Abstract
Introduction:
Cardiopulmonary resuscitation (CPR) is a method to improve survival of patients with cardiac arrest. This study aimed to identify the key genes affected five minutes after cardiac arrest, hoping to elevate the efficacy of CPR.
Methods:
In this bioinformatics study differentially expressed genes of six pigs were downloaded from GEO and screened. The significant and characterized genes were analyzed via calculating fold change and protein-protein interaction (PPI) networks. The crucial nodes were determined based on centrality parameters and their related biological processes were investigated via ClueGO.
Results:
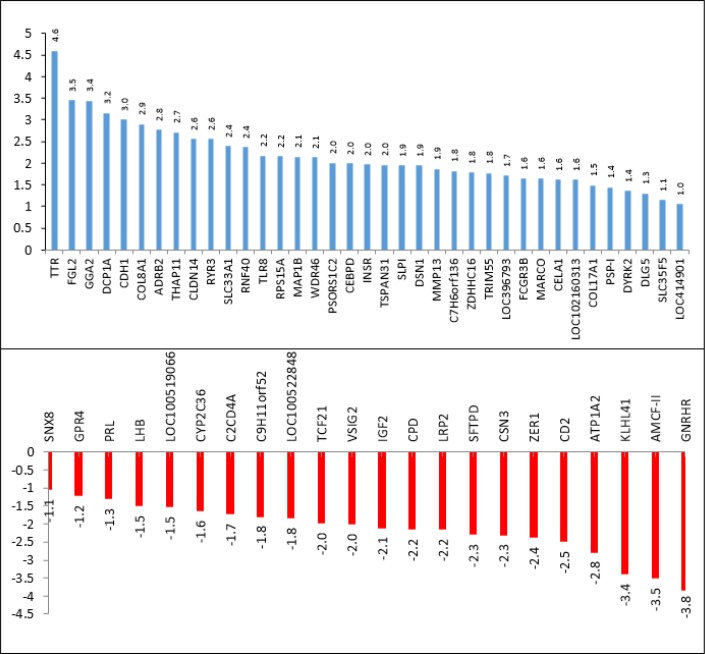
17 significant up-regulated (LogFC ≥ 2) and 22 down-regulated (LogFC < -0.5) genes were detected. Transthyretin (TTR logFC = 4.59) and Gonadotropin-releasing hormone receptor (GNRHR logFC = 3.84) had higher logFC among up-regulated and down-regulated genes, respectively. The critical genes including four up-regulated and five down-regulated genes were detected from network analysis. GNRHR and Prolactin precursor (PRL) were among the most important down res 5 minutes after cardiac arrest and Beta-2 adrenergic receptor and Cadherin-1 were among the most important up regulated gens.
Conclusion:
The introduced potential biomarkers could reveal a new molecular aspect for CPR performance and pituitary gland protection was highlighted in this respect.
Key Words: Cardiac Arrest, Cardiopulmonary Resuscitation, Protein Interaction Maps, Gene Ontology, Biomarkers
Introduction:
Cardiopulmonary resuscitation (CPR) is established and developed to increase perfusion in cardiac arrest patients and improve their survival (1). Although resuscitation science has progressed remarkably, poor survival of cardiac arrest patients is a still a problem in medicine (2). There are evidence that the majority of survivors are neurologically damaged in the short term, which can lead to long term disorders (3). Post cardiac arrest syndrome as well as high rate of in hospital mortality after CPR are reported and discussed (4).
Attempts have been made to understand the molecular and cellular aspects of CPR, which can help to improve the quality of life in patients who survive (5). In addition, treatment with chemical reagents could improve the outcome of CPR performance. For instance, it has been reported that nitric oxide inhalation improves CPR outcome in animal model (6). Recently, high throughput molecular investigations including proteomics and genomics have provided large amounts of data and have attracted the attention of researchers in various fields of medicine and pharmacology. Martijn and Wiklund published valuable microarray data about CPR in pigs, which is a useful molecular source about CPR (7). There are various methods that are useful for decreasing the volume of data and highlighting the remarkable components (8). Protein-protein interaction (PPI) networks analysis is an attractive method to screen disease-related genes (9, 10). In this approach, the genes or proteins are organized in an interactome unit and the critical elements are identified (11, 12).
In this study, the aim was to identify crucial CPR related genes among the large number of genes introduced by Martijn and Wiklud (7) using PPI network analysis. The findings will promote molecular aspects of CPR, which is important to elevate the efficacy of CPR methods.
Methods:
Study design and setting
This bioinformatics study was conducted via evaluating gene expression changes in the animal model of cardiac arrest 5 minutes after its incidence, for better programing of treatment approaches and CPR performance. The selected groups were from one genomic study conducted by Martijn and Wiklud (7). In the mentioned original study, three groups are available with different time courses and treatments. The protocol of the present study was approved by ethics committee of Shahid Beheshti University of Medical Sciences.
Participants
Data of differentially expressed genes of six pigs’ brain samples (Sus scrofa) were downloaded from Gene Expression Omnibus (GEO). The animals were cardiac arrested as described in the report of Miclescu, Basu, and Wiklund (13). 3 animals in control group (0 minute after cardiac arrest) and 3 in case group (5 minutes after cardiac arrest) were considered for analysis.
Procedure
After downloading and screening 250 differentially expressed genes from GEO, the next step was to construct a network of interactions.
The hub-nodes were identified via mean ± 2 standard deviation cut off on degree values and the top 5% of the nodes based on betweenness centrality were considered as bottleneck-genes. Common hub and bottleneck genes were introduced as hub-bottleneck nodes (the crucial genes).
Detail of gene expression assay is explained in the report of Martijn and Wiklud (7). Box plot finding indicates that the mid points of data are in the same range; therefore, samples are comparable in terms of gene expression.
Statistical analysis
The differentially expressed genes with a fold change (FC) above 2 (for up-regulated genes) and below -0.5 (for down-regulation) were selected for analysis. Considering p ≤ 0.01 and elimination of uncharacterized genes, the candidate genes were determined. The PPI network was constructed using Cytoscape software version 3.6.0 via its STRING plugin (14). ClueGO was used for gene ontology analysis of the introduced key genes.
Results:
Fold change analysis
LogFC of the 59 differentially expressed genes is calculated and shown in figure 1 (37 up-regulated and 22 down-regulated). Based on fold change analysis, 17 significant up-regulated (LogFC ≥ 2) and 22 significant down-regulated (LogFC < -0.5) genes were detected. Transthyretin (TTR logFC = 4.59) and Gonadotropin-releasing hormone receptor (GNRHR logFC = 3.84) had higher logFC among up-regulated and down-regulated genes, respectively.
Figure 1.
Log fold change (FC) of the 59 differentially expressed genes. Blue color: up-regulated genes; red color: down-regulated genes.
PPI network analysis
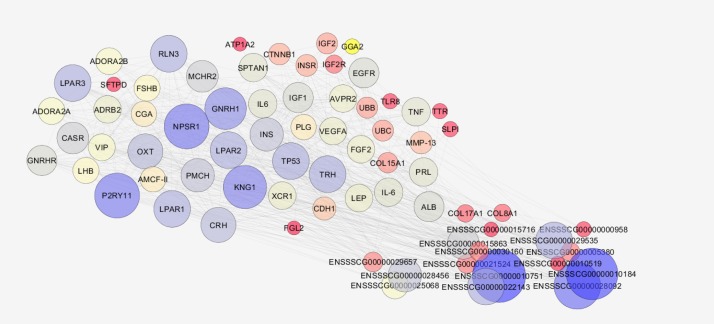
The PPI network contained 29 isolated nodes and a main connected component including 72 linked genes with 634 edges (figure 2). The critical genes including four up-regulated and five down-regulated genes were detected via network analysis and listed in table 1. GNRHR and Prolactin precursor (PRL) were among the most important down-regulated genes 5 minutes after cardiac arrest and Beta-2 adrenergic receptor and Cadherin-1 were among the most important up-regulated genes based on this analysis.
Figure 2.
The main connected component of Protein-protein interaction network of the cardiac arrested pigs. The bigger size and the blue color nodes refer to higher degree value. The uncharacterized nodes are shown in the bottom right corner.
Table 1.
List of central genes (hub-bottleneck nodes) of the main connected component (only including query genes)
| Genes | Description | Degree | Centrality * |
|---|---|---|---|
| GNRHR | Gonadotropin-releasing hormone receptor | 22 | 0.05 |
| PRL | Prolactin precursor | 21 | 1.00 |
| ADRB2 | Beta-2 adrenergic receptor | 20 | 0.77 |
| LHB | Lutropin subunit beta | 18 | 0.04 |
| AMCF-II | Alveolar macrophage chemotactic factor 2 | 17 | 0.19 |
| CDH1 | Cadherin-1 | 14 | 0.02 |
| MMP-13 | Matrix Metalloproteinase-13 | 13 | o.57 |
| INSR | Insulin receptor | 12 | 0.00 |
| IGF2 | Insulin-like growth factor II | 11 | 0.13 |
Normalized betweenness centrality; Red color: up-regulation; Green color: down-regulation.
GNRHR is highlighted as the top central gene following PPI network analysis; however, TTR was not remarkable in this analysis.
Discussion:
There are many investigations about events occurring after a cardiac arrest and intervention approaches in this regard. Many factors could play a role in the quality of life after CPR. One of the key factors is timing (7). To improve the medical care in this state, understanding the pathophysiology of CA is critical. Investigating molecular mechanisms could assist in reaching this goal. Molecular changes are the main corresponding features of any organism’s profile (11, 12). In this respect, one way is to analyze gene expression profile of patients with cardiac arrest to set new diagnosis and treatment strategies. In our bioinformatics study, animal model of cardiac arrest was selected for further evaluations to provide a molecular view of cardiac arrest for promoting CPR procedure. Comparison of samples depicted that there are genes with significant expression values, in which up-regulation is dominant. These vast expression changes within 5 minutes post cardiac arrest express that there are complex molecular changes in this state. Further analysis evaluates the role and contribution of these genes to pathophysiology 5 minutes after cardiac arrest, which can lead to organ injury. TTR and GNRHR were the most up-regulated and down-regulated genes in the study, respectively.
Plasma TTR with high concentration level is responsible for transportation of thyroxine and retinol, but within the mammalian central nervous system it may play another role (16). It seems that, the vast expression change of TTR may be correlated to the possible decrement of thyroxin hormone, the concentration of which is controlled by the pituitary gland.
GNRHR and its GNRH are important endocrine elements in regulation of reproduction (17). Nine crucial genes are recognized as vital elements of cardiac arrest PPI network, which are divided in the two sub-groups of up-regulated and down-regulated genes (see table 1). It seems that biological interactions between the nine highlighted genes may play significant roles in cardiac arrest and could be important for CPR. It is reasonable to consider ADRB2, CDH1, IGF2, INSR, PRL, LHB, and GNRHR as candidate biomarker panel of cardiac arrest after 5 minutes.
It is reported that ADRB2 affects the lungs to change fluid clearance (18). Various types of activity such as role in different cancers, axon growth, and brain metabolism are reported for CDH1 gene (19-22). Insulin-like growth factor II is an embryonic growth factor and its expression is reported in most tissues. The relationship between IGF2 and a number of cancers and cardiomyopathy has been investigated (23, 24). A glucose-insulin-potassium infusion is considered for cardiac arrest; however, it does not have an effect on reduction of mortality (25). It seems that insulin (or its receptor) is linked to cardiac arrest (26). PRL and GNRH are the two important hormones of pituitary gland and their well-known function is to play a critical role in lactation and fertility (27, 28). Subunit beta of gonadal lutropin is responsible for receptor binding of the hormone (29).
As mentioned, the crucial genes are directly related to pituitary gland. Thus, this finding implies that the protection of this vital gland could be critical during CPR. Hence, simultaneous treatment intervention is suggested for CPR promotion. On the other hand, the biological processes that are related to the pituitary gland may be damaged in the survivors. It is possible that a young survivor loses fertility or experiences some related disorders. The other significant point is the possible role of these critical genes in increasing the percentage of successful administration of CPR. Complementary research on survivors is required to support the findings of this study.
Conclusion:
The finding indicates that pituitary gland is a sensitive part during cardiac arrest and its protection should be considered in CPR guidelines.
Acknowledgment
This project is supported by Shahid Beheshti University of Medical Sciences.
Conflict of interests
The authors declare that they have no conflict of interest.
Author contribution
All authors meet the standard criteria of authorship based on the recommendations of the international committee of medical journal editors.
Funding and support
This project is supported by Shahid Beheshti University of Medical Sciences.
References
- 1.Cave DM, Gazmuri RJ, Otto CW, Nadkarni VM, Cheng A, Brooks SC, et al. Part 7: CPR techniques and devices. Circulation. 2010;122(18 suppl 3):S720–S8. doi: 10.1161/CIRCULATIONAHA.110.970970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung J, Meeks R, Edelson D, Gao F, Soar J, Perkins GD. The use of CPR feedback/prompt devices during training and CPR performance: a systematic review. Resuscitation. 2009;80(7):743–51. doi: 10.1016/j.resuscitation.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Zhao H, Li C-S, Gong P, Tang Z-R, Hua R, Mei X, et al. Molecular mechanisms of therapeutic hypothermia on neurological function in a swine model of cardiopulmonary resuscitation. Resuscitation. 2012;83(7):913–20. doi: 10.1016/j.resuscitation.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Minamishima S, Bougaki M, Sips PY, De Yu J, Minamishima YA, Elrod JW, et al. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3–dependent mechanism in mice. Circulation. 2009;120(10):888–96. doi: 10.1161/CIRCULATIONAHA.108.833491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böttiger BW, Motsch J, Braun V, Martin E, Kirschfink M. Marked activation of complement and leukocytes and an increase in the concentrations of soluble endothelial adhesion molecules during cardiopulmonary resuscitation and early reperfusion after cardiac arrest in humans. Critical care medicine. 2002;30(11):2473–80. doi: 10.1097/00003246-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Minamishima S, Kida K, Tokuda K, Wang H, Sips PY, Kosugi S, et al. Inhaled Nitric Oxide Improves Outcomes After Successful Cardiopulmonary Resuscitation in MiceClinical Perspective. Circulation. 2011;124(15):1645–53. doi: 10.1161/CIRCULATIONAHA.111.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martijn C, Wiklund L. Effect of methylene blue on the genomic response to reperfusion injury induced by cardiac arrest and cardiopulmonary resuscitation in porcine brain. BMC medical genomics. 2010;3(1):27. doi: 10.1186/1755-8794-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navlakha S, Kingsford C. The power of protein interaction networks for associating genes with diseases. Bioinformatics. 2010;26(8):1057–63. doi: 10.1093/bioinformatics/btq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen T-P, Liu W-c, Jordán F. Inferring pleiotropy by network analysis: linked diseases in the human PPI network. BMC systems biology. 2011;5(1):179. doi: 10.1186/1752-0509-5-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karbalaei R, Allahyari M, Rezaei-Tavirani M, Asadzadeh-Aghdaei H, Zali MR. Protein-protein interaction analysis of Alzheimers disease and NAFLD based on systems biology methods unhide common ancestor pathways. Gastroenterology and Hepatology from bed to bench. 2018;11(1) [PMC free article] [PubMed] [Google Scholar]
- 11.Safari-Alighiarloo N, Taghizadeh M, Rezaei-Tavirani M, Goliaei B, Peyvandi AA. Protein-protein interaction networks (PPI) and complex diseases. Gastroenterology and Hepatology from bed to bench. 2014;7(1) [PMC free article] [PubMed] [Google Scholar]
- 12.Peyvandi H, Peyvandi AA, Safaei A, Azodi MZ, Rezaei-Tavirani M. Introducing Potential Key Proteins and Pathways in Human Laryngeal Cancer: A System Biology Approach. Iranian Journal of Pharmaceutical Research: IJPR. 2018;17(1):415. [PMC free article] [PubMed] [Google Scholar]
- 13.Miclescu A, Basu S, Wiklund L. Cardio-cerebral and metabolic effects of methylene blue in hypertonic sodium lactate during experimental cardiopulmonary resuscitation. Resuscitation. 2007;75(1):88–97. doi: 10.1016/j.resuscitation.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Bai B, Xie B, Pan Z, Shan L, Zhao J, Zhu H. Identification of candidate genes and long non-coding RNAs associated with the effect of ATP5J in colorectal cancer. International journal of oncology. 2018;52(4):1129–38. doi: 10.3892/ijo.2018.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mlecnik B, Galon J, Bindea G. Comprehensive functional analysis of large lists of genes and proteins. Journal of proteomics. 2018;171:2–10. doi: 10.1016/j.jprot.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Herbert J, Wilcox JN, Pham K-TC, Fremeau RT, Zeviani M, Dwork A, et al. Transthyretin A choroid plexus‐specific transport protein in human brain: The 1986 S Weir Mitchell Award. Neurology. 1986;36(7):900. doi: 10.1212/wnl.36.7.900. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Z, Gibson JP, Archibald AL, Haley CS. The porcine gonadotropin-releasing hormone receptor gene (GNRHR): genomic organization, polymorphisms, and association with the number of corpora lutea. Genome. 2001;44(1):7–12. doi: 10.1139/gen-44-1-7. [DOI] [PubMed] [Google Scholar]
- 18.Snyder EM, Turner ST, Johnson BD. β2-adrenergic receptor genotype and pulmonary function in patients with heart failure. Chest. 2006;130(5):1527–34. doi: 10.1378/chest.130.5.1527. [DOI] [PubMed] [Google Scholar]
- 19.Graziano F, Humar B, Guilford P. The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice. Annals of oncology. 2003;14(12):1705–13. doi: 10.1093/annonc/mdg486. [DOI] [PubMed] [Google Scholar]
- 20.Pannone G, Santoro A, Feola A, Bufo P, Papagerakis P, Lo Muzio L, et al. The role of E-cadherin down-regulation in oral cancer: CDH1 gene expression and epigenetic blockage. Current Cancer Drug Targets. 2014;14(2):115–27. doi: 10.2174/1568009613666131126115012. [DOI] [PubMed] [Google Scholar]
- 21.Konishi Y, Stegmüller J, Matsuda T, Bonni S, Bonni A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303(5660):1026–30. doi: 10.1126/science.1093712. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Rodriguez P, Almeida A, Bolaños JP. Brain energy metabolism in glutamate-receptor activation and excitotoxicity: role for APC/C-Cdh1 in the balance glycolysis/pentose phosphate pathway. Neurochemistry international. 2013;62(5):750–6. doi: 10.1016/j.neuint.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, Steegers EA, et al. Periconceptional maternal folic acid use of 400 µg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One. 2009;4(11):e7845. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brade T, Kumar S, Cunningham TJ, Chatzi C, Zhao X, Cavallero S, et al. Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial Igf2. Development. 2011;138(1):139–48. doi: 10.1242/dev.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta SR, Yusuf S, Díaz R, Zhu J, Pais P, Xavier D, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. Jama. 2005;293(4):437–46. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 26.Stellpflug SJ, Harris CR, Engebretsen KM, Cole JB, Holger JS. Intentional overdose with cardiac arrest treated with intravenous fat emulsion and high-dose insulin. Clinical toxicology. 2010;48(3):227–9. doi: 10.3109/15563650903555294. [DOI] [PubMed] [Google Scholar]
- 27.La Rosa S, Celato N, Uccella S, Capella C. Detection of gonadotropin-releasing hormone receptor in normal human pituitary cells and pituitary adenomas using immunohistochemistry. Virchows Archiv. 2000;437(3):264–9. doi: 10.1007/s004280000247. [DOI] [PubMed] [Google Scholar]
- 28.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiological reviews. 2000;80(4):1523–631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 29.Cosowsky L, Rao SV, Macdonald GJ, Papkoff H, Campbell RK, Moyle WR. The groove between the α-and β-subunits of hormones with lutropin (LH) activity appears to contact the LH receptor, and its conformation is changed during hormone binding. Journal of Biological Chemistry. 1995;270(34):20011–9. doi: 10.1074/jbc.270.34.20011. [DOI] [PubMed] [Google Scholar]