groups had no significant difference regarding analgesia during endometrial biopsy (13). As can be seen, most studies expressed that combination of propofol and ketamine or propofol and midazolam are better regimens for PSA induction compared to propofol-fentanyl. Other observations emphasize that ketamine is a safe drug and a proper sedative for use in EDs (14). The advantage of the present study over other similar ones is using low doses of multiple sedatives for PSA induction. Most study protocols have been based on using 2 or at most 3 drugs, while this study designed a 5-drug protocol. Confirmation of the results of the current study requires carrying out further studies with stronger methodologies and considering different racial characteristics.
Limitations
The study being single-blind is among the most important limitations of this study.
Conclusion:
Based on the findings of the present study, low-dose fentanyl, propofol, midazolam, ketamine and lidocaine combination was more successful in induction of deep sedation compared to regular dose of propofol and fentanyl combination and was associated with higher satisfaction among both patients and physicians. Recovery time was a little longer in this group and the 2 groups were not significantly different regarding drug side effects and effect on vital signs.
Acknowledgement:
The present article is derived from Dr. Shahram Shokrzadeh’s thesis, numbered 105, to achieve his specialist degree in emergency medicine from Shahid Beheshti University of Medical Sciences. Hereby, we sincerely thank all the staff of emergency department of Imam Hossein Hospital for assisting us in all stages of the study.
Authors’ contribution:
All the authors meet the 4 criteria for authorship based on recommendations of the International Committee of Medical Journal Editors.
Conflict of interest:
Hereby, the authors declare that there is no conflict of interest regarding the present study.
Funding:
No financial support has been received for this project.
References
- 1.Karceski S, Morrell M, Carpenter D. The expert consensus guideline series: treatment of epilepsy. Epilepsy & Behavior. 2001;2(6):A1–A50. doi: 10.1016/j.yebeh.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Group TC. Rapid tranquillisation for agitated patients in emergency psychiatric rooms: a randomised trial of midazolam versus haloperidol plus promethazine. BMJ: British Medical Journal. 2003;327(7417):708–14. doi: 10.1136/bmj.327.7417.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esmailian M, Moshiri R, Zamani M. Comparison of the Analgesic Effect of Intravenous Acetaminophen and Morphine Sulfate in Rib Fracture; a Randomized Clinical Trial. Emerg (Tehran) 2014;3(3) [PMC free article] [PubMed] [Google Scholar]
- 4.Faridaalaee G, Rahmani SH, Mehryar H, Bina Shishavan S, Merghati SZ, Valizade Hasanloei MA, et al. Comparison of Intravenous Metoclopramide and Acetaminophen in Primary Headaches: a Randomized Controlled Trial. Emerg (Tehran) 2014;3(2) [PMC free article] [PubMed] [Google Scholar]
- 5.Alimohammadi H, Shojaee M, Samiei M, Abyari S, Vafaee A, Mirkheshti A. Nerve Stimulator Guided Axillary Block in Painless Reduction of Distal Radius Fractures; a Randomized Clinical Trial. Emerg (Tehran) 2013;1(1) [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman GM, Nowakowski R, Troshynski TJ, Berens RJ, Weisman SJ. Risk reduction in pediatric procedural sedation by application of an American Academy of Pediatrics/American Society of Anesthesiologists process model. Pediatrics. 2002;109(2):236–43. doi: 10.1542/peds.109.2.236. [DOI] [PubMed] [Google Scholar]
- 7.Apfelbaum JL, Silverstein JH, Chung FF, Connis RT, Fillmore RB, Hunt SE, et al. Practice Guidelines for Postanesthetic CareAn Updated Report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. The Journal of the American Society of Anesthesiologists. 2013;118(2):291–307. doi: 10.1097/ALN.0b013e31827773e9. [DOI] [PubMed] [Google Scholar]
- 8.Tosun Z, Esmaoglu A, Coruh A. Propofol–ketamine vs propofol–fentanyl combinations for deep sedation and analgesia in pediatric patients undergoing burn dressing changesa. Pediatric Anesthesia. 2008;18(1):43–7. doi: 10.1111/j.1460-9592.2007.02380.x. [DOI] [PubMed] [Google Scholar]
- 9.Tosun Z, Aksu R, Guler G, Esmaoglu A, Akin A, Aslan D, et al. Propofol‐ketamine vs propofol‐fentanyl for sedation during pediatric upper gastrointestinal endoscopy. Pediatric Anesthesia. 2007;17(10):983–8. doi: 10.1111/j.1460-9592.2007.02206.x. [DOI] [PubMed] [Google Scholar]
- 10.Correia LM, Bonilha DQ, Gomes GF, Brito JR, Nakao FS, Lenz L, et al. Sedation during upper GI endoscopy in cirrhotic outpatients: a randomized, controlled trial comparing propofol and fentanyl with midazolam and fentanyl. Gastrointestinal endoscopy. 2011;73(1):45–51. doi: 10.1016/j.gie.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Sajedi PAY, Ahmad A Bigi, Ali Akbar A Rezaie, Mahin Mehrabi, Kooshki Ali. Comparative Evaluation of Vital Signs Stability, Sedation and Analgesia Scores with Two Methods of Sedation: Propofol+Fentanyl and Ketamine+Fentanyl during Perm Cath Insertion. Research in Medicine. 2010;34(1):13–9. [Google Scholar]
- 12.Aydin Erden I, Gulsun Pamuk A, Akinci SB, Koseoglu A, Aypar U. Comparison of propofol‐fentanyl with propofol‐fentanyl‐ketamine combination in pediatric patients undergoing interventional radiology procedures. Pediatric Anesthesia. 2009;19(5):500–6. doi: 10.1111/j.1460-9592.2009.02971.x. [DOI] [PubMed] [Google Scholar]
- 13.Akin A, Guler G, Esmaoglu A, Bedirli N, Boyaci A. A comparison of fentanyl-propofol with a ketamine-propofol combination for sedation during endometrial biopsy. Journal of clinical anesthesia. 2005;17(3):187–90. doi: 10.1016/j.jclinane.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Treston G, Bell A, Cardwell R, Fincher G, Chand D, Cashion G. What is the nature of the emergence phenomenon when using intravenous or intramuscular ketamine for paediatric procedural sedation? Emergency medicine Australasia : EMA. 2009;21(4):315–22. doi: 10.1111/j.1742-6723.2009.01203.x. [DOI] [PubMed] [Google Scholar]