Abstract
Tuberculosis has been reaffirmed as the infectious disease causing most deaths in the world. Co-infection with HIV and the increase in multi-drug resistant Mycobacterium tuberculosis strains complicate treatment and increases mortality rates, making the development of new drugs an urgent priority. In this study we have identified a promising candidate by screening antimicrobial peptides for their capacity to inhibit mycobacterial growth. This non-toxic peptide, NZX, is capable of inhibiting both clinical strains of M. tuberculosis and an MDR strain at therapeutic concentrations. The therapeutic potential of NZX is further supported in vivo where NZX significantly lowered the bacterial load with only five days of treatment, comparable to rifampicin treatment over the same period. NZX possesses intracellular inhibitory capacity and co-localizes with intracellular bacteria in infected murine lungs. In conclusion, the data presented strongly supports the therapeutic potential of NZX in future anti-TB treatment.
Keywords: Mycobacterium tuberculosis, Antimicrobial peptides, Tuberculosis treatment
1. Introduction
Despite the availability of antibiotics and the extensive use of the live-attenuated vaccine Bacille Calmette-Guérin (BCG), tuberculosis (TB) remains a major health concern with an estimated 10.4 million new cases every year. Current treatment and vaccination programmes have failed to make a significant impact on either transmission or protection against disease, while co-infection with HIV and the emergence of drug resistant strains has further undermined TB control programmes [1,2]. Isoniazid and Rifampicin form an essential part of the standard four-drug treatment for drug-sensitive infections, with a cure rate of 95% under optimal conditions [3]. However, the duration of treatment is long, varying from six months for drug susceptible TB, to more than two years for multi-drug resistant (MDR) TB, where bacteria are resistant to the first-line anti-TB drugs, and extensively drug resistant (XDR) TB in which strains have acquired additional resistance to fluoroquinolone and any one of the three injectable second-line anti-TB drugs [4]. Prolonged treatment contributes to poor patient compliance and the emergence of antibiotic resistance. In 2016, the WHO estimated 600,000 new cases of MDR-TB, of which 6.2% were XDR-TB [5,6].
Antimicrobial peptides (AMPs) have gained interest as potential therapeutics to treat mycobacterial infections. Among the antimicrobial peptides, human defensins and cathelicidins play an important role linking innate and adaptive immune responses [7]. Defensins are a family of naturally occurring cysteine-rich peptides found in higher plants and animals that display activity against a wide variety of microbes, making them attractive drug candidates. The majority of defensins act through disruption of microbial membranes, although they may have additional host-related immune-modulating activities [8]. Most defensins are amphipathic molecules with clusters of positively charged and hydrophobic amino-acid side chains that interact with microbial membranes. It has been proposed that the cationic portion targets the peptide to the negatively charged bacterial membrane, while the hydrophobic portion intercalates into the membrane [9,10].
Many naturally occurring AMPs have been tested for activity against Mycobacterium tuberculosis, including human and rabbit defensins and porcine protegrins [[11], [12], [13], [14]]. In addition to naturally occurring AMPs, synthetic libraries have been tested for activity against M. tuberculosis [15]. Suboptimal efficacy, instability and/or toxicity have so far precluded testing of most AMPs in animal models of M. tuberculosis infection. Until now, only the peptides LL-37, innate defense regulators and ecumicin showed significant results in experimental TB murine models after 25–28-days of treatment [16,17]. In this study we investigated NZX, a novel derivative of plectasin, which is the first fungal defensin-like AMP with proven activity against Streptococcus pneumoniae and methicillin-resistant Staphylococcus aureus [18,19]. Plectasin peptides can be produced as a recombinant protein expressed in fungi and obtained at high purity, thereby avoiding the high manufacturing costs of AMPs [18,19]. The current study presents data on the effect of NZX on M. tuberculosis both in vitro and in a murine model of acute M. tuberculosis infection. We found NZX is proteolytically stable and non-toxic to eukaryotic cells but kills M. tuberculosis at concentrations comparable to conventional antibiotics in both in vitro and in vivo models of infection. In the murine TB-model, we found that NZX associated with mycobacteria inside macrophages, and substantially lowered the bacterial load with five days of treatment, comparable to the reduction seen with rifampicin treatment over the same time period.
2. Materials and methods
2.1. Peptides
Four of the investigated peptides were previously reported to possess activity against M. tuberculosis in vitro or in vivo (Table 1). LL-37 and the synthetic variants of tryptophan-rich peptides were previously shown to eliminate M. tuberculosis at low concentrations in vitro [14,16,20,21]. Plectasin as a potential TB therapeutic although mentioned in the literature has not been tested experimentally to-date [22,23].
Table 1.
Screening of peptides against mycobacteria.
| Name | Peptide |
|---|---|
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES-NH2 |
| WKWLKKWIK-OHxTFA | |
| WKWLKKWIKG-NH2xTFA | |
| WKWLKKWIKG-NH2xHOAc | |
| NZ2114 | GFGCNGPWNEDDLRCHNHCKSIKGYKGGYCAKGGFVCKCYa |
| NZX | GFGCNGPWSEDDIQCHNHCKSIKGYKGGYCARGGFVCKCYa |
Disulfide bonds at position C4-C30, C15-C37, C19-C39 and differences in amino acid sequence N9S, L13I, K32R (bold).
NZ2114 is a variant of plectasin, originally isolated from Pseudoplectania nigrella [18,24,25]. Numerous publications report NZ2114 to exhibit improved activity compared to plectasin against staphylococci, including Staphylococcus aureus, as well as Streptococci pneumonia [18,24,25] (Table 1). The peptide NZX was manufactured by solid phase peptide synthesis, followed by cyclisation of the three naturally occurring disulphide bonds and purification by sequential chromatography steps (PolyPeptide Laboratories AB, Limhamn, Sweden). The peptide NZ2114 was provided by Adenium ApS, Denmark. The purity (97.3%) of the peptides was confirmed by high-performance liquid chromatography.
2.2. Bacteria
For screening experiments and the time kill assay, Mycobacterium bovis bacillus Calmette-Guerin (BCG) Montreal containing the pSMT1-luxAB plasmid was prepared as previously described [26]. Briefly, BCG was grown in Middlebrook 7H9 broth, supplemented with 10% ADC enrichment (Middlebrook Albumin Dextrose Catalase Supplement, Becton Dickinson, Oxford, UK) and hygromycin (50 mg/l; Roche, Lewes, UK), the culture was washed twice with sterile PBS, and re-suspended in broth and then dispensed into vials. Glycerol was added to a final concentration of 25% and the vials were frozen at −80 °C. Prior to each experiment, a vial was defrosted, added to 9 ml of 7H9/ADC/hygromycin medium, and incubated with shaking for 72 h at 37 °C. Mycobacteria were then centrifuged for 10 min at 3000×g, washed twice with PBS, and re-suspended in 10 ml of PBS.
For murine TB experiments, we used M. tuberculosis H37Rv with known expression of the surface lipid phthiocerol dimycocerosate (PDIM) (a kind gift from Christophe Guilhot, Institut de Pharmacologie et de Biologie Structurale (IPBS), Toulouse, France). The strain was cultured to mid-log phase in Middlebrook 7H9 culture medium, supplemented with 0.05% Tween 80, 0.2% glycerol and 10% oleic acid-albumin-dextrose-catalase (OADC) enrichment (Becton Dickinson, Oxford, UK).
In preparation for MIC-determination and electron microscopy studies of the effect of NZX on M. tuberculosis in vitro, H37Rv (ATCC 27294) and three clinical strains isolated from pleural effusions (TB2016/268 (clinical isolate 1), TB1298 (clinical isolate 2) and TB4001 (clinical MDR isolate)) were cultured in MGIT960 according to manufacturer's instructions. TB4001 is an MDR-TB strain, resistant to Rifampicin and Isoniazid (Supplementary Table 2). The other two strains were fully susceptible to first-line antibiotics and were verified to be M. tuberculosis using standard methods at Clinical Microbiology, Regional Laboratories Skåne, Lund, Sweden (data not shown). Mycobacterium smegmatis mc2155 (a kind gift from Prof. Leif Kirsebom, Department of Cell and Molecular Biology, Box 596, Biomedical Centre, Uppsala, Sweden).
For the intracellular experiment, M. tuberculosis H37Rv (ATCC 27294) transformed with a Live-Dead reporter plasmid [27] was used for infecting primary human macrophages. The bacteria were grown to mid-log phase at 37 °C in Middlebrook 7H9 medium (BD Biosciences, San Diego, CA, USA) with 0.05% Tween-80, 0.05% glycerol and albumin-dextrose-catalase enrichment (ADC, Becton Dickinson) in the presence of 50 μg/ml hygromycin B (Sigma-Aldrich, St Louis, MO) as a selective antibiotic. The bacteria were passaged at 1:9 in the medium and incubated for one more week before use in experiments.
2.3. Cells
Human venous blood mononuclear cells were obtained from healthy volunteers using a Lymphoprep density gradient (Axis-Shield, Oslo, Norway) according to the manufacturer's instructions. To obtain pure monocytes, CD14 micro beads were applied to the cell suspension, washed and passed through a LS-column according to manufacturer's description (130-050-201, 130-042-401, Miltenyi Biotec, USA). The monocytes were counted (Sysmex), diluted in RPMI 1640 supplemented with 5% FCS, NEAA, 1 mM Sodium Pyruvate, 0.1 mg/ml Gentamicin (11140-035, 111360-039, 15710-49, Gibco, Life Technologies) and 50 ng/ml GM-CSF (215-GM, R&D systems) and seeded in 96-well plates (105/well) for a week to differentiate into macrophages. Infection experiments were performed in RPMI 1640 without Gentamicin.
The human monocyte cell line, THP-1-XBlue™-CD14 (Invivogen, San Diego, USA) were cultured in RPMI 1640 supplemented with 10% FCS, Antibiotic-Antimycocytic, Zeocin, and Geneticin (15240062, R25005, 10131035, Gibco, Life Technologies).
2.4. Screening studies
To measure peptide activity against mycobacteria, BCG expressing luxAB was diluted in Middlebrook 7H9 medium (104 CFU; 150 μl/well) in 96-well opaque white plates (Corning). Peptides (0, 6.3, 12.5, 25, 50 or 100 μM) were added to the wells. Growth controls containing no peptide and peptide without bacteria were also prepared. The plates were incubated at 37 °C for 24 h before adding 0.1% n-decyl aldehyde (Decanal, Sigma), a substrate for bacterial luciferase. Bioluminescence was measured as relative luminescence unit (RLU) [26] for 1s using a TriStar2 microplate reader (Berthold Technologies).
2.5. Cytotoxicity assays
For the peptide-screening assay, primary macrophages were prepared from whole blood (see above). The medium was replaced with fresh medium containing 0, 6.3, 12.5, 25 or 100 μM peptides and incubated for 0, 1, 4 and 24 h in 5% CO2 atmosphere. For cytotoxicity measurements, 10 μl 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) solution (Sigma) was added to each well according to manufacturer's instructions and analysed in a spectrophotometer at 580 nm.
NZX cytotoxicity was further examined by PrestoBlue® and ATPlite™ assays. Primary macrophages were treated with 100 μM NZX or 50 μM Staurosporine (S-4400, Sigma) for 24 h. Cell viability was assessed with PrestoBlue® fluorescence (A13261, Thermo Scientific) and cellular ATP levels using ATPlite™ kit (6016943, Perkin Elmer) compared to untreated controls, according to the manufacturer's instructions.
NF-κB activation was measured in human THP-1-XBlue™-CD14 monocytes following addition of 12.5 μM NZX or 10 ng/ml LPS as positive control. QUANTI-Blue™ assay was performed according to manufacturer's instructions (Invivogen, San Diego, USA). Briefly, supernatants from the cells were added to QUANTI-Blue™ substrate for one hour and absorbance was measured at 620 nm. In parallel, the ATPlite™ assay was performed to ensure that the activation was not due to toxicity.
2.6. Protease sensitivity assay
NZX (1 μg) was incubated at 37 °C with human neutrophil elastase (HNE, 20 μg/ml, 29 units/mg; Calbiochem (La Jolla, CA)), Cathepsin G (20 μg/ml, EMD Millipore) and human α-thrombin (20 μg/ml Innovative research) for 6 h. The materials were analysed on 10–20% precast SDS-PAGE Tris-Tricine gels (Life Technologies) and stained with Coomassie Blue R-250.
2.7. MIC
To assess NZX for anti-mycobacterial activity we measured the minimal inhibitory concentration (MIC) against three strains of M. tuberculosis (H37Rv and the clinical isolate 1 (TB1298) and the clinical MDR isolate (TB4001)) using the MGIT960-culture system (BACTEC MGIT 960, Becton Dickinson, Franklin Lakes, NJ, USA) following previously validated methods [28]. Briefly, NZX diluted in PBS was added to MGIT960-culture tubes in increasing log2-concentrations. M. tuberculosis in log phase (0.5 McFarland, ∼1.5 × 108 CFU/ml) were added to the MGIT960-tubes and the lowest NZX concentration with no detected growth was determined as the MIC using a MGIT-tube with bacteria diluted 1:100 as growth control. The MIC was determined as the antibiotic concentration where there was less growth compared to 1:100 or 1:10 diluted controls of corresponding strain, i.e. the lowest concentration that inhibited >99% or >90% of the bacterial population respectively. The MIC-determinations for all strains were performed twice on separate occasions.
2.8. Time kill assay
BCG was grown to logarithmic phase (103 CFU/ml) and treated with NZX (0.8, 1.6 and 3.2 μM). Duplicate samples were taken daily from treated bacteria and the non-treated bacteria (negative control). 0.1% n-decyl aldehyde (Decanal, Sigma) was added and bioluminescence was measured as RLU for 1s using a TriStar2 microplate reader (Berthold Technologies). The results are representative of two biological repeats.
2.9. Intracellular MIC
Infection of primary human macrophages was done with a protocol modified from a previously described method [29,30]. Briefly, macrophages and M. tuberculosis H37Rv strain expressing m-Cherry [27] were mixed in a tube at a multiplicity of infection (MOI) of 1:1 and seeded in 384-well plates. Isoniazid at a concentration of 0.1 mg/ml (0.7 μM) was used as a positive control. After 6 days of incubation, the infected cells were fixed with paraformaldehyde, stained with nuclear stain DAPI and analysed using ImageXpress (Molecular Devices). Bacterial numbers were estimated by enumerating the particles with red fluorescence in the obtained images.
2.10. Murine treatment model
All animal procedures were performed under the license issued by the UK Home Office and in accordance with the Animal Scientific Procedures Act of 1986. Six to eight-week-old female BALB/c mice (Charles River Ltd, UK) were maintained in biosafety containment level 3 (BSL3) facilities at Imperial College London, London, United Kingdom according to institutional protocols [31]. Mice were infected with 7 × 103 CFU/ml of M. tuberculosis H37Rv via the intranasal route (control group (n = 15, plus 3 mice to check bacterial numbers implanted in the lungs on day 2), NZX group (n = 5) and rifampicin group (n = 5). The experiment was repeated twice. Two days after infection, 3 control mice were euthanized to determine the actual dose implanted in the lungs. Five mice from the control group were euthanized prior to the start of treatment in order to determine the bacterial load in the lungs. Following 19 days of infection, the NZX groups were treated for five consecutive days with 0.83 mg NZX (33 mg/kg) diluted in 50 μl PBS by intra-tracheal administration. The control group received 50 μl PBS by the same route. As an additional treatment control, five mice were dosed intra-tracheally with rifampicin at a concentration of 20 mg/kg for 5 days. An additional group of mice (n = 5) was treated with gold-labelled NZX. Following treatment, mice were culled and the lungs were aseptically removed. The left lobe of the lung was placed in 10% buffered formalin for 24 h, for histology. The remaining tissue were homogenized in PBS containing 0.05% Tween-80, serially diluted and plated on Middlebrook 7H11 agar plates supplemented with 0.5% glycerol and 10% OADC. The number of CFU from all mice was enumerated 21 days later.
2.11. Histology and immunohistochemistry
Formalin fixed tissue was transferred to 70% ethanol overnight, then embedded and frozen in optimal cutting temperature compound (Sakura Finetek USA) for cryosectioning (8 μm; Leica microtome). Sections were collected on positively charged microscope slides (Superfrost/Plus, Thermo Fisher Scientific), fixed in acetone-methanol (1:1, 10 min), dried, permeabilized (0.2% Triton X-100, 5% normal goat serum/PBS), and stained with primary rat anti-neutrophil antibody (NIMP-R14) (1:200; Abcam, ab2557), rabbit monoclonal anti–M. tuberculosis antibody (1:100; Lionex, NB200-579) and mouse anti-neutrophil (1:50; Abcam, ab119352), followed by Alexa 488 or Alexa 568–labelled rabbit anti-rat or goat anti-mouse immunoglobulin G secondary antibodies (Molecular Probes; A-21210, A-11001, and A-11011). Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole) (0.05 mM; Sigma-Aldrich). Slides were examined by fluorescence microscopy (AX60, Olympus Optical). Richard-Allan Scientific Signature Series Hematoxylin 7211 and Eosin-Y 7111 (Thermo Scientific) were used to counterstain the tissue sections.
2.12. Transmission electron microscopy
Lung samples from infected mice treated with gold-labelled NZX and gold treated controls were embedded in Epon 812 resin according to routine protocols [32]. Specimens were observed in a Philips/FEI CM100 transmission electron microscope (Philips, Eindhoven, Holland) operated at 80 kV accelerating voltage, and images were recorded with a side-mounted Olympus Veleta camera (Olympus, Münster, Germany) with a resolution of 2048 × 2048 pixels.
2.13. Statistical analysis
Graphs and statistics were generated using the Prism software (version 6.1). Significance, where indicated, was calculated using the unpaired Student's t-test or ANOVA. For the bacterial growth inhibition screening tests, ANOVA followed by Dunnett's multiple comparison between NZX and the other peptides were performed. For the murine experiments based on two groups, untreated and treated, we analysed first the results for normal distribution (Shapiro-Wilk test) and then performed Student's t-test as recommended by Festing et al. and Morgan et al., [33,34]. For the murine experiment comparing three groups, untreated and treated with NZX of rifampicin, we performed ANOVA followed by Dunnett's multiple comparison and Mann-Whitney between groups. Significance was accepted at *p < 0.05, **p < 0.01, or ***p < 0.001.
2.14. Study approval
The animal studies have been approved (PPL 70/7160 and 70/8653) by the Local Animal Welfare and Ethical Review Board (London, UK). The Local Ethical Review Board Dnr 2011/403 and 2014/35 approved the donation of blood from human volunteers for the in vitro studies (Lund, Sweden), and the Lund district court approved the control animal studies (Dnr M 7–15). The blood for monocyte isolation for the toxicity analysis was donated by healthy volunteers (Local Ethical Review Board Dnr 2011/403 and 2014/35). No personal data was collected from the volunteers and the blood was pooled for the isolation of the monocytes. For the intracellular assays, human donor blood was purchased from the blood bank of Linköping University hospitals and blood donors gave written informed consent for research use of the blood.
3. Results
3.1. NZX demonstrates anti-mycobacterial activity
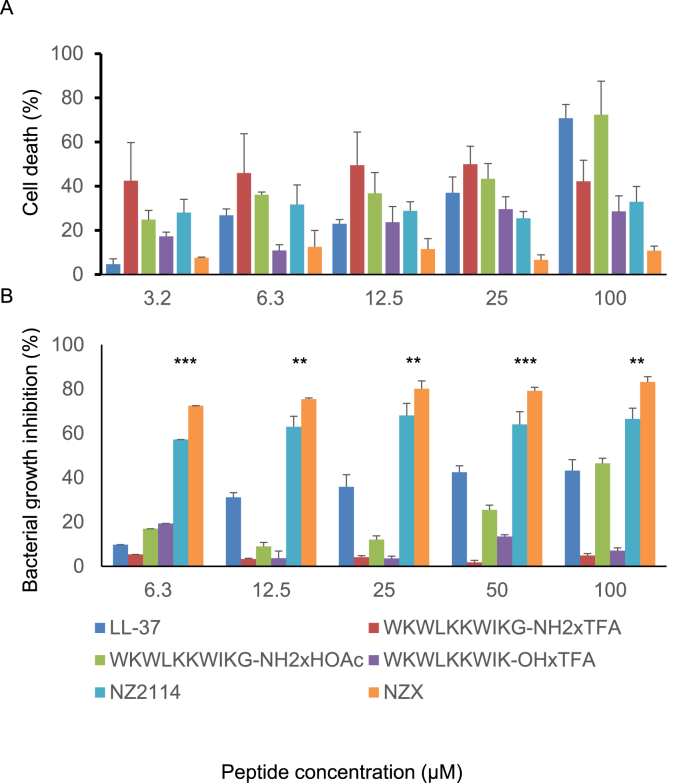
We investigated six peptides (Table 1) of which four were previously reported to possess activity against M. tuberculosis in vitro or in vivo [16] We evaluated peptide toxicity against primary human macrophages (Fig. 1A) and peptide dose response curves against BCG (Fig. 1B). Of the peptides tested, LL-37 is the best-known antimicrobial compound and was used as a benchmark for the other peptides. At the lowest concentration tested (6.3 μM) LL-37 inhibited up to 9.7% of mycobacteria growth (Fig. 1B) but was toxic at higher concentrations (Fig. 1A).
Fig. 1.
Screening analysis of the six peptides in the study. Different concentrations of the peptides were analysed for cell toxicity and for mycobacterial growth inhibition. (A) Cell toxicity was analysed with MTT on human primary macrophages and shown as percentage of untreated control. (B) Bacterial growth inhibition is shown as percentage of untreated bacteria. Inhibition was evaluated with recombinant BCG expressing luxAB and bioluminescence (RLU). All P values were calculated by ANOVA and post hoc with Dunnet's correction and shown for NZX and NZ2114 (*p < 0.05, **p < 0.01, ***p < 0.001). Experiments were repeated three times for each peptide.
The peptide WKWLKKWIKG, previously shown to kill mycobacteria [14], was tested in three different versions (Table 1). Of these, WKWLKKWIKG-NH2xHOAc showed the best activity. At a concentration of 6.3 μM, this peptide inhibited 19.2% of the mycobacterial growth, showing dose-dependent inhibition (Fig. 1B). The toxicity analysis revealed that peptide version WKWLKKWIKG-OHxTFA was least toxic to primary macrophages, but bacterial killing capacity was overall lower than that of WKWLKKWIKG-NH2xHOAc. The peptides WKWLKKWIKG-NH2xHOAc and WKWLKKWIKG-NH2xTFA showed dose-dependent toxicity to macrophages, similar to LL-37 (Fig. 1A).
The best antimicrobial activity was obtained with the plectasin derivatives NZ2114 and NZX, which both possessed high mycobacterial inhibitory capacity after 24 h of incubation (Fig. 1B). Furthermore, the toxicity analysis revealed that NZX was less toxic to human cells than LL-37 or the W-rich peptides (Fig. 1A). Comparing the two plectasin derivatives, NZX was the most effective as this peptide inhibited up to 74% of the mycobacterial growth at a concentration of 6.3 μM (Fig. 1B).
3.2. NZX inhibits M. tuberculosis
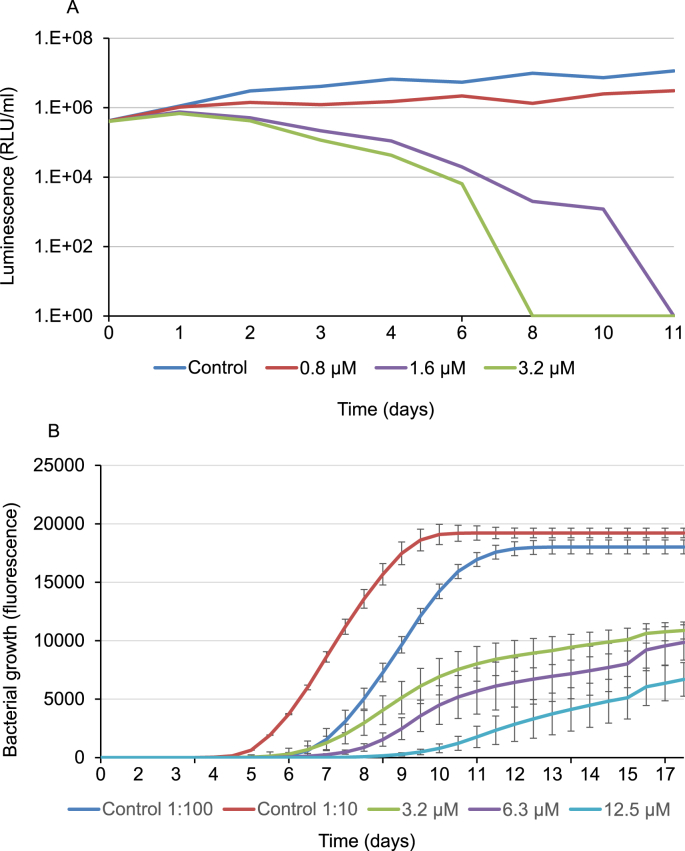
M. tuberculosis H37Rv and three clinical isolates were treated with NZX. The median 99% MIC concentrations were 6.3 μM for H37Rv, 6.3 μM and 3.2 μM for two clinical M. tuberculosis isolates (clinical isolate 1 and 2) and 6.3 μM for the clinical MDR isolate (Table 2). The MIC concentrations for BCG and M. smegmatis were also 6.3 μM. The potency of NZX compared to front-line drugs, ethambutol, rifampicin and isoniazid are shown in Supplementary Table 1. The time kill assay revealed that a single dose of NZX at 1.6 or 3.2 μM killed BCG at days eleven and eight respectively (Fig. 2A). This assay also indicated that low doses of NZX could kill BCG. The influence of NZX on mycobacterial growth was further analysed by growth kinetics of NZX treated M. tuberculosis H37Rv (Fig. 2B). NZX treatment induced concentration-dependent reduction of initial bacterial growth.
Table 2.
NZX MIC values determined for different mycobacteria spp. and clinical isolates.
| MIC (μM)a |
||
|---|---|---|
| Mean (±SD) | ||
| H37Rv | 6.3 | 3.4 |
| Clinical isolate 1 | 5.3 | 1.8 |
| Clinical isolate 2 | 3.2 | 0 |
| Clinical MDR isolate | 6.3 | 0 |
| M. smegmatis | 6.3 | 0 |
| BCG | 6.3 | 4.7 |
Experiments were repeated three times for each strain.
Fig. 2.
Effect of NZX on growth kinetics of M. tuberculosis. (A) Time-kill assay. BCG treated once with 0.8, 1.6 or 3.2 μM NZX at day 0. Graph depict relative luminescence units (RLU) after sampling each condition twice per time point. (B) Graph depicts growth kinetics of M. tuberculosis H37Rv using the MGIT960-culture system for a period of 18 days. Bacteria were treated once with 3.2, 6.3 or 12.5 μM NZX at day 0. Data depicts an average of three replicates.
3.3. NZX is not toxic to human cells
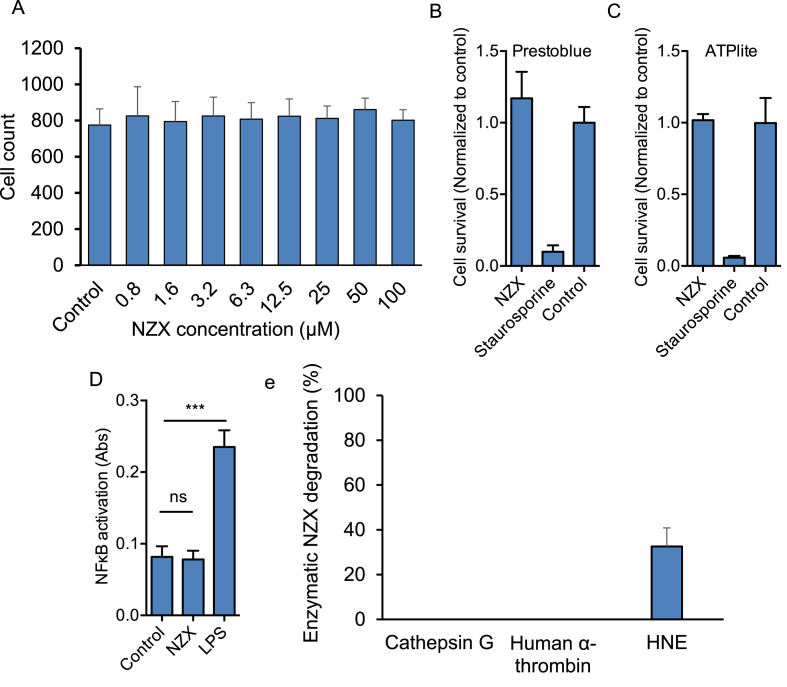
As several AMPs have been reported as toxic in vitro [35], we further investigated if NZX showed cytotoxic effects on human cells. No toxicity was detected for NZX concentrations up to 100 μM in any of the three assays (Fig. 3A–C). In addition, analysis of NF-κB activation after exposure of a monocyte cell line to NZX, revealed that the peptide does not induce inflammation (Fig. 3D).
Fig. 3.
NZX characteristics. (A) Concentration related MTT cytotoxicity analysis of NZX in M. tuberculosis H37Rv-infected primary macrophages measured 6 days post treatment. Cytotoxicity assays of NZX (100 μM) treated primary macrophages as determined by (B) ATPlite and (C) Prestoblue. (D) Inflammatory response was measure by NF-κB-activation in monocytes after addition of NZX (12.5 μM) or 10 ng/ml LPS (mean +sd, N = 3, ***p < 0.001, ns = not significant.). (E) Enzymatic degradation of NZX by the human proteases Cathepsin G, alpha-Thrombin and Human Neutrophil Elastase (HNE) after six hours of incubation. Results are depicted as mean ± 95% CI of three independent experiments.
3.4. NZX is resistant to degradation by proteases
A major barrier limiting the clinical application of AMPs is their susceptibility to degradation in biological fluids, by proteases such as the neutrophil elastase [36]. To investigate NZX stability, the peptide was incubated with human neutrophil elastase (HNE), cathepsin G and human α-thrombin. Of the investigated proteases, only HNE at a concentration of 20 μg/ml degraded the NZX peptide, with approximately 33% breakdown after 6 h (Fig. 3E).
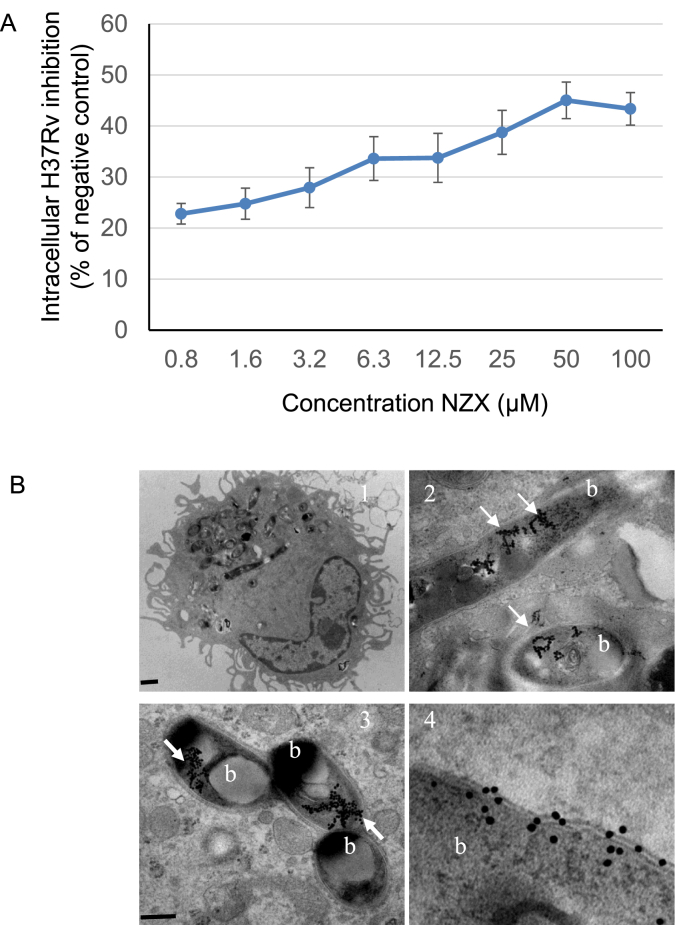
3.5. NZX induces intracellular killing of virulent M. tuberculosis
Intracellular anti-mycobacterial capacity of NZX was determined after 6 days using primary human macrophages infected with M. tuberculosis H37Rv. NZX mediated a concentration-dependent reduction in intracellular bacterial load with a maximum of 45% at 50 μM (Fig. 4A). Isoniazid (0.1 μg/ml, 0.7 μM), used as a positive control, resulted in the same intracellular killing as 50 μM of NZX (not shown). For in vivo experiments, NZX was labelled with gold particles and used to treat M. tuberculosis H37Rv infected mice. TEM visualization of sections from murine lungs demonstrated the presence of NZX on M. tuberculosis within macrophages (Fig. 4B). Gold particles without NZX was used as control to treat M. tuberculosis infected mice, but no gold particles were found in the lungs (data not shown).
Fig. 4.
(A) Intracellular NZX activity analysed in M. tuberculosis infected human macrophages. Results shown are from two separate experiments using two different donors. The positive control was isoniazid. The intracellular MIC is calculated by the comparing the number of bacteria per cell. (B) Lung sections from infected mice treated with gold-labelled NZX peptide (arrows) around M. tuberculosis H37Rv (marked b) in lung macrophages. Scale bars: 1 = 2 μm; 2 and 3 = 500 nm; 4 = 200 nm.
3.6. NZX treatment efficacy in the M. tuberculosis mouse infection model
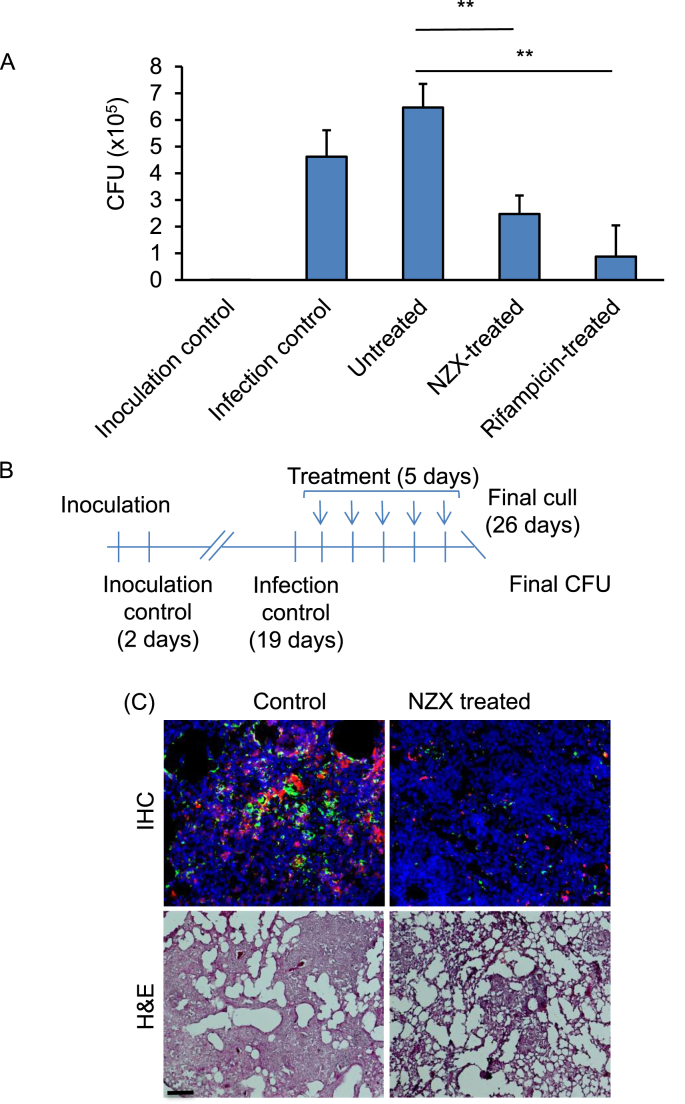
Bactericidal activity experiments were performed in a murine TB model with M. tuberculosis H37Rv and repeated two times [31] (Fig. 5A and B). The mean bacterial implantation dose in the lungs, measured two days after infection, was 677 CFU/ml, and the animals received five doses of NZX or rifampicin through intra-tracheal administration. In both experiments, we observed an CFU reduction by 46% after five days (Fig. 5A, p = 0.0079) in the lungs of mice treated with NZX compared to the control animals. Comparing the untreated group with NZX group or rifampicin group, we found significant differences (p < 0.001). Both rifampicin and NZX were significantly lower compared to untreated control (p = 0.0079 in both), and we found no significant difference between rifampicin and NZX treatment (p = 0.0556).
Fig. 5.
Treatment efficacy of NZX. (A) Daily endotracheal administration of NZX or rifampicin for days reduced lung CFU. Results showing one representative of two independent experiments. Data are presented as mean ± sd. All P values were calculated by unpaired Student's t-test, Mann-Whitney or ANOVA (**p < 0.01). (B) Schematic representation of experimental setup for murine pulmonary TB with M. tuberculosis H37Rv. (C) Representative immunohistochemistry (IHC) and eosin (H&E) staining showing lung sections from M. tuberculosis H37Rv infected or control mice. Neutrophil infiltration (red) and bacteria (green) is abundant in untreated mice. H&E staining of untreated lungs showed tissue destruction and granuloma formation. Mice treated for five days with NZX showed lower counts of both neutrophils and bacteria in the lungs. H&E of treated lungs showed decreased tissue destruction. Scale bar 50 μm.
3.7. NZX preserves alveolar structure during acute tuberculosis
NZX treatment abrogated tissue destruction in infected mice as shown by immunohistochemistry (Fig. 5C). Lung tissue sections from NZX treated M. tuberculosis-infected mice showed less tissue damage, with lower bacterial and neutrophil counts than infected controls. Reduced inflammation and preserved alveolar structure was further confirmed by hematoxylin and eosin staining, which showed cellular infiltrates and consolidation of the lung in infected but untreated lungs, both of which were absent in the lungs of NZX-treated animals (Fig. 5C).
4. Discussion
The present study has identified a non-human peptide, NZX, that inhibits M. tuberculosis in vitro at concentrations comparable with standard anti-mycobacterial drugs [37]. Our murine TB model supports these observations, indicating that NZX may be useful as an adjunct therapy to treat TB. Of the peptides investigated, NZX had highest capacity to inhibit mycobacterial growth at a low concentration and was found to be non-toxic even at high concentrations. Our study challenge though previous publications on LL-37 and the synthetic variants of tryptophan-rich peptides that were all reported to effectively eliminate M. tuberculosis at low concentrations [14,16,20]. This discrepancy could result from the cell lines and species of mycobacteria used in different studies. We followed the toxicity guidelines and used primary cells [36,38], with additional toxicity assays on NZX. For antimicrobial activity, this peptide was assayed against M. tuberculosis H37Rv, but also three clinical isolates, of which one was resistant to the first-line antibiotics rifampicin and isoniazid. MIC values were similar for all species and strains investigated including the fast-growing M. smegmatis and the bovine vaccine strain BCG.
Antimicrobial peptides are generally easily degradable, which could pose a treatment problem, but we demonstrated that NZX was not easily degraded by a range of proteases. In addition, NZX reduced the burden of M. tuberculosis in the lungs of infected mice, further evidence that NZX is not readily degraded in these compartments. Interestingly, Grosset et al. investigated early bactericidal activity of the first-line TB drugs [39], and showed that after two days of rifampin/isoniazid/pyrazinamide/ethambutol treatment the mean CFU counts were reduced by 0.25 log10 on day 2 and by 0.96 log10 on day 7. Applying the same calculations to our data, we observed a 0.45 log10 reduction of M. tuberculosis in the lungs after five days of NZX-treatment compared with the untreated mice. No statistical difference was obtained between NZX and rifampicin treated mice in our study, but because this is a short-term treatment we cannot distinguish between NZX-mediated killing of bacteria and suppression of bacterial growth in vivo. However, these data suggest that NZX could be a useful addition to the current drug regimen used to treat TB.
During the different stages of infection M. tuberculosis survives both intracellularly and extracellularly [40]. Susceptibility to first-line anti-TB drugs correlates poorly for M. tuberculosis residing in macrophages compared to extracellular bacteria [41,42]. In our study, NZX mediated a concentration-dependent reduction of intracellular M. tuberculosis and we observed gold-labelled NZX inside alveolar macrophages from M. tuberculosis infected mice. However, the antimycobacterial mechanism of NZX is yet unknown. NZ2114 and NZX contain the same cysteine residues as plectasin suggesting structural similarity. These peptides also share histidine residues with plectasin. At physiological conditions, these histidines are largely unprotonated and uncharged with weak lytic properties [43]. However, NZ2114 and NZX could kill mycobacteria by other mechanisms, as plectasin was found to specifically target cell wall precursors in Staphylococcus aureus by binding to the peptidoglycan precursor lipid II N-terminal amino groups [44]. Taken together with the toxicity data, NZX appears to be able to target intracellular bacteria without lysing human cells. We also observed that NZX by reducing bacterial load in the lungs of infected mice, dampened leukocyte recruitment, which probably lead to the observed preserved tissue integrity. Whether NZX has immunomodulatory effect, in addition to its activity towards M. tuberculosis, needs further investigation.
In this study, we present a novel AMP that effectively kills M. tuberculosis in vitro and decreases the bacterial load in a murine TB infection model. The MIC and therapeutic dosage were comparable in their effectiveness to conventional TB-antibiotics, with no evidence of toxicity in our model systems. In addition, we found evidence that NZX has intracellular activity towards M. tuberculosis, further supporting NZX as a possible future treatment candidate.
Conflicts of interest
All authors declare no conflict of interest.
Acknowledgements
GG conceived and designed the study. ET, NK, AR, SK, MM, MP, MD, MO and IG-M developed the methodology and acquired data. GG, ET, NK, ML and BR analysed and interpreted the data. GG, ET, BR, NK, AS, ML and ES wrote, reviewed, and/or revised the manuscript. NA provided administrative support. GG and ET supervised the study. All authors contributed significantly to the final version of the manuscript.
NK, IGM and BR thank the UK Medical Research Council for support in the MRC Centre for Molecular Bacteriology and Infection, Imperial College London. The research was funded by the Swedish Heart-Lung Foundation (20150733), Alfred Österlunds Foundation, Royal Physiographic Society of Lund, Swedish Research Council and European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no 604182, FORMAMP-Innovative Nanoformulation of Antimicrobial Peptides to Treat Bacterial Infectious Diseases (http://ec.europa.eu.research). The funding sources had no involvement in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tube.2018.10.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO. reportGlobal tuberculosis report 2017. http://wwwwhoint/tb/publications/global_report/en/. 2017;World Health Organization.
- 2.Zumla A., Chakaya J., Centis R., D'Ambrosio L., Mwaba P., Bates M. Tuberculosis treatment and management–an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir Med. 2015;3(3):220–234. doi: 10.1016/S2213-2600(15)00063-6. [DOI] [PubMed] [Google Scholar]
- 3.Koul A., Arnoult E., Lounis N., Guillemont J., Andries K. The challenge of new drug discovery for tuberculosis. Nature. 2011;469(7331):483–490. doi: 10.1038/nature09657. [DOI] [PubMed] [Google Scholar]
- 4.Adane K., Ameni G., Bekele S., Abebe M., Aseffa A. Prevalence and drug resistance profile of Mycobacterium tuberculosis isolated from pulmonary tuberculosis patients attending two public hospitals in East Gojjam zone, northwest Ethiopia. BMC Publ Health. 2015;15:572. doi: 10.1186/s12889-015-1933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jassal M., Bishai W.R. Extensively drug-resistant tuberculosis. Lancet Infect Dis. 2009;9(1):19–30. doi: 10.1016/S1473-3099(08)70260-3. [DOI] [PubMed] [Google Scholar]
- 6.Kieser K.J., Baranowski C., Chao M.C., Long J.E., Sassetti C.M., Waldor M.K. Peptidoglycan synthesis in Mycobacterium tuberculosis is organized into networks with varying drug susceptibility. Proc Natl Acad Sci U S A. 2015;112(42):13087–13092. doi: 10.1073/pnas.1514135112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3(9):710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 8.Hilchie A.L., Wuerth K., Hancock R.E. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol. 2013;9(12):761–768. doi: 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- 9.Yeaman M.R., Yount N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55(1):27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 10.Wimley W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol. 2010;5(10):905–917. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linde C.M., Hoffner S.E., Refai E., Andersson M. In vitro activity of PR-39, a proline-arginine-rich peptide, against susceptible and multi-drug-resistant Mycobacterium tuberculosis. J Antimicrob Chemother. 2001;47(5):575–580. doi: 10.1093/jac/47.5.575. [DOI] [PubMed] [Google Scholar]
- 12.Miyakawa Y., Ratnakar P., Rao A.G., Costello M.L., Mathieu-Costello O., Lehrer R.I. In vitro activity of the antimicrobial peptides human and rabbit defensins and porcine leukocyte protegrin against Mycobacterium tuberculosis. Infect Immun. 1996;64(3):926–932. doi: 10.1128/iai.64.3.926-932.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Z., Higgins M.P., Whitehurst J., Kisich K.O., Voskuil M.I., Hodges R.S. Anti-tuberculosis activity of alpha-helical antimicrobial peptides: de novo designed L- and D-enantiomers versus L- and D-LL-37. Protein Pept Lett. 2011;18(3):241–252. doi: 10.2174/092986611794578288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramon-Garcia S., Mikut R., Ng C., Ruden S., Volkmer R., Reischl M. Targeting Mycobacterium tuberculosis and other microbial pathogens using improved synthetic antibacterial peptides. Antimicrob Agents Chemother. 2013;57(5):2295–2303. doi: 10.1128/AAC.00175-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson C.S., Kloos Z., Murray B., Tabe E., Gupta M., Kwak J.H. Combined bioinformatic and rational design approach to develop antimicrobial peptides against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2016;60(5):2757–2764. doi: 10.1128/AAC.00940-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivas-Santiago B., Castaneda-Delgado J.E., Rivas Santiago C.E., Waldbrook M., Gonzalez-Curiel I., Leon-Contreras J.C. Ability of innate defence regulator peptides IDR-1002, IDR-HH2 and IDR-1018 to protect against Mycobacterium tuberculosis infections in animal models. PloS One. 2013;8(3) doi: 10.1371/journal.pone.0059119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao W., Kim J.Y., Anderson J.R., Akopian T., Hong S., Jin Y.Y. The cyclic peptide ecumicin targeting ClpC1 is active against Mycobacterium tuberculosis in vivo. Antimicrob Agents Chemother. 2015;59(2):880–889. doi: 10.1128/AAC.04054-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mygind P.H., Fischer R.L., Schnorr K.M., Hansen M.T., Sonksen C.P., Ludvigsen S. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437(7061):975–980. doi: 10.1038/nature04051. [DOI] [PubMed] [Google Scholar]
- 19.Xiong Y.Q., Hady W.A., Deslandes A., Rey A., Fraisse L., Kristensen H.H. Efficacy of NZ2114, a novel plectasin-derived cationic antimicrobial peptide antibiotic, in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55(11):5325–5330. doi: 10.1128/AAC.00453-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres-Juarez F., Cardenas-Vargas A., Montoya-Rosales A., Gonzalez-Curiel I., Garcia-Hernandez M.H., Enciso-Moreno J.A. LL-37 immunomodulatory activity during Mycobacterium tuberculosis infection in macrophages. Infect Immun. 2015;83(12):4495–4503. doi: 10.1128/IAI.00936-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivas-Santiago B., Rivas Santiago C.E., Castaneda-Delgado J.E., Leon-Contreras J.C., Hancock R.E., Hernandez-Pando R. Activity of LL-37, CRAMP and antimicrobial peptide-derived compounds E2, E6 and CP26 against Mycobacterium tuberculosis. Int J Antimicrob Agents. 2013;41(2):143–148. doi: 10.1016/j.ijantimicag.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Padhi A., Sengupta M., Sengupta S., Roehm K.H., Sonawane A. Antimicrobial peptides and proteins in mycobacterial therapy: current status and future prospects. Tuberculosis (Edinb) 2014;94(4):363–373. doi: 10.1016/j.tube.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Silva J.P., Appelberg R., Gama F.M. Antimicrobial peptides as novel anti-tuberculosis therapeutics. Biotechnol Adv. 2016;34(5):924–940. doi: 10.1016/j.biotechadv.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Sandvang D, Mygind PH, Jones ME, Sahm DF, Kristensen H. 47th Intersci. Conf. Antimicrob. Agents Chemother. 2007:F1-F1663.
- 25.Andes D., Craig W., Nielsen L.A., Kristensen H.H. In vivo pharmacodynamic characterization of a novel plectasin antibiotic, NZ2114, in a murine infection model. Antimicrob Agents Chemother. 2009;53(7):3003–3009. doi: 10.1128/AAC.01584-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snewin V.A., Gares M.P., Gaora P.O., Hasan Z., Brown I.N., Young D.B. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect Immun. 1999;67(9):4586–4593. doi: 10.1128/iai.67.9.4586-4593.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin C.J., Booty M.G., Rosebrock T.R., Nunes-Alves C., Desjardins D.M., Keren I. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe. 2012;12(3):289–300. doi: 10.1016/j.chom.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturegard E., Angeby K.A., Werngren J., Jureen P., Kronvall G., Giske C.G. Little difference between minimum inhibitory concentrations of Mycobacterium tuberculosis wild-type organisms determined with BACTEC MGIT 960 and Middlebrook 7H10. Clin Microbiol Infect. 2015;21(2) doi: 10.1016/j.cmi.2014.08.021. 148 e5-7. [DOI] [PubMed] [Google Scholar]
- 29.Kalsum S., Braian C., Koeken V., Raffetseder J., Lindroth M., van Crevel R. The cording phenotype of Mycobacterium tuberculosis induces the formation of extracellular traps in human macrophages. Front Cell Infect Microbiol. 2017;7:278. doi: 10.3389/fcimb.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raffetseder J., Pienaar E., Blomgran R., Eklund D., Patcha Brodin V., Andersson H. Replication rates of Mycobacterium tuberculosis in human macrophages do not correlate with mycobacterial antibiotic susceptibility. PloS One. 2014;9(11):e112426. doi: 10.1371/journal.pone.0112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquina-Castillo B., Garcia-Garcia L., Ponce-de-Leon A., Jimenez-Corona M.E., Bobadilla-Del Valle M., Cano-Arellano B. Virulence, immunopathology and transmissibility of selected strains of Mycobacterium tuberculosis in a murine model. Immunology. 2009;128(1):123–133. doi: 10.1111/j.1365-2567.2008.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stahl A.L., Arvidsson I., Johansson K.E., Chromek M., Rebetz J., Loos S. A novel mechanism of bacterial toxin transfer within host blood cell-derived microvesicles. PLoS Pathog. 2015;11(2):e1004619. doi: 10.1371/journal.ppat.1004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Festing M.F., Altman D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002;43(4):244–258. doi: 10.1093/ilar.43.4.244. [DOI] [PubMed] [Google Scholar]
- 34.Morgan C.J. Use of proper statistical techniques for research studies with small samples. Am J Physiol Lung Cell Mol Physiol. 2017;313(5):L873–L877. doi: 10.1152/ajplung.00238.2017. [DOI] [PubMed] [Google Scholar]
- 35.Abedinzadeh M., Gaeini M., Sardari S. Natural antimicrobial peptides against Mycobacterium tuberculosis. J Antimicrob Chemother. 2015;70(5):1285–1289. doi: 10.1093/jac/dku570. [DOI] [PubMed] [Google Scholar]
- 36.Assays for predicting acute toxicity. Application of modern toxicology approaches for predicting acute toxicity for chemical defense: National Academies Press (US); 2015. [PubMed]
- 37.WHO. Treatment of tuberculosis - guidelines http://apps.who.int/iris/bitstream/10665/44165/1/9789241547833_eng.pdf2009 [fourth ed.].
- 38.Ekwall B, Silano V, Paganuzzi-Stammati A, Zucc F. Toxicity tests with mammalian cell cultures. Short-term toxicity tests for non-genotoxic effects: John Wiley & Sons Ltd; 1990. p. 75-93.
- 39.Grosset J., Almeida D., Converse P.J., Tyagi S., Li S.Y., Ammerman N.C. Modeling early bactericidal activity in murine tuberculosis provides insights into the activity of isoniazid and pyrazinamide. Proc Natl Acad Sci U S A. 2012;109(37):15001–15005. doi: 10.1073/pnas.1203636109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warner D.F., Mizrahi V. The survival kit of Mycobacterium tuberculosis. Nat Med. 2007;13(3):282–284. doi: 10.1038/nm0307-282. [DOI] [PubMed] [Google Scholar]
- 41.Hartkoorn R.C., Chandler B., Owen A., Ward S.A., Bertel Squire S., Back D.J. Differential drug susceptibility of intracellular and extracellular tuberculosis, and the impact of P-glycoprotein. Tuberculosis (Edinb) 2007;87(3):248–255. doi: 10.1016/j.tube.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Aljayyoussi G., Jenkins V.A., Sharma R., Ardrey A., Donnellan S., Ward S.A. Pharmacokinetic-Pharmacodynamic modelling of intracellular Mycobacterium tuberculosis growth and kill rates is predictive of clinical treatment duration. Sci Rep. 2017;7(1):502. doi: 10.1038/s41598-017-00529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kashiwada A., Mizuno M., Hashimoto J. pH-Dependent membrane lysis by using melittin-inspired designed peptides. Org Biomol Chem. 2016;14(26):6281–6288. doi: 10.1039/c6ob01002d. [DOI] [PubMed] [Google Scholar]
- 44.Schneider T., Kruse T., Wimmer R., Wiedemann I., Sass V., Pag U. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science. 2010;328(5982):1168–1172. doi: 10.1126/science.1185723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.