Abstract
Introduction:
Appropriate pain relief enhances patient satisfaction and reduces patient anxiety. This study aimed to compare oral oxycodone with intravenous (IV) morphine sulfate (MS) in pain management of acute limb trauma.
Method:
In this randomized double-blind clinical trial, patients over 14 years old, with acute isolated limb trauma were randomized to receive either 5mg IV MS or 5 mg oral oxycodone. Pain intensity and adverse effects of medications were recorded 0, 30 and 60 minutes after drug administration and compared between the groups.
Result:
58 patients were studied. Pain intensity was similar between the two studied groups at 30 minutes (P = 0.834) and 60 minutes (P = 0.880) after drug administration. Furthermore, there was no significant difference between the two groups regarding decrease in pain within the defined time interval. Drowsiness was reported more frequently in MS group after 30 minutes (p = 0.006). Patients in MS group asked for more rescue analgesia. Other adverse effects were similar in both groups.
Conclusion:
Oral oxycodone is as effective as IV morphine sulfate in treatment of acute musculoskeletal pain following blunt limb trauma.
Key Words: Oxycodone, morphine, wounds and injuries, acute pain
Introduction:
Pain is an unpleasant emotional and sensory experience and the most common cause of emergency department (ED) visits. In the United States, almost 40 million ED visitors looked for analgesics for relief of acute pain in one year [1, 2].
Appropriate pain relief enhances patient satisfaction and reduces patient anxiety [2, 3]. Hence, effective management of acute pain is one of the most important aspects of emergency physicians’ practice [2, 4].
One acceptable approach for pain management in the ED is administration of effective drugs with minimal side effects, through an appropriate route. Several therapeutic agents have been used including acetaminophen (Paracetamol), non-steroidal anti-inflammatory drugs (NSAIDs), opioids and synthetic drugs with narcotic properties [3]. Mild to moderate pain is usually controlled by non-opioid agents, while opioid analgesics are used to reduce moderate-to-severe pain [5].
Intravenous (IV) morphine sulfate (MS) is a common and effective analgesic agent, used for management of moderate to severe pain in ED [6-8]. However, some potential side effects such as respiratory and central nervous system depression, nausea and vomiting and pruritus have been reported [6-8]. Yet another feature that can limit the utility of MS as an ideal analgesic in the ED is its IV form of administration. Therefore, agents that do not require IV line placement could potentially be the preferred approach for initiation of pain treatment [7].
Oxycodone is a semisynthetic analgesic opioid and an agonist of mu, kappa and delta receptors [1, 5, 6, 9]. Mu receptors are known to be responsible for analgesia (µ1), sedation, euphoria and its side effects include pruritus, vomiting and respiratory depression (µ2). Furthermore, kappa receptors are responsible for analgesia, dyspnea, dysphoria, urinary retention, and meiosis while delta receptors have spinal analgesic effects [10]. Oxycodone is a potent opioid with high oral bioavailability, rapid absorption and predictable effects [1, 6, 9]. Compared to morphine, oxycodone has a greater analgesic potency and a shorter half-life. Its onset of action is 1 hour after administration and peak plasma concentration is achieved within 90 minutes [1, 5, 6].
Although oxycodone and morphine share some characteristics such as increased tolerance and addictive potential, [1] easy titration, predictable metabolism, less toxicity and less sedation distinguish oxycodone from morphine [6, 9, 11]. Oxycodone produces less hallucination, nausea and pruritus compared to morphine and it does not affect arterial pressure and heart rate [1, 5, 6].
An overview of prior literature highlighted the lack of studies about use of oral oxycodone in the field of acute trauma. The majority of reports are about chronic pain or in settings other than the ED [12].
This study was designed to compare ease of administration, efficacy and safety of oral oxycodone with those of IV morphine sulfate in sequential time points after drug administration in patients with acute limb trauma.
Method:
Study design and settings
This is a randomized, double-blind placebo controlled clinical trial that was approved and monitored by the ethics committee of Tehran University of Medical Sciences (ethics reference number: 54352). The study was conducted in the emergency department of a tertiary teaching hospital (Imam Khomeini), Tehran, Iran, From July 2014 to March 2015. The trial was officially registered in IRCT.ir (registration number: IRCT201204089387N2). Written informed consent was obtained from all patients before enrolment.
Participants
Eligible participants were adult patients aged 14 or above with moderate to severe pain (Numerical Rating Scale [NRS] more than 3) following isolated limb trauma. We excluded patients with altered consciousness, severe chronic disease (liver, kidney and respiratory), previous history of allergy to opioids, recent opioid use and pregnant patients.
Intervention:
Participants were randomized to receive either 5 mg oral oxycodone hydrochloride plus 5ml IV injection of normal saline or 5 ml equivalent to 5 mg injection of IV MS and oral placebo. Block randomization using computer generated blocks of four was used to assign patients to each group. Drugs were prepared and sealed in consecutively numbered envelops by a research assistant who was not involved in drug administration. Patients’ enrollment, drug administration and data collection were done by another trained research assistant. Research assistants were medical students and they were trained for the study objectives. Patients and assistants who administrated drugs and collected data remained blinded to study groups during the study.
Data Gathering
Prior to drug administration demographic features and mechanism of trauma were registered in data collection sheets. Participants were asked to score the pain severity from 0 to 10. Eligible patients received either drug. Data were collected at 0, 30 and 60 minutes after patients received analgesics.
Outcome assessment
Primary outcome of this study was pain relief and was assessed by NRS at exact time points. Secondary outcomes including changes in blood pressure, dizziness, pruritus, nausea and vomiting were also monitored and registered simultaneously.
Statistical analysis
Based on previous studies, which showed standard deviation of 1.7 in the population [2], and considering an effect size of 1.3, we estimated that sample size of 28 patients in each group will give a power of 80% and two side type one error of 5% to detect a significant difference. Descriptive analysis was used to compare basic features in the two groups. One way ANOVA and general linear mode were used to analyze outcomes between and within groups. The study result analysis was based on per protocol method. Data were analyzed using SPSS 20 statistical software and intension to treat analysis approach.
Results:
Characteristics of the study population
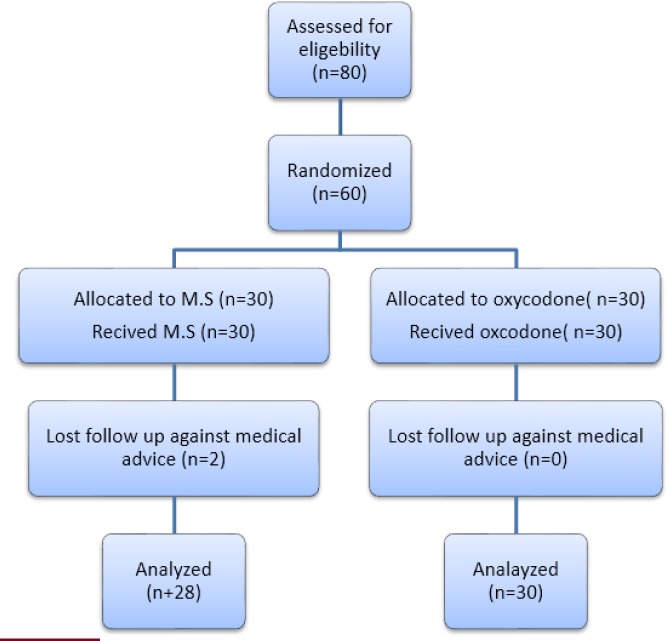
80 patients were assessed for eligibility; 20 of which were excluded from the study. Sixty patients were enrolled and randomly allocated to either oxycodone or MS group. Trial subjects flow is shown in figure 1. Participants’ main characteristics are shown in Table 1. Baseline characteristics were similar between the two groups.
Figure 1.
study participants' flow diagram.
Table 1.
Baseline Characteristics of studied participants
| Characteristics | Morphine sulfate (n=28) | Oxycodone (n=30) | P Value |
|---|---|---|---|
| Age | |||
| Mean ± standard deviation | 32.86 ± 15.39 | 29.27 ±9.35 | 0.432 |
| Sex | |||
| Male | 23 (82.10) | 20 (66.67) | 0.179 |
| Female | 5 (17.90) | 10(33.33) | |
| Mechanism of injury | |||
| Direct trauma | 1 (3.60) | 5 (16.70) | 0.226 |
| MVA | 10 (35.70) | 13 (43.30) | |
| Falling | 14 (50.00) | 11 (36.70) | |
| CPA | 3 (10.7) | 1 (3.30) |
Data were presented as mean ± standard deviation or frequency (%).
MVA: motor vehicle accidents; CPA: car pedestrian accident
Comparison of two groups
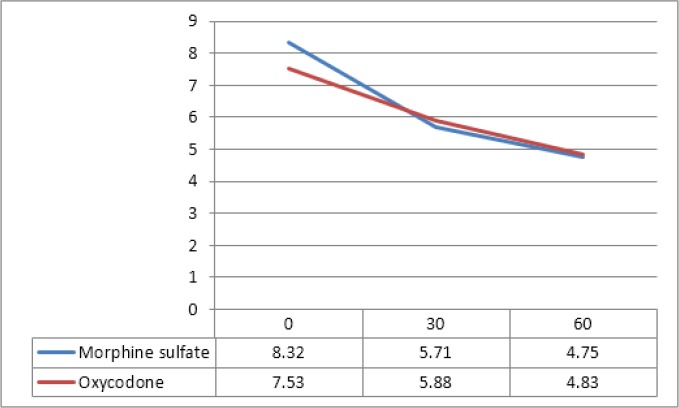
Mean pain score in MS group was 8.32 ± 1.36 at the time 0, reached 5.71 ± 2.39 at 30 minutes, and 4.75 ± 2.24 at 60 minutes after drug administration (p < 0.001). Also mean pain score in oxycodone group was 7.35 ± 1.55 at the time 0, reached 5.83 ± 1.89 at 30 minutes, and 4.83 ± 1.93 at 60 minutes after drug administration (p < 0.001). Although there was a 0.81 difference in pain score between the two study groups at the time 0, there was no significant difference between groups 30 and 60 minutes after administration of medications (Table 2, figure 2).
Table 2.
Comparison of pain severity between groups in 0, 30, and 60 minutes after drug administration
| Time (minute) | Median | Mean ± SD | SEM | P value |
|---|---|---|---|---|
| 0 | ||||
| Morphine Sulfate | 9 | 8.32 ± 1.36 | 0.257 | 0.045 |
| Oxycodone | 8 | 7.53 ± 1.55 | 0.282 | |
| 30 | ||||
| Morphine Sulfate | 6 | 5.71 ± 2.39 | 0.450 | 0.834 |
| Oxycodone | 6 | 5.83 ± 1.89 | 0.346 | |
| 60 | ||||
| Morphine Sulfate | 4 | 4.75 ± 2.24 | 0.422 | 0.880 |
| Oxycodone | 5 | 4.83 ± 1.93 | 0.352 | |
| Differences 0-30 | ||||
| Morphine Sulfate | -2.8214 | 1.96362 | .37109 | .078 |
| Oxycodone | -1.7333 | 1.55216 | .28338 | |
| Differences 30-60 | ||||
| Morphine Sulfate | -0.7857 | .99469 | .18798 | 0.903 |
| Oxycodone | -0.9667 | .92786 | .16940 | |
| Differences 0-60 | ||||
| Morphine Sulfate | -3.5000 | 1.68874 | .31914 | 0.110 |
| Oxycodone | -2.7000 | 1.91455 | .34955 |
SEM, standard error of mean; SD: standard deviation.
Figure 2.
Pain score changes during study period (p > 0.05).
Secondary outcomes
Dizziness was reported more frequently in morphine sulfate group compared to oxycodone group. Eight participants asked for rescue analgesic in morphine group, while only one patient asked for more analgesia in oxycodone group. Other adverse effects were similar in both groups (Table 3). There were no cases of seizure, respiratory depression or loss of consciousness. No naloxone was administrated during the study.
Table 3.
Frequency of adverse event occurrence in the studied groups
| Adverse event | Oxycodone | Morphine sulfate | P value |
|---|---|---|---|
| Hypotension | |||
| 30 minute | 7 (23.30) | 3 (10.70) | 0.301 |
| 60 minute | 8 (26.70) | 4 (14.30) | 0.336 |
| Nausea | |||
| 30 minute | 2 (6.70) | 6 (21.40) | 0.138 |
| 60 minute | 2 (6.70) | 4 (14.30) | 0.415 |
| Dizziness | |||
| 30 minute | 3 (10.00) | 12 (42.90) | 0.006 |
| 60 minute | 11 (36.70) | 11 (39.30) | 1.000 |
| Rescue analgesic | |||
| 30 minute | - | - | - |
| 60 minute | 1(3.30) | 8(28.60) | 0.011 |
Discussion:
The results of the current study demonstrate that oral oxycodone is as effective as IV MS in relieving pain of patients with acute limb trauma. Considering pain score at the time points of 30 minutes and 60 minutes after drug administration, no significant differences were observed between the two groups. To minimize the effect of the mentioned disparity, we analyzed the amount of decrease in pain score in specific time intervals. The result was similar and the analgesic effects of these two opioids were comparable.
A limited number of studies have compared oxycodone with morphine sulfate. The findings of our study are compatible with the study conducted by Miner et al. In their study, patients received either 0.125 mg/kg oral solution of oxycodone or 0.1 mg/kg IV morphine sulfate. Pain score, adverse effects, onset of action and time to administration of drugs were assessed in patients with acute musculoskeletal pains in sequential time intervals [7]. Result of that study, similar to our findings, highlighted that the pain scores of the two study groups were similar 30 minutes after drug administration. The results of our study are also similar to the study by Pedersen et al. Although the results were comparable, the design of their study was different from ours. They studied analgesic effects of morphine and oxycodone, both in dose of 0.1 mg/kg, 4 hours after percutaneous kidney stone surgery [11]. Pain score and side effects of medications were assessed every 15 minutes. Oxycodone appeared to be similar to morphine in analgesia 4 hours after surgery.
In our study, drowsiness was reported more frequently at time point of 30 minutes by patients who received morphine sulfate while the distinction between the two groups was less obvious at time point of 60 minutes. Kalso, in a study in 1991, mentioned more drowsiness in patients who received morphine for post-surgical pain treatment compared to patients who received oxycodone [13]. Other adverse effects were similar in both groups according to our study. Patients in morphine sulfate group asked for more rescue analgesic.
Limitations
We believe that our study faced a number of limitations. Firstly, the sampling of our patients could have been affected by the following conditions: in crowded EDs most patients with acute isolated limb trauma are managed as outpatients and they receive prescribed analgesics at home. Our study population consisted of admitted patients and hence the results may not be applicable to an outpatient population. Furthermore, we conducted the study in a single center. Secondly, we administerated a single dose of 5mg for both morphine sulfate and oxycodone groups instead of adjusting the dosage to the weight. Although the dosage effectively reduced pain; the fact is that the side effects of opioids occur in higher doses. For more accurate comparison of side effects between the two drugs, higher doses should be administrated. Thirdly, we used NRS for pain scoring. It is possible that some patients mentioned higher pain score in order to accelerate the services they would receive. Finally, although we had randomly allocated the patients to either group, the final groups were significantly different regarding sex. As a result of our randomization, there were significant discordance between sex distribution of the two groups and this can be considered as a confounding factor. Considering the fact that there is potential sex difference in analgesic effects of opioids [14-18], result of this study should be interpreted cautiously.
Conclusion:
To sum up, oral oxycodone is as effective as IV morphine sulfate in management of acute pain following limb trauma. It can be considered as an appropriate alternative for IV morphine sulfate in crowded EDs, where placing an IV line may be time-consuming.
Acknowledgment
This study has been supported by Tehran University of Medical Sciences and Health Service (Grant No: 91-1-30-15974). We would like to thank Dr. Ghazale Keshvadi and Dr. Amir Eslami for assistance in data Gathering. We would also like to show our gratitude to Shafa Pharmaceutical and Hygienic Company for providing us with placebos.
Conflict of interest
The authors of this study certify that they have NO involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Author contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other journal.
Specific contributions made by each author are listed as below:
Mohammad Jalili: study concept and design, critical revision of the manuscript for important intellectual content, and acquisition of funding
Ahmad Reza Dehpour: study concept and design, critical revision of the manuscript for important intellectual content
Parisa Eizadi: study concept and design, acquisition of the data, analysis and interpretation of the data, statistical expertise, drafting of the manuscript
References
- 1.V Oldfield, CM Perry. Oxycodone/Ibuprofen Combination Tablet. Drugs. 2005;65:2337–2354. doi: 10.2165/00003495-200565160-00011. [DOI] [PubMed] [Google Scholar]
- 2.CA Marco, MC Plewa, N Buderer C, Black A. Roberts, Comparison of Oxycodone and Hydrocodone for the Treatment of Acute Pain Associated with Fractures: A Double‐blind, Randomized, Controlled Trial. Academic emergency medicine. 2005:282–288. doi: 10.1197/j.aem.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 3.ND Moore. In search of an ideal analgesic for common acute pain. Acute Pain. 2009;11:129–137. [Google Scholar]
- 4.SJ Lovell, T Taira, E Rodriguez, A Wackett, J Gulla, AJ Singer. Comparison of valdecoxib and an oxycodone–acetaminophen combination for acute musculoskeletal pain in the emergency department: a randomized controlled trial. Academic emergency medicine. 2004:1278–1282. doi: 10.1197/j.aem.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 5.IA Dhalla, MM Mamdani, ML Sivilotti. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. Canadian Medical Association Journal. 2009:891–896. doi: 10.1503/cmaj.090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MA Zare, AH Ghalyaie M, Fathi D, Farsi S, Abbasi P. Hafezimoghadam, Oral oxycodone plus intravenous acetaminophen versus intravenous morphine sulfate in acute bone fracture pain control: a double-blind placebo-controlled randomized clinical trial. European Journal of Orthopaedic Surgery & Traumatology. 2014:1305–1309. doi: 10.1007/s00590-013-1392-x. [DOI] [PubMed] [Google Scholar]
- 7.JR Miner, J Moore, RO Gray. Oral versus intravenous opioid dosing for the initial treatment of acute musculoskeletal pain in the emergency department. Academic Emergency Medicine. 2008:1234–1240. doi: 10.1111/j.1553-2712.2008.00266.x. [DOI] [PubMed] [Google Scholar]
- 8.O Ahmadi, MN Isfahani, A Feizi. Comparing low-dose intravenous ketamine-midazolam with intravenous morphine with respect to pain control in patients with closed limb fracture. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences. 2014;19:502. [PMC free article] [PubMed] [Google Scholar]
- 9.F Franceschi, M Marini, S Ursella. Use of oxycodone in polytrauma patients: the``Gemelli''experience. European review for medical and pharmacological sciences. 2008;12:123. [PubMed] [Google Scholar]
- 10.AM Trescot, S Datta. Opioid pharmacology. Pain physician. 2008:S133–153. [PubMed] [Google Scholar]
- 11.KV Pedersen, AE Olesen, AM Drewes. Morphine versus oxycodone analgesia after percutaneous kidney stone surgery. Urolithiasis. 2013:423–430. doi: 10.1007/s00240-013-0587-2. [DOI] [PubMed] [Google Scholar]
- 12.Z Al Dabbagh. No signs of dose escalations of potent opioids prescribed after tibial shaft fractures: a study of Swedish National Registries. BMC anesthesiology. 2014;14:1. doi: 10.1186/1471-2253-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.E Kalso, R Pöyhiä, P Onnela, K Linko I. Intravenous morphine and oxycodone for pain after abdominal surgery. Acta anaesthesiologica scandinavica. 1991;35:642–646. doi: 10.1111/j.1399-6576.1991.tb03364.x. [DOI] [PubMed] [Google Scholar]
- 14.M Niesters, A Dahan, B Kest, J Zacny, T Stijnen, L Aarts, E Sarton. Do sex differences exist in opioid analgesia? .A systematic review and meta-analysis of human experimental and clinical studies. Pain. 2010;151:61–68. doi: 10.1016/j.pain.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 15.E Sarton, E Olofsen, R Romberg, J den Hartigh, B Kest. Sex Differences in Morphine AnalgesiaAn Experimental Study in Healthy Volunteers. The Journal of the American Society of Anesthesiologists. 2000;93:1245–1254. [Google Scholar]
- 16.JK Zubieta, RF Dannals, JJ Frost. Gender and age influences on human brain mu-opioid receptor binding measured by PET. American Journal of Psychiatry. 1999;156:842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 17.JK Zubieta, YR Smith, JA Bueller, Y Xu, MR Kilbourn, DM Jewett, CR Meyer, RA Koeppe, CS Stohler. μ-Opioid receptor-mediated antinociceptive responses differ in men and women. Journal of Neuroscience. 2002:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.TN Andreassen, P Klepstad, A Davies, K Bjordal, S Lundström, S Kaasa, O Dale. Influences on the pharmacokinetics of oxycodone: a multicentre cross-sectional study in 439 adult cancer patients. European journal of clinical pharmacology. 2011;67:493–506. doi: 10.1007/s00228-010-0948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]