Abstract
Purpose
The distinction of patients with symptomatic multiple myeloma (MM) from those with smoldering MM poses a challenge for researchers who use administrative databases. Historically, researchers either have included all patients or used treatment receipt as the distinguishing factor; both methods have drawbacks. We present an algorithm for distinguishing between symptomatic and smoldering MM using ICD-9-CM (International Classification of Diseases, Ninth Revision, Clinical Modification) codes for the classic defining events of symptomatic MM commonly referred to as the CRAB criteria (hypercalcemia, renal impairment, anemia, and bone lesions).
Patients and Methods
SEER-Medicare–linked data from 4,187 patients with MM diagnosed between 2007 and 2011 were used for this analysis.
Results
Eighty-four percent had ICD-9-CM codes consistent with CRAB criteria, whereas only 57% received treatment. Overall survival of patients with symptomatic MM defined as receipt of treatment was 32.3 months versus 26.6 months for the overall population and 22.9 months for patients with symptomatic MM defined by CRAB criteria. Conceptually, removal of patients with smoldering MM should result in a reduction in overall survival; however, the cohort of patients who received treatment tended to be younger and healthier than the overall population, which could have skewed the results.
Conclusion
The algorithm we present resulted in a larger and more representative sample than classification by treatment status and reduced potential bias that could result from including all patients with smoldering MM in the analysis. Although this study was performed using the SEER-Medicare database, the methodology was broad enough that the algorithm could be extended to additional claims-based data sets with relative ease.
INTRODUCTION
Multiple myeloma (MM) is a rare hematologic malignancy. An estimated 30,000 new cases will have been diagnosed in the United States in 2017.1 Like many less common cancers, the low incidence rate poses a challenge to researchers. Because each center sees only a relatively small number of patients with MM, population-based databases such as SEER allow for larger sample sizes and more generalizable findings than single-institution studies.
The use of population-based databases for MM research poses a specific challenge: the distinction of symptomatic MM from smoldering MM, which does not require treatment. In smoldering MM, aberrant plasma cells have begun to accumulate in the bone marrow but have not resulted in clinical symptoms or organ damage.2 An estimated 15% of patients with suspected MM are in the smoldering phase3; only approximately one half of these patients will progress to symptomatic MM over 5 years.4 Currently, treatment is not recommended for patients with smoldering MM, although research in ongoing to identify high-risk groups and to determine a possible role for early intervention before the development of symptomatic MM.
Because diagnostic codes do not distinguish between smoldering MM and symptomatic MM that requires treatment, previous population-based studies either have predominantly been in all patients with MM, including smoldering MM, or have used treatment administration as the defining factor, which assumes that all patients who do not receive treatment have smoldering MM.5-7 Both strategies have weaknesses. For example, the inclusion of patients with smoldering MM could underestimate associations between clinical features and outcomes. The exclusion of patients with MM who do not receive treatment may overestimate treatment effects at the population level. To address this methodological gap, we developed an algorithm to distinguish smoldering from symptomatic MM using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), claims data.
PATIENTS AND METHODS
The source of data for this study was the National Cancer Institute SEER-Medicare–linked database. We chose the SEER-Medicare database because it is a popular choice for researchers; however, we aimed to keep the methodology broad enough that the algorithm could be extended to additional claims-based data sets.
The SEER database collects demographic, tumor characteristic, and survival data from 18 cancer registries throughout the United States and covers approximately 26% of the US population.8 In the SEER-Medicare–linked database, the SEER registry data are linked to Medicare enrollment and claims data. The SEER-Medicare database has been described in detail elsewhere.9 At the time of this study, the SEER-Medicare linkage included all Medicare-enrolled people who appear in the SEER data through 2011 and their Medicare claims through 2013.
Inclusion and Exclusion Criteria
Eligible patients were diagnosed with MM between 2007 and 2011. Identification of MM was made using the WHO International Classification of Diseases for Oncology, Third Edition (histology code 9732).10 We excluded patients with duplicate or incomplete records, including those identified by death certificates or autopsy reports; those not enrolled in Medicare parts A, B, and D the 12 months before and after MM diagnosis; and those enrolled in managed care plans (ie, a health maintenance organization). We also excluded all patients who did not have one or more claims within 1 year before diagnosis because this is another indicator of a possible incomplete record. Patients diagnosed with MM before age 65 years were excluded. Medicare eligibility before age 65 years is restricted to those with severe illness or disability, which limits the generalizability of this subset of patients.
Variables
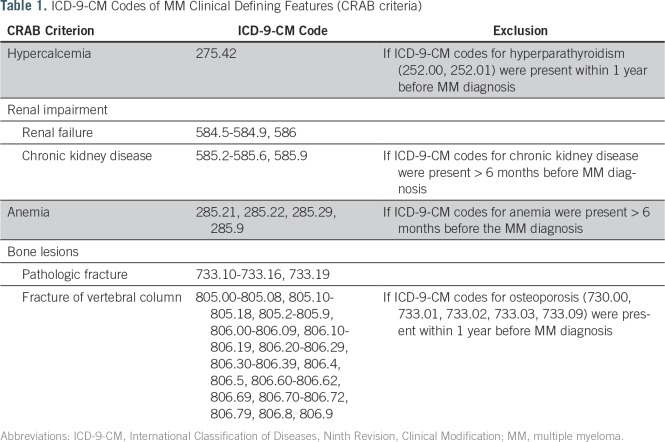
The classic distinguishing clinical features of symptomatic MM relative to smoldering MM are hypercalcemia, renal impairment, anemia, and bone lesions, otherwise known as CRAB criteria.2 To determine the presence or absence of these features, we extracted the relevant ICD-9-CM codes from all claims data, including inpatient (Medicare Provider and Analysis Review), outpatient, and provider (National Claims History) claims. Table 1 lists all the ICD-9-CM codes used in this study.
Table 1.
ICD-9-CM Codes of MM Clinical Defining Features (CRAB criteria)
These clinical features are not specific to MM. Hypercalcemia can be secondary to hyperparathyroidism, anemia to iron deficiency, chronic kidney disease to comorbidities such as hypertension and diabetes, and bone loss or fracture to osteoporosis. Therefore, we created an algorithm to exclude codes not related to MM on the basis of proximity to diagnosis and the presence of other possible causes. Only codes that occurred within the 6 months before and after the MM diagnosis were considered. We then excluded hypercalcemia if codes for hyperparathyroidism also existed and vertebral fractures if codes for osteoporosis also existed. We excluded codes for anemia and chronic kidney disease if the code originated > 6 months before MM diagnosis, which assumes that if these were not related to MM, their diagnostic codes would have been present before the 6 months that preceded MM diagnosis. A macro was developed by using SAS Enterprise Guide 5.1 (SAS Institute, Cary, NC) based on the commonly used SEER-Medicare Charlson comorbidity weights macro.11 A copy of the macro is included in the Data Supplement.
MM treatment was determined by using the Medicare claims for inpatient (Medicare Provider and Analysis Review), outpatient, provider (National Claims History), and prescription (part D) coverage using relevant ICD-9-CM and Healthcare Common Procedure Coding System codes for injectable drugs and generic names for prescription drugs. MM treatment was defined as receipt of any of the following within the 6 months after MM diagnosis: bortezomib, cyclophosphamide, doxorubicin, lenalidomide, melphalan, thalidomide, vincristine, unspecified antineoplastic chemotherapy or immunotherapy, or autologous or allogeneic stem-cell transplant. Radiation therapy and corticosteroids were not included as MM treatment because these were not considered adequate systemic therapy in the modern era but rather as symptom-directed therapy.
Analysis
We created three cohorts of patients: all with smoldering or symptomatic MM, symptomatic MM as determined by CRAB criteria, and symptomatic MM as determined by treatment receipt. Bivariable analyses were used to compare the characteristics of these three cohorts and included age; ethnicity; sex; Medicaid beneficiary status; and previously validated measures of overall health, such as performance status and Charlson comorbidity index (CCI).12-14 Overall survival, defined as the number of months from MM diagnosis to death as a result of any cause, was compared using Kaplan-Meier method with log-rank tests.
RESULTS
A total of 4,187 patients were included in the analyses. The median age was 76 years at MM diagnosis, and 77% of the cohort was white, 16% black, 7% another ethnicity, and 52% female. Thirty-two percent were Medicaid beneficiaries. Twenty-three percent had one or more indicators of poor performance status, and the mean CCI score was 1.9. At the time of the analysis, 68% of the patients had died (median estimated overall survival, 26.6 months; 95% CI, 25.2 to 28.1 months).
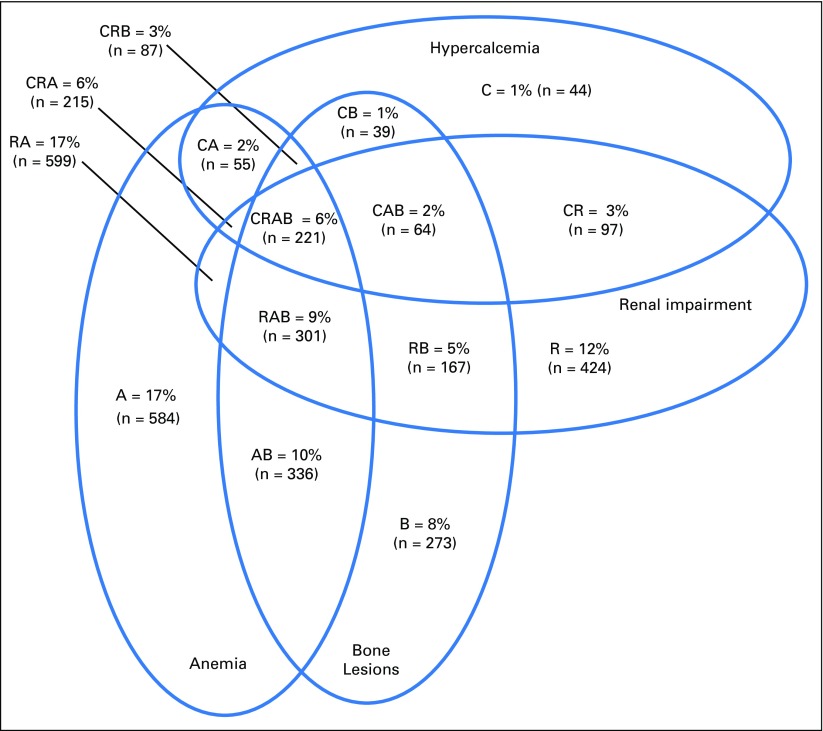
Eighty-four percent of patients (n = 3,506) had ICD-9-CM codes consistent with CRAB criteria. The most common CRAB criterion was anemia, which occurred in 57% of all patients. The least common criterion was hypercalcemia, which occurred in 20% of all patients. The majority of patients had more than one of the four possible CRAB criteria; only 5% had all four. Figure 1 shows a Venn diagram that displays the intersections of CRAB criteria.
Fig 1.
The intersections of CRAB symptoms (C, hypercalcemia; R, renal impairment; A, anemia; B, bone lesions) among patients with symptomatic multiple myeloma as defined by the newly established algorithm that uses International Classification of Diseases, Ninth Revision, Clinical Modification codes.
Fifty-seven percent of patients (n = 2,369) received MM treatment; 53% (n = 2,201) received a novel agent, including bortezomib, lenalidomide, and thalidomide. Of patients who received MM treatment, 87% met CRAB criteria. Conversely, only 59% of patients with CRAB criteria received MM treatment. Each of the four CRAB criteria had similar specificity for MM treatment. Sixty-one percent of patients with ICD-9-CM codes consistent with hypercalcemia received MM treatment, as did 57% with renal impairment, 62% with anemia, and 64% with bone lesions. Forty-five percent of patients without diagnostic codes consistent with CRAB criteria received MM treatment.
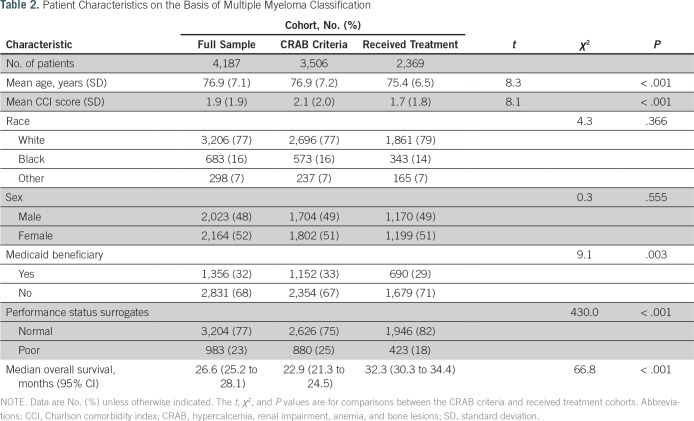
Compared with the cohort of patients with CRAB criteria, the cohort who received MM treatment tended to be younger (mean age, 75.4 v 76.9 years; P < .001), were less likely to be Medicaid beneficiaries (29% v 33%; P = .003), were less likely to have indicators of poor performance status (18% v 25%; P < .001), and had a lower CCI score on average (mean, 1.7 v 2.1; P < .001). The characteristics of the study cohorts are listed in Table 2.
Table 2.
Patient Characteristics on the Basis of Multiple Myeloma Classification
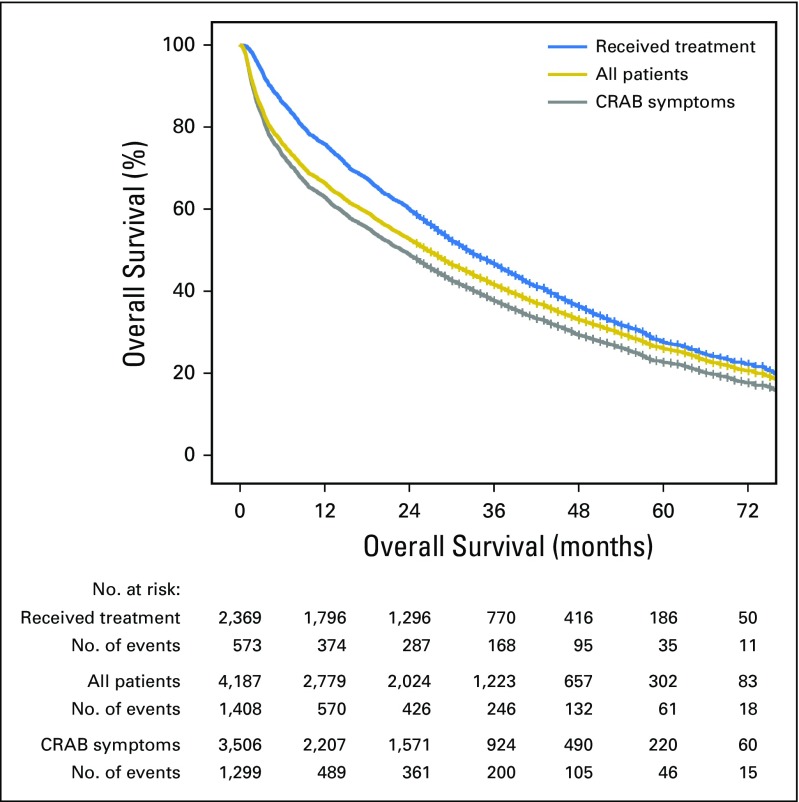
The estimated median overall survival of patients who received treatment was 32.3 months (95% CI, 30.3 to 34.4 months) versus 22.9 months for those with CRAB criteria (95% CI, 21.3 to 24.5 months; P < .001). Conversely, the estimated median overall survival of smoldering MM cases as determined by the absence of CRAB symptoms was 51.5 months (95% CI, 44.7 to 57.7 months) versus 16.4 months (95% CI, 13.0 to 19.0 months; P < .001) when smoldering MM was defined as a lack of treatment. Overall survival by cohort is shown in Figure 2.
Fig 2.
Overall survival by cohort. The median overall survival of all patients was 26.6 versus 22.9 months for those with CRAB criteria (hypercalcemia, renal impairment, anemia, and bone lesions) and 32.3 months for those who received treatment for multiple myeloma (MM; P < .001). Conceptually, the removal of patients with smoldering MM should be associated with reduced overall survival, but this was only true when smoldering MM was defined by CRAB criteria. The removal of patients with smoldering MM as defined by treatment receipt was associated with an improvement in overall survival.
DISCUSSION
On the basis of the algorithm we established, 16% of patients with MM were considered to have smoldering disease. In a study of the National Cancer Database, Ravindran et al3 estimated that 14% of patients with MM were classified as having smoldering disease on the basis of the treatment recommendations of the attending physician. A Swedish population study also reported a smoldering MM rate of 14%.15 Thus, our algorithm illustrates a prevalence of smoldering MM similar to that identified in other population-based studies.
Classification of symptomatic MM by CRAB criteria led to a larger sample size, and the demographics more closely resembled the overall population than classification by receipt of treatment. Conceptually, the removal of patients with smoldering MM should result in a symptomatic MM cohort with a lower overall survival compared with the original cohort. In this study, we found that classification by CRAB criteria did just that. Moreover, the overall survival of the patients with symptomatic and smoldering MM was similar to those previously published.3 Conversely, a distinction by treatment status resulted in a symptomatic MM cohort with improved overall survival compared with the smoldering MM and overall cohorts. Because older patients and those with more comorbidities and poorer performance status are less likely to receive treatment, the outcomes of patients who received treatment may not reflect the overall symptomatic MM population. For some studies, such as outcomes research that compares treatment regimens, the elimination of patients who do not receive MM treatment would be appropriate, but it should be done cautiously when estimating the effect of demographics or treatment regimens at the population level.
In this study, approximately one half of patients without CRAB ICD-9-CM codes received MM treatment. CRAB criteria possibly were undercoded in these patients. Providers who have already coded for myeloma in billing may not have believed it necessary to also code for CRAB criteria. In addition, these patients may have had some other manifestation of MM disease. Additional biomarkers, including high bone marrow–plasma cell percentage or light-chain ratio and lesions on magnetic resonance imaging of the spine, have been added as myeloma-defining events that warrant treatment initiation.16 Such findings on a patient’s work-up could have prompted a physician to treat despite the lack of CRAB criteria. Other nonspecific symptoms, such as peripheral neuropathy, also are used when making treatment recommendations but, again, are not captured under CRAB criteria. One center reported that 20% of patients treated for MM did not have the presence of any of the classic CRAB symptoms.17
To increase the sensitivity of the algorithm we present, treatment receipt can be added with CRAB criteria. In this study, 91% of the entire population had ICD-9-CM codes for CRAB criteria or receipt of MM treatment. This strategy was previously used in a study of monoclonal gammopathy of unknown significance complications in the SEER-Medicare data set. The researchers found a similar rate of patients with smoldering disease (15%), but the study included Waldenström macroglobulinemia and lymphoplasmacytic lymphoma in addition to MM; thus, the results are not directly comparable. Many MM regimens are administered orally on an outpatient basis; therefore, prescription information, which is absent from many administrative databases, is required to comprehensively integrate treatment receipt. Thus, our algorithm included only the CRAB criteria to maximize its utility and the generalizability of results.
Another finding of this study is that 41% of patients with symptomatic MM classified by CRAB criteria did not receive systemic treatment. A limitation of our work is the possibility that some of the patients with ICD-9-CM codes for CRAB criteria may have had smoldering MM and that the ICD-9-CM codes were for unrelated conditions (ie, hypertensive chronic kidney disease, a scenario in which a patient should not receive MM therapy). That said, we believe that the algorithm should minimize such cases and that the results imply that a large number of patients with symptomatic MM are being undertreated. Similar findings have been seen in older adults with other hematologic malignancies. Medeiros et al18 reported that approximately 50% of patients in the SEER-Medicare database diagnosed with acute myeloid leukemia did not receive chemotherapy.
Newer MM therapies, such as proteasome inhibitors and immunomodulatory drugs, have greater tolerability and efficacy than traditional cytotoxic chemotherapy, and the overwhelming majority of patients should be fit enough for these treatments; thus, these results in MM are surprising but not unfounded. Warren et al19 reported that < 50% of patients older than 60 years received novel agents in 2007. In the current study, we found that 54% of patients received novel therapies from 2007 to 2011, which suggests a marginal improvement. More research is needed to determine what factors are associated with undertreatment in MM.
Of note, we did not include radiation therapy or corticosteroids as systemic treatment because they alone are not considered systemic MM therapy per National Comprehensive Cancer Network guidelines.20 The inclusion of these therapies would have likely increased the proportion of patients considered to have received treatment. For studies that identify symptomatic MM on the basis of treatment receipt, the ambiguity of what constitutes treatment adds to the complexity of study design and may hinder interstudy comparisons.
Use of administrative databases such as SEER-Medicare for this type of analysis has several strengths, including the provision of a large sample size from a diverse geographic area, but their application is limited by possible incomplete or inaccurate data and the use of claims data as surrogates for important information (eg, the distinction between smoldering and symptomatic MM). The current study was performed in part to improve the application of such databases; still, inherent limitations exist. For example, CRAB criteria ICD-9-CM codes could be under- or over-reported as a result of miscoding in the claims data. ICD-9-CM codes have been shown to have only approximately 70% predictive value for bone metastases21; therefore, we excluded these codes from the algorithm. The predictive value of the included ICD-9-CM codes for their corresponding CRAB criteria is unclear, but unlike codes for bone metastases, the included codes are tied to reimbursement and, theoretically, have higher predictive values.22 In addition, biomarkers and nonmyeloma-specific symptoms that determine treatment recommendations often are not identifiable by claims codes. On the basis of these limitations, results from studies that use an administrative database should be interpreted with caution.
In conclusion, we present a methodology for distinguishing smoldering from symptomatic MM in claims-based data. This methodology results in a larger and more representative sample than classification by treatment status and reduces the potential bias that could result from including all patients with smoldering MM in the analysis. Although this study was performed by using the SEER-Medicare database, the methodology can be translated to other claims-based databases with relative ease.
Acknowledgment
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services; and the SEER program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Supported by National Cancer Institute Grant No. K12 CA167540. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or National Institutes of Health. The Center for Administrative Data Research is supported in part by Washington University Institute of Clinical and Translational Sciences Grant No. UL1 TR000448 from the National Center for Advancing Translational Sciences, Agency for Healthcare Research and Quality Grant No. R24 HS19455, and National Cancer Institute Grant No. KM1 CA156708.
AUTHOR CONTRIBUTIONS
Conception and design: Mark A. Fiala, James Dukeman, Sascha A. Tuchman, Tanya M. Wildes
Financial support: Tanya M. Wildes
Provision of study material or patients: Tanya M. Wildes
Collection and assembly of data: Mark A. Fiala, James Dukeman, Matt Keller, Sascha A. Tuchman, Tanya M. Wildes
Data analysis and interpretation: Mark A. Fiala, James Dukeman, Ravi Vij, Sascha A. Tuchman, Tanya M. Wildes
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwcorascopubs.org/jco/site/ifc.
Mark A. Fiala
No relationship to disclose
James Dukeman
No relationship to disclose
Sascha A. Tuchman
Honoraria: Takeda Pharmaceuticals, Celgene, Alnylam Pharmaceuticals
Speakers’ Bureau: Takeda Pharmaceuticals, Celgene
Research Funding: Takeda Pharmaceuticals (Inst), Celgene (Inst), Alnylam Pharmaceuticals (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Alnylam Pharmaceuticals, Karyopharm Therapeutics
Matt Keller
No relationship to disclose
Ravi Vij
Consulting or Advisory Role: Bristol-Myers Squibb, Celgene, Janssen Pharmaceuticals, Karyopharm Therapeutics, Jazz Pharmaceuticals, Amgen, Takeda Pharmaceuticals, AbbVie
Research Funding: Amgen, Takeda Pharmaceuticals, Celgene, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Celgene, Bristol-Myers Squibb, Sanofi, Janssen Pharmaceuticals, Dava Oncology, Karyopharm Therapeutics, Amgen, Takeda Pharmaceuticals, AbbVie
Tanya M. Wildes
Honoraria: Carevive Systems
Research Funding: Janssen Pharmaceuticals (Inst)
REFERENCES
- 1.National Cancer Institute : Cancer stat facts: Myeloma, 2017. https://seer.cancer.gov/statfacts/html/mulmy.html
- 2.International Myeloma Working Group : Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br J Haematol 121:749-757, 2003 [PubMed] [Google Scholar]
- 3.Ravindran A, Bartley AC, Holton SJ, et al. : Prevalence, incidence and survival of smoldering multiple myeloma in the United States. Blood Cancer J 6:e486, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyle RA, Remstein ED, Therneau TM, et al. : Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med 356:2582-2590, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Fiala MA, Wildes TM: Racial disparities in treatment use for multiple myeloma. Cancer 123:1590-1596, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winn AN, Shah GL, Cohen JT, et al. : The real world effectiveness of hematopoietic transplant among elderly individuals with multiple myeloma. J Natl Cancer Inst 107:djv139, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanfilippo KM, Keller J, Gage BF, et al. : Statins are associated with reduced mortality in multiple myeloma. J Clin Oncol 34:4008-4014, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Cancer Institute : About the SEER program, 2015. http://seer.cancer.gov/about
- 9.Warren JL, Klabunde CN, Schrag D, et al. : Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care 40:IV-3-IV-18, 2002(suppl 8) [DOI] [PubMed] [Google Scholar]
- 10.North American Association of Central Cancer Registries : Guidelines for ICD-O-3 implementation, 2015. http://www.facs.org/cancer/coc/naaccr.pdf
- 11.National Cancer Institute : Comorbidity SAS macro (2000 version), 2017. https://healthcaredelivery.cancer.gov/seermedicare/considerations/macro-2000.html
- 12.Griffiths R, Mikhael J, Gleeson M, et al. : Addition of rituximab to chemotherapy alone as first-line therapy improves overall survival in elderly patients with mantle cell lymphoma. Blood 118:4808-4816, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40:373-383, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Potosky AL, Legler JM, et al. : Development of a comorbidity index using physician claims data. J Clin Epidemiol 53:1258-1267, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Kristinsson SY, Holmberg E, Blimark C: Treatment for high-risk smoldering myeloma. N Engl J Med 369:1762-1763, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. : International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15:e538-e548, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Talamo G, Farooq U, Zangari M, et al. : Beyond the CRAB symptoms: A study of presenting clinical manifestations of multiple myeloma. Clin Lymphoma Myeloma Leuk 10:464-468, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Medeiros BC, Satram-Hoang S, Hurst D, et al. : Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol 94:1127-1138, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren JL, Harlan LC, Stevens J, et al. : Multiple myeloma treatment transformed: A population-based study of changes in initial management approaches in the United States. J Clin Oncol 31:1984-1989, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network : NCCN clinical practice guidelines in oncology: Multiple myeloma version 3.2017, 2016. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf [DOI] [PubMed]
- 21.Onukwugha E, Yong C, Hussain A, et al. : Concordance between administrative claims and registry data for identifying metastasis to the bone: An exploratory analysis in prostate cancer. BMC Med Res Methodol 14:1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Institute : Measures that are limited or not available in the data, 2017. https://healthcaredelivery.cancer.gov/seermedicare/considerations/measures.html#16